Abstract
Aims
The objectives were to investigate the pharmacokinetics, pharmacodynamics and safety of ilaprazole infusion in healthy subjects and patients with esomeprazole as positive control, and then recommend the dosage regimen for Phase 2b/3 studies.
Methods
Three clinical studies were performed. First, 16 healthy subjects received infusion of ilaprazole 30 mg or esomeprazole 80 mg. Second, 12 healthy subjects received ilaprazole 20 mg followed by 10 mg once daily for 2 days. Finally, 20 patients with duodenal ulcers received ilaprazole 20 mg followed by 10 mg for 2 days or esomeprazole 40 mg twice daily for 3 days. Serial blood samples were collected and intragastric pH was recorded.
Results
The mean percentages time of intragastric pH >6 was 63.6 and 51.7% for healthy subjects after receiving ilaprazole 30 mg and esomeprazole 80 mg. Linear pharmacokinetics was observed when the dose was increased to 30 mg but the effect was saturated. Ilaprazole 20 mg followed by 10 mg for 2 days provided higher plasma exposure in healthy subjects than patients, but the effect was comparable. After multiple administrations, ilaprazole provided similar effect to esomeprazole. Ilaprazole infusion was safe and well tolerated without serious adverse events.
Conclusions
Ilaprazole provided comparable effect of pH control to esomeprazole, with lower dose and fewer times of administration. There was no significant difference of ilaprazole between healthy subjects and patients regarding intragastric acid inhibition. A loading dose of ilaprazole 20 mg followed by 10 mg once daily for 2 days was recommended for Phase 2b/3 studies.
Keywords: efficacy, ilaprazole, intravenous, pharmacokinetics, proton‐pump inhibitor
What is already known about this subject
Ilaprazole is a novel proton‐pump inhibitor (PPI) of the third generation. Ilaprazole infusion is developed for the patients who are unable to take oral medications, or for some emergent situations such as non‐varicella upper gastrointestinal bleeding, in which a rapid onset of action is desired.
In healthy subjects, ilaprazole exhibited linear pharmacokinetics over the dose of 5–20 mg.
The potency of intragastric acid pH control and onset of action were closely related to the dose and ilaprazole 20 mg provided the most significant effect in healthy subjects.
What this study adds
Ilaprazole was able to provide comparable intragastric acid inhibition to that of esomeprazole, with lower dose and fewer times of administration.
There was no significant efficacy difference of ilaprazole infusion between healthy subjects and patients, and the effect was saturated when dose was increased to 30 mg.
An optimum dose regimen of 20 mg followed by 10 mg for 2 days was recommended for Phase 2b/3 studies.
To reduce the impact of food intake and circadian rhythm, baseline pH–time profile should be acquired when assessing the efficacy of PPIs. Furthermore, the saturated effect should be determined. These can be utilized in guiding studies of other PPIs.
1. INTRODUCTION
Proton‐pump inhibitors (PPIs) are the most effective agents for suppressing gastric acid secretion and are the mainstay of the treatment of acid‐related diseases including gastro‐oesophageal reflux disease, peptic ulcer disease, Zollinger–Ellison syndrome and Helicobacter pylori infection.1 PPIs are generally safely and effectively used; however, several challenges remain, including high individual variability caused by CYP2C19 polymorphisms, short duration of action and reduced acid inhibition during the night.2, 3 Current effort is focused on the development of the third‐generation PPIs, which, at least ideally, should be more potent, rapid and prolonged in action with lower interindividual variability.
Ilaprazole is a novel PPI that is developed by Livzon Pharmaceutical Co., Ltd. (Zhuhai, China). Ilaprazole http://www.baidu.com/link?url=vN1B9JxnrWxLr1O6iSMTUrk-DTvtl779nJ3hR6odIK4KSBNCbhpPMnLGRMkAdV52O-MFfz2HJZJylfJ5jJN-_vD7GRLEJo_QJ-Fajpn1S6vAwZfhM45gvDSjIbLqTbO8 has been approved for the treatment of gastric and duodenal ulcer, as well as erosive oesophagitis. Ilaprazole belongs to a class of substituted benzimidazole molecules, chemically related to omeprazole.4 At variance with other PPIs, ilaprazole was predominantly metabolized by CYP3A and partly by CYP 2C195 and hence the pharmacokinetics (PK) and pharmacodynamics (PD) of ilaprazole were not significantly influenced by CYP 2C19 polymorphism.6, 7 Besides ilaprazole displayed a t1/2 (3–6 h) longer than that of other PPIs (0.5–2 h).8, 9 These features translated into lower interindividual variability and long‐lasting acid inhibition in clinical application. It was reported that ilaprazole tablet 20 mg provided significantly higher mean 24‐hour pH than omeprazole tablet 20 mg in healthy subject10 and in patients with gastroesophageal reflux disease, ilaprazole 5 mg was comparable to omeprazole 20 mg; ilaprazole 10 and 20 mg thus provided a more potent and prolonged intragastric acid inhibition.11 Recently, a meta‐analysis involving 1481 patients concluded that ilaprazole was a highly effective and safe PPI in the treatment of acid‐related disease.12
Intravenous PPI therapy is critical for the patients who are unable to take oral medications, or for some emergent situations such as non‐varicella upper gastrointestinal bleeding, in which a rapid onset of action is desired.13, 14, 15 Currently ilaprazole infusion is being evaluated in China. The PK, PD and safety of ilaprazole infusion have been investigated in healthy subjects. It was reported that intravenous ilaprazole exhibited dose‐related effect of action and ilaprazole 20 mg provided the most significant pH control effect.16, 17 However, there are problems remaining unsolved: (i) is 20 mg the right dose producing the best effect; (ii) is there a gap when extrapolating the PK and PD of ilaprazole from healthy subjects to patients; (iii) what is the optimum dose regimen for clinic? Therefore, the present study was designed to explore the potential PK and PD differences of ilaprazole infusion between healthy subjects and patients, to compare the efficacy of ilaprazole with esomeprazole in healthy subjects and patients with duodenal ulcers, and then determine the optimum dose regimen for Phase 2b/3 studies.
