Abstract
Acute and advanced heart failure are associated with substantial adverse short- and longer-term prognosis. Both conditions necessitate complex treatment choices to restore haemodynamic stability and organ perfusion, relieve congestion, improve symptoms and allow the patient to leave the hospital and achieve an adequate quality of life. Among the available intravenous vasoactive therapies, inotropes constitute an option when an increase in cardiac contractility is needed to reverse a low output state. Within the inotrope category, levosimendan is well suited to the needs of both sets of patients since, in contrast to conventional adrenergic inotropes, it has not been linked in clinical trials or wider clinical usage with increased mortality risk and retains its efficacy in the presence of beta-adrenergic receptor blockade; it is further believed to possess beneficial renal effects. The overall haemodynamic profile and clinical tolerability of levosimendan, combined with its extended duration of action, have encouraged its intermittent use in patients with advanced heart failure. This paper summarises the key messages derived from a series of 12 tutorials held at the Heart Failure 2019 congress organised in Athens, Greece, by the Heart Failure Association of the European Society of Cardiology.
Keywords: Acute heart failure, advanced heart failure, cardiorenal syndrome, inotropes, inodilators, levosimendan
The use of IV vasoactive drugs, diuretics, vasodilators and inotropes for correcting haemodynamic dysfunction in patients with decompensated heart failure has been described over many decades.[1] However, data on their effects on prognosis do not offer a convincing picture of clinical benefit.[2] This is particularly true regarding IV inotropes. Clinical data collected on the effects of cardiac glycosides, catecholamines and phosphodiesterase inhibitors indicate an overall increase in mortality risk.[3,4] Increased cardiomyocyte oxygen consumption in ischaemically jeopardised myocardium, plus a heightened propensity to cardiac arrhythmias, have been proposed as possible explanations for these findings.[5]
The calcium sensitiser and potassium channel opener levosimendan has emerged in recent years as potentially a safer inotropic option than the traditional classes of cardio-mobilising drugs by virtue of its different mechanism of action.[6,7] Levosimendan delivers inotropy via a broadly energy-neutral route, and vasodilation, including reduction of central venous pressure, relief of hepatic congestion and indications of improvement in renal function.[8] Taken in combination with an extended duration of effect ascribable to a long-acting metabolite, this profile identifies levosimendan as a unique inotrope for the management of acute heart failure (AHF) and advanced heart failure (AdHF).[9–12]
This article presents some views on the use of vasoactive drugs in the management of AHF and AdHF that emerged during a series of tutorials held in conjunction with the annual congress of the Heart Failure Association of the European Society of Cardiology (ESC), in Athens, Greece in May 2019. Twelve speakers (from Austria, Cyprus, Finland, Germany, Greece, Hungary, Italy, Spain, Sweden and Switzerland) delivered the tutorials and collaborated in the development of this text.
Levosimendan in Acute Heart Failure
The assessment and management of AHF have been set on a robust practical footing by the most recent ESC guidelines, to which readers are referred for a comprehensive statement on this subject.[13] Summarising broadly, AHF may be described as a situation of rapid onset or worsening of the signs and symptoms of HF. AHF must, inter alia, be characterised as a life-threatening medical condition that requires urgent evaluation and management and frequently leads to hospitalisation.
AHF may present de novo or as a deterioration in chronic HF. Many cases will arise from primary cardiac dysfunction, notably MI, but extrinsic precipitants, such as infection or anaemia, may play a role, along with an extensive range of triggering factors.[13] Other high-risk cohorts include patients with severe aortic stenosis, mitral regurgitation, acute pulmonary embolism or serious cardiac arrhythmias.
An immediate priority in the work-up of a case of suspected AHF is to identify patients with either cardiogenic shock (CS) and/or respiratory failure. These are among the approximately 10% of patients who are critically ill and require intensive care.
Systemic blood pressure is an important guide to the classification and management of AHF. A systolic blood pressure level <90 mmHg is encountered in about 10% of patients, but the occurrence of hypotension of this degree, in conjunction with evidence of inadequate peripheral perfusion, identifies those who are candidates for inotropic therapy and, possibly, vasopressors. These patients usually correspond to the ‘wet and cold’ quadrant of the AHF clinical classification, which is associated with notably poor prognosis.[14]
From a pathophysiological perspective, a key aspect of HF is that it flattens the increase in cardiac output to a given afterload, giving rise to ‘forward’ failure. Use of inotropic drugs can be a valid response to this situation, but the repertoire of available agents is restricted. Indeed, it may be argued that levosimendan is one of the few inotropes for which a compelling justification of use can be provided, and in some circumstances it is perhaps the only one.
Clinical trials and meta-analyses conducted over the past quarter of a century have repeatedly indicated that conventional adrenergic inotropes and phosphodiesterase (PDE)-3 inhibitors increase cellular energy consumption and are sometimes associated with increased mortality, arrhythmias, or other safety concerns. By contrast, levosimendan does not cause an increase in cellular oxygen demand or calcium content, thus having a more favourable safety profile, as seen in an overview of the long-term mortality outcome of the regulatory clinical trials (Figure 1). Levosimendan, which has been in clinical use for more than 20 years and has been evaluated in controlled clinical trials involving >3,000 HF patients, represents an established inotropic therapy in AHF.[15]
Figure 1: Effect of Levosimendan on Survival.
