Abstract
Aims
Drug‐induced aseptic meningitis (DIAM) is an adverse drug reaction of exclusion; only few studies have addressed this iatrogenic disease. The aim was to characterize DIAM and to identify suspected drugs.
Methods
Data were collected from the analysis of the French Pharmacovigilance Database from inception (1 January 1985) to 8 March 2017. All cases were initially analysed according to the French imputability method by institutional pharmacologists (clinicians or pharmacists). Further analyses of well documented cases were then performed.
Results
In this study, 329 cases of aseptic meningitis were retrieved from the French Pharmacovigilance Database for a total of 429 suspected drugs. Analysis of 203 well documented cases, including 282 drugs, showed that the main reported classes were intravenous polyvalent immunoglobulin, nonsteroidal anti‐inflammatory drugs (NSAIDs), vaccines, antimicrobials, intrathecal antimetabolites, corticosteroids and antalgics/anaesthetics (except NSAIDs). Lymphocytic (33.0%) and purulent (44.8%) meningitis represented the majority of cases of aseptic meningitis. In other cases, the cerebrospinal fluid was mixed (45–55% of neutrophils +45–55% of lymphocytes) or data about cerebrospinal fluid composition were lacking. Most DIAM cases (96%) had a favourable reported outcome with full recovery or minimal residual symptoms.
Conclusion
The most frequently involved drugs in DIAM were intravenous polyvalent immunoglobulin, NSAIDs, vaccines, and antimicrobials and this without being able to differentiate them in terms of biological characteristics. Although further studies are needed to better understand the pathophysiological mechanisms of DIAM, a continuous enrichment of pharmacovigilance databases is essential to identify new signals and to help clinicians in the understanding of DIAM.
Keywords: aseptic meningitis, drug‐induced, pharmacoviglance, adverse effect, meningitis
What is already known about this subject
Drug causality may be difficult to establish in the case of aseptic meningitis with mechanisms poorly understood.
Mistaken diagnosis of drug‐induced aseptic meningitis (DIAM) could lead to delay administration of antimicrobials or intravenous polyvalent immunoglobulin that could be major treatment options in the other types of aseptic meningitis.
This study is the first and the largest analysis of DIAM cases from a national pharmacovigilance database.
What this study adds
The main suspected classes were human intravenous polyvalent immunoglobulin, nonsteroidal anti‐inflammatory drugs, vaccines, antimicrobials, analgesics, antimetabolites and disease‐modifying anti‐rheumatic drugs.
Lymphocytic (33.0%) and purulent (44.8%) meningitis represented the majority of cases of DIAM.
The management of aseptic meningitis was essentially based on the discontinuation of the suspected drug(s), sometimes with symptomatic treatment (analgesic).
1. INTRODUCTION
Meningitis is a major neurological emergency requiring rapid diagnosis of causes that can be treated such as bacterial meningitis and drug‐induced aseptic meningitis (DIAM). An urgent management is mandatory especially to confirm or exclude bacterial meningitis.1, 2 Once cerebrospinal fluid (CSF) is negative for classical microbial agents, aseptic meningitis can be considered according to the MESH definition of aseptic meningitis. Their aetiologies may be classified as follows: (i) systemic diseases with meningeal involvement, (ii) neoplastic or paraneoplastic meningitis; (iii) DIAM; and (iv)—paradoxically—infections (virus principally but also some intracellular bacteria, Mycoplasma spp., Rickettsia spp.).2, 3
The diagnosis of DIAM is important to make because it clearly modifies prognostic information that will be given to the patient or their relatives. The diagnosis is, however, challenging. Several drugs can induce meningeal inflammation. Drug causality may be difficult to establish in the case of aseptic meningitis at least for 2 reasons: DIAM is mostly an exclusion diagnosis and a protopathic bias can be discussed in some situations when drugs are administered or taken to treat the first symptoms of meningitis.4 Mistaken diagnosis of DIAM could lead to delayed administration of antimicrobials or intravenous immunoglobulin (IVIG) that could be major treatment options in the other types of aseptic meningitis. Thus, it appears of major importance to better describe DIAM to improve its early recognition.
The aims of this study were to characterize DIAM and to identify the main drugs involved by means of French Pharmacovigilance Database (FPDB) analysis.