2. METHODS
2.1. Ethics
The studies were conducted in accordance with Good Clinical Practice regulations, the ethical principles stated in the Declaration of Helsinki. The studies were registered on the official website of the China Food and Drug Administration: http://www.chinadrugtrials.org.cn/ (registration number: CTR20132846, CTR20150685 and CTR20150686). Approval of the study was obtained from the Ethical Committee of Peking Union Medical College hospital (Beijing, China) and Jiangsu Province Hospital (Nanjing, China). Written informed consent was obtained from all subjects prior to the start of the study.
2.2. Subjects
The healthy subjects were assessed for eligibility based on the following inclusion criteria: men or women aged 18–45 years with a body mass index of 19–25 kg m−2. Key exclusion criteria were: history of allergy to drugs or alcohol abuse; participation in a clinical drug study or blood donation within a period of 3 months; positive for hepatitis B virus (HBV), hepatitis C virus (HCV), or human immunodeficiency virus (HIV); H pylori‐positive as determined by from the 13C urea breath test; administration of any prescription or over‐the‐counter medication within 14 days prior to the first dose of study drug. Pregnant and lactating women were excluded from this study.
Eligible patients were those with endoscopically diagnosed active duodenal ulcers within 1 week before entry and with ulcer diameter ≤ 15 mm, aged 18–65 years with a body mass index of 19–30 kg m−2. Exclusion criteria included evidence of complex and multiple ulcers, Zollinger–Ellison syndrome, or oesophageal erosion/ulcer; pregnant and lactating women; evidence of clinically significant abnormalities in cardiac, hepatic, renal, pulmonary, neurological, gastrointestinal, haematological and psychiatric function; history of allergy to drugs; a history of alcohol abuse; participation in a clinical drug study or blood donation within a period of 3 months.
2.3. Study design
Three clinical studies were designed and performed in 2 sites in Jiangsu and Beijing (Figure 1). Study A was an open‐label, randomized, positive‐control (esomeprazole) and parallel study. Sixteen healthy subjects were randomized to receive infusion of ilaprazole 30 mg or esomeprazole 80 mg at 08:00 on day 1 after an overnight fasting. Study B was a multiple‐dose study in which 12 healthy subjects received ilaprazole 20 mg at 08:00 on day 1 after an overnight fasting, and followed by 10 mg once daily on day 2 and day 3 Study C was an open‐label, randomized, positive‐control (esomeprazole) and parallel study. Twenty eligible patients with duodenal ulcers were randomized ilaprazole 20 mg at 08:00 on day 1 after an overnight fasting and followed by 10 mg once daily on day 2 and day 3, or f esomeprazole 40 mg twice daily (08:00 and 20:00) on day 1, day 2 and day 3. The subjects were admitted to the phase I ward on day −1 and stayed throughout the treatment periods. Drinking water was not permitted until 2 h after dosing and standard meals were provided at 4 and 10 hours on each study day.
Figure 1.
