Abstract
Aims
Vancomycin is frequently used in critically ill children in whom the drug pharmacokinetics are significantly altered as a result of changes in renal clearance and volume of distribution. Therapeutic drug monitoring (TDM) is recommended to achieve vancomycin trough concentrations between 10 and 20 mg/L. In this study we reviewed vancomycin dosing, TDM and treatment outcomes in paediatric and neonatal intensive care unit patients.
Methods
We reviewed the medical records of all patients receiving intravenous vancomycin in a tertiary paediatric and neonatal intensive care unit over a 10‐month period. Demographic, vancomycin dosing, TDM and drug‐related adverse effects data were collected.
Results
In total, 115 children received 126 courses of vancomycin and had at least 1 TDM blood sample taken at steady state. In only 38/126 (30%) courses was the target concentration (10–20 mg/L) achieved at the initial steady state trough sample. Of the 88 courses that had initial trough concentrations outside the target range, the dose was adjusted in only 49 (56%). Overall, minimum doses of 30 mg/kg/day in neonates with a corrected gestational age of <35 weeks, and 50 mg/kg/day in older children, were required to achieve target vancomycin concentrations. Vancomycin‐attributable nephrotoxicity occurred in 10/126 (8%) courses and there were no episodes of red man syndrome.
Conclusion
In critically ill children, individualised dosing is needed. In the absence of Bayesian model‐based dosing, in children with normal renal function, empiric vancomycin doses of at least 30 mg/kg/day in neonates of <35 weeks corrected gestational age, and 50 mg/kg/day in older children, should be considered. Optimisation of TDM practices through the development of protocols, ideally built into electronic medical records, should be considered.
Keywords: children, dosing, glycopeptide, neonates, pharmacokinetics, therapeutic drug monitoring
What is already known about this subject
Vancomycin is used as empiric therapy for septic children in the intensive care setting.
Vancomycin pharmacokinetics are significantly altered in critically ill children.
Data on whether current empiric dosing recommendations effectively achieve target concentrations in this population are limited.
What this study adds
Empiric vancomycin doses of at least 30 mg/kg/day in neonates with a corrected gestational age < 35 weeks, and 50 mg/kg/day in older children, should be considered
Interventions to improve therapeutic drug monitoring and dose adjustment practices are required in the intensive care setting.
There is marked interindividual variability in the vancomycin doses required to achieve target concentrations in critically ill children.
1. INTRODUCTION
Critically ill children are at an increased risk of invasive infections, particularly with Gram‐positive bacteria. This is due to indwelling plastic and other foreign‐material devices, as well as the underlying medical condition rendering these patients critically ill.1 The glycopeptide antibiotic, vancomycin, is often used for both the empiric and targeted treatment of invasive Gram‐positive infections in this setting.
The recommended target serum trough concentrations for vancomycin are 10–20 mg/L: 10–15 mg/L for uncomplicated infections and 15–20 mg/L for serious infections (i.e. endocarditis, meningitis, osteomyelitis, pneumonia, sepsis).2, 3 Vancomycin concentrations <10 mg/L are associated with an increased risk of both therapeutic failure and antimicrobial resistance2, 4 while concentrations > 20 mg/L increase the likelihood of nephrotoxicity.5, 6 In children, attaining and maintaining therapeutic serum concentrations of vancomycin is difficult due to variability in renal clearance and volume of distribution. This is particularly challenging in critically ill children as a result of fluctuations in fluid balance and renal function secondary to both the underlying disease (e.g. congenital heart disease, renal impairment, sepsis), and treatment of their condition, which may involve fluid resuscitation or restriction.7, 8, 9 Studies that form the basis of current standard vancomycin dosing guidelines in children were not specifically validated in critically ill children.2
Therapeutic drug monitoring (TDM) is recommended in all children receiving intravenous vancomycin. Early TDM, by measuring trough vancomycin concentrations, is crucial to ensure therapeutic concentrations are achieved as rapidly as possible, thereby minimising the risk of treatment failure or toxicity associated with sub‐ and supratherapeutic concentrations, respectively.
There is currently no consensus paediatric dosing guidelines for vancomycin use in critically ill children. At present, the current empiric dosing recommendations range between 15 mg/kg 8‐, 12‐ or 24‐hourly according to corrected gestational age (CGA) in neonates (15–45 mg/kg/day) and 10–20 mg/kg 6‐ to 8‐hourly in older children (30–80 mg/kg/day).2, 3, 10 There are few studies investigating whether these dosing recommendations are adequate to attain therapeutic trough concentrations in critically ill children. We describe our experience with intravenous vancomycin dosing and attainment of therapeutic trough concentrations in a tertiary paediatric and neonatal intensive care unit, with particular focus on dosing regimens, TDM, dose adjustments, adverse effects, and outcomes of treatment.