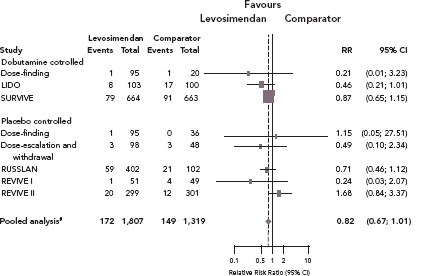
Meta-analysis of the results of the phase II and III clinical trials considered in the regulatory process. These trials included the Dose-finding study by Slawsky et al.,[72] the Dose-escalation and withdrawal study by Nieminen et al.,[73] the LIDO study by Follath et al.,[74] the RUSSLAN study by Moiseyev et al.,[33] the SURVIVE study by Mebazaa et al.[75] and the REVIVE I and II studies by Packer et al.[76] Pooled statistics were calculated using the Cochran–Mantel–Haenszel test, controlling for the study. Graphic rendition from data by Pollesello et al.[15]
Of course, these remarks should not be regarded as carte blanche for the use of levosimendan or any other specific inotrope. Indeed, it may be argued on the basis of various sources of clinical evidence that inotropes are, in general, overused in AHF, whereas vasodilators are possibly underused.[16–19] Appreciation of causative pathophysiology is central to correcting this situation. AHF is a phenotype suitable for treatment with vasodilators, as the product of vasoconstriction with increase in venous return, increased left ventricular pressure and fluid redistribution leading to pulmonary congestion. Inotrope therapy is properly confined to AHF arising from a low cardiac output condition. A few observations highlight the need to improve the identification of patients who really need inotropic support (and perhaps the selection of the most appropriate inotrope for any particular case).[13]
However, within that qualifying population, inodilators, such as levosimendan, should be the therapy of preference for patients already receiving beta-blockers, those with AHF of ischaemic aetiology and those experiencing cardiorenal syndrome. Aspects of renal function in AHF and AdHF are considered later in this article.
Levosimendan in Acute Heart Failure or Cardiogenic Shock Arising from Acute Coronary Syndromes
AHF in the context of acute coronary syndromes (ACS) is an urgent situation that requires early identification and treatment, not least because AHF can deteriorate into CS. Risk factors for the emergence of AHF in ACS include advanced age, previous MI or chronic HF, diabetes, hypertension and female sex.
More than 40% of cases of AHF were encountered with episodes of ACS in the EuroHeart Failure Survey II, and the combination of ACS with AHF has been associated with very poor survival prospects.[18] The Finnish Acute Heart Failure (FINN-AKVA) study documented an almost twofold higher 30-day mortality in AHF patients with ACS than in non-ACS cases (13% versus 8%; p=0.03).[20] ACS–AHF was also associated with prolonged hospitalisation and with more costly treatment in the intensive care unit. Similar adverse findings for the interplay between ACS and AHF have been recorded in the CardShock study and other investigations.[21]
As evidenced by the Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies (SWEDEHEART) registry, both the incidence of AHF as a complication of ACS and the mortality associated with ACS–AHF have decreased in recent years: between 1996 and 2008, the incidence of AHF as a sequel to ACS declined from 46% to 28% (p<0.001).[22] This downward trend has been particularly marked in patients with ST-segment elevation MI and is very likely attributable to a more frequent use of primary percutaneous coronary intervention (PCI), which assures early reperfusion and salvage of jeopardised myocardium, thereby averting the emergence of AHF.[23] The use of more high-sensitivity troponin testing to enhance detection of minor evolving ischaemia may also have contributed to this trend.
The results of the Culprit Lesion Only PCI versus Multivessel PCI in Cardiogenic Shock (CULPRIT-SHOCK) trial make a strong case for favouring a culprit-lesion-only strategy in most patients when performing PCI for ACS–AHF.[24] The short-term risks associated with longer procedure times, more complex interventions and higher doses of contrast agents seem to outweigh any potential benefits of a multivessel approach.
There is extensive polypharmacy in ACS–AHF, with widespread use of inotropes, vasopressors and other classes of drugs, but many of these practices are empirical and pragmatic rather than evidence based.[20] Formal structured research into the relative merits of different drug therapies in ACS–AHF is lacking and there is insufficient reliable information regarding the comparative efficacy of different agents.[25,26]
Some broad principles of therapy may nevertheless be identified. Several of these apply with special force to the management of CS, the emergence of which is identified in the 2016 ESC guidelines as warranting consideration of inotrope use.[13] The percentage of ACS episodes that progress to CS is relatively low (≤10%), but short-term (in-hospital) mortality in CS is exceptionally high (40% in CardShock, higher in other reports) and CS is the leading cause of death in patients with acute MI.[21,23,27]
The management of CS includes haemodynamic support with inotropes and vasopressors to increase cardiac output and blood pressure in order to restore tissue perfusion. Inotropes as a broad class are endorsed to support the circulation of patients who are demonstrably hypotensive and/or hypoperfused despite adequate filling pressures. This circumscribed indication reflects concerns that conventional adrenergic inotropes (and PDE-3 inhibitors) increase cellular energy demands and oxygen consumption in a situation of ischaemic compromise and may exert undesirable tachycardic or pro-arrhythmic effects. Levosimendan, by virtue of its calcium-sensitising action, does not exert untoward effects of this kind to the same degree and, moreover, exhibits anti-stunning and condition effects that may be relevant and advantageous in states of ischaemia.[28–32] The survival benefit of levosimendan in the Randomized Study on Safety and Effectiveness of Levosimendan in Patients with Left Ventricular Failure after an Acute Myocardial Infarct (RUSSLAN) trial supports those considerations, as do the findings of a meta-analysis of six studies (n=1,065), which documented improvements in various indices of haemodynamic function in ACS patients treated with IV levosimendan, with no adverse effect on mortality in AHF–CS patients and a strong signal for a survival benefit in AHF–ACS patients (RR 0.74, 95% CI [0.58–0.93]; p=0.01).[33,34]
The Survival of Patients with Acute Heart Failure in Need of Intravenous Inotropic Support (SURVIVE) trial compared levosimendan and dobutamine in AHF.[25] For the subset of patients who had acute MI as a cause of AHF, mortality in both treatment groups was two to three times that in the non-ischaemic subset and 31-day mortality was 4% lower in the levosimendan ACS–AHF group (28% versus 32%), a notable, although not statistically significant, survival gain.