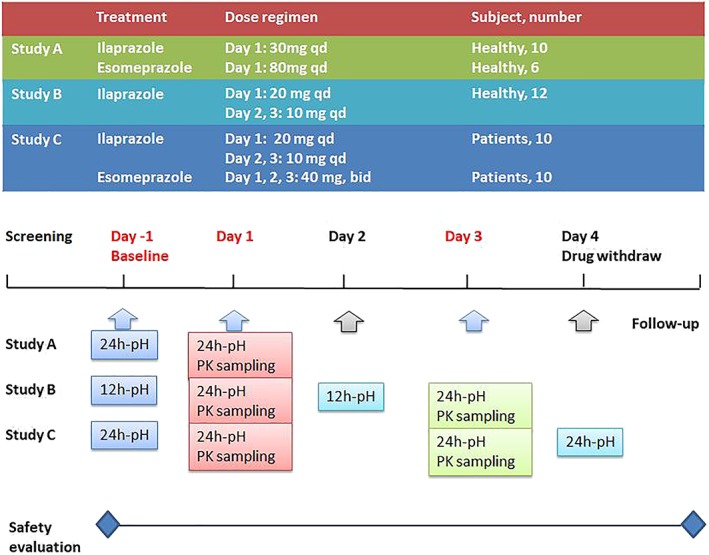
Study design: study A, an open‐label, randomized, positive‐control and parallel study; study B, an open‐label, randomized and parallel study; study C, an open‐label, randomized, positive‐control and parallel study. qd, once daily; bid, twice daily; PK, pharmacokinetics
2.4. Study medications
Ilaprazole (batch number: S131201, Livzon Pharmaceutical Co., Ltd.,Zhuhai, China) and esomeprazole (batch number: PAMT1310025/PAMS131002, AstraZeneca AB, Sweden) lyophilized powder were freshly reconstituted with saline (0.9%, g 100 mL−1) to produce the following solutions: ilaprazole, 0.1 mg mL−1 and 0.15 mg mL−1; esomeprazole, 0.4 mg mL−1. The solution was given to the subjects using a calibrated infusion pump and the infusion time was 45 minutes. The following medications were prepared: (i) 30 mg ilaprazole: 0.15 mg mL−1, 200 mL; 80 mg esomeprazole: 0.4 mg mL−1, 200 mL; (ii) 10 mg ilaprazole: 0.1 mg mL–1, 100 mL; 20 mg ilaprazole: 0.1 mL−1, 200 mL; 40 mg esomeprazole: 0.4 mg mL−1, 100 mL; (iii) the same as dose regimen ii.
2.5. PK measurement
Serial blood samples (3 mL) were collected for PK evaluation. In study A, the blood samples were drawn on day 1 at the following time points: 0 (predose), 15, 30, 45, 50 minutes, 1, 2, 3, 4, 5, 8, 12, 16 and 24 hours. In study B, the blood samples were drawn on day 1 and 3 as follows: 0 (predose), 15, 30, 45, 50 minutes, 1, 2, 3, 4, 5, 8, 12, 16 and 24 hours, and were collected prior to dose (0 hours) and 45 minutes after dose on day 2. In study C, the blood samples were drawn on day 1 as follows: 0 (predose), 15, 30, 45, 50 minutes, 1, 2, 3, 4, 5, 8, 12, 16 and 24 hours for ilaprazole; 0 (predose), 25, 45, 55 minutes, 1.5, 2, 3, 5, 8 and 12 hours for esomeprazole. The blood samples were collected into EDTA‐K2 vacutainers and immediately placed on ice. After being centrifuged at 1500× g for 10 minutes at 4°C, the plasma was collected and stored at −80°C until analysis. The plasma concentrations of ilaprazole and esomeprazole were determined in using a validated ultra‐performance liquid chromatography–tandem mass spectrometry method (Supplementary Text 1).
The PK parameters were calculated by noncompartmental analysis using WinNonlin software version 6.3 (Pharsight, St Louis, MO, USA). Cmax was the maximum plasma concentration in a profile and tmax was the time to Cmax. The elimination half‐life (t1/2) was estimated by linear regression of log‐transformed concentration–time data. The area under the plasma concentration–time curve from time zero to t (AUC0–t), where t was the time of last measurable sample, was calculated according to the linear trapezoidal rule. The AUC from time zero to infinity (AUC0–∞) was estimated as AUC0–t + Ct/λz, where Ct was the plasma concentration of the last measurable sample. Clearance (CL) was calculated as dose/AUC0–∞ and apparent volume of distribution (V) as calculated as CL/λz.
2.6. PD measurement
A calibrated pH microelectrode (MMS, the Netherlands) was inserted transnasally into the stomach, fixed approximately 10 cm below the lower oesophageal sphincter, and connected to a pH data recorder (Orion II‐Ohmega, MMS, the Netherlands). The antimony electrode was calibrated using commercial buffer solutions (pH 1.07 and 7.01) at room temperature pre‐ and postmeasurement. Intragastric pH was automatically recorded every 4 seconds during the 12‐ or 24‐hour recording period.
The subjects underwent intragastric pH monitoring at baseline (day –1) and during the treatment. In study A, intragastric pH was monitored for 24 hours on day −1 and day 1. In study B, intragastric pH was monitored for 12 hours on day −1, day 2 and for 24 hours day 1. In study C, 24 hours intragastric pH was monitored on day −1, day 1, day 3 and day 4 after drug withdrawal.
For PD evaluation, the intragastric pH–time profile of each subject, percentages of time intragastric pH >4, >5 and > 6 over the treatment period, the nocturnal pH (20:00–08:00 next day), as well as the nocturnal time at pH < 4 were recorded. The time to first reach pH > 6 and maintained for over 0.25 hours after dosing was also recorded, representing the onset of action. Among these parameters, the percentages of time intragastric pH > 6 was as major index generally used to evaluate the clinical effects of intravenous PPIs for patients with acid‐related disease.