2. METHODS
2.1. Study design
We retrospectively reviewed the medical records of children who were treated with intravenous vancomycin in the paediatric intensive care unit (PICU) and neonatal intensive care unit of the Royal Children's Hospital (RCH) Melbourne over a 10‐month period (April 2016–February 2017). Eligible patients were identified using the electronic medical records and the pharmacy database. Patients were included if they received intravenous vancomycin administered as intermittent infusions and had at least 1 blood sample taken for TDM. Patients with renal impairment at the time of vancomycin commencement were excluded.11, 12
Demographic data, together with information on vancomycin therapy and treatment outcomes, were collected. Patients were stratified by age correlating with maturation of renal function: (i) CGA <29 weeks (ii) CGA 29–<35 weeks (iii) CGA 35–≤44 weeks (iv) CGA >44 weeks–<2 years, (v) age ≥2 years.13, 14 Neonates were defined as aged up to CGA 44 weeks, and older children were defined as those aged 29 days or above. The study was approved by the RCH Human Research Ethics Committee (HREC 36177C).
2.2. Vancomycin administration and TDM
In the absence of local guidelines for vancomycin dosing in critically ill children, dosing regimens were determined by the treating clinicians, who were guided by hospital dosing protocols. In neonates, the vancomycin dosing regimen recommended by the British National Formulary for Children was used (i.e. 15 mg/kg/dose 24‐, 12‐, and 8‐hourly for CGA <29 weeks, 29–≤35 weeks, and 35–≤ 44 weeks respectively).10 Older children received 10–20 mg/kg 6‐ to 8‐hourly.3 Vancomycin concentrations were determined using high performance liquid chromatography. Timing of vancomycin concentrations were deemed to be appropriate if a trough concentration was taken at steady state. This was defined as a vancomycin concentration taken after at least 2 doses, and immediately prior (within 1 hour) to the third or subsequent dose, as per local guidelines. Only trough concentrations taken at steady state were included in the study. Trough concentrations of 10–20 mg/L were considered therapeutic, regardless of illness.2
A vancomycin‐attributable clinical adverse effect was defined according to the criteria outlined by Naranjo et al. for adverse drug reactions.15 Baseline, end‐of‐treatment, and 48‐hour post end‐of‐treatment serum creatinine levels were reviewed where these data were available. Nephrotoxicity was defined as an increase in the serum creatinine by a magnitude of 1.5 times the baseline level, as per the International Society of Nephrology guidelines.16 Concomitant nephrotoxic medications were defined as those administered during the same treatment period as vancomycin. Data were collected on the most commonly used nephrotoxic agents in the intensive care setting.
For those patients with a positive blood culture at the time of initiation of vancomycin therapy, the outcome of treatment was classified as cure if there was clearance of blood cultures with associated clinical improvement. Treatment failure was defined as persistent bacteraemia or death due to Gram‐positive infection.
Data were analysed using Stata v15.1.17 The difference in median values were compared using Bonnett‐Price confidence intervals.
3. RESULTS
Overall, there were 171 courses of vancomycin prescribed in 141 patients during the study period. Of these, 38 courses (in 19 children) were excluded due to renal impairment at the time of commencement of vancomycin. Of the 133 remaining vancomycin courses, at least 1 trough sample was taken for TDM at steady state in 126 courses, in a total of 115 patients. Demographic data are summarised in Table 1. Of the 126 courses, 53 were in neonates, 41 in children aged 29 days to <2 years and 32 in those aged 2–18 years.
Table 1.
Demographic details
| Demographics | Neonates (CGA) | Infants and children | |||||
|---|---|---|---|---|---|---|---|
| <29 w | 29–≤35 w | 35–≤44 w | Total | 29 d–23 mo | 2–18 y | Total | |
| No. of courses, n (%) | 6 (5%) | 13 (10%) | 34 (27%) | 53 (42%) | 41 (33%) | 32 (25%) | 73 (58%) |
| CGA, median (range) | 26.5 (25.3–28.5) | 31.6 (29.2–34.4) | 40.4 (35.3–43.4) | 38.1 (25.3–43.4) | 6 m (29 d–23 mo) | 9 y 5 mo (25 mo–16 y 3 mo) | 1 y 4 mo (29 d–16 y 3 mo) |
| Vancomycin therapy, TDM and toxicity | |||||||
| Duration of vancomycin course, median (range) | 6 d (2–11 d) | 5 d (2–12 d) | 4 d (1–42 d) | 5 d (1–42 d) | 5 d (1–53 d) | 3 d (1–23 d) | 4 d (1–53 d) |
| Target trough attained at any stage during the course, n (%) | 3 (50%) | 10 (77%) | 29 (85%) | 42 (79%) | 31 (76%) | 21 (66%) | 52 (71%) |
| Number of dose adjustments, median (range) | 2 (0–3) | 1 (0–3) | 1 (0–5) | 1 (0–5) | 1 (0–7) | 1 (0–9) | 1 (0–9) |
| Vancomycin‐attributable nephrotoxicity, n (%) | 1 (17%) | 0 (0%) | 2 (6%) | 3 (6%) | 3 (7%) | 4 (12%) | 7 (10%) |
CGA, corrected gestational age; w, weeks; TDM, therapeutic drug monitoring.