2. METHODS
2.1. Cases identification process
All cases recorded in the FPDB from the database creation date (1 January 1985) to 8 March 2017 (date of the query) were considered. Briefly, the FPDB gathers spontaneous reports of adverse drug reactions from French health practitioners or patients. Each report is validated by clinical pharmacologists in the relevant regional pharmacovigilance centre—according to the French drug causality method of imputability—before being recorded in the database. Briefly, the French drug causality method consists to take into account the following parameters: (i) intrinsic imputability—ranging from I0 (no association between the reaction and a drug) to I6 (strong association between the events)—combining a chronological score (temporal link) and a semiological score (etiological link), each ranging from 0 to 3; and (ii) an extrinsic score based on previously published similar cases (bibliographic documentation), which ranges from B1 (no published case) to B4 (expected adverse event).5 The FPDB is administered by the Agence Nationale de Sécurité du Médicament et des Produits de Santé (ANSM), the French Medicine Agency. All data were registered anonymized.
Inclusion criteria for the final analysis were as follows: (i) MedDRA Preferred Term aseptic meningitis; (ii) available results of lumbar puncture; (iii) >10 cells/μL with no evidence of the presence of any microorganism (either bacterial or viral) by direct examination, usual cultures or polymerase chain reaction (PCR) techniques of CSF.
Cases with missing data were excluded.
2.2. Definitions
Meningitis was defined by the following clinical symptoms: headache, nausea and/or vomiting, meningeal stiffness, photophobia and/or phonophobia and/or photo‐phonophobia and a fever >38.5°C.
Aseptic CSF was defined as a CSF with no evidence of the presence of any microorganism (either bacterial or viral) by direct examination, usual cultures or PCR techniques.
According to the biological characteristics of the CSF, aseptic meningitis was classified as follows:
Lymphocytic meningitis: >10 elements/μL with >60% lymphocytes. Also included in this category were cases of lymphocytic meningitis or where lymphocytes were predominant.
Purulent meningitis: >10 elements/μL with >60% neutrophils. Also included in this category were cases reported as purulent meningitis or where neutrophils were predominant.
Mixed meningitis: >10 elements/μL with an equitable distribution (between 40 and 60%) of lymphocytes and neutrophils.
Meningitis other: >10 elements/μL with a majority of nonlymphocytic and non‐neutrophilic cells (except tumoral cells).
Meningitis NC (not communicated): when the information was not provided.
2.3. Analysis
All cases were initially analysed according to the French imputability method (intrinsic imputation ≥ I1) and industrial cases after excluding duplicates (overall analysis).5 We then analysed only well‐documented cases including a narrative with a least a clinical symptoms description, CSF analysis with at least 10 cells/μL and negative infectious results (final analysis).
These cases were compared with cases published in PubMed (search terms “Meningitis, Aseptic [Mesh]” and “Drug Induced [all fields]” and “French” AND “English” without date restriction) and specialized books.2, 6, 7, 8
2.4. Statistical description
Data are presented as total (n), mean (± standard deviation), median (with 1st and 3rd quartiles), percentage (%), and minimum and maximum values.
2.4.1. Data availability policy
The use of confidential, electronically processed patient data was approved by the French national commission for data protection and liberties (Commission Nationale de l'Informatique et des Libertés; reference number, 1922081).
3. RESULTS
3.1. Overall analysis
In total, 329 cases of DIAM were included in the FPDB over 32 years. Mean age was 40 ± 21 years and sex ratio was 1.5 women for 1 man. Those cases corresponded to 429 suspected drugs (1.3 drugs/case), among which 132 substances with different international nonproprietary names were found. The flow chart of the study is shown in Figure 1.
Figure 1.
