Abstract
Biosimilars of low molecular weight heparins (LMWHs) are more alike the originator than different branded LMWHs. The latter differ largely in molecular weight, anti‐FXa/anti‐FIIa ratio and antithrombin binding. The Food and Drug Administration and European Medicines Agency guidelines are sufficient for the clinical use of high quality LMWHs. However, the Food and Drug Administration guideline lacks the results of a phase I clinical trial in the approval process. Most information about biosimilars is available for enoxaparin given that many biosimilars of enoxaparin have received market access. The guidelines of many International Thrombosis Societies for LMWH biosimilars are too stringent, not updated and impractical for formulary uptake discussions. This review gives background information on critical factors for the formulary uptake process of LMWHs with special attention for the use of the System of Objectified Judgment Analysis/Infomatrix model.
Keywords: anticoagulant, biosimilar, formulary, generics, low molecular weight heparin
1. INTRODUCTION
Heparin was discovered nearly 100 years ago by Howell (Baltimore, USA).1 Heparin has to be administered intravenously with the need of frequent activated partial thromboplastin time monitoring. The effect of heparin is almost entirely due to its antithrombin action. It lasted until 1976 before it was elucidated that a specific pentasaccharide structure of the heparin molecule binds specific to antithrombin.
The discovery of low molecular weight heparin (LMWH), a small part of the large heparin molecule, in 1976 introduced the use of a new and more patient friendly subcutaneous administration for acute therapeutic interventions without the need of frequent activated partial thromboplastin time monitoring. Heparin inhibits the activated factors X and II, but LMWH inhibits only activated factor X. The effect of LMWH therapy can be monitored by an anti‐factor Xa (FXa) test. This test is not appropriate for heparin and measures partly the effect of an LMWH.2
Nowadays LMWHs are prescribed for treatment of a broad array of high‐risk potentially life‐threatening thromboembolic complications: acute coronary syndrome, acute deep vein thrombosis and pulmonary embolism. The use of LMWHs is also fully documented in thromboprophylaxis after surgery, in cancer patients and during pregnancy.
The introduction of direct‐acting oral anticoagulants has broadened the choice in anticoagulants for the practitioner. The LMWHs however still have an established and important role in the prevention and treatment of acute thromboembolic complications. In cancer patients with venous thromboembolism and prophylaxis during pregnancy, LMWHs remains the drugs of choice.3, 4
The first LMWHs were approved by the Food and Drug Administration (FDA) in the early 1990s. Their approval relied on analytical, biological and pharmacological data limited by the technology available that time. Nowadays, the US Pharmacopeia (USP), the European Pharmacopeia (EuPh) and others have monographs to test the quality of LMWHs by standard biological and analytical methods. Currently there are 8 LMWHs available with their own individual international nonproprietary name (INN) as approved by the World Health Organization. This indicates that the active ingredients of these branded products are not the same, given their difference in molecular weight distribution [see Table 1]. Enoxaparin is currently the LMWH with the highest market share and highest citation score in the Medline medical database (5071 hits, 28 July 2019). In contrast, biosimilar enoxaparin has only 21 hits (28 July 2019).
Table 1.
Specific characteristics of 8 branded originator low molecular weight (MW) heparins registered as international nonproprietary name (INN) by the World Health Organization (WHO) [Gray 2009; Minghetti 20139]
| INN WHO product name | Average MW (D) | EuPh Range MW (D) | Ratio anti‐FXa/anti‐FIIa | EuPh Range for ratio anti‐FXa/anti‐FIIa |
|---|---|---|---|---|
| Bemiparin | 3800 | 8.1–9.7 | ||
| Certoparin | 5400 | 1.5–2.5 | ||
| Dalteparin | 6000 | 5600–6400 | 2.5–4 | 1.9–3.2 |
| Enoxaparin a | 4500 | 3800–5000 | 3.3–5.3 | 3.3–5.3 |
| Nadroparin | 4300 | 3600–5000 | 2.5–4 | 2.5–4.0 |
| Parnaparin | 5000 | 4000–6000 | 1.5–3 | 1.5–3.0 |
| Reviparin | 4400 | 3000–4500 | 3.6–6.1 | |
| Tinzaparin | 6500 | 5500–7500 | 1.5 | 1.5–2.5 |
Reference product in this review; EuPh: European Pharmacopoeia.
In the last 10 years many biosimilar or generic variants of branded LMWHs were introduced. Most of the information on biosimilar LMWHs is available for enoxaparin. [http://www.guidetopharmacology.org; ID 6811: Clexane, enoxaparin sodium, Lovenox, PK‐10169, RP‐54563].
This review will focus on the regulatory aspects, quality aspects and clinical aspects of LMWHs. These data may be helpful for data collection of interchangeability and substitution between originator and biosimilar, and to enhance the selection process of biosimilar LMWHs for uptake in the National or Local Drug Formularies.