It has been proposed that levosimendan may be considered in four clinical AHF–CS scenarios based on a patient’s haemodynamic status and Killip classification.[25] In the lower Killip classes, and in patients with relatively well-sustained blood pressure (systolic >110 mmHg), levosimendan may be used as monotherapy to enhance urinary output if the response to diuretics is inadequate. In the more advanced stages with pulmonary oedema or frank CS, levosimendan may be combined with a vasopressor such as noradrenaline to augment cardiac output and raise blood pressure.
When vasopressors are used to support blood pressure there should be a strong presumption for noradrenaline over adrenaline, based on data from a head-to-head controlled comparison in CS and findings from CardShock.[35,36]
The combination of vasopressors plus inodilators may offer better short-term prognosis than vasopressor therapy alone (HR 0.66, 95% CI [0.55–0.80]). This proposition is based on a pooled analysis from three observational studies and requires confirmation in a suitably powered randomised controlled trial.[37] The Acute Heart Failure Global Survey of Standard Treatment (ALARM-HF) registry, which contributed data to this analysis, indicated within a single dataset of reasonable size (n=4,953) that inodilatation as delivered by levosimendan was associated with substantially better survival than inopressors or adrenergic inotropes (Figure 2).[38] This identifies, subject to confirmation, a niche for levosimendan, which may be used in combination with noradrenaline as an alternative to dobutamine. Of note in this context, the blood pressure-lowering effect of levosimendan does not appear to require excessive increases in vasopressor dosage in CS.[39] Because its inotropic effect is independent of beta-adrenoceptor stimulation, levosimendan is an appropriate haemodynamic support for ACS–AHF or CS patients on chronic beta-blocker therapy.
Figure 2: In-hospital Mortality Rates of Inotropes.
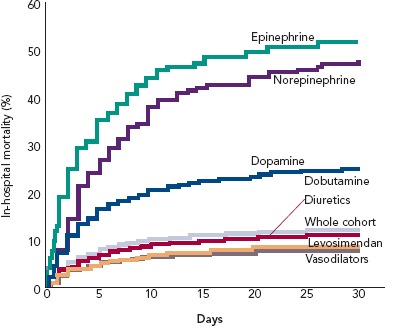
In the ALARM-HF registry, use of the inodilator levosimendan was linked with a notably lower mortality rate than traditional adrenergic inotropes. Graphic rendition from data by Mebazaa et al.[38]
All inotropes and vasopressors should be used at the lowest dose and for the shortest time possible. Levosimendan should be administered at an individualised infusion rate in the range 0.05–0.2 μg/kg/min. Loading dose is to be used when in need for immediate effect, and if systolic blood pressure exceeds 100 mmHg. Ideally, the infusion rate should be closely monitored and individualised in dependency of tolerability and haemodynamic response. Hypovolaemia and/or hypokalemia must be corrected before and during treatment. The effects of a 24-hour infusion of levosimendan persist for up to 2 weeks due to the long-lasting effect of its active metabolite, but the haemodynamic effects may be longer. Thereafter, treatment may safely be repeated.
Levosimendan in Advanced Heart Failure
Patients with AdHF suffer from severe and persistent symptoms that are often intractable to recommended drug therapies; they typically have marked limitation of exercise capacity and accompanying impaired QoL and are likely to have undergone repeated hospitalisations.[40] AdHF is also widely associated with progressive deterioration in the function of multiple organ systems, including the kidneys and liver. AdHF may affect up to 10% of patients with HF and this prevalence may be expected to increase in future because of growth in the HF population and improved survival among AdHF patients.
Some published studies and some preliminary observations on the physiological effects of levosimendan in AdHF provide a starting point for an appraisal of the drug’s use in this context. A series of recent studies has examined the impact of levosimendan treatment on the lungs, heart and skeletal muscle.[41–43] Collectively, these studies provided evidence that single-dose levosimendan administration to AdHF patients was accompanied by:
improved peak oxygen uptake and amelioration of ventilation efficiency;
reduced brain natriuretic peptide (BNP);
increased cardiac output at rest and during exercise;
improved lung mechanics and diaphragm function;
restoration of the normal function of alveolar capillary cells (but not of alveolar capillary gas diffusion); and
improved oxygen delivery to the muscle and muscle oxygen utilisation.
The Heart Failure Association of the ESC reviewed its definition of AdHF in 2018.[44] In our collective opinion, this revised definition provides the best available starting point for a consideration of treatment options, with the proviso that it is not a guideline and that it offers neither classes of recommendation nor formal, structured levels of evidence.
Heart transplantation (HTx) remains the definitive intervention in AdHF and delivers very good outcomes.[45] However, donor shortage limits this option to a minority of patients who must be carefully selected from those who are simultaneously at high risk of dying without a transplant and who may be expected to have good prognosis after receiving a donor heart.[46]
For the many patients rendered ineligible for HTx by virtue of age and/or co-morbidities or by the absence of a donor heart, long-term mechanical circulatory support (MCS) with continuous flow left ventricular assist devices (LVADs) may now be a valid alternative destination therapy (DT). About half of the >2,500 LVADs implanted annually in the US are intended as DT measures. Contemporary registries report good survival with LVADs as DT (78% and 68% at 1 and 2 years, respectively between 2013 and 2016 in the International Society for Heart and Lung Transplantation Mechanically Assisted Circulatory Support [INTERMACS] registry).[47]
Complication rates with MCS remain tangible, but the risk of death, disabling stroke and device reoperation has been substantially reduced with the advent of newer devices.[48,49]
Many AdHF patients falling outside the parameters for HTx or MCS receive inotropes to stabilise their haemodynamic status and relieve symptoms. Repeated scheduled infusions of drugs, such as dobutamine or PDE-3 inhibitors, should be avoided because of concerns about malignant arrhythmias and increased mortality.[50–52]
In contrast, the intermittent use of levosimendan has been shown to be safe and well tolerated; neither the LEVO-Rep nor LION-HEART randomised controlled trials produced indications of increases in all-cause mortality or sudden cardiac death during four and six cycles, respectively, of levosimendan therapy.[53,54] In addition, levosimendan offers persistent haemodynamic improvement thanks to a pharmacologically active metabolite with a long half-life.