2.7. Safety evaluation
Safety and tolerance were evaluated based on occurrence, frequency and severity of adverse events (AEs). Routine clinical laboratory tests (biochemistry, haematology and urinalysis), 12‐lead electrocardiograms, physical examinations and vital signs (blood pressure, pulse rate and body temperature) were performed at initial screening, day –1, treatment period to discharge or upon early termination. The AEs were monitored from the initial administration to a follow‐up phase of 14 days after last treatment. The AEs were assessed by the investigators with regard to severity (mild, moderate, severe and life‐threatening) and the relationship to study treatment (reasonably or possibly related, not reasonably or not possibly related).
2.8. Statistical analysis
Statistical analysis was performed using SAS version 9.1.3 (SAS Institute, Cary, NC, USA). The safety analysis set comprised all the subjects who received ilaprazole or esomeprazole. The PK and PD analysis set comprised the subjects who received the study drugs and had sufficient data to derive parameters. The parameters were tabulated and summarized using descriptive statistics by treatment group and period. Dose proportionality of intravenous ilaprazole was assessed using the power model: ln(y) = β0 + β1ln (dose), where y represents the AUC and Cmax. 18 The potential PK and PD differences between populations and study drugs were evaluated using a mixed‐model analysis of variance (ANOVA), and P value <.05 was considered statistically significant.
3. RESULTS
3.1. Subject demographics
In total, 60 healthy subjects and 46 patients were screened, and of them 28 eligible healthy subjects and 20 patients were enrolled. All the subjects completed the study and were included in the PK and PD analysis, as well as safety analysis. The subject demographics are shown in Table 1.
Table 1.
Subject demographics
| Study A | Study B | Study C | |||
|---|---|---|---|---|---|
| Ilaprazole (n = 10) | Esomeprazole (n = 6) | Ilaprazole (n = 12) | Ilaprazole (n = 10) | Esomeprazole (n = 10) | |
| Age (y) | 25 (22–31) | 25 (23–29) | 25 (20–29) | 45 (25–63) | 49 (39–63) |
| Height (m) | 1.7 (1.5–1.8) | 1.6 (1.5–1.8) | 1.7 (1.6–1.8) | 1.67 (1.6–1.7) | 1.7 (1.6–1.8) |
| Weight (kg) | 58.0 (50.0–67.8) | 60.8 (50.0–69.3) | 61.4 (54.7–72.4) | 61.9 (53.0–70.0) | 63.5 (47.0–75.0) |
| BMI (kg m−2) | 21.2 (19.0–23.6) | 22.5 (21.4–23.7) | 22.1 (19.7–25.0) | 22.4 (19.2–25.7) | 22.8 (19.3–27.3) |
| Sex, male (%) | 5 (50%) | 3 (50%) | 6 (50%) | 5 (50%) | 6 (60%) |
Data are represented as median (range).
BMI, body mass index.
3.2. PK
Mean plasma concentration–time curves of infusions of ilaprazole and esomeprazole were shown in Figure 2 and the PK parameters were shown in Table 2. In study A, the mean t1/2, CL and V of ilaprazole (30 mg) were 3.0 h, 3.1 L h−1 and 11.5 L h−1, and these parameters were comparable to those from the previous study using 5, 10 and 20 mg.16 Dose‐proportionality over 5–30 mg was assessed using power model analysis. The point estimate and 90% CI for the ratio of dose‐normalized, geometric mean values of Cmax, AUC0–t and AUC0–∞ were 0.865 (0.787, 0.950) ng mL−1, 1.021 (0.949, 1.098) μg h mL−1 and 1.019 (0.946, 1.099) μg h mL−1, respectively, indicating that intravenous ilaprazole exhibited linear PK property over the dose of 5–30 mg. Compared with esomeprazole, ilaprazole eliminated much slower from plasma (t1/2, ilaprazole vs esomeprazole, 3.0 vs 1.5 hours). In study B, 12 healthy subjects received ilaprazole infusion 20 mg followed by 10 mg once daily for 2 days. After dose correction the accumulation index was 0.92 ± 0.10 based on AUC0–t and 0.93 ± 0.06 based on Cmax, indicating that there was no accumulation after 3 days of multiple infusions. In study C, 10 patients received the same dose regimen of ilaprazole as study B. However, the plasma exposure of patients was lower than those of healthy subject in part B (Cmax, 1540 vs 1871.3 ng mL−1; AUC0‐–∞, 4.9 μg h ml−1 vs 7.4 μg h mL−1). There were statistical differences between healthy subjects and patients, in terms of Cmax and AUC, as well as CL and V (P < .01).
Figure 2.