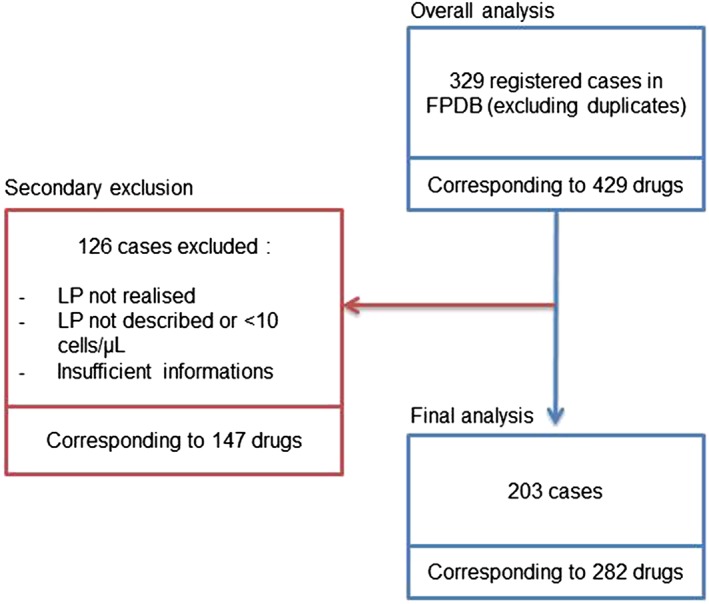
Flow chart of the study. FPDB: French Pharmacovigilance Database; LP: lumbar puncture
Among these 132 substances, only 16 drugs are reported at least 4 times over the period that represents 64% of the total of suspected drugs. IVIG, anti‐inflammatory drugs (NSAIDs), antimicrobials, vaccines and antimetabolites together accounted for >69% of suspected drugs.
3.2. Final analysis of well documented cases
Well‐documented data were available in 203 of 329 cases (63.4%). The main meningitis symptoms (headache, neck stiffness, nausea/vomiting, photophobia‐phonophobia, fever >38.5°C) and the patient's characteristics are summarized in Tables 1 and 2, respectively.
Table 1.
Description of the main meningitis symptoms (final analysis)
| Present | Absent | NC | ||||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Headache | 145 | 71.4 | 5 | 2.5 | 53 | 26.1 |
| Neck stiffness | 64 | 31.5 | 26 | 12.8 | 113 | 55.7 |
| Nausea/vomiting | 80 | 39.4 | 6 | 3 | 117 | 57.6 |
| Phono‐photosensitivity | 43 | 21.2 | 12 | 5.9 | 148 | 72.9 |
| Fever | 84 | 41.4 | 32 | 15.8 | 87 | 42.9 |
NC: not communicated.
Table 2.
Population characteristics (sex and age)
| No. (%) | Total | Female | Male | |
|---|---|---|---|---|
| 203 (100%) | 122 (58.4%) | 81 (41.6%) | ||
| Age (y) | Mean | 42.2 | 40.0 | 45.6 |
| Standard deviation | 21.0 | 19.6 | 22.8 | |
| Median (1st–3rd quartile) | 40 (28–58) | 38 (26–54) | 47 (29–65) | |
| Minimum | 0.6 | 3 | 0.6 | |
| Maximum | 86 | 85 | 86 | |
Other reported symptoms were: altered consciousness going from delirium to coma; meningeal syndrome without details; sensitivo‐motor impairment (motor weakness encompassing facial palsy, trismus, cervicalgia, back pain, myalgia); dermatological manifestations (rash, purpura); gastrointestinal symptoms (diarrhoea).
In the cases with detailed information, 282 suspected drugs were retrieved. The main drugs involved were IVIG (30%), NSAIDs (14%), antimicrobials (11% with amoxicillin representing 5% of the total of suspected drugs) and sulfamethoxazole/trimethoprim (2% of the total of suspected drugs), some monoclonal antibodies (8%, including antineoplastic and immunomodulating agents) and various vaccines (7%) detailed in Table 3 and Figure 2.
Table 3.