The median duration of vancomycin therapy was 4 (range 1–53) days. The indications for vancomycin were empiric treatment of suspected sepsis in 80/126 (63%), treatment of proven Gram‐positive infection in 38/126 (30%), and prophylaxis as part of the RCH organ transplantation protocol in 8/126 (6%). A Gram‐positive bacterium was isolated in 49 courses, comprising coagulase‐negative staphylococci (CONS; n = 29), Staphylococcus aureus (n = 7), Enterococcus spp. (n = 4), α‐haemolytic streptococci (n = 4), Bacillus spp. (n = 2), Micrococcus spp. (n = 2) and β‐haemolytic streptococci (n = 1).
3.1. Dosing regimens
Of the 126 courses, 53 were given to neonates. The median dose required to achieve target concentrations was 37 mg/kg/day in neonates with a CGA of <29 weeks, 43 mg/kg/day for CGA of 29–≤35 weeks and 47 mg/kg/day for CGA of 35–≤44 weeks (Figure 1). The final dose required to attain therapeutic trough concentrations was significantly higher than the empiric dosing recommendations in neonates whose CGA was <29 weeks and CGA of 29–≤35 weeks, with doses of 2 and 1.5 times the empiric dosing recommendation respectively required to achieve target concentrations (Table 2).
Figure 1.
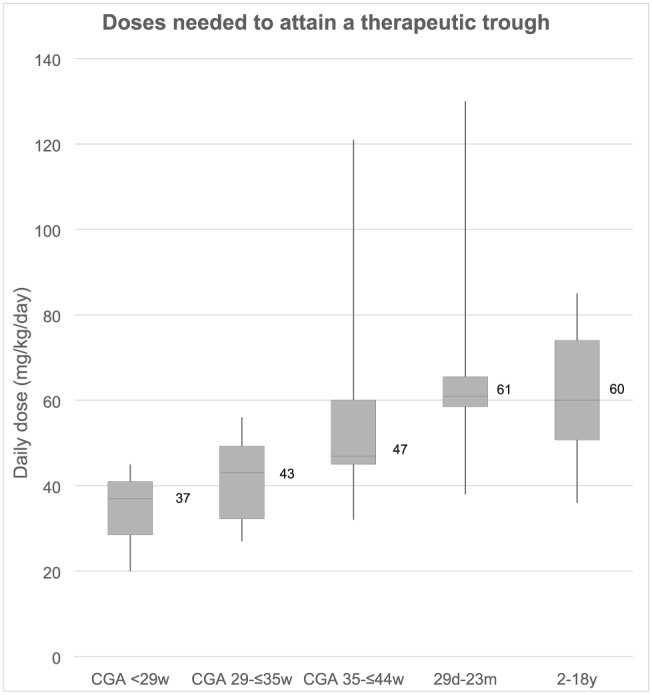
Box plot denotes median, with box depicting 25th and 75th percentiles, whiskers depicting maximum and minimum values. CGA, corrected gestational age; w, weeks; m, months
Table 2.
Vancomycin doses required to attain target therapeutic concentration
| Neonates | ||||
|---|---|---|---|---|
| Corrected gestational age | Standard empiric dosing guidelines (for comparison) | Dosing | Daily dosing (mg/kg/day), median (IQR) | P value |
| <29 w | 15 | Empiric dosing | 14 (13–15) | <.05 |
| Dose required | 37 (29–41) | |||
| 29–≤35 w | 30 | Empiric dosing | 29 (27–30) | |
| Dose required | 43 (32–49) | <.01 | ||
| 35–≤44 w | 45 | Empiric dosing | 45 (44–47) | .62 |
| Dose required | 47 (45–60) | |||
| Infants and children | ||||
|---|---|---|---|---|
| Age | Standard empiric dosing guidelines (for comparison) | Dosing | Daily dosing (mg/kg/day), median (IQR) | P value |
| 29 d–23 mo | 30–80 | Empiric dosing | 59 (45–60) | .60 |
| Dose required | 61 (58–66) | |||
| 2–18 y | 30–80 | Empiric dosing | 45 (41–58) | <.05 |
| Dose required | 60 (51–74) | |||
IQR, interquartile range; w, weeks.
The median dose required to attain therapeutic trough concentrations in older children was 60 (IQR 50–69, range 36–130) mg/kg/day (Figure 1). There were 6 older children who required daily vancomycin doses exceeding 80 mg/kg/day (up to 130 mg/kg/day) to attain target concentrations. The underlying diagnoses for these patients were: haematological malignancy (n = 3), congenital heart disease (n = 1), chronic lung disease of prematurity (n = 1) and chronic liver disease (n = 1).