A survival effect of intermittent levosimendan has not been demonstrated in a properly powered randomised controlled trial, but the results of the Pulsed Infusions of Levosimendan in Outpatients With Advanced Heart Failure (Levo-Rep) and Intermittent IV Levosimendan in Ambulatory Advanced Chronic Heart Failure Patients (LION-HEART) trials make a persuasive case for further evaluation of levosimendan in this context.[53,54] The Repetitive Levosimendan Infusion for Patients With Advanced Chronic Heart Failure (LeoDOR) trial is currently recruiting patients for this purpose (NCT03437226). This multicentre randomised controlled trial is designed to explore the safety and efficacy of repetitive levosimendan infusions (seven cycles at 0.2 μg/kg/min for 6 hours every 2 weeks or five cycles at 0.1 μg/kg/min for 24 hours every 3 weeks) administered to AdHF patients following a recent HF-related hospitalisation.
As many as 80% of AHF hospitalisations are the product of acute-on-chronic deterioration in haemodynamic status; this may include cases where AHF is superimposed on AdHF.[40] As was exemplified in the findings of the Efficacy of Vasopressin Antagonism in Heart Failure: Outcome Study with Tolvaptan (EVEREST) study, congestion and dyspnoea precede the emergence of AHF; more generally, haemodynamic congestion precedes symptomatic congestion, which in turn precedes hospitalisation for AHF.[55] As described by Zile et al. and conceptualised by Adamson, the phase of presymptomatic congestion may precede the emergence of overt clinical symptoms by several days to weeks.[56,57]
The existence of this period of preclinical decline represents an opportunity for intervention that may avert unplanned hospitalisation due to haemodynamic crisis. Given that repeat hospitalisation for AHF is associated with progressively deteriorating survival prospects, identifying and exploiting this opportunity for pre-emptive treatment is clearly in the interests of patients. Observations on the feasibility of pre-symptomatic intervention to avert hospitalisation add weight to observations in the LEVO-Rep and LION-HEART trials that use of intermittent levosimendan in outpatients with AdHF was associated with marked improvement in event-free survival (LEVO-Rep) or a reduction in HF hospitalisation (LION-HEART).[53,54,58,59] A recent meta-analysis of six studies of intermittent levosimendan in chronic HF has produced an estimated risk ratio of 0.40 (95% CI [0.27–0.59]; p<0.00001), with consistency of effect in all the contributing studies.[60]
Differential Renal Effects of Levosimendan
Kidney dysfunction is encountered in a substantial proportion of patients with AHF or AdHF.[61] In this setting, it is usually secondary to impaired cardiac function, conforming to the definition of type 1 cardiorenal syndrome (CRS). Various pathophysiological mechanisms contribute to kidney damage in CRS, including hypoperfusion, renal venous congestion and neurohormonal activation.
Renal dysfunction has repeatedly been shown to be one of the most adverse prognostic indicators for patients with HF and to be linked with prolonged hospitalisation.[62–65] Therefore, pharmacological and non-pharmacological interventions for AHF or AdHF need to be shaped by the ambition to preserve or rectify renal perfusion, the deterioration of which underlies the emergence of kidney dysfunction.
The use of inodilators or inotropes to avert or correct CRS may be particularly apt in patients with low blood pressure or hypoperfusion and the specific effects of levosimendan on renal vasculature and haemodynamics highlight its potential in these cases.[66–68] Those effects include selective vasodilation of the renal glomerular afferent arterioles, thereby enhancing renal filtration directly as well as via its effect on cardiac output.
Lannemyr et al. recently reported that both levosimendan (loading dose of 12 μg/kg for 10 minutes, then infusion at 0.1 μg/kg/min for 65 minutes; n=16) and dobutamine (continuous infusion started at 5.0 μg/kg/min for 10 minutes, then 7.5 μg/kg/min for 65 minutes; n=16) improve systemic haemodynamics and renal blood flow to a similar extent in patients with chronic HF (mean baseline left ventricular ejection fraction 27%) and impaired renal function (mean eGFR <80 ml/min/1.73 m2).[69] However, only levosimendan increased eGFR (Figure 3), supporting the proposition that levosimendan causes selective vasodilation of afferent renal arterioles whereas dobutamine dilates both afferent and efferent vessels. These data indicate that the similarity of effect on systemic haemodynamic indices may not translate into correspondingly favourable effects on renal perfusion and signal that levosimendan may be a preferred inotropic agent for the management of CRS in the setting of low-output AHF or AdHF.
Figure 3: Effects of Levosimendan and Dobutamine in Chronic Heart Failure.