Description of the main involved drugs in aseptic meningitis
| IVIG (n = 84, 29.8%) | n | Antalgic/anaesthetic (n = 23, 8.2%) | n | SAID (n = 11, 3.9%) | n |
|---|---|---|---|---|---|
| Privigen | 31 | Paracetamol | 5 | Hydrocortisone (IT and IV) | 3 |
| Tegeline | 21 | Sufentanil | 4 | Methylprednisolone (IT) | 3 |
| Clairyg | 15 | Bupivacaine (IR and NC) | 4 | Prednisone (PO) | 3 |
| Octagam | 7 | Morphine | 2 | Betamethasone (PO) | 1 |
| Sandoglobulin | 5 | Articaine (DEN) | 1 | Prednisolone (ID) | 1 |
| Gammagard | 2 | Codeine | 1 | Sexual hormone (n = 4, 1.4%) | N |
| Kiovig | 2 | Dextropropoxyphene | 1 | Estradiol | 1 |
| Vivaglobin | 1 | Paracetamol/ascorbic acid/pheniramine | 1 | Follitropin | 1 |
| NSAID (n = 39, 13.8%) | n | Oxycodone | 1 | Oxytocin | 1 |
| Ibuprofen | 17 | Ibuprofen/pseudoephedrine | 1 | Progesterone | 1 |
| Sulfasalazine | 6 | Ropivacaine (IS) | 1 | Other antineoplastic drugs (n = 3, 1.1%) | n |
| Ketoprofen | 5 | Tramadol | 1 | Carmustine | 1 |
| Naproxen | 3 | Vaccine (n = 21, 7.4%) | n | Cisplatin | 1 |
| Tiaprofenic acid | 2 | Yellow fever vaccine | 5 | Cyclophosphamide | 1 |
| Celecoxib | 2 | DTCP vaccine | 3 | Immunosuppressive drugs (n = 2, 0.7%) | n |
| Diclofenac | 2 | Typhoid fever vaccine | 2 | Azathioprine | 1 |
| Aspirin | 1 | Influenza vaccine | 2 | Sirolimus | 1 |
| Rofecoxib | 1 | Meningococcal A C W Y vaccine | 2 | Antiemetic drugs (n = 1, 0.4%) | n |
| Antimicrobials (n = 31, 11.0%) | n | HVA vaccine | 2 | Ondansetron | 1 |
| Amoxicillin (± clavulanic acid) | 14 | Influenza vaccine | 1 | Antihistaminic drugs (n = 1, 0.4%) | n |
| Sulfamethoxazole/trimethoprim | 5 | H1N1 vaccine | 1 | Dexchlorpheniramine | 1 |
| Aciclovir | 1 | Meningococcal A B vaccine | 1 | Contrast agents (n = 1, 0.4%) | n |
| Amikacin | 1 | Meningococcal B vaccine | 1 | Iopamidol | 1 |
| Aztreonam | 1 | HVB vaccine | 1 | DMARD (n = 1, 0.4%) | n |
| Bismuth/metronidazole/tetracycline | 1 | Neuro‐ and psychotropic drugs (n = 16, 5.7%) | n | Leflunomide | 1 |
| Cefazolin | 1 | Lamotrigine | 4 | Human fibrinogen (n = 1, 0.4%) | n |
| Ceftazidime | 1 | Zolpidem | 2 | Human fibrinogen | 1 |
| Ceftriaxone | 1 | Alprazolam | 1 | Alpha 1 adrenergic drugs (n = 1, 0.4%) | n |
| Fumagillin | 1 | Amitryptiline | 1 | Phenylephrine | 1 |
| Linezolide | 1 | Carbamazepine | 1 | PPI (n = 1, 0.4%) | n |
| Norfloxacin | 1 | Citalopram | 1 | Esomeprazole | 1 |
| Tetracycline | 1 | Clozapine | 1 | Sympathomimetic drugs (n = 1, 0.4%) | n |
| Valaciclovir | 1 | Diazepam | 1 | Adrenalin | 1 |
| Monoclonal antibodies (n = 23, 8.2%) | n | Lithium | 1 | Other (n = 3, 1.1%) | n |
| Adalimumab | 5 | Lormetazepam | 1 | Allopurinol | 1 |
| Cetuximab | 4 | Tetrazepam | 1 | Mizolastine | 1 |
| Infliximab | 4 | Venlafaxine | 1 | Folic acid | 1 |
| Efalizumab | 3 | Antimetabolite (n = 14, 5.0%) | n | ||
| Rituximab | 3 | Methotrexate (IT, IV, PO, NC) | 9 | ||
| Golimumab | 1 | Cytarabine (IT and IV) | 5 | ||
| Ipilimumab | 1 | ||||
| Nivolumab | 1 | ||||
| Tocilizumab | 1 |
DEN, loco‐regional anaesthesia in dental procedures; DMARD, disease‐modifying antirheumatic drug; HVA, hepatitis virus A; HVB, hepatitis virus B; ID, intradural; IS, intraspinal; IT, intrathecal; IVIG, intravenous immunoglobulins; IV, intravenous; NSAID, nonsteroidal anti‐inflammatory; NC, not communicated; PO, per os; PPI, proton pump inhibitors; SAID, steroidal anti‐inflammatory.