2. SCOPE OF THE REVIEW
This review will highlight:
Regulatory aspects of biosimilar and originator LMWHs: European Medicines Agency (EMA) vs US FDA/EuPh vs USP
Quality aspects. Molecular structure of LMWHs and new sophisticated methods for in vitro/in vivo characteristics of LMWHs
Clinical aspects of biosimilars with special attention for heparin‐induced thrombocytopenia (HIT)
Guidelines and recommendations for generic or biosimilar LMWHs for uptake in the Formulary
3. METHODS
Search Strategy Medline (no starting date and ended by 31 March 2019):
Mesh terms (all fields):
Biosimilar and/or LMWH
Biosimilar and/or enoxaparin
This is not a formal meta‐analysis of clinical studies because in the field of biosimilars only limited clinical data are needed and mostly not or not yet published for approval by EMA or FDA.
3.1. Regulatory aspects
The European Commission prepared jointly with the EMA a guideline on “Non‐clinical and clinical development of similar biological medicinal products containing low‐molecular‐weight‐heparins”.5 The comparison of data required for approval of a biosimilar vs a reference medicine is qualitatively nearly the same. However, quantitatively, the LMWH biosimilar authorization process is less expanded than guidelines for non‐LMWH biosimilars.6
The EMA guideline for a biosimilar LMWH asks for the approval of specific aspects of the quality comparison: e.g. molecular weight distribution and overall chemical composition, starting material (e.g. porcine) and mode of depolymerization, disaccharide building blocks, fragment mapping profiles and sequences of selected unfragmented oligosaccharides, and biological and biochemical assays. Nonclinical studies need: in vitro assays for evaluation of anti‐FXa and anti‐FIIa, an appropriate in vivo pharmacodynamics model (anti‐FXa, anti‐FIIa and release of tissue factor pathway inhibitor [TFPI]). Immunogenicity does not have to be evaluated in a clinical trial because of the low predictability.
Pharmacodynamic tests for LMWHs are available to test the equivalence and some aspects of safety and efficacy. In HIT, anti‐PF4/heparin antibodies play a dominant role in unfractionated heparin and to a lesser extent in LMWHs.7 The incidence of HIT is low (<1%) in LMWHs and valuable comparative data on LMWH biosimilars are lacking in the registry files because of the low incidence of this serious complication.
For clinical studies, the EMA states that conventional pharmacokinetic studies cannot be performed, instead pharmacodynamics activities (anti‐XFXa, anti‐FIIa activity and TFPI) are most relevant. A small 2‐way, double blind study with subcutaneous LMWH in 20 healthy volunteers is needed. Within the authorization procedure a pharmacovigilance/risk management plan is needed.
Most striking are the differences in assessment of comparability of LMWHs in Europe and the USA. The FDA made a statement that if a generic product e.g. enoxaparin contained the same active ingredient, it is marked as a generic drug. This is reflected in 5 criteria by the FDA: (i) the physical and chemical characteristics of enoxaparin; (ii) the nature of heparin material and the chemical process to break up heparin into smaller pieces; (iii) the nature and arrangement of components that constitute enoxaparin; (iv) specific laboratory measurements of the product's anticoagulant activity; and (v) specific aspects (e.g. HIT) of the drug effects in humans. These 5 criteria should ensure that a generic enoxaparin product will have the same drug effects when administered to the patient. As such, in the USA, there is no need for additional clinical trials in patients to demonstrate equivalence of the clinical effectiveness and safety of generic enoxaparin to the branded formulations (Lovanex, Clexane).8, 9
The monographs of LMWHs in the US Pharmacopeia (USP) and Europe (PhEu) are based on long‐term experience with normal unfractionated heparin. However, after the 2006 contamination scandal of oversulfated chondroitin in the unfractionated heparin, which is the raw starting material for LMWHs, more alertness for contaminants in pharmacopeia is urgent needed.10, 11 Monographs of USP and PhEu have been adapted since that event to ensure better quality of enoxaparin generic or biosimilar formulations.
In conclusion, the FDA has a different approach for authorization of LMWHs. The FDA states that LMWHs are semisynthetic drugs (generics) while the EMA classifies them as biological drugs (biologicals). We argue that the authorization process by EMA is more appropriate than the FDA process. However, the lack of need for phase III clinical studies in the approval process by FDA and EMA makes high quality post‐marketing studies on safety issues more urgent. In section 4, this is illustrated by uptake of phase III trials the criteria for formulary implementation of LMWHs.