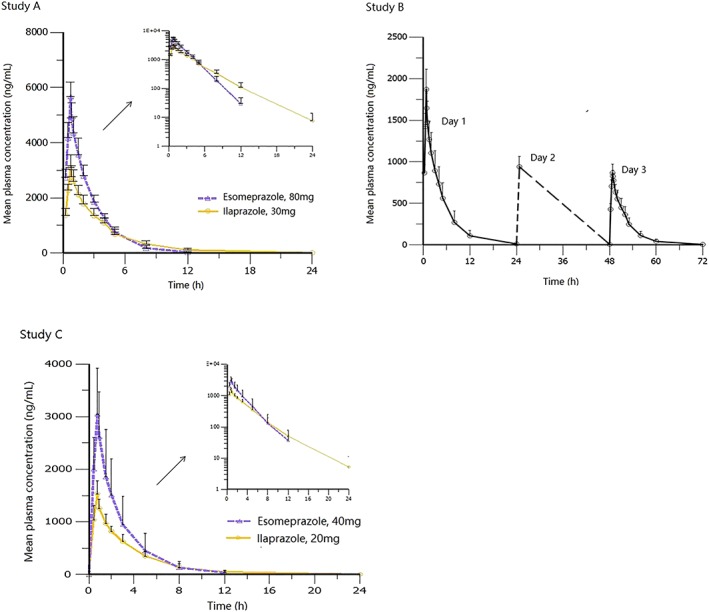
Mean plasma concentration–time curves of ilaprazole and esomeprazole in healthy subjects and patients with duodenal ulcers: (1) study A (n = 16); (2) study B (n = 12); (3) study C, day 1 (n = 20)
Table 2.
Mean pharmacokinetic parameters of ilaprazole and esomeprazole in healthy subjects and patients with duodenal ulcers (mean ± standard deviation)
| Parameters | Study A | Study B | Study C | |||
|---|---|---|---|---|---|---|
| Ilaprazole | Esomeprazole | Ilaprazole | Ilaprazole | Ilaprazole | Esomeprazole | |
| (30 mg, day 1) | (80 mg, day 1) | (20 mg, day 1) | (10 mg, day 3) | (20 mg, day 1) | (40 mg, day 1)b | |
| (n = 10) | (n = 6) | (n = 12) | (n = 12) | (n = 10) | (n = 10) | |
| t1/2 (h) | 3.0 ± 0.5 | 1.5 ± 0.2 | 3.3 ± 0.6 | 3.1 ± 0.7 | 3.1 ± 0.6 | 1.6 ± 0.4 |
| tmax(h)a | 0.75 (0.75–0.75) | 0.75 (0.75–0.76) | 0.75 (0.75–0.83) | 0.75 (0.75–0.75) | 0.75 (0.75–0.78) | 0.75 (0.75–0.77) |
| Cmax (ng mL−1) | 3101.4 ± 468.7 | 5680.2 ± 524.9 | 1871.3 ± 242.0 | 876.9 ± 84.4 | 1540.3 ± 241.0 | 3040.1 ± 875.1 |
| AUC0–t (μg mL h−1) | 10.3 ± 2.0 | 13.5 ± 1.4 | 7.4 ± 2.1 | 3.5 ± 0.8 | 4.7 ± 0.9 | 7.4 ± 3.9 |
| AUC0–∞ (μg mL h−1) | 10.5 ± 2.1 | 13.6 ± 1.6 | 7.4 ± 2.2 | 3.5 ± 0.9 | 4.9 ± 1.1 | 7.5 ± 3.8 |
| V (L) | 11.5 ± 1.4 | 12.5 ± 1.6 | 12.9 ± 2.1 | 11.3 ± 1.2 | 17.6 ± 2.7 | 14.0 ± 3.4 |
| CL (L h−1) | 3.0 ± 0.6 | 5.9 ± 0.7 | 2.9 ± 0.8 | 3.1 ± 0.7 | 4.1 ± 0.9 | 6.7 ± 2.6 |
Data are represented as mean ± standard deviation.
For t max, median (range);
For esomeprazole cohort in study C, the given dose was 40 mg twice daily and the last time point was 12 h (i.e. AUC0–t = AUC0–12).
3.3. PD
Mean 12‐ or 24‐hour intragastric pH–time profiles of ilaprazole and esomeprazole acquired from healthy subjects and patients were shown in Figure 3. The mean percentages of time intragastric pH > 4, > 5 and > 6, mean nocturnal pH (20:00–08:00 next day), and the time to first reach pH > 6 and maintained for >0.25 hours were summarized in Table 3.
Figure 3.