3.2. Therapeutic drug monitoring and dose adjustments
In only 38 of the 126 (30%) courses was the initial vancomycin trough within target range. Of the 88 courses that had initial trough concentrations outside the target range, the was dose adjusted in only 49 (56%). In 3 courses, therapeutic trough concentrations were only attained by repeatedly withholding doses, without altering the prescribed daily dosing regimen. The median number of dose adjustments in those with initial concentrations outside the target range was 1 (range 1–4), with associated median dose adjustment increments required to attain target concentrations of 73% (range 3–173%) of the original dose.
Overall, the initial trough concentration of vancomycin was in target range in approximately one‐third (19/53, 39%) of courses in neonates and one‐quarter (19/73, 26%) in older children. Of those courses in which initial therapeutic concentrations were not attained, most had subtherapeutic trough concentrations at first trough sampling: 29/53 (55%) neonates, and 49/73 (67%) older children. A total of 10 courses had supratherapeutic concentrations at initial trough sampling: 5/53 (9%) neonates, and 5/73 (7%) older children (Table 3). The underlying diagnoses of these children included: congenital heart disease (n = 6), persistent pulmonary hypertension of the newborn (n = 1), septic shock (n = 1), post liver transplant (n = 1) and post haematopoietic stem cell transplant (n = 1).
Table 3.
Trough concentrations attained at initial monitoring with empiric dosing
| Initial vancomycin concentration (mg/L) | Neonates | Infants and children | |||
|---|---|---|---|---|---|
| CGA <29 w | CGA 29–≤35 w | CGA 35–≤44 w | 29 d–23 mo | 2–18 y | |
| <10 | 6 (100%) | 10 (77%) | 13 (38%) | 27 (66%) | 22 (69%) |
| 10–20 | 0 | 3 (23%) | 16 (47%) | 13 (32%) | 6 (19%) |
| >20 | 0 | 0 | 5 (15%) | 1 (2%) | 4 (13%) |
CGA, corrected gestational age; w, weeks.
Overall, a therapeutic trough concentration of vancomycin was not attained at any stage during therapy in 11/53 (21%) courses in neonates, and 21/73 (29%) courses in older children.
3.3. Adverse effects
In 107 of the 126 (85%) courses, patients received at least 1 concomitant nephrotoxic medication (Table S1). The median number of concomitant nephrotoxic medications was 2 (range 0–4). In 29 of the 126 (23%) courses of vancomycin, the trough concentration exceeded 20 mg/L during the course of treatment. In these 29 courses, patients received a median vancomycin dose of 60 (range 41–130) mg/kg/day. Of these, 10 children experienced vancomycin‐attributable nephrotoxicity (3 neonates, 7 older children) with a median vancomycin dose of 63 (range 45–130) mg/kg/day. Time to resolution of renal impairment ranged from 1–18 days. None required renal replacement therapy. All those who experienced nephrotoxicity received at least 1 concomitant nephrotoxic medication, with a median of 2 (range 1–4). There were no documented cases of red man syndrome in the study population.
3.4. Outcomes
Outcome data were analysed for the 49 courses with an isolated Gram‐positive bacterium. Of these, 18 patients were changed to targeted therapy following susceptibility results, and 2 ceased treatment due to the high likelihood of the isolate being a contaminant. One patient died as a result of their underlying disease (unrelated to infection). Two patients were changed to continuous infusions of vancomycin due to persistent subtherapeutic concentrations in the setting of Staphylocococus epidermidis sepsis and mastoiditis with Staphylococcus capitis isolated from pus. Of the remaining 26 patients with an isolated Gram‐positive bacterium, all achieved cure with a course of vancomycin for infections caused by CONS (n = 20), Bacillus spp. (n = 2), S. aureus (n = 2), Enterococcus spp. (n = 1) and Micrococcus spp. (n = 1). Of note, 2 of these 26 courses were for treatment of CONS infections that were flucloxacillin sensitive.
4. DISCUSSION
Our study highlights the challenges in attaining target vancomycin concentrations in critically ill neonates and children as well as the wide interindividual variability in vancomycin doses required to achieve target concentrations.