3.2. Quality aspects: molecular structure of LMWHs and new sophisticated methods for characterization
Heparin is the raw material for the production of LMWHs. The source for heparin is lung tissue or porcine mucosa. Today, porcine intestinal mucosa is the preferred raw material for unfractionated heparin (UFH) as raw material for production of LMWHs. LMWHs are produced by partially depolymerizing UFH to exhibit a molecular weight of 3–7 kD.
LMWHs can be produced by using a variety of means of depolymerization (e.g. physical, chemical or enzymatic) of UFH. This can cause small structural differences. Dalteparin is produced using nitrous acid digestion, which produces an anhydromanno group at the reducing end of the LMWH chain. The structural characterization of dalteparin is well documented by combining different analytical strategies.12 Tinizaparin is produced by enzymatic cleavage of UFH with heparinase‐I, which induces a double band at the reducing end of the LMWH chain.13, 14 Most of the experimental work on quality control and structural characterization has been done with enoxaparin.15 Enoxaparin is derived from heparin extracted from porcine intestinal mucosa, by depolymerization that leads to lower molecular weight fractions. Enoxaparin is further obtained via alkaline β‐elimination of the UFH benzylester. Most interesting are research data from China that the antithrombin binding sites for different sample lots of enoxaparin (Teva; Sandoz, USA) may differ. Although they have a similar disaccharide and 3‐O‐sulfogroup containing tetrasaccharide composition, they were different in antithrombin‐III binding.16 There may be potential clinical differences in effect if fractions containing larger sized chains and have a higher antitrombin binding. Overall the similarity of generic enoxaparin (Teva) has good lot‐to‐lot consistency. The US‐marketed generic enoxaparins from Sandoz/Momenta, Winthrop and Amphastar, compared to the originator from Sanofi, exhibit dissimilarities in terms of their composition, but the clinical relevance of this differences in unclear and possible irrelevant.17, 18 Furthermore, many of the publications or authors are sponsored by the originator, so one cannot exclude conflict of interest.19, 20
Small differences in chemical structure may result from the production process or the porcine heparin source.17 Figure 1 presents the synthesis and schematic structure of enoxaparin and the specific aspects of different domains in the molecule.
Figure 1.
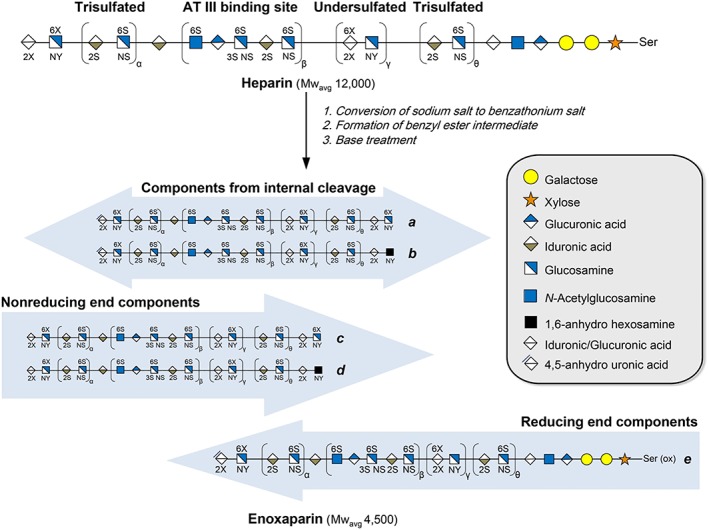
Synthesis of enoxaparin from heparin with schematic structures. Chains generated from the internal structure of parent heparin chain are the most abundant components. (with permission Elsevier and author: Liu et al. J Chromatography A 2017;1480:32040)
The main chemical characteristics of European and US LMWHs are presented in Table 2.
Table 2.
Main chemical characteristics of low molecular weight (MW) heparins (LMWHs) [Minghetti et al.9]
| Depolarization method | LMWH | Representative and residues | MW mean | Xa/IIa ratio | Pharmacopeia specific assays | Sulfatation degree |
|---|---|---|---|---|---|---|
|
Deaminative cleavage Nitrous acid |
Nadroparin § |
Nonreducing; 2‐O‐sulfo‐alfa‐L‐ idopyranosuric acid Reducing |
See Table 1 |
See Table 1 |
Ethanol; N‐NO groups; free sulfates
Nitrite, boron |
2.5
1.8 |
|
Dalteparin # |
6‐O‐sulfo‐2,5‐anhydro‐D‐mannitol | 2.5 | ||||
| Isoamyl‐nitrate |
Reviparin § |
2–2.6 | ||||
|
Certoparin § |
2 | |||||
|
Beta‐eliminative cleavage benzylation and alkaline treatment |
Enoxaparin # |
Nonreducing: 4‐enopyranose urinate Reducing: 1,6‐anhydro derivate |
See Table 1 | See Table 1 |
Benzylalcohol Content + % oligosaccharide chains that are cylizied in 1,6‐anhydro ring |
|
|
Alkaline treatment with quarternary NH4‐salt |
Bemiparin § |
‐ | ‐ |
‐ |
||
|
Enzymatic treatment Heparinase |
Tinzaparin # |
Nonreducing end: 2‐O‐sulfo‐4 enepyrano‐suric acid Reducing end: 2‐N,6‐O‐disulfo‐D‐glucosamine |
See Table 1 | See Table 1 | ||
|
Radical‐catalysed depolymerization: peroxide + copper salt |
Parnaparin § |
Nonreducing end: 2‐O‐sulafo‐alfa‐L‐idopyrano‐suric acid Reducing end: 2‐N,6‐O‐disulfo‐D‐glucosamine |
Copper | |||
| Market | #USA + EU §EU |
To have full access to the physicochemical properties of biosimilar or generic LMWHs it is essential to have access to the Investigator Medical Product dossier that is used for the regulatory approval process. We note that only for enoxaparin are there sufficient data in the public domain for full physicochemical characterization of biosimilar of generic LMWHs.