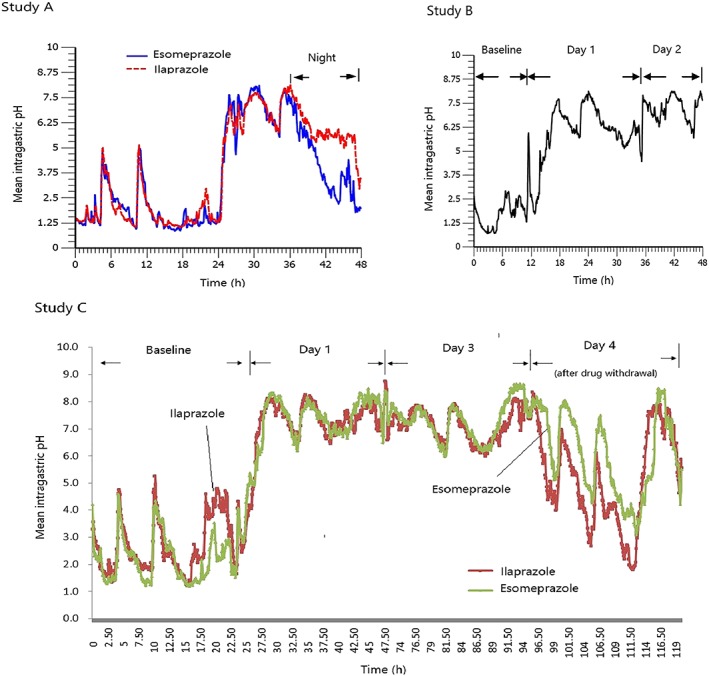
Mean 12‐ or 24‐hour intragastric pH–time profiles of ilaprazole and esomeprazole in healthy subjects and patients with duodenal ulcers: (1) study A, healthy subjects (n = 16); (2) study B, healthy subjects (n = 12); (3) study C, patients (n = 20)
Table 3.
Mean pharmacodynamics parameters of ilaprazole and esomeprazole according to dose regimens
| Study A | Study Bb | |||||||
|---|---|---|---|---|---|---|---|---|
| Ilaprazole 30 mg (n = 10) | Esomeprazole 80 mg (n = 6) | Day 1 (20 mg) | Day 2(10 mg) | |||||
| Baseline | Day 1 | Baseline | Day 1 | Baseline | (n = 12) | (n = 12) | ||
| Mean 24‐h pH | 1.8 ± 0.3 | 6.2 ± 0.6 | 1.8 ± 0.4 | 5.6 ± 0.5 | 1.8 ± 1.0‡ | 6.1 ± 0.9 | 7.2 ± 0.3 | |
| pH > 4 (%) | 6.9 ± 5.1 | 85.6 ± 9.0 | 7.9 ± 6.8 | 69.2 ± 8.2 | 12.3 ± 8.1 | 80.2 ± 13.2 | 98.9 ± 2.2 | |
| pH > 5 (%) | 3.8 ± 3.2 | 79.6 ± 12.0 | 4.6 ± 4.3 | 62.8 ± 7.3 | 10.3 ± 7.9 | 78.6 ± 16.2 | 96.1 ± 4.8 | |
| pH > 6 (%) | 1.7 ± 1.6 | 65.6 ± 12.3 | 1.4 ± 1.3 | 51.7 ± 8.3 | 8.9 ± 6.9 | 69.1 ± 17.0 | 88.6 ± 11.1 | |
| First time to pH > 6 (h)a | 0.8 (0.5–1.9) | 1.1 (0.5–1.8) | 3.1 (0.3–4.5) | |||||
| Mean nocturnal pH | 1.7 ± 0.4 | 5.9 ± 0.7 | 1.7 ± 0.6 | 4.2 ± 0.6 | 1.6 ± 1.1 | 6.3 ± 1.3 | ||
| Night‐time of pH < 4 (h) | 1.9 (0.0–4.4) | 6.0 (3.3–8.4) | ||||||
| Study C | ||||||||
|---|---|---|---|---|---|---|---|---|
| Ilaprazole (n = 10) | Esomeprazole (n = 10) | |||||||
| Baseline | Day 1 (20 mg) | Day 3 (10 mg) | Day 4 (10 mg) | Baseline | Day 1(40 mg) | Day 3 (40 mg) | Day 4(40 mg) | |
| Mean 24‐h pH | 2.7 ± 1.3 | 7.0 ± 1.1 | 7.1 ± 1.5 | 5.1 ± 1.2 | 2.2 ± 0.7 | 7.1 ± 0.6 | 7.3 ± 0.3 | 6.0 ± 0.8 |
| pH > 4 (%) | 22.7 ± 23.1 | 89.3 ± 11.3 | 94.4 ± 15.5 | 59.6 ± 20.2 | 12.6 ± 11.3 | 92.1 ± 5.1 | 99.8 ± 0.2 | 75.5 ± 14.7 |
| pH > 5 (%) | 18.8 ± 19.43 | 84.7 ± 17.3 | 92.0 ± 18.9 | 51.2 ± 19.7 | 7.8 ± 6.8 | 88.7 ± 7.7 | 99.3 ± 0.8 | 68.4 ± 15.9 |
| pH > 6 (%) | 14.3 ± 14.9 | 79.5 ± 24.8 | 86.8 ± 22.4 | 41.9 ± 21.3 | 4.5 ± 3.5 | 82.2 ± 12.1 | 91.1 ± 5.3 | 57.0 ± 14.2 |
| First time to pH > 6 (h)a | 2.5(1.4–4.3) | 2.7(0.2–4.1) | ||||||
| Mean nocturnal pH | 2.8 ± 1.5 | 7.4 ± 1.5 | 7.0 ± 1.7 | 4.9 ± 1.2 | 2.0 ± 0.7 | 7.5 ± 0.7 | 7.3 ± 0.3 | 5.6 ± 1.5 |
Data are represented as mean ± standard deviation.
