To the editor:
The human airway plays an essential role in oxygen exchange and is subjected to airborne environmental, allergen, and infectious exposures. These agents incite oxidative stress either directly or indirectly through recruitment of inflammatory cells which release reactive oxygen species1. Oxidative stress has been linked to disease severity of lower airway chronic inflammatory disorders such as asthma and COPD, however, its role in upper airway (sinonasal) chronic inflammatory disorders is less clear1–2. Interestingly, increased expression of cytoprotective enzymes has been reported in chronic rhinosinusitis (CRS) suggesting that oxidative stress may play a role in CRS pathophysiology3.
Nuclear erythroid 2 p45-related factor (Nrf2) is a transcription factor that upon activation invokes an anti-oxidant response pathway via nuclear translocation and up-regulation of cytoprotective genes4. Disruption of the Nrf2 pathway in mice has been reported to enhance susceptibility to Ovalbumin (Ova)-induced asthma5. Interestingly, activation of Nrf2 has recently been reported to stabilize sinonasal epithelial barrier function in vitro4, 6. We sought to determine whether sinonasal barrier stabilization through Nrf2 pathway activation could stabilize barrier function in vivo and reduce allergen-induced inflammation in a mouse model of rhinosinusitis.
Under normal circumstances, Nrf2 is sequestered in the cytoplasm and inhibited through binding Kelch-like ECH-associated protein 1 (Keap1)7. Disruption of this interaction results in Nrf2 activation and nuclear translocation. Therefore we tested whether Nrf2 activation via tamoxifen inducible Keap1flox/flox; ER-cre mice may reverse Ova-induced sinonasal barrier dysfunction. Indeed, deletion of Keap1 significantly reduced sinonasal barrier breakdown as assessed by serum albumin accumulation in lavage fluid and zonula occludens (ZO-1) cell surface localization on immunofluorescence (Figure 1a-b). Furthermore, genetic activation of the Nrf2 pathway through Keap1 deletion significantly reduced Ova-induced accumulation of eosinophils (Figure 1c-d) and goblet cell hyperplasia (Figure 1e-f). Details regarding the materials and methods may be found in this article’s online supporting information (Data S1).
Figure 1.
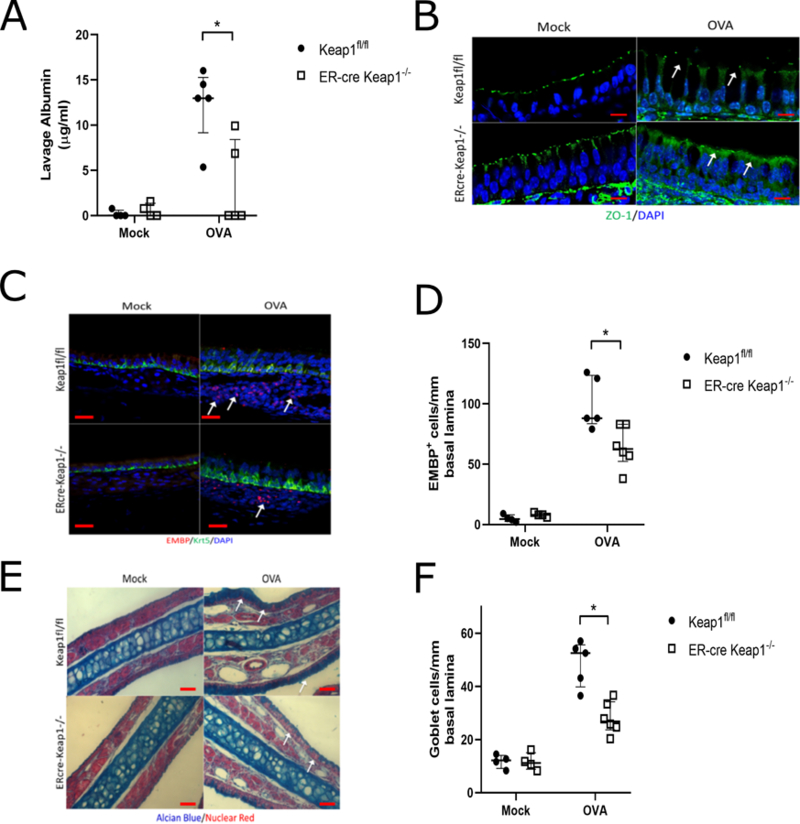