Figure 2.
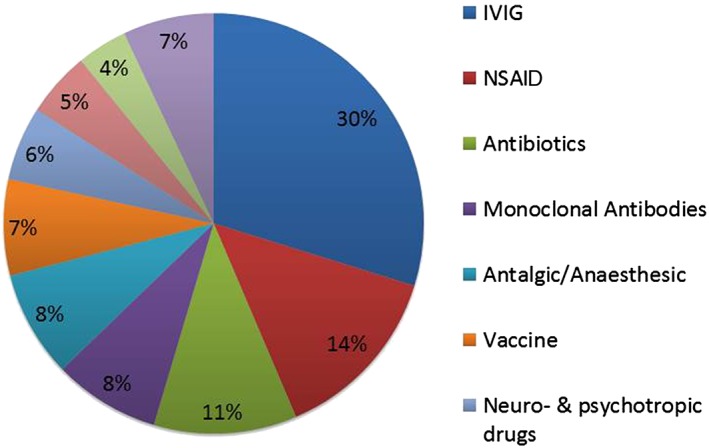
Main classes of involved drugs in aseptic meningitis. Among 282 suspect drugs, the main drugs involved were intravenous immunoglobulin (IVIG: 30%), nonsteroidal anti‐inflammatory drugs (NSAIDs: 14%), antimicrobials (11% with amoxicillin representing 5% of the total of suspected drugs) and sulfamethoxazole/trimethoprim (2% of the total of suspected drugs), some monoclonal antibodies (8%, including antineoplasic and immunomodulating agents) and various vaccines (7%)
When information was available, lymphocytic (33.0%) and purulent (44.8%) meningitis represented the majority of cases of aseptic meningitis (158 cases, 77.8%; Table 4). In other cases, the CSF was mixed (45–55% of neutrophils +45–55% of lymphocytes) or data about CSF composition were lacking (45 cases, 22.2%). The biochemical profiles of CSF were similar between the different classes of drugs with a moderate hyperproteinorachia and a glycorachia that can be considered as normal under the assumption of normal blood glucose (data generally not communicated).
Table 4.
Description of the biochemical profiles of cerebrospinal fluid
| Elements (/μL) | Neutrophils (%) | Lymphocytes (%) | Red cells (/μL) | Protein (g/L) | Glucose (mmol/L) | |
|---|---|---|---|---|---|---|
| n | 171 | 104 | 74 | 44 | 136 | 77 |
| Mean | 921.8 | 71.0 | 55.1 | 152.4 | 1.3 | 2.7 |
| Standard deviation | 1710.8 | 27.2 | 35.9 | 294.1 | 1.7 | 1.1 |
| Median (1st–3rd quartile) | 260.0 (63–995) | 80.0 (63.75–92) | 63.5 (16.5–89.75) | 42.0 (7.75–132.5) | 0.8 (0.6–1.3) | 2.8 (2.2–3.4) |
| Minimum | 10.0 | 2.0 | 1.0 | 1.0 | 0.2 | 0.2 |
| Maximum | 11 500.0 | 100.0 | 100.0 | 1200.0 | 14.6 | 5.6 |
According to the French drug imputability method used in the FPDB, the majority of suspect drugs involved in aseptic meningitis are considered chronologically (74.8%) and semiologically (58.9%) plausible or probable. Thirty‐three drugs (among 282 reported suspect drugs, 11.7%) were re‐introduced with recurrence of symptoms.
Reported time from drug initiation to onset of symptoms was highly variable (mean of 62 days, standard deviation of 268 days, coefficient of variation of 434%, range from 0.125 to 2555 days) depending on several criteria such as route of drug administration, administration plan, drug type.
Most reported suspect drugs had been administered intravenously (41.3%) or orally (35.0%) followed by the subcutaneous or intramuscular route for 11.0%. Direct administrations in the central nervous system (intrathecal, subarachnoidal, intradural) represented 9.2% of the routes of administration.
Most DIAM cases (96%) had a favourable outcome with full or progressing recovery with residual symptoms. Excluding 2 unrelated deaths (one cardiorespiratory arrest with a postmortem diagnosis of aseptic meningitis and 1 death in a context of non‐Hodgkin's B lymphoma relapse), no case of aseptic meningitis had a direct fatal outcome.