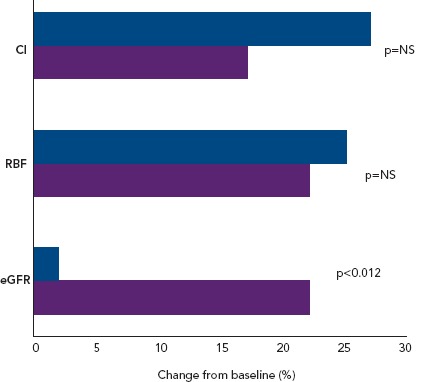
Increase of eGFR by levosimendan, but not dobutamine, in patients with chronic heart failure.[69] Percentage changes from baseline in cardiac index, renal blood flow and eGFR after administration of IV levosimendan or dobutamine. CI = cardiac index; RBF = renal blood flow. Purple bars = levosimendan; blue bars = dobutamine. From data by Lannemyr et al.[69]
Case studies reviewed at Heart Failure 2019 illustrate that levosimendan may also be appropriate as part of a bridge to transplant strategy for preserving renal function in patients with AdHF and restrictive cardiomyopathy. A series of 35 repeat courses of levosimendan therapy delivered over 20 months was associated with large and sustained improvements in a series of indicators of renal function, including creatinine, N-terminal pro-BNP and the need for oral potassium supplementation. This intervention brought creatinine levels, the most responsive and most quickly reacting indicator of haemodynamic effects on kidney function in CRS, into the normal range for six consecutive months before further clinical deterioration necessitated HTx. These experiences are consistent with an earlier report of long-term improvement in renal function in a prospective study of 40 patients with AdHF treated with levosimendan while awaiting HTx.[70]
Conclusion
The appropriate, effective and successful use of IV vasoactive drugs in AHF and AdHF is founded on accurate assessment of the aetiology of decompensation and the broader patient profile. Where congestion or hypertension predominate, and patients present with either fluid accumulation or fluid redistribution, the management emphasis should favour vasodilators and diuretics to unload the heart and mobilise fluid. Inotropes and/or vasopressors are indicated for ‘wet and cold’ patients who exhibit inadequate peripheral perfusion despite adequate filling status. These patients usually present with low blood pressure (systolic <90 mmHg), but it should be kept in mind that hypoperfusion is not synonymous with hypotension; hypoperfusion may not always be followed by significant hypotension, as in the presence of sympathetic overactivation causing peripheral vasoconstriction. Levosimendan is an inodilator with a unique pharmacology (Table 1), and may be appropriate for similar patients with higher blood pressure if they are refractory to vasodilator and diuretic therapy.[71] A series of clinical scenarios warranting the use of inotropes/inodilators and/or vasopressors is shown in Table 2 and illustrates the wide-ranging utility of levosimendan as an intervention in these situations.
Table 1: Molecular Targets and Pharmacological Effects of Levosimendan.
| Molecular Targets | Pharmacological Effects |
|---|---|
|
|
|
|
|
|
Table 2: Indications for IV Vasoactive Drugs in Clinical Scenarios in Heart Failure.
| Clinical Setting | Agent |
|---|---|
| Increased pulmonary artery pressure |
|
| Need for beta-blocker |
|
| Hypotension |
|
| Worsening renal function |
|
| Ischaemic disease |
|
Acknowledgments
The authors acknowledge Hughes Associates, Oxford, UK, for assistance in the preparation and editing of the manuscript.
References
- 1.Sinnenberg L, Givertz MM. Acute heart failure. Trends Cardiovasc Med. 2019. April 2; Epub ahead of print. [DOI] [PubMed]
- 2.Rayner-Hartley E, Virani S, Toma M. Update on the management of acute heart failure. Curr Opin Cardiol. 2018;33:225–31. doi: 10.1097/HCO.0000000000000485. [DOI] [PubMed] [Google Scholar]
- 3.Belletti A, Castro ML, Silvetti S et al. The effect of inotropes and vasopressors on mortality: a meta-analysis of randomized clinical trials. Br J Anaesth. 2015;115:656–75. doi: 10.1093/bja/aev284. [DOI] [PubMed] [Google Scholar]
- 4.Gong B, Li Z, Yat Wong PC. Levosimendan treatment for heart failure: A systematic review and metanalysis. J Cardiothorac Vasc Anesth. 2015;29:1415–25. doi: 10.1053/j.jvca.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 5.Teerlink JR, Metra M, Zacà V et al. Agents with inotropic properties for the management of acute heart failure syndromes. Traditional agents and beyond. Heart Fail Rev. 2009;14:243–53. doi: 10.1007/s10741-009-9153-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pollesello P, Papp Z, Papp JG. Calcium sensitizers: What have we learned over the last 25 years? Int J Cardiol. 2016;203:543–8. doi: 10.1016/j.ijcard.2015.10.240. [DOI] [PubMed] [Google Scholar]
- 7.Papp Z, Édes I, Fruhwald S et al. Levosimendan: molecular mechanisms and clinical implications: consensus of experts on the mechanisms of action of levosimendan. Int J Cardiol. 2012;159:82–7. doi: 10.1016/j.ijcard.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 8.Nieminen MS, Fruhwald S, Heunks LM et al. Levosimendan: current data, clinical use and future development. Heart Lung Vessel. 2013;5:227–45. [PMC free article] [PubMed] [Google Scholar]
- 9.Najjar E, Stålhberg M, Hage C et al. Haemodynamic effects of levosimendan in advanced but stable chronic heart failure. ESC Heart Fail. 2018;5:302–8. doi: 10.1002/ehf2.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Altenberger J, Gustafsson F, Harjola VP et al. Levosimendan in acute and advanced heart failure: An appraisal of the clinical database and evaluation of its therapeutic applications. J Cardiovasc Pharmacol. 2018;71:129–36. doi: 10.1097/FJC.0000000000000533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asif M. A review on role of the calcium sensitive inotropic agent, levosimendan and its metabolites. Mini Rev Med Chem. 2018;18:1354–62. doi: 10.2174/1389557516666160905094721. [DOI] [PubMed] [Google Scholar]