Keap1−/−; ER-cre mice demonstrate reduced barrier dysfunction in response to chronic Ova exposure. (A) Analysis of nasal lavage fluid for serum albumin (n= 4–6 per group). Mock is vehicle control. (B) Immunofluorescence for ZO-1 (green) demonstrates reduced barrier disruption. DAPI (blue). Images are at 100x magnification and scale bar is 10μm. (C) Immunofluorescence for eosinophil major basic protein (EMBP, red) and Keratin-5 (Krt5, green). White arrows indicate areas of increased eosinophil accumulation. Images are at 40x magnification and scale bar is 20μm. (D) Eosinophil counts per mm of basal lamina from imaging of the entire nasal septum (n= 4–6 per group). Goblet cell via Alcian blue stain (E-F) counts per mm of basal lamina (n= 4–6 per group). DAPI (blue). Images are at 20x magnification and scale bar is 50μm. * P<0.05, ** P<0.01, *** P<0.001, **** P<0.0001 Data are represented as median with interquartile ranges.
Activation of Nrf2 and stabilization of the sinonasal epithelial barrier can also be achieved through stimulation with the small molecule Sulforaphane (SFN)4. We therefore hypothesized that SFN treatment may limit Ova-induced sinonasal barrier disruption and sought to assess pre-clinical therapeutic potential. Wildtype mice were subjected to the Ova-induced model of chronic sinonasal inflammation and treated with SFN at a dose found to increase Nrf2 downstream target expression (Figure S1-S2). SFN administration significantly reduced Ova-induced accumulation of serum albumin in nasal lavage fluid as well as disruption of cell surface ZO-1 localization (Figure 2a-b). SFN administration also reduced disruption of cell surface epithelial cadherin (E-cadherin) localization although this improvement appeared to a lesser degree than ZO-1 (Figure 2c). SFN administration was also found to reduce eosinophil accumulation in sinonasal tissue (Figure 2d-e). Furthermore, Nrf2 activation via SFN treatment reduced eotaxin-1 levels in the nasal lavage fluid of these mice (Figure 2f).
Figure 2.
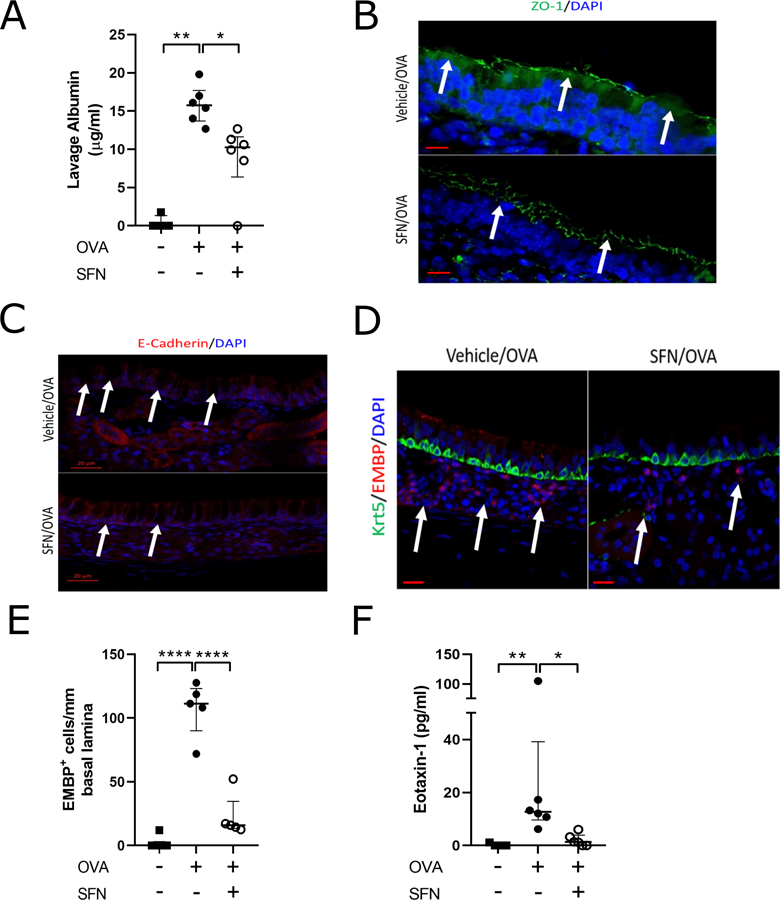
Nrf2 activation via Sulforaphane treatment reduces Ova-induced sinonasal barrier dysfunction. (A) Analysis of nasal lavage fluid for serum albumin (n= 4–6 per group). (B) Immunofluorescence for ZO-1 (green) demonstrates reduced barrier disruption. Images are at 100x magnification and scale bar is 10μm. (C) Immunoflourescence for E-cadherin (red) demonstrates reduced barrier disruption. Images are at 25x