The management of aseptic meningitis was essentially based on the discontinuation of the suspected drug(s), sometimes with symptomatic treatment (analgesic).
4. DISCUSSION
This study is the first analysis of DIAM cases from a national pharmacovigilance database of quality. It is the world's largest series over >30 years with a classification of reported suspect drug and with a clinical and biological description of the DIAM.
Results of the overall and final analysis were similar for population characteristics (sex and age) and for type of suspected drugs. As shown in Table 3, although many drug classes were involved, the main suspected classes were human IVIG, NSAIDs, vaccines, antimicrobials, analgesics, antimetabolites and disease‐modifying antirheumatic drug (DMARD). Data from the literature also mainly report cases of aseptic meningitis with NSAIDs, antimicrobials, certain monoclonal antibodies (such as adalimumab, cetuximab, infliximab and efalizumab), IVIG and some antiepileptics.6, 7, 8, 9 In contrast, vaccines, other monoclonal antibodies (such as rituximab, golimumab, nivolumab, ipilimumab and tocilizumab), antimetabolites, analgesics and DMARDs are more sparsely reported—or even absent—in the literature. Although no clear protopathic bias could be identified in our series, such bias remains possible for certain drug classes such as antimicrobials, NSAIDs and some analgesics. Also, some reported DIAM—principally the oldest ones—would possibly be classified as viral with modern techniques of PCR.10 The recent increase in the diagnosis of either paraneoplastic or autoimmune encephalitis by specific autoantibody detection constitutes another possible source of misdiagnosis.
As defined by the World Health Organization, meningitis is an absolute emergency and CSF analysis is fundamental to make the diagnosis of meningeal inflammation.11
In this study, among the 329 cases registered in the FPDB, 203 (63.4%) were reported clinically well documented and provided results of a lumbar puncture. The CSF results allowed the characterisation of aseptic meningitis in 84% of these well documented cases. Unfortunately, even among the cases with CSF analysis, it is difficult to identify a specific profile for DIAM. Indeed, lymphocytic meningitis is almost as common as purulent meningitis, and both together account for more than 68% of explored meningitis. No particular profile in terms of cellularity or biochemistry of CSF seems to be associated with DIAM due to a particular drug class and/or route of administration. The heterogeneity of the data precluded statistical comparisons to be made between drugs classes and between types of meningitis.
A clear meningeal syndrome—involving headache, neck stiffness and nausea/vomiting—is reported in only 15.5% of cases in this DIAM series. Among symptoms typically found in the meningeal triad, only headache is present in >2/3 of DIAM cases. All other symptoms are reported in only 20–40% of cases. However, as in all pharmacovigilance databases, an unknown number of symptoms may not have been reported of recorded.
Indeed, current postmarketing pharmacovigilance is strongly based on spontaneous notification and presents well‐known bias. Among these, the main bias concerns the information of cases, the notorious of adverse effects or drugs, the protopathic bias and the under notification.
Moreover, the coding habits of the pharmacovigilance centres having recorded the cases may also have had an impact on our results. Indeed, the calculated time to onset depends on the date of initiation of treatment.12 This date is sometimes difficult to interpret in the database. For example, during repeated IVIG treatments, the date of treatment initiation in the database may be that of the last administration or of the first administration even if the effects started during a subsequent cure.
It must be emphasized that outcome of aseptic meningitis is generally favourable with spontaneous recovery, without sequelae, after stopping the suspect drugs. Mortality appears exceptional. Among these cases, no death was reported.
Unfortunately, the FPDB does not allow a reliable analysis of the possible role of associated diseases preceding the occurrence of these meningitis.
According to our literature research made in PubMed, >100 cases of DIAM were found, for which more than 130 drugs were cited as suspect drugs. The main drug classes reported in the literature—by decreasing frequency—are NSAIDs (ibuprofen, naproxen), antimicrobials (mainly sulfamethoxazole/trimethoprim), monoclonal antibodies (adalimumab, cetuximab, infliximab, tocilizumab), antiepileptic drugs (lamotrigine and carbamazepine) and IVIG.9, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24
Analysis of the summaries of product characteristics (SPC) of the first 16 drugs among the 416 suspect drugs in the FPDB shows that the term aseptic meningitis appears as a potential adverse effect in most of these SPC. Nevertheless, on June 2019, this effect is not mentioned in the SPC of amoxicillin, Sandoglobuline (no longer available), and bupivacaine (epidural route). It is interesting to note that the SPC of adalimumab reports the occurrence of viral meningitis but not aseptic meningitis per se.