For time to first reach pH > 6 and nocturnal pH < 4 (h), median (range).
For study B, the pH value was monitored for 12 h on day −1 (baseline) and day 3.
It is well known that intragastric acid secretion shows a circadian rhythm and is influenced by buffering meals and nocturnal duodenogastric reflux.19 Therefore these factors should be taken into account when evaluating the efficacy of a PPI treatment. In the present 3 studies, the baseline pH–time profiles were acquired for each subject on day −1 before administration. It was clearly indicated that the basal pH values were significantly affected by meals and circadian rhythm. Compared with baseline (day −1) all the treatment cohorts in the studies displayed significant pH control effect.
In study A, the PD of ilaprazole (30 mg) was compared with esomeprazole (80 mg, the maximum single infusion dose of esomeprazole20) in healthy subjects. This indicated that the mean 24‐hour intragastric pH–time profile of ilaprazole (30 mg) was comparable to that of esomeprazole (80 mg) in healthy subjects except the nocturnal stage (20:00–08:00 next day; Figure 3‐1). The mean 24‐hour pH values were not statistically different (P > .05); however, during the night (20:00–08:00 next day), ilaprazole showed better effect than esomeprazole regarding mean night‐time pH and the time of pH < 4 (P < .05). Individual data for the percentage time of intragastric pH >4, >5 and > 6 of ilaprazole and esomeprazole was illustrated in Figure 4. Mean percentages time of intragastric pH >4 and >5 of ilaprazole were significantly higher than those of esomeprazole; however, there was no statistical difference between the 2 treatments in the mean percentages time of intragastric pH > 6 (p = 0.052). The time to first reach pH > 6 was 0.8 hours and 1.1 hours for ilaprazole and esomeprazole, respectively, indicating that the onset of action of ilaprazole was similar to that of esomeprazole. Overall it was demonstrated that ilaprazole 30 mg exhibited comparable intragastric acid inhibition to esomeprazole 80 mg in healthy subjects; moreover, ilaprazole might have better effect in overcoming the nocturnal acid breakthrough, which is always accompanied with the treatment of PPIs.
Figure 4.
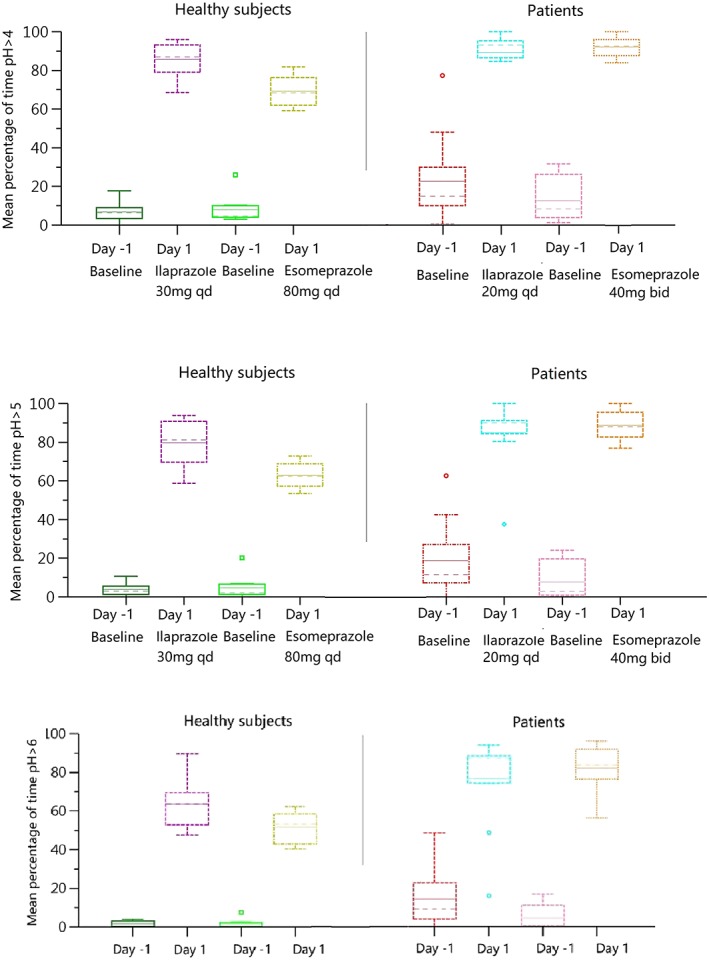
Individual data for the percentage time of pH >4, pH >5 and pH >6 in healthy subjects (study A) and patients (study C) after administration of ilaprazole or esomeprazole. qd, once daily; bid, twice daily
In study B, during the 3‐day treatment, the mean percentages time of intragastric pH >4, >5 and >6 were, respectively, 80.2, 78.6 and 69.1% on day 1 at the dose of 20 mg, and were increased to 98.9, 96.1 and 88.6% on day 2 at the following dose of 10 mg. This indicated that this dosing regimen provided stable and sustained pH control effect (Figure 3‐2).