magnification and scale bar is 20μm. (D) Immunofluorescence for eosinophil major basic protein (EMBP, red) and Keratin-5 (Krt5, green). White arrows indicate areas of increased eosinophil accumulation. Images are at 40x magnification and scale bar is 20μm. (E) Eosinophil counts per mm of basal lamina from imaging of the entire nasal septum (n= 4 per group). (F) Analysis of nasal lavage fluid via ELISA for eotaxin-1 (n= 4–6 per group). DAPI (blue) * P<0.05, ** P<0.01, **** P<0.0001, Data are represented as median with interquartile ranges.
These data demonstrate that Nrf2 activation via genetic and therapeutic approaches can stabilize barrier function and reduce sinonasal inflammation in a mouse model of rhinosinusitis. The Nrf2 pathway therefore represents a potential therapeutic target in CRS and chronic sinonasal inflammatory disorders8. These results are in line with previous reports in the lower airway that place Nrf2 activity as a key regulator of allergen-induced inflammation5, 9. Interestingly, while activation of Nrf2 through either Keap1 deletion and SFN treatment profoundly reduced sinonasal barrier dysfunction in vivo (Fig 1a, Fig 2a), the effect on eosinophil accumulation in Keap1 tamoxifen-inducible knockout mice was much less than SFN treatment (Fig 1d, Fig 2e). The explanation for this finding is unknown, but may perhaps be related to incomplete Keap1 knockout in this tamoxifen-inducible system.
It remains unknown the mechanism whereby Nrf2 activation stabilizes sinonasal barrier function and promotes cell surface localization of ZO-1 and the adherens junction protein E-cadherin. A potential explanation given the known function of Nrf2 in a cytoprotective response includes an overall reduction of oxidative stress. If this were the sole mechanism, one may therefore hypothesize that any pathway or means in reduction of oxidative stress may have a similar effect in this mouse model of rhinosinusitis. Additional possibilities include an undefined effect on cell junction protein cell surface localization or an indirect mechanism. Previous studies in vitro have demonstrated that SFN can stabilize human sinonasal epithelial cell dysfunction in the presence of multiple stimuli including particulate matter, cigarette-smoke extract, and house dust mite antigen4, 6. Future studies are necessary to clarify the mechanism whereby Nrf2 stabilizes barrier function.