Early attainment of therapeutic vancomycin concentrations is important in optimising patient outcomes from both an individual‐ and population‐based perspective. Studies show that attaining therapeutic concentrations early (within 1 day of commencing vancomycin) improves patient outcomes, with lower rates of treatment failure in those patients with methicillin‐resistant S. aureus bacteraemia.4 Furthermore, attainment of concentrations within target range, and specifically >10 mg/L, minimises emergence of vancomycin‐intermediate S. aureus and has been associated with a shorter duration of bacteraemia in a multicentre retrospective study of children with MRSA bacteraemia.18 Clinical decision support aids for TDM and dose adjustment, with automated alerts in‐built into electronic medical records, have been proposed as a tool to optimise dosing. Such interventions have been shown to improve compliance with dosing recommendations, and decrease dosing errors when prescribing antiemetics and antithrombotic agents in the critical care setting.19 Similar tools have also been suggested for antimicrobial stewardship to improve attainment of therapeutic concentrations of antimicrobials.20, 21, 22
Recently, there has been a shift from targeting vancomycin trough concentrations of 10–20 mg/L to targeting the ratio of the area under the concentration–time curve over a 24‐hour period (AUC24) to the minimum inhibitory concentration (MIC) of the bacteria (i.e. AUC24/MIC).23 This is based on studies of S. aureus bacteraemia and pneumonia showing that a vancomycin AUC24/MIC>373–400 was associated with improved clinical outcomes.2, 24, 25 In children, trough vancomycin concentrations are routinely measured and, therefore, understanding the relationship between trough concentrations and AUC24 is important to guide therapy for S. aureus infection. In children aged >2 months–18 years, a trough concentration of 11 mg/L was shown to correlate with an AUC24 > 400 for 6‐hourly vancomycin dosing.26 Also, a population pharmacokinetic modelling study of children aged ≥3 months showed that doses of 60–70 mg/kg/day would be required to achieve an AUC24/MIC ≥ 400 in three‐quarters of children.27 In young infants aged 0–90 days, trough concentrations >20 mg/L and >15 mg/L for 6‐ and 12‐hourly dosing, respectively, were required to achieve an AUC24 ≥ 400 for a S. aureus infection with an MIC of 1 mg/L.28 It is important to note that the pharmacodynamic target, AUC24/MIC ≥ 400, is derived from studies of S. aureus infection. However, in neonates with late‐onset sepsis, infections due to CONS occur more frequently and, to our knowledge, there are no studies in humans determining the pharmacodynamic target for vancomycin for CONS infection.29
Our finding that only one‐third of critically ill children and neonates attained therapeutic vancomycin concentrations with empiric dosing guidelines is in keeping with studies in critically ill adults.30, 31 As few as 20% of adults attain therapeutic concentrations with initial empiric dosing, with wide interindividual variability in pharmacokinetic parameters (volume of distribution and clearance).30, 31 These variations in pharmacokinetic parameters reflect the underlying illnesses of the patients. In particular, trauma patients have consistently been found to have augmented renal clearance of vancomycin.30, 32 While data on vancomycin dosing and TDM in critically ill children are sparse, in the neonatal intensive care setting it has been shown that only 25–51% of trough concentrations are within target range with empiric dosing, despite both these studies targeting lower trough concentrations of 5–15 mg/L.33, 34 A more recent study, targeting trough concentrations of 10–20 mg/L found that only 25% of neonates attained therapeutic trough concentrations with empiric dosing, consistent with our findings.35 These findings are also consistent with data for other drugs that are renally cleared, such as gentamicin, where increased doses are required in the intensive care setting secondary to an illness‐induced hyperdynamic state and consequent increased volume of distribution.36 Continuous infusions of vancomycin have been proposed as a solution to the poor attainment of therapeutic vancomycin concentrations with intermittent dosing.37, 38, 39, 40 Overall, our data suggest that a more aggressive approach to vancomycin dosing in critically ill children and neonates is necessary. Doses at the higher end of daily recommendations were required in older children, with 75% of children attaining therapeutic concentrations with doses >50 mg/kg/day. In neonates, doses of 1.5–2 times the recommended dose were required to attain therapeutic concentrations in those with a CGA of <29 and 29–≤35 weeks respectively.
Vancomycin therapy in critically ill children is further complicated by an increased risk of supratherapeutic concentrations with resultant vancomycin‐associated renal impairment. This increased risk is related to the severity of the underlying illness, shock, concomitant medications, and organ failure.41 In our study, there was a similar proportion of children with supratherapeutic vancomycin concentrations compared to previously published paediatric data from outside the PICU setting. These studies reported that between 2.2 and 6.3% of patients had initial concentrations exceeding 20 mg/L42, 43 Accordingly, the overall rate of nephrotoxicity seen in our study (8%) is comparable with previously reported rates of 9–14% in the paediatric population.44, 45, 46 All vancomycin‐associated renal impairment that occurred in our cohort of children was mild and self‐limiting, as was also seen in other paediatric studies.44, 45, 46
The wide variability in dosing requirements seen in critically ill children suggests that a standard empiric vancomycin dose is not always appropriate in this population. In critically ill patients with sepsis or septic shock, there is a need to rapidly attain therapeutic vancomycin concentrations. This is likely only to be achieved with individualised vancomycin dosing algorithms, based on specific patient characteristics (i.e. creatinine clearance, weight, age).47, 48 In the intensive care setting, individualised dosing using Bayesian forecasting has been shown to improve attainment of target vancomycin concentrations when compared to standard empiric dosing in adults.49, 50 In neonates in intensive care, better attainment of target vancomycin concentrations has been shown with individualisation of dosing using a pharmacokinetic model, compared with dosing following standard guidelines (63 vs 39%, P < .02).51 Ideally, a population‐based Bayesian dosing algorithm, designed and validated specifically within critically ill children, would improve attainment of target concentrations while minimising toxicity.