It is widely accepted that individual LMWHs are chemically unique agents and the therapeutic interchangeability of different brands has been questioned, although they are approved for the same indications.14 We have to realize that the differences between different biosimilar/generic enoxaparin presentations are much smaller than the differences between the different 8 brands of LMWHs. Nowadays, the use of enoxaparin biosimilar or generics is fully accepted in clinical practice.
Note that because of the foreseeable shortage of intestinal porcine mucosa as source for heparin (the raw product for the production of LMWHs), expansion to other animal tissues seems necessary. Currently, porcine intestine is the only approved source for producing LMWHs in most countries.21 Enoxaparin prepared from ovine heparin closely resembles branded enoxaparin and will soon be tested clinically.22
The development of new synthetic oligosaccharides, in addition to pentasaccharides, were published, which can possibly replace biological LMWHs in the future.23
3.3. Clinical aspects of LMWHs with special attention for HIT
The guidelines of the US FDA and EMA are leading the clinical development plan of biosimilar/generic LMWHs. The US FDA approval does not require comparative clinical trials between the originator and the similar LMWH. The EMA has an updated guideline which came into effect in June 2017.5 This guideline states that conventional pharmacokinetic studies cannot be performed. Instead, pharmacodynamic activities, most importantly anti‐Xa, anti‐F IIa and TFPI between biosimilar LMWH and reference brand should be performed. The study design should be a randomized, single dose, 2‐way cross‐over and preferably double‐blind in healthy volunteers.
Studies for intravenous or intra‐arterial use are not needed, since subcutaneous administration covers both adsorption and elimination of the LMWH. The selected dose should be in the sensitive (steep) part of the dose–response curve. A dedicated comparative efficacy trial is not considered necessary. For the detection of clinical safety, a comparative safety/immunogenicity study in patients is needed. The immunogenicity assessment includes determination of HPF4 antibodies and platelet count for early detection of HIT II events. Major bleedings have to be documented. A risk management plan after market access and authorization in accordance with current European legislation and pharmacovigilance guidelines is obligatory.
Although antithrombin binding of LMWHs is not part of the clinical data asked for by FDA and EMA assessment process, studies have been performed that detected small differences between the original enoxaparin (Sanofi) and the generic LMWH (Teva). It is unlikely that the differences found between 1 batch of Sanofi enoxaparin and 2 batches of Teva enoxaparin have any clinical consequences.24
There are 3 biosimilarity studies with enoxaparin published from 2008 up to July 2018. Most are Phase I cross over studies in healthy volunteers, with subcutaneous single doses of 100, 400 and 600 mg enoxaparin or comparator. The general conclusions from these studies is that they are bioequivalent.25, 26, 27 One study has the focus on immunogenicity of a product from Brazil compared to the originator enoxaparin. There was a difference in AHPF4 antibodies for the biosimilar, but the authors made no conclusions what should be the consequence of this finding.28 Another explorative trial with the same Brazilian enoxaparin in 200 patients with venous thromboembolism showed no difference in effect.29 The pharmacovigilance of biosimilars in the European has not yet lead to safety signals.30
Specific attention on the occurrence of HIT should be a more prominent part of the discussion. HIT is a thrombotic disorder caused by immune complexes containing platelet factor 4 antibodies (PF4) and heparin or cellular glycoseaminoglycans. From clinical studies with UFH and LMWHs the incidence of clinical relevant HIT is respectively <1 and 5%.31 PF4‐heparin antibodies are detected in 2–8% of the LMWH treated patients.32 The reason for only some patients with antibodies acquiring HIT has not been clarified. It has been suggested that a particular fragment of heparin to PF4 is needed to develop the antigen epitope.33 However, it should be noted that diagnostic specificity of widely applied PF4 dependent immunoassays is not very high.32 Commercial immunoassays detect PF4/heparin antibodies in 1–4.3% of healthy subjects. This background prevalence overlaps the seropositive rates in LMWH‐ and heparin treated patients, so the normal cut‐off may require refinement. Although active surveillance of enoxaparin (generic/biosimilar) is useful and encouraged, the current spontaneous reporting system will not distinguish product‐specific safety signals such as HIT incidence. Comparative clinical trials are not feasible, because >1000 patients in both groups are needed given the low incidence of HIT. Clinicians have to use the most practical approach: regular thrombocyte counts during therapy with LMWHs for early detection of HIT, although in clinical practice, this is mostly neglected.