- 12.Farmakis D, Alvarez J, Gal TB et al. Levosimendan beyond inotropy and acute heart failure: Evidence of pleiotropic effects on the heart and other organs: An expert panel position paper. Int J Cardiol. 2016;222:303–12. doi: 10.1016/j.ijcard.2016.07.202. [DOI] [PubMed] [Google Scholar]
- 13.Ponikowski P, Voors AA, Anker SD et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 14.Nohria A, Tsang SW, Fang JC et al. Clinical assessment identifies hemodynamic profiles that predict outcomes in patients admitted with heart failure. J Am Coll Cardiol. 2003;41:1797–804. doi: 10.1016/S0735-1097(03)00309-7. [DOI] [PubMed] [Google Scholar]
- 15.Pollesello P, Parissis J, Kivikko M et al. Levosimendan meta-analyses: Is there a pattern in the effect on mortality? Int J Cardiol. 2016;209:77–83. doi: 10.1016/j.ijcard.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Yancy CW, Lopatin M, Stevenson LW et al. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol. 2006;47:76–84. doi: 10.1016/j.jacc.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 17.Gheorghiade M, Abraham WT, Albert NM et al. Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. JAMA. 2006;296:2217–26. doi: 10.1001/jama.296.18.2217. [DOI] [PubMed] [Google Scholar]
- 18.Nieminen MS, Brutsaert D, Dickstein K et al. EuroHeart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J. 2006;27:2725–36. doi: 10.1093/eurheartj/ehl193. [DOI] [PubMed] [Google Scholar]
- 19.Follath F, Yilmaz MB, Delgado JF et al. Clinical presentation, management and outcomes in the Acute Heart Failure Global Survey of Standard Treatment (ALARM-HF) Intensive Care Med. 2011;37:619–26. doi: 10.1007/s00134-010-2113-0. [DOI] [PubMed] [Google Scholar]
- 20.Tarvasmäki T, Harjola VP, Nieminen MS et al. Acute heart failure with and without concomitant acute coronary syndromes: patient characteristics, management, and survival. J Card Fail. 2014;20:723–30. doi: 10.1016/j.cardfail.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 21.Harjola VP, Lassus J, Sionis A et al. Clinical picture and risk prediction of short-term mortality in cardiogenic shock. Eur J Heart Fail. 2015;17:501–9. doi: 10.1002/ejhf.260. [DOI] [PubMed] [Google Scholar]
- 22.Desta L, Jernberg T, Löfman I et al. Incidence, temporal trends, and prognostic impact of heart failure complicating acute myocardial infarction. The SWEDEHEART Registry (Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies): a study of 199,851 patients admitted with index acute myocardial infarctions, 1996 to 2008. JACC Heart Fail. 2015;3:234–42. doi: 10.1016/j.jchf.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Mebazaa A, Combes A, van Diepen S et al. Management of cardiogenic shock complicating myocardial infarction. Intensive Care Med. 2018;44:760–73. doi: 10.1007/s00134-018-5214-9. [DOI] [PubMed] [Google Scholar]
- 24.Thiele H, Akin I, Sandri M et al. One-year outcomes after PCI strategies in cardiogenic shock. N Engl J Med. 2018;379:1699–710. doi: 10.1056/NEJMoa1808788. [DOI] [PubMed] [Google Scholar]
- 25.Nieminen MS, Buerke M, Cohen-Solál A et al. The role of levosimendan in acute heart failure complicating acute coronary syndrome: A review and expert consensus opinion. Int J Cardiol. 2016;218:150–7. doi: 10.1016/j.ijcard.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Schumann J, Henrich EC, Strobl H et al. Inotropic agents and vasodilator strategies for the treatment of cardiogenic shock or low cardiac output syndrome. Cochrane Database Syst Rev. 2018;1:CD009669. doi: 10.1002/14651858.CD009669.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah AH, Puri R, Kalra A. Management of cardiogenic shock complicating acute myocardial infarction: A review. Clin Cardiol. 2019;42:484–93. doi: 10.1002/clc.23168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sonntag S, Sundberg S, Lehtonen LA et al. The calcium sensitizer levosimendan improves the function of stunned myocardium after percutaneous transluminal coronary angioplasty in acute myocardial ischemia. J Am Coll Cardiol. 2004;43:2177–82. doi: 10.1016/j.jacc.2004.02.052. [DOI] [PubMed] [Google Scholar]
- 29.De Luca L, Sardella G, Proietti P et al. Effects of levosimendan on left ventricular diastolic function after primary angioplasty for acute anterior myocardial infarction: a Doppler echocardiographic study. J Am Soc Echocardiogr. 2006;19:172–7. doi: 10.1016/j.echo.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 30.Hönisch A, Theuring N, Ebner B et al. Postconditioning with levosimendan reduces the infarct size involving the PI3K pathway and KATP-channel activation but is independent of PDE-III inhibition. Basic Res Cardiol. 2010;105:155–67. doi: 10.1007/s00395-009-0064-9. [DOI] [PubMed] [Google Scholar]
- 31.Meyer K, Klocke RC, Schipke JD et al. Ca2+ sensitizer superior to catecholamine during myocardial stunning? Eur J Cardiothorac Surg. 2008;34:326–31. doi: 10.1016/j.ejcts.2008.04.042. [DOI] [PubMed] [Google Scholar]
- 32.Meyer K, Schipke JD, Klocke RC et al. Inotropic, vasodilating and preconditioning actions of levosimendan in the heart. Thorac Cardiovasc Surg. 2008;56:379–85. doi: 10.1055/s-2008-1038729. [DOI] [PubMed] [Google Scholar]
- 33.Moiseyev VS, Põder P, Andrejevs N et al. Safety and efficacy of a novel calcium sensitizer, levosimendan, in patients with left ventricular failure due to an acute myocardial infarction. A randomized, placebo-controlled, double-blind study (RUSSLAN) Eur Heart J. 2002;23:1422–32. doi: 10.1053/euhj.2001.3158. [DOI] [PubMed] [Google Scholar]