Our study is limited by the retrospective nature of its design. As a result, data relating to creatinine levels were not available for some patients. TDM was performed as part of routine care, and the timing in relation to the dose varied. Because only trough concentrations were determined, the AUC could not be reliably calculated. Also, in only 49/126 (39%) courses, was a Gram‐positive bacterium isolated. Despite the large overall sample size for this study, data within each individual age strata were limited. Finally, a potential limitation of this study is that the achievement of steady state was not confirmed. However, paediatric drug formularies recommend determining vancomycin concentrations between 24–48 hours based on the fact that steady state is likely to have occurred.52, 53
It is clear that individualised dosing is needed in critically ill children and our study adds further weight to this argument. However, in the absence of model‐based dosing, in septic patients with normal renal function, vancomycin doses of at least 30 mg/kg/day in neonates with a CGA up to 35 weeks, and 50–60 mg/kg/day in all other children could be considered. Further studies to determine the optimal dosing regimen for critically ill patients is required. Optimisation of TDM practices through the development of protocols, and ideally built into electronic medical records, should be considered, particularly in a busy clinical setting such as PICU. Further research is needed to identify those subgroups of patients who require daily vancomycin doses exceeding the maximum daily recommendation of 80 mg/kg/day.
COMPETING INTERESTS
This study was conducted as part of routine work. There are no competing interest to declare.
5.
Supporting information
TABLE S1 Use of concomitant nephrotoxic medications
ACKNOWLEDGEMENTS
Dr Gwee conceptualised the study, supervised the analyses, and reviewed and revised the manuscript. Dr Sosnin collected and analysed the data, drafted the initial version of the manuscript and reviewed and revised the manuscript. A/Prof Cranswick, Dr Chiletti and Prof Curtis conceptualised the study and reviewed and revised the manuscript. All authors approved the final manuscript. We thank Christine Plover and the Royal Children's Hospital Pharmacy Department for providing the vancomycin dispensing data. We thank Poh Chua for her assistance with the literature review.
Sosnin N, Curtis N, Cranswick N, Chiletti R, Gwee A. Vancomycin is commonly under‐dosed in critically ill children and neonates. Br J Clin Pharmacol. 2019;85:2591–2598. 10.1111/bcp.14084
The authors confirm that the PIs for this paper are Dr Natasha Sosnin and Dr Amanda Gwee.
Data Availability Statement:The data that support the findings of this study are available from the corresponding author upon reasonable request.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Gentile LF, Cuenca AG, Efron PA, et al. Persistent inflammation and immunosuppression: a common syndrome and new horizon for surgical intensive care. J Trauma Acute Care Surg. 2012;72(6):1491‐1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin‐resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52(3):285‐292. [DOI] [PubMed] [Google Scholar]
- 3. Royal Children's Hospital . Vancomycin. In, Melbourne, Australia.
- 4. Jung Y, Song KH, Cho J, et al. Area under the concentration‐time curve to minimum inhibitory concentration ratio as a predictor of vancomycin treatment outcome in methicillin‐resistant Staphylococcus aureus bacteraemia. Int J Antimicrob Agents. 2014;43(2):179‐183. [DOI] [PubMed] [Google Scholar]
- 5. Jeffres MN, Isakow W, Doherty JA, Micek ST, Kollef MH. A retrospective analysis of possible renal toxicity associated with vancomycin in patients with health care‐associated methicillin‐resistant Staphylococcus aureus pneumonia. Clin Ther. 2007;29(6):1107‐1115. [DOI] [PubMed] [Google Scholar]
- 6. Elyasi S, Khalili H, Dashti‐Khavidaki S, Mohammadpour A. Vancomycin‐induced nephrotoxicity: mechanism, incidence, risk factors and special populations. A literature review. Eur J Clin Pharmacol. 2012;68(9):1243‐1255. [DOI] [PubMed] [Google Scholar]
- 7. Roberts JA, Lipman J. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit Care Med. 2009;37(3):840‐851. quiz 59 [DOI] [PubMed] [Google Scholar]
- 8. Blot SI, Pea F, Lipman J. The effect of pathophysiology on pharmacokinetics in the critically ill patient‐‐concepts appraised by the example of antimicrobial agents. Adv Drug Deliv Rev. 2014;77:3‐11. [DOI] [PubMed] [Google Scholar]
- 9. Pinder M, Bellomo R, Lipman J. Pharmacological principles of antibiotic prescription in the critically ill. Anaesth Intensive Care. 2002;30(2):134‐144. [DOI] [PubMed] [Google Scholar]
- 10. Paediatric Formulary Committee . British national formulary for children. London: BMJ Group and Pharmaceutical Press; 2016. [Google Scholar]
- 11. Safer Care Victoria . Normal laboratory values for neonates. In: Neonatal eHandbook.