Clinical use of LMWHs is restricted in patients with renal failure, because LMWHs have renal clearance. Dose adjustments and dosing guided by anti‐FXa is required for safety reasons in order to prevent bleeding complications.34
3.4. Guidelines and formulary considerations for biosimilar LMWH
Many recommendations for the development and use of biosimilar LMWHs have been published. A summary of these recommendations is presented by the International Society on Thrombosis and Haemostasis,35 The North American Thrombosis Forum, The Scientific Committee of the International Union of Angiology, South Asian Society of Artherosclerosis and Thrombosis, The American Society of Chest Physicians, The Society of Hospital Medicine, the American Pharmacists Association, American College of Cardiology, American Health Association, Austrian Society of Hematology and Oncology,36 and the Italian Society for Haemostasis and Thrombosis.37
In summary, most of these guidelines refer preferably to the EMA, World Health Organization and FDA guidance and their shortcomings.
The recommendation and guidelines of different societies refer to the heparin source and production process, analytical methods, lower limits for impurities that influence the coagulation system, information on lot‐to lot variation, toxicology studies, animal pharmacodynamic studies including thrombosis and bleeding models, phase I and III studies, and pharmacodynamics studies in renally impaired persons. The file should also include clinical safety and efficacy of the generic LMWH and a pharmacovigilance programme on the level of traceability of the generic.
The recommendations are very detailed and many of them have not been revised over the last 5 years. Many of the proposals are relevant for improving the process for regulators and only partly for the formulary uptake process.
Furthermore, many guidelines have nonrealistic proposals or have authors with conflict of interest as they have received consultancy or speaker fees from companies who promote the originator LMWHs.36, 37
We present some of the nonrealistic proposals from the guidelines36:
-
1
Biosimilar follow up‐on LMWHs must be produced exactly as described in the monograph of the originator product.
Comment: This seems impossible because the production data are mostly confidential and modern production methods are often more precise than the older methods used for the originator.
-
2
Batch‐to‐batch analysis demonstrating no difference between biosimilar follow‐on and originator LMWH.
Comment: This also seems impossible given that fact that biosimilars are characterized nowadays with different analytical strategies than those used when the originator was launched.12, 13
-
3
A minimum of 2 double blind randomized parallel group clinical phase III trials for noninferiority for each indication and therapeutic equivalence studies in sensitive indications such as venous thromboembolism or acute coronary syndrome.
Comment: This is not realistic. For such a phase III trial, over 1000 patients in each arm are needed to power the trial sufficiently, with extreme high costs.
The formulary decision making is a complex process, practical skills are needed to make a high‐quality formulary decision.
Taking into account the critical factors in the choice of LMWH in the formulary decision process we state that a set of minimal requirements to warrant appropriate standards of quality and of LMWHs is needed. Also, a strategy for reduction of prices and to enhance access to LMWH treatment worldwide is needed.
Dutch clinical pharmacists and researchers from Queens University of Belfast made a model for formulary decision that is applicable for choices within a therapeutic group e.g. different LMWHs and/or different biosimilar LMWHs: System of Objectified Judgment Analysis and the Infomatrix method: http://www.sojaonline.com.38, 39, 40
This model has proven to fulfil criteria for drug selection in the formulary uptake process and for pharmacy benefit managers and payers.
Table 3 shows an example of key factors and weight factors that have to be determined and approved by voting in advance by the Pharmacy and Therapeutic committee members. The biosimilar LMWH with the highest score is the preferential drug for formulary uptake.
Table 3.