Unfortunately, the mechanisms of DIAM are poorly understood and have precluded the identification of any biomarker. Two categories of mechanisms can be proposed: (i) hypersensitivity reactions; and (ii) direct inflammation of the meninges.13, 25
5. CONCLUSION
This retrospective study is the first analysis of DIAM from a national pharmacovigilance database. The main classes of drugs involved in this type of adverse effect are IVIG, NSAIDs, vaccines and antimicrobials.
Another message about this study is that, before aseptic meningitis, it is important to always think of a drug aetiology whatever the nature of the CSF (lymphocytic, purulent, mixed or other). This is why detailed analysis of the drug history is essential.
Continuous enrichment of pharmacovigilance databases through the recording of well‐documented suspected adverse reactions allowing good‐quality analysis is essential for the emergence of relevant signals, to improve knowledge about suspected adverse drug reactions and analyse their causes and the relationship with some factors (predisposing or confounding).
Further studies are required to examine the pathophysiology of DIAM.
Aseptic meningitis should be added in the sections adverse reactions and warnings and precautions of the SPC of drugs for which the risk of aseptic meningitis has been established, according to our results and to the literature (i.e. amoxicillin, adalimumab).
COMPETING INTERESTS
There are no competing interests to declare.
CONTRIBUTORS
K.B. and B.L.‐V. were involved in the conception and the design of this retrospective study. K.B. collected and organized the data from the French pharmacovigilance database and performed the analysis. N.W., H.T., C.F.‐B. and B.L.‐V. contributed to the data interpretation and provided clinical and/or pharmacological expertise. K.B. was the writer of the final version. All authors reviewed and approved the manuscript.
ACKNOWLEDGEMENTS
This publication uses data collected from all Regional Centers of Pharmacovigilance—organized in a French network of RCPVs—that belong to the ANSM. The views expressed in this article are those of the authors and do not necessarily represent the ANSM position.
K.B. belongs to the working group of neuropsychopharmacology of the French society of pharmacology and therapeutics (SFPT).
Bihan K, Weiss N, Théophile H, Funck‐Brentano C, Lebrun‐Vignes B. Drug‐induced aseptic meningitis: 329 cases from the French pharmacovigilance database analysis. Br J Clin Pharmacol. 2019;85:2540–2546. 10.1111/bcp.14073
REFERENCES
- 1. Bamberger DM. Diagnosis, initial management, and prevention of meningitis. Am Fam Physician. 2010;82(12):1491‐1498. [PubMed] [Google Scholar]
- 2. Adams RD, Victor M, Ropper AH. Principles of Neurology. 6th ed. New York: McGraw‐Hill, Health Professions Division; 1997. [Google Scholar]
- 3. Mount HR, Boyle SD. Aseptic and bacterial meningitis: evaluation, treatment, and prevention. Am Fam Physician. 2017;96(5):314‐322. [PubMed] [Google Scholar]
- 4. Feinstein AR. Clinical Epidemiology: The Architecture of Clinical Research. 2nd ed. Philadelphia, PA: W. B. Saunders Company; 1985. [Google Scholar]
- 5. Bégaud B, Evreux JC, Jouglard J, Lagier G. Imputation of the unexpected or toxic effects of drugs. Actualization of the method used in France. Therapie. 1985;40(2):111‐118. [PubMed] [Google Scholar]
- 6. Jain KK. Drug‐Induced Neurological Disorders. Cambridge, MA: Hogrefe Pub; 2012. [Google Scholar]
- 7. Massaro EJ. Handbook of Neurotoxicology. Totowa, NJ: Humana Press; 2002. https://link.springer.com/openurl?genre=book&isbn=978-0-89603-795-3. Accessed March 1, 2019. [Google Scholar]