- 12. The Royal College of Pathologists of Australasia . Creatinine In: RCPA Manual, 7th ed. Surry Hills, Australia: NSW; 2015. [Google Scholar]
- 13. Kearns GL, Abdel‐Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology‐‐drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349(12):1157‐1167. [DOI] [PubMed] [Google Scholar]
- 14. Rodieux F, Wilbaux M, van den Anker JN, Pfister M. Effect of kidney function on drug kinetics and dosing in neonates, infants, and children. Clin Pharmacokinet. 2015;54(12):1183‐1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239‐245. [DOI] [PubMed] [Google Scholar]
- 16. Summary of Recommendation Statements. Kidney International Supplements; 2: 8–12. [DOI] [PMC free article] [PubMed]
- 17. StataCorp . Stata Statistical Software: Release 15. In, College Station, TX: StataCorp LLC, 2017.
- 18. Hsu AJ, Hamdy RF, Huang Y, et al. Association between vancomycin trough concentrations and duration of methicillin‐resistant Staphylococcus aureus bacteremia in children. J Pediatric Infect Dis Soc. 2018;7:338‐341. [DOI] [PubMed] [Google Scholar]
- 19. Teich JM, Merchia PR, Schmiz JL, Kuperman GJ, Spurr CD, Bates DW. Effects of computerized physician order entry on prescribing practices. Arch Intern Med. 2000;160(18):2741‐2747. [DOI] [PubMed] [Google Scholar]
- 20. Evans RS, Pestotnik SL, Classen DC, Burke JP. Evaluation of a computer‐assisted antibiotic‐dose monitor. Ann Pharmacother. 1999;33(10):1026‐1031. [DOI] [PubMed] [Google Scholar]
- 21. Ferrandez O, Urbina O, Grau S, et al. Computerized pharmacy surveillance and alert system for drug‐related problems. J Clin Pharm Ther. 2017;42(2):201‐208. [DOI] [PubMed] [Google Scholar]
- 22. Hall AB, Montero J, Cobian J, Regan T. The effects of an electronic order set on vancomycin dosing in the ED. Am J Emerg Med. 2015;33(1):92‐94. [DOI] [PubMed] [Google Scholar]
- 23. Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health‐System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health System Pharm. 2009;66(1):82‐98. [DOI] [PubMed] [Google Scholar]
- 24. Moise‐Broder PA, Forrest A, Birmingham MC, Schentag JJ. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet. 2004;43(13):925‐942. [DOI] [PubMed] [Google Scholar]
- 25. Holmes NE, Turnidge JD, Munckhof WJ, et al. Vancomycin AUC/MIC ratio and 30‐day mortality in patients with Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2013;57(4):1654‐1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kishk OA, Lardieri AB, Heil EL, Morgan JA. Vancomycin AUC/MIC and Corresponding troughs in a pediatric population. J Pediatr Pharmacol Ther. 2017;22(1):41‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Le J, Bradley JS, Murray W, et al. Improved vancomycin dosing in children using area under the curve exposure. Pediatr Infect Dis J. 2013;32(4):e155‐e163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gwee A, Cranswick N, McMullan B, et al. Defining target vancomycin trough concentrations for treating Staphylococcus aureus infection in infants aged 0 to 90 days. JAMA Pediatr. 2019;173(8):791‐793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hornik CP, Fort P, Clark RH, et al. Early and late onset sepsis in very‐low‐birth‐weight infants from a large group of neonatal intensive care units. Early Hum Dev. 2012;88(Suppl 2):S69‐S74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bakke V, Sporsem H, Von der Lippe E, et al. Vancomycin levels are frequently subtherapeutic in critically ill patients: a prospective observational study. Acta Anaesthesiol Scand. 2017;61(6):627‐635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Obara VY, Zacas CP, Carrilho CM, Delfino VD. Currently used dosage regimens of vancomycin fail to achieve therapeutic levels in approximately 40% of intensive care unit patients. Rev Bras Ter Intensiva. 2016;28:380‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Udy AA, Roberts JA, Shorr AF, Boots RJ, Lipman J. Augmented renal clearance in septic and traumatized patients with normal plasma creatinine concentrations: identifying at‐risk patients. Crit Care. 2013;17:R35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mehrotra N, Tang L, Phelps SJ, Meibohm B. Evaluation of vancomycin dosing regimens in preterm and term neonates using Monte Carlo simulations. Pharmacotherapy. 2012;32(5):408‐419. [DOI] [PubMed] [Google Scholar]
- 34. Badran EF, Shamayleh A, Irshaid YM. Pharmacokinetics of vancomycin in neonates admitted to the neonatology unit at the Jordan University hospital. Int J Clin Pharmacol Ther. 2011;49(04):252‐257. [DOI] [PubMed] [Google Scholar]