Example of critical factors for formulary uptake of biosimilar low molecular weight heparin by the use of the System of Objectified Judgment Analysis/Infomatrix system. Note: Weight factor and key factors are in real life determined by Pharmacy and Therapeutic members
| Key factora | Weight factor | Originator‐enoxaparin | Biosimilar A enoxaparin | Biosimilar B enoxaparin |
|---|---|---|---|---|
| Quality | ||||
|
10 | 10 | 10 | 10 |
|
10 | 5 | 10 | 10 |
| Nonclinical | ||||
| 5 | 5 | 0 | 5 | |
| Clinical | ||||
|
10 | 10 | 0 | 10 |
|
5 | 5 | 5 | 0 |
|
5 | 5 | 5 | 5 |
|
5 | 5 | 0 | 0 |
|
5 | 5 | 0 | 0 |
|
5 | 5 | 0 | 0 |
| Other | ||||
dosage forms available |
20 | 20 | 10 | 20 |
|
10 | 10 | 10 | 0 |
| Cost considerations | ||||
| Price | 30 | 0 | 30 | 30 |
| Total score | 120 (max) | 85 | 80 | 90 |
Items needed for the European Medicines Agency approval process are performed and thus there are no key factors.
NNH = number needed to harm; HIT, heparin‐induced thrombocytopenia.
The LMWH biosimilars that have the most extensive documentations and scientific publications are enoxaparine TEVA (FDA), enoxaparin Momenta‐Sandoz (FDA), enoxaparin Techdow UK (EMA) and enoxaparine Chemi s.p.a.Italy (EMA).
4. CONCLUSION
Biosimilar LMWHs are competitive and less expensive than the originator. Most of the documentation by far is available for the originator and the biosimilar of enoxaparin. Since the first introduction in clinical practice, new and better analytical methods are available to identify more precisely the biosimilar/generic LMWH‐characteristics from the originator.
For formulary uptake of LMWHs, straightforward instruments and models to enhance policy making in a transparent, rational way are needed. The System of Objectified Judgment Analysis/Infomatrix model is based on transparent and clinical relevant selection items and applicable for payers and formulary uptake of LMWHs.40, 43 A proposal for critical key factors for uptake of a biosimilar LMWH in the formulary and/or electronic prescribing module is presented.
Pharmacovigilance programmes and traceability are essential for the further evaluation of LMWH‐biosimilar differences.44
COMPETING INTERESTS
M.J.B. and J.E.R.v.L. have no competing interests to declare. J.R.B.J.B. received an unrestricted grant from Techdow London UK, 1 of the distributors in Europe of a LMWH biological.
Brouwers JRBJ, Roeters van Lennep JE, Beinema MJ. Biosimilars of low molecular weight heparins: Relevant background information for your drug formulary. Br J Clin Pharmacol. 2019;85:2479–2486. 10.1111/bcp.14081
The authors confirm the Principal Investigator of this paper is Jacobus Brouwers and that he had direct responsibility for the initiation of this subject and the selection of substances described
REFERENCES
- 1. Wardrop D, Keeling D. The story of the discovery of heparin and warfarin. Br J Haematol. 2008;141(6):757‐763. [DOI] [PubMed] [Google Scholar]
- 2. Hemker HC. A century of heparin: past, present and future. J Thromb Haemost. 2016;14(12):2329‐2338. [DOI] [PubMed] [Google Scholar]
- 3. Young AM, Marshal A, Thirlwall J, et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with thromboembolism: results of a randomized trial (SELECT‐D). J Clin Oncol. 2018;36(20):2017‐2023. [DOI] [PubMed] [Google Scholar]
- 4. Fogerty AE. Management of venous thromboembolism in pregnancy. Curr Treat Options Cardiovac Med. 2018;20(8):69. [DOI] [PubMed] [Google Scholar]
- 5. EMA ‐ Guideline on non‐clinical and clinical development of similar biological medicinal products containing low‐molecular‐weight‐ heparins 10 Nov 2016 EMA/CHMP/BMWP/118264/2007 revision 1 (into effect 01‐June 2017) p 1–8. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-non-clinical-clinical-development-similar-biological-medicinal-products-containing-low_en.pdf. Accessed August 17, 2019.