- 8. Blum K, Manzo L. (Eds). Neurotoxicology. New York: M. Dekker; 1985. [Google Scholar]
- 9. Moris G, Garcia‐Monco JC. The challenge of drug‐induced aseptic meningitis. Arch Intern Med. 1999;159(11):1185‐1194. [DOI] [PubMed] [Google Scholar]
- 10. Steiner I, Schmutzhard E, Sellner J, Chaudhuri A, Kennedy PGE. EFNS‐ENS guidelines for the use of PCR technology for the diagnosis of infections of the nervous system. Eur J Neurol. 2012;19(10):1278‐1291. 10.1111/j.1468-1331.2012.03808.x [DOI] [PubMed] [Google Scholar]
- 11. Organisation mondiale de la SantÈ . Maladies Émergentes et Autres Maladies Transmissibles. Plan Stratégique 1996–2000. Genève: WHO. [Google Scholar]
- 12. Klepper MJ, Edwards B. Individual case safety reports‐‐how to determine the onset date of an adverse reaction: a survey. Drug Saf. 2011;34(4):299‐305. 10.2165/11588490-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 13. Marinac JS. Drug‐ and chemical‐induced aseptic meningitis: a review of the literature. Ann Pharmacother. 1992;26(6):813‐822. 10.1177/106002809202600613 [DOI] [PubMed] [Google Scholar]
- 14. Chapuis C, Guy N, Besson G. Adverse effects of intravenous human polyvalent immunoglobulins. Rev Neurol (Paris). 2008;164 Spec No 3:F203‐F210. 10.1016/S0035-3787(08)74100-6 [DOI] [PubMed] [Google Scholar]
- 15. Hamrock DJ. Adverse events associated with intravenous immunoglobulin therapy. Int Immunopharmacol. 2006;6(4):535‐542. 10.1016/j.intimp.2005.11.015 [DOI] [PubMed] [Google Scholar]
- 16. Bonnel RA, Villalba ML, Karwoski CB, Beitz J. Aseptic meningitis associated with rofecoxib. Arch Intern Med. 2002;162(6):713‐715. [DOI] [PubMed] [Google Scholar]
- 17. Murphy E, Martin S, Patterson JV. Developing practice guidelines for the administration of intravenous immunoglobulin. J Infus Nurs off Publ Infus Nurses Soc. 2005;28(4):265‐272. [DOI] [PubMed] [Google Scholar]
- 18. Hopkins S, Jolles S. Drug‐induced aseptic meningitis. Expert Opin Drug Saf. 2005;4(2):285‐297. [DOI] [PubMed] [Google Scholar]
- 19. Kepa L, Oczko‐Grzesik B, Stolarz W, Sobala‐Szczygiel B. Drug‐induced aseptic meningitis in suspected central nervous system infections. J Clin Neurosci off J Neurosurg Soc Australas. 2005;12(5):562‐564. 10.1016/j.jocn.2004.08.024 [DOI] [PubMed] [Google Scholar]
- 20. Holle D, Obermann M. Headache in drug‐induced aseptic meningitis. Curr Pain Headache Rep. 2015;19(7):29 10.1007/s11916-015-0505-0 [DOI] [PubMed] [Google Scholar]
- 21. Peck MG, Joyner PU. Ibuprofen‐associated aseptic meningitis. Clin Pharm. 1982;1(6):561‐565. [PubMed] [Google Scholar]
- 22. Simms KM, Kortepeter C, Avigan M. Lamotrigine and aseptic meningitis. Neurology. 2012;78(12):921‐927. 10.1212/WNL.0b013e31824c4634 [DOI] [PubMed] [Google Scholar]
- 23. DeStefano F. Vaccine safety datalink research group. The vaccine safety datalink project. Pharmacoepidemiol Drug Saf. 2001;10(5):403‐406. 10.1002/pds.613 [DOI] [PubMed] [Google Scholar]
- 24. Joffe AM, Farley JD, Linden D, Goldsand G. Trimethoprim‐sulfamethoxazole‐associated aseptic meningitis: case reports and review of the literature. Am J Med. 1989;87(3):332‐338. [DOI] [PubMed] [Google Scholar]
- 25. Yelehe‐Okouma M, Czmil‐Garon J, Pape E, Petitpain N, Gillet P. Drug‐induced aseptic meningitis: a mini‐review. Fundam Clin Pharmacol. 2018;32(3):252‐260. 10.1111/fcp.12349 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The use of confidential, electronically processed patient data was approved by the French national commission for data protection and liberties (Commission Nationale de l'Informatique et des Libertés; reference number, 1922081).