- 35. Ringenberg T, Robinson C, Meyers R, et al. Achievement of therapeutic vancomycin trough serum concentrations with empiric dosing in neonatal intensive care unit patients. Pediatr Infect Dis J. 2015;34(7):742‐747. [DOI] [PubMed] [Google Scholar]
- 36. Kraus DM, Dusik CM, Rodvold KA, Campbell MM, Kecskes SA. Bayesian forecasting of gentamicin pharmacokinetics in pediatric intensive care unit patients. Pediatr Infect Dis J. 1993;12(9):713‐718. [DOI] [PubMed] [Google Scholar]
- 37. Cristallini S, Hites M, Kabtouri H, et al. New regimen for continuous infusion of vancomycin in critically ill patients. Antimicrob Agents Chemother. 2016;60(8):4750‐4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Van Herendael B, Jeurissen A, Tulkens PM, et al. Continuous infusion of antibiotics in the critically ill: the new holy grail for beta‐lactams and vancomycin? Ann Intensive Care. 2012;2(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cies JJ, Moore WS 2nd, Conley SB, et al. Continuous infusion vancomycin through the addition of vancomycin to the continuous renal replacement therapy solution in the PICU: a case series. Pediatric Crit Care Med. 2016;17(4):e138‐e145. [DOI] [PubMed] [Google Scholar]
- 40. Gwee A, Cranswick N, McMullan B, et al. Continuous versus intermittent vancomycin infusions in infants: a randomized controlled trial. Pediatrics. 2019;143(2):e20182179. [DOI] [PubMed] [Google Scholar]
- 41. Lacave G, Caille V, Bruneel F, et al. Incidence and risk factors of acute kidney injury associated with continuous intravenous high‐dose vancomycin in critically ill patients: a retrospective cohort study. Medicine (Baltimore). 2017;96(7):e6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Balch AH, Constance JE, Thorell EA, et al. Pediatric vancomycin dosing: trends over time and the impact of therapeutic drug monitoring. J Clin Pharmacol. 2015;55(2):212‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rajon K, Vaillancourt R, Varughese N, Villarreal G. Vancomycin use, dosing and serum trough concentrations in the pediatric population: a retrospective institutional review. Pharm Pract (Granada). 2017;15(2):887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McKamy S, Hernandez E, Jahng M, Moriwaki T, Deveikis A, Le J. Incidence and risk factors influencing the development of vancomycin nephrotoxicity in children. J Pediatr. 2011;158(3):422‐426. [DOI] [PubMed] [Google Scholar]
- 45. Constance JE, Balch AH, Stockmann C, et al. A propensity‐matched cohort study of vancomycin‐associated nephrotoxicity in neonates. Arch Dis Child Fetal Neonatal Ed. 2016;101(3):F236‐F243. [DOI] [PubMed] [Google Scholar]
- 46. Oudin C, Vialet R, Boulamery A, Martin C, Simon N. Vancomycin prescription in neonates and young infants: toward a simplified dosage. Arch Dis Child Fetal Neonatal Ed. 2011;96(5):F365‐F370. [DOI] [PubMed] [Google Scholar]
- 47. Shahrami B, Najmeddin F, Mousavi S, et al. Achievement of vancomycin therapeutic goals in critically ill patients: early individualization may be beneficial. Crit Care Res Pract. 2016;2016:1245815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pai MP, Neely M, Rodvold KA, Lodise TP. Innovative approaches to optimizing the delivery of vancomycin in individual patients. Adv Drug Deliv Rev. 2014;77:50‐57. [DOI] [PubMed] [Google Scholar]
- 49. Llopis‐Salvia P, Jimenez‐Torres NV. Population pharmacokinetic parameters of vancomycin in critically ill patients. J Clin Pharm Ther. 2006;31(5):447‐454. [DOI] [PubMed] [Google Scholar]
- 50. Pea F, Bertolissi M, Di Silvestre A, Poz D, Giordano F, Furlanut M. TDM coupled with Bayesian forecasting should be considered an invaluable tool for optimizing vancomycin daily exposure in unstable critically ill patients. Int J Antimicrob Agents. 2002;20:326‐332. [DOI] [PubMed] [Google Scholar]
- 51. Crumby T, Rinehart E, Carby MC, Kuhl D, Talati AJ. Pharmacokinetic comparison of nomogram‐based and individualized vancomycin regimens in neonates. American Journal of Health‐System Pharmacy: AJHP: Official Journal of the American Society of Health‐System Pharmacists. 2009;66(2):149‐153. [DOI] [PubMed] [Google Scholar]
- 52. Kim J, Walker SA, Iaboni DC, et al. Determination of vancomycin pharmacokinetics in neonates to develop practical initial dosing recommendations. Antimicrob Agents Chemother. 2014;58(5):2830‐2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. British National Formulary for Children. London, United Kingdom.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1 Use of concomitant nephrotoxic medications
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