- 34.Shang G, Yang X, Song D et al. Effects of levosimendan on patients with heart failure complicating acute coronary syndrome: A meta-analysis of randomized controlled trials. Am J Cardiovasc Drugs. 2017;17:453–63. doi: 10.1007/s40256-017-0237-0. [DOI] [PubMed] [Google Scholar]
- 35.Levy B, Clere-Jehl R, Legras A et al. Epinephrine versus norepinephrine for cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol. 2018;72:173–82. doi: 10.1016/j.jacc.2018.04.051. [DOI] [PubMed] [Google Scholar]
- 36.Tarvasmäki T, Lassus J, Varpula M et al. Current real-life use of vasopressors and inotropes in cardiogenic shock—adrenaline use is associated with excess organ injury and mortality. Crit Care. 2016;20:208. doi: 10.1186/s13054-016-1387-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pirracchio R, Parenica J, Resche Rigon M et al. The effectiveness of inodilators in reducing short term mortality among patients with severe cardiogenic shock: a propensity-based analysis. PLoS One. 2013;8:e71659. doi: 10.1371/journal.pone.0071659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mebazaa A, Parissis J, Porcher R et al. Short-term survival by treatment among patients hospitalized with acute heart failure: the global ALARM-HF registry using propensity scoring methods. Intensive Care Med. 2011;37:290–301. doi: 10.1007/s00134-010-2073-4. [DOI] [PubMed] [Google Scholar]
- 39.Russ MA, Prondzinsky R, Christoph A et al. Hemodynamic improvement following levosimendan treatment in patients with acute myocardial infarction and cardiogenic shock. Crit Care Med. 2007;35:2732–9. doi: 10.1097/00003246-200712000-00010. [DOI] [PubMed] [Google Scholar]
- 40.Braunwald E. Heart failure. JACC Heart Fail. 2013;1:1–20. doi: 10.1016/j.jchf.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 41.Mushtaq S, Andreini D, Farina S et al. Levosimendan improves exercise performance in patients with advanced chronic heart failure. ESC Heart Fail. 2015;2:133–41. doi: 10.1002/ehf2.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Campodonico J, Mapelli M, Spadafora E et al. Surfactant proteins changes after acute hemodynamic improvement in patients with advanced chronic heart failure treated with levosimendan. Respir Physiol Neurobiol. 2018;252(253):47–51. doi: 10.1016/j.resp.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 43.Magrì D, Brioschi M, Banfi C et al. Circulating plasma surfactant protein type B as biological marker of alveolar-capillary barrier damage in chronic heart failure. Circ Heart Fail. 2009;2:175–80. doi: 10.1161/CIRCHEARTFAILURE.108.819607. [DOI] [PubMed] [Google Scholar]
- 44.Crespo-Leiro MG, Metra M, Lund LH et al. Advanced heart failure: a position statement of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2018;20:1505–35. doi: 10.1002/ejhf.1236. [DOI] [PubMed] [Google Scholar]
- 45.Khush KK, Cherikh WS, Chambers DC et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-fifth Adult Heart Transplantation Report-2018; Focus Theme: Multiorgan Transplantation. J Heart Lung Transplant. 2018;37:1155–68. doi: 10.1016/j.healun.2018.07.022. [DOI] [PubMed] [Google Scholar]
- 46.Mehra MR, Canter CE, Hannan MM et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: A 10-year update. J Heart Lung Transplant. 2016;35:1–23. doi: 10.1016/j.healun.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 47.Kirklin JK, Xie R, Cowger J et al. Second annual report from the ISHLT Mechanically Assisted Circulatory Support Registry. J Heart Lung Transplant. 2018;37:685–91. doi: 10.1016/j.healun.2018.01.1294. [DOI] [PubMed] [Google Scholar]
- 48.Mehra MR, Goldstein DJ, Uriel N et al. Two-year outcomes with a magnetically levitated cardiac pump in heart failure. N Engl J Med. 2018;378:1386–95. doi: 10.1056/NEJMoa1800866. [DOI] [PubMed] [Google Scholar]
- 49.Kormos RL, Cowger J, Pagani FD et al. The Society of Thoracic Surgeons Intermacs Database Annual Report: Evolving Indications, Outcomes, and Scientific Partnerships. Ann Thorac Surg. 2019;107:341–53. doi: 10.1016/j.athoracsur.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 50.Tacon CL, McCaffrey J, Delaney A. Dobutamine for patients with severe heart failure: a systematic review and meta-analysis of randomised controlled trials. Intensive Care Med. 2012;38:359–67. doi: 10.1007/s00134-011-2435-6. [DOI] [PubMed] [Google Scholar]
- 51.Amsallem E, Kasparian C, Haddour G et al. Phosphodiesterase III inhibitors for heart failure. Cochrane Database Syst Rev. 2005;1:CD002230. doi: 10.1002/14651858.CD002230.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Metra M, Eichhorn E, Abraham WT et al. Effects of low-dose oral enoximone administration on mortality, morbidity, and exercise capacity in patients with advanced heart failure: the randomized, double-blind, placebo-controlled, parallel group ESSENTIAL trials. Eur Heart J. 2009;30:3015–26. doi: 10.1093/eurheartj/ehp338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Altenberger J, Parissis JT, Costard-Jaeckle A et al. Efficacy and safety of the pulsed infusions of levosimendan in outpatients with advanced heart failure (LevoRep) study: a multicentre randomized trial. Eur J Heart Fail. 2014;16:898–906. doi: 10.1002/ejhf.118. [DOI] [PubMed] [Google Scholar]
- 54.Comín-Colet J, Manito N, Segovia-Cubero J et al. Efficacy and safety of intermittent intravenous outpatient administration of levosimendan in patients with advanced heart failure: the LION-HEART multicentre randomised trial. Eur J Heart Fail. 2018;20:1128–36. doi: 10.1002/ejhf.1145. [DOI] [PubMed] [Google Scholar]