- 6. Kurki P, van Aerts L, Wolff‐Holz E, Giezen T, Skibeli V, Weise M. Interchangeability of biosimilars: a European perspective. BioDrugs. 2017;31(2):83‐91. [DOI] [PubMed] [Google Scholar]
- 7. Junqueira DR, Zorzela LM, Penini E. Unfractionated heparin versus low molecular weight heparins for avoiding heparin‐induced thrombocytopenia in postoperative patients. Cochrane Database Syst Rev. 2017;4:CD007557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Courage N, Parsons A. The comparability conundrum: biosimilars in the United States, Europe and Canada. Food Drug Law J. 2011;66:203‐224. [PubMed] [Google Scholar]
- 9. Minghetti P, Cilurzo F, Franzé S, Musazzi UM, Itri M. Low molecular weight heparins copies: are they considered to be generics or biosimilars? Drug Disc Today. 2013;18(5/6):305‐311. [DOI] [PubMed] [Google Scholar]
- 10. Fareed J, Hoppenstaedt DA, Ramacciotti E, Hull RD. Contaminants in heparins: are all facts known? Clin Appl Thromb Hemost. 2010;16(3):242‐243. [DOI] [PubMed] [Google Scholar]
- 11. Ramacciotti E, Clark M, Sadeghi N, et al. Contaminants in heparin: review of the literature, molecular profiling, and clinical applications. Clin Appl Thromb Hemost. 2011;17(2):126‐135. [DOI] [PubMed] [Google Scholar]
- 12. Bisio A, Urso E, Guerrini M, de Wit P, Torri G, Naggi A. Structural characterization of the LMWH dalteparin combining different analytical strategies. Molecules. 2017;22:1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maddineni J, Walenga JM, Jeske WP, et al. Product individuality of commercial available LMWHs and their generic versions: therapeutic implications. Clin Appl Thromb Hemostas. 2006;12(3):267‐276. [DOI] [PubMed] [Google Scholar]
- 14. Merli G, Vanscoy G, Rihn T, Groce JB 3rd, McCormick W. Applying scientific criteria to therapeutic interchange: a balanced analysis of low‐molecular‐weight heparins. J Thromb Thrombolysis. 2001;11(3):247‐259. [DOI] [PubMed] [Google Scholar]
- 15. Gupta R, Ponnusamy MP. Analysis of sulfates on low molecular weight heparin using mass spectrometry: structural characterization of enoxaparin. Expert Rev Proteomics. 2018;15(6):503‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen Y, Zhao J, Yu Y, et al. Antitrombin III‐binding site analysis of LMWHs. J Pharm Sci. 2018;107:1290‐1295. [DOI] [PubMed] [Google Scholar]
- 17. Liu X, St Ange K, Lin L, Zhang F, Chi L, Linhardt RJ. Top‐down and bottom up analysis of commercial enoxaparins. J Chromatogr A. 2017;1480:32‐40. [DOI] [PubMed] [Google Scholar]
- 18. Guerrini M, Rudd TR, Mauri L, et al. Differentiation of generic enoxiparins marketed in the United States by employing NMR and multivariate analysis. Anal Chem. 2015;87(16):8275‐8283. [DOI] [PubMed] [Google Scholar]
- 19. Mourier PAJ, Herman F, Sizun P, Viskov C. Analytical comparison of a US generic enoxaparin with the originator product: the focus on comparative assessment of antihtrombin‐binding components. J Pharm Biomed Anal. 2016;129:542‐550. [DOI] [PubMed] [Google Scholar]
- 20. Cohen M, Jeske WP, Nicolau JC, Montalescot G, Fareed J. US food and dug Administration approvel of generic versions of complex biologics: implications for the practicing physician using LMWHs. J Thromb Thrombol. 2012;33(3):230‐238. [DOI] [PubMed] [Google Scholar]
- 21. Guan Y, Xu X, Liu X, et al. Comparison of LMWHs prepared from bovine lung heparin and porcine instestine heparin. J Pharm Sci. 2016;105(6):1843‐1850. [DOI] [PubMed] [Google Scholar]
- 22. Chen J, Yanlei Y, Fareed J, et al. Comparison of low molecular weight heparins prepared from ovine heparins with enoxaparin. Clin Appl Thromb/Hemost. 2019;25:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xu Y, Chandrarajoti K, Zhang X, et al. Synthetic oligosaccharides can replace animal‐sourced low‐molecular weight heparins. Sci Transl Med. 2017;9(406):eaan5954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mourier PAJ, Herman F, Sizun P, Viskov C. Analytical comparison of a US generic enoxaparin with the originator product: the focus on comparative assessment of anti‐thrombin binding components. J Pharm Biomed Anal. 2016;129:542‐550. [DOI] [PubMed] [Google Scholar]
- 25. Kuczka K, Harder S, Picard‐Willems B, et al. Biomarkers and coagulation tests for assessing the biosimilarity of generic low‐molecular‐weight heparin: results of a study in healthy subjects with enoxaparin. J Clin Pharmacol. 2008;48(10):1189‐1196. [DOI] [PubMed] [Google Scholar]
- 26. Feng L, She‐Tu J, Liu J, Chen J, Wu L, Huang M. Bioequivalence of generic and branded subcutaneous enoxaparin: a single dose: randomized‐sequence, open‐label, two period cross over study in healthy male subjects. Clin Ther. 2009;31(7):1559‐1567. [DOI] [PubMed] [Google Scholar]