Supplementary Material
Supplementary Figure 1. Wildtype mice were treated with SFN at a dose found to increase Nrf2 downstream target expression. mRNA expression of Nrf2 target gene NQO1 16 hours after intraperitoneal injection of vehicle (0.1% ethanol) or 20 mg/kg SFN (n=4 per group p<0.01).
Supplementary Figure 2. Schematic representation of OVA model. Mice were given daily intranasal instillation of OVA for 2 weeks starting on day 14. Mice were sacrificed on day 29.
Acknowledgements
This study was funded by NIH ES020859 and FAMRI Clinician Innovator Award (M.R.). This study was presented as an oral presentation at the National American Rhinologic Society Meeting October 6, 2018.
Footnotes
Declaration of Interests
N.R.L. is a patent co-inventor for methods treating vascular barrier dysfunction licensed to Navigen Pharmaceuticals which is unrelated to the Nrf2 pathway. N.R.L. holds a small amount of stock in Navigen Pharmaceuticals which is currently of no value. N.R.L. is a consultant for Cooltech Inc. The remaining authors declare no competing financial interests.
References
- 1.Bowler R Oxidative stress in the pathogenesis of asthma. Curr. Allergy Asthma Rep 2004; 4:116–122. [DOI] [PubMed] [Google Scholar]
- 2.Loukides S, Bakakos P, Kostikas K Oxidative stress in patients with COPD. Curr. Drug Targets 2011; 12:469–477. [DOI] [PubMed] [Google Scholar]
- 3.Mrowicka M, Zielinska-Blizniewska H, Milonski J, Olszewski J, Majsterek I Evaluation of oxidative DNA damage and antioxidant defense in patients with nasal polyps. Redox Rep 2015; 20:177–183. doi: 10.1179/1351000215Y.0000000001. Epub 2015 Jan 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.London NR, Tharakan A, Rule AM, Lane AP, Biswal S, Ramanathan M Air pollutant mediated disruption of sinonasal epithelial cell barrier function is reversed by activation of the Nrf2 pathway. J. Allergy Clin. Immunol 2016; 138(6):1736–1738.e4. doi: 10.1016/j.jaci.2016.06.027. Epub 2016 Jul 27. [DOI] [PubMed] [Google Scholar]
- 5.Rangasamy T, Guo J, Mitzner WA, Roman J, Singh A, Fryer AD, et al. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J. Exp. Med 2005; 202(1):47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.London NR, Tharakan A, Lane AP, Biswal S, Ramanathan M Nuclear erythroid 2-related factor 2 activation inhibits house dust mite-induced sinonasal epithelial cell barrier dysfunction. Int Forum Allergy Rhinol 2017; 7(5):536–541. doi: 10.1002/alr.21916. Epub 2017 Feb 2. [DOI] [PubMed] [Google Scholar]
- 7.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, and Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev 13(1):76–86 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steelant B, Seys SF, Boeckxstaens G, Akdis CA, Ceuppens JL, Hellings PW. Restoring airway epithelial barrier dysfunction: a new therapeutic challenge in allergic airway disease. Rhinology 2016; 54(3):195–205. doi: 10.4193/Rhin15.376. [DOI] [PubMed] [Google Scholar]
- 9.Sussan TE, Gajghate S, Chatterjee S, Mandke P, McCormick S, Sudini K, et al. Nrf2 reduces allergic asthma in mice through enhanced epithelial cytoprotective function. Am J Physiol Lung Cell Mol Physiol 2015; 309(1):L27–36. doi: 10.1152/ajplung.00398.2014. Epub 2015 May 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Wildtype mice were treated with SFN at a dose found to increase Nrf2 downstream target expression. mRNA expression of Nrf2 target gene NQO1 16 hours after intraperitoneal injection of vehicle (0.1% ethanol) or 20 mg/kg SFN (n=4 per group p<0.01).
Supplementary Figure 2. Schematic representation of OVA model. Mice were given daily intranasal instillation of OVA for 2 weeks starting on day 14. Mice were sacrificed on day 29.