- 55.Ambrosy AP, Pang PS, Khan S et al. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: findings from the EVEREST trial. Eur Heart J. 2013;34:835–43. doi: 10.1093/eurheartj/ehs444. [DOI] [PubMed] [Google Scholar]
- 56.Zile MR, Bennett TD, St John Sutton M et al. Transition from chronic compensated to acute decompensated heart failure: pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation. 2008;118:1433–41. doi: 10.1161/CIRCULATIONAHA.108.783910. [DOI] [PubMed] [Google Scholar]
- 57.Adamson PB. Pathophysiology of the transition from chronic compensated and acute decompensated heart failure: new insights from continuous monitoring devices. Curr Heart Fail Rep. 2009;6:287–92. doi: 10.1007/s11897-009-0039-z. [DOI] [PubMed] [Google Scholar]
- 58.Abraham WT, Adamson PB, Bourge RC et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658–66. doi: 10.1016/S0140-6736(11)60101-3. [DOI] [PubMed] [Google Scholar]
- 59.Costanzo MR, Stevenson LW, Adamson PB et al. Interventions linked to decreased heart failure hospitalizations during ambulatory pulmonary artery pressure monitoring. JACC Heart Fail. 2016;4:333–44. doi: 10.1016/j.jchf.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 60.Silvetti S, Belletti A, Fontana A et al. Rehospitalization after intermittent levosimendan treatment in advanced heart failure patients: a meta-analysis of randomized trials. ESC Heart Fail. 2017;4:595–660. doi: 10.1002/ehf2.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reid R, Ezekowitz JA, Brown PM et al. The prognostic importance of changes in renal function during treatment for acute heart failure depends on admission renal function. PLoS One. 2015;10:e0138579. doi: 10.1371/journal.pone.0138579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fonarow GC, Adams KF Jr, Abraham WT et al. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005;293:572–80. doi: 10.1001/jama.293.5.572. [DOI] [PubMed] [Google Scholar]
- 63.Hillege HL, Nitsch D, Pfeffer MA et al. Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation. 2006;113:671–8. doi: 10.1161/CIRCULATIONAHA.105.580506. [DOI] [PubMed] [Google Scholar]
- 64.Smith GL, Lichtman JH, Bracken MB et al. Renal impairment and outcomes in heart failure: systematic review and meta-analysis. J Am Coll Cardiol. 2006;47:1987–96. doi: 10.1016/j.jacc.2005.11.084. [DOI] [PubMed] [Google Scholar]
- 65.Agostoni P, Corrà U, Cattadori G et al. Metabolic exercise test data combined with cardiac and kidney indexes, the MECKI score: a multiparametric approach to heart failure prognosis. Int J Cardiol. 2013;167:2710–8. doi: 10.1016/j.ijcard.2012.06.113. [DOI] [PubMed] [Google Scholar]
- 66.Zager RA, Johnson AC, Lund S et al. Levosimendan protects against experimental endotoxemic acute renal failure. Am J Physiol Renal Physiol. 2006;290:F1453–62. doi: 10.1152/ajprenal.00485.2005. [DOI] [PubMed] [Google Scholar]
- 67.Rehberg S, Ertmer C, Vincent JL et al. Effects of combined arginine vasopressin and levosimendan on organ function in ovine septic shock. Crit Care Med. 2010;38:2016–23. doi: 10.1097/CCM.0b013e3181ef4694. [DOI] [PubMed] [Google Scholar]
- 68.Grossini E, Molinari C, Pollesello P et al. Levosimendan protection against kidney ischemia/reperfusion injuries in anesthetized pigs. J Pharmacol Exp Ther. 2012;342:376–88. doi: 10.1124/jpet.112.193961. [DOI] [PubMed] [Google Scholar]
- 69.Lannemyr L, Ricksten SE, Rundqvist B et al. Differential effects of levosimendan and dobutamine on glomerular filtration rate in patients with heart failure and renal impairment: A randomized double-blind controlled trial. J Am Heart Assoc. 2018;7:e008455. doi: 10.1161/JAHA.117.008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zemljic G, Bunc M, Yazdanbakhsh AP et al. Levosimendan improves renal function in patients with advanced chronic heart failure awaiting cardiac transplantation. J Card Fail. 2007;13:417–21. doi: 10.1016/j.cardfail.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 71.Bouchez S, Fedele F, Giannakoulas G et al. Levosimendan in acute and advanced heart failure: an expert perspective on posology and therapeutic application. Cardiovasc Drugs Ther. 2018;32:617–24. doi: 10.1007/s10557-018-6838-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Slawsky MT, Colucci WS, Gottlieb SS et al. Acute hemodynamic and clinical effects of levosimendan in patients with severe heart failure. Circulation. 2000;102:2222–7. doi: 10.1161/01.CIR.102.18.2222. [DOI] [PubMed] [Google Scholar]
- 73.Nieminen MS, Akkila J, Hasenfuss G et al. Hemodynamic and neurohumoral effects of continuous infusion of levosimendan in patients with congestive heart failure. J Am Coll Cardiol. 2000;36:1903–12. doi: 10.1016/S0735-1097(00)00961-X. [DOI] [PubMed] [Google Scholar]
- 74.Follath F, Cleland JG, Just H et al. Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low-output heart failure (the LIDO study): a randomised double-blind trial. Lancet. 2002;360:196–202. doi: 10.1016/S0140-6736(02)09455-2. [DOI] [PubMed] [Google Scholar]
- 75.Mebazaa A, Nieminen MS, Packer M et al. Levosimendan vs dobutamine for patients with acute decompensated heart failure: the SURVIVE Randomized Trial. JAMA. 2007;297:1883–91. doi: 10.1001/jama.297.17.1883. [DOI] [PubMed] [Google Scholar]
- 76.Packer M, Colucci W, Fisher L et al. Effect of levosimendan on the short-term clinical course of patients with acutely decompensated heart failure. JACC Heart Fail. 2013;1:103–11. doi: 10.1016/j.jchf.2012.12.004. [DOI] [PubMed] [Google Scholar]