- 27. Martínez González J, Monreal M, Ayani Almagia I, Llaudó Garín J, Ochoa Díaz de Monasterioguren L, Gutierro Adúriz I. Bioequivalence of a biosimilar enoxaparin sodium of Clexane after single 100mg subcutaneous dose: results of a randomized, double‐blind, cross over study in healthy volunteers. Drug des Devel Ther. 2018;12:575‐582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gomes M, Ramacciotti E, Hoppenstaedt D, et al. An open label, non‐randomized, prospective clinical trial evaluating the immunogenecity of branded enoxaparin versus biosimilars in healthy volunteers. Clin Appl Thromb Hemost. 2011;17(1):66‐69. [DOI] [PubMed] [Google Scholar]
- 29. Gomes M, Ramcciotti E, Henriques AC, et al. Generic versus branded enoxaparin in the prevention of venous thromboembolism following major abdominal surgery: report of an exploratory clinical trial. Clin Appl Thromb Hemost. 2011;17(6):633‐639. [DOI] [PubMed] [Google Scholar]
- 30. Casadevall GM, Ortel TL. Clinical practice: heparin induced thrombocytopenia. N Engl J Med. 2006;355(8):809‐817. [DOI] [PubMed] [Google Scholar]
- 31. Junqueira DR, Zorzela LM, Perini E. Unfractionated heparin versus low molecular weight heparins for avoiding heparin induced thrombocytopenia in postoperative patients. Cochrane Database Syst Rev. 2017;4:CD007557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Arepally GM, Hursting MJ. Platelet factor4/heparin antibody (IgG/M/a) in healthy subjects: a literature analysis of commercial immunoassay results. J Thromb Thrombolysis. 2008;26(1):55‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jeske W, Walenga JM, Hoppensteadt D, Fareed J. Update on the safety and bioequivalence of biosimilars ‐ focus on enoxaparin. Drug Healthc Patient Saf. 2013;5:133‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. DeCarolis DD, Thornson JG, Clairmont MA, Leuthner AM, Rector TS, Johnson GJ. Enoxaparin outcomes in patients with moderate renal impairment. Arch Intern Med. 2012;172(22):1713‐1718. [DOI] [PubMed] [Google Scholar]
- 35. Harenberg J, Walenga J, Torri G, et al. Update of the recommendations on biosimilar low molecular weight heparins from the scientific committee on control of anticoagulation of the international society on thrombosis and Haemostasis. J Thromb Haemost. 2013;11(7):1421‐1425. [DOI] [PubMed] [Google Scholar]
- 36. Kalodiki E, Leong W, on behalf of the SASAT task force on generic LMWHs . SASAT (South Asian Soc Artherosclerosis & Thrombosis) proposal for regulatory guidelines for generic LMWHs. Cin Appl Thromb Hemost. 2009;15(1):8‐11. [DOI] [PubMed] [Google Scholar]
- 37. Imberti D, Marietta M, Polo Friz H, Cimminiello C. The introduction of biosimilars of LMWHs in Europe: a critical review and reappraisal endorsed by the Italian Society for Haemostasis and Thrombosis (SISET) and the Italian Society for Angiology and Vascular Medicine (SIAPAV). Thromb J. 2017;15:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Janknegt R, Scott M, Mairs J, Timoney M, McElnay J, Brenninkmeijer R. System of objectified judgment analysis (SOJA) as a tool for rational and transparent drug decision making. Exp Opion Pharmacother. 2007;8(Supp 1):S5‐S14. [DOI] [PubMed] [Google Scholar]
- 39. Brenninkmeijer R, Janknegt R. Application of SOJA and Infomatrix in practice: interactive web and workshop tools. Exp Opion Pharmacother. 2007;8(Supp1):S49‐S55. [DOI] [PubMed] [Google Scholar]
- 40. Scott M, Timoney M, Mairs J, et al. Matrix models and STEPS: concluding remarks. Exp Opinion Pharmacother. 2007;8(Suppl1):S65‐S67. [DOI] [PubMed] [Google Scholar]
- 41. Oliveira S‐N, Santos GR, Glauser BF, et al. Structural and functional analysis of biosimilar enoxaparins available in Brazil. Thromb Haemost. 2015;113(01):53‐65. [DOI] [PubMed] [Google Scholar]
- 42. Jeske W, Litinas E, Khan H, Hoppensteadt D, Fareed J. A comparison of pharmacodynamic behavior of branded and biosimilar enoxaparin in primates. Clin Appl Thromb Hemost. 2012;18(3):294‐298. [DOI] [PubMed] [Google Scholar]
- 43. Smeeding J, Malone DC, Ramchandani M, Stolshek B, Green L, Schneider P. Biosimilars: considerations for payers. P&t. 2019;44:54‐63. [PMC free article] [PubMed] [Google Scholar]
- 44. Chao J, Skup M, Alexander E, et al. Nomenclature and tracebility debate for biosimilars: small molecule surrogates lend support for distinguishable nonproprietary names. Adv Ther. 2015;32(3):270‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]