In study C, the PD of ilaprazole (a bolus of 20 mg on day 1 followed by 10 mg once daily for 2 days) was compared with esomeprazole (40 mg twice daily for 3 days, the prescribed dose of esomeprazole infusion20) in patients. The time to first reach pH > 6 was 2.6 and 2.4 hours for ilaprazole and esomeprazole, respectively, indicating that the onset of action of ilaprazole in patients was comparable to that of esomeprazole. During the continuous infusions, the nocturnal (20:00–08:00 next day) pH value was maintained over 6.0 both for ilaprazole and esomeprazole. Individual data for the percentage time of intragastric pH >4, >5 and >6 of ilaprazole and esomeprazole was illustrated in Figure 3‐3. Statistical analysis indicated that there was no significant difference in the mean percentages time of intragastric pH >4, >5 and >6, as well as mean 24‐hour pH between the 2 treatments. After drug withdrawal (day 4), the mean percentages time of intragastric pH >6 were 41.9% and 57.0% for ilaprazole and esomeprazole, indicating that the acid suppression was maintained. Overall, the present dosing regimen of ilaprazole provided significant and sustained pH control effect, comparable to that of esomeprazole with lower dose and less times of administration.
3.4. H. pylori status and pH value
H. pylori infection is known to be the main aetiology of gastritis and peptic ulcers. It was reported that in PPIs (pantoprazole, lansoprazole, omeprazole, esomeprazole and rabeprazole) treatment, H. pylori‐positive individuals needed only about 20% of the dose to achieve a similar effect compared with healthy volunteers.21 So far, the effect of H. pylori infection on the control of intragastric pH by ilaprazole is unclear.
Of the patients receiving ilaprazole in study C, 8 patients were H. pylori positive and 2 subjects were negative, determined by 13C urea breath test. The mean 24‐hour pH–time profiles of H. pylori‐positive and ‐negative patients are shown in Figure 5. The mean percentages time of intragastric pH > 6 in H. pylori‐positive and ‐negative subjects were as follows: 16.6 ± 15.8% vs 5.2 ± 7.3% (day −1,baseline); 82.5 ± 14.1% vs 52.8 ± 52.2% (day 1); 90.5 ± 10.6% vs 61.9 ± 49.2% (day 3); 45.1 ± 19.5% vs 29.3 ± 32.1% (day 3), respectively. It was indicated that H. pylori‐positive subjects were much more sensitive to intragastric acid inhibition effect of ilaprazole, however more data would be required.
Figure 5.
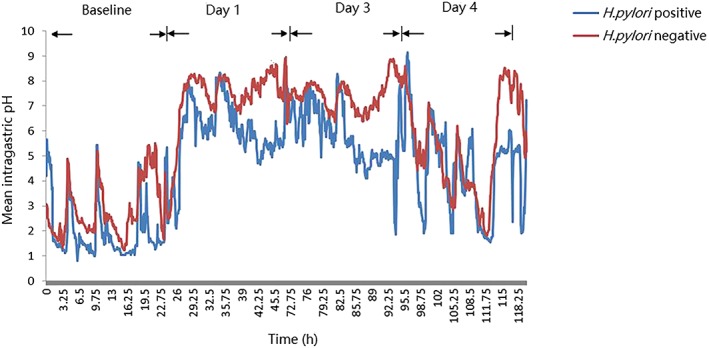
Mean 24‐hour intragastric pH–time profiles of Helicobacter pylori‐positive and ‐negative patients before and after intravenous infusion of ilaprazole
3.5. Safety
A total of 13 (27%) subjects experienced AEs considered by the investigator to be possibly, probably or definitely drug related. No subjects withdrew from the study due to AEs. AEs observed included stool occult blood positive (study B, ilaprazole, 8%), urinary albumin positive (study C, ilaprazole, 10%), abnormal http://www.baidu.com/link?url=FzSMeiUCP9PNysivK4xTsnReyyESkD5RxOeEW54vl9_whtvTrsl8Rsg6c4rnVkNQN_oVDnTCuukUP6LIT4jrq7szG94lgcOl9rPX8gsIWRK6hARuCw-N_YADvh2NYZhP8yrj8N2D4ThfwV5hUv7Eva (study A: ilaprazole, 12.5%; esomeprazole, 12.5%), http://www.baidu.com/link?url=FzSMeiUCP9PNysivK4xTsnReyyESkD5RxOeEW54vl9_whtvTrsl8Rsg6c4rnVkNQN_oVDnTCuukUP6LIT4jrq7szG94lgcOl9rPX8gsIWRK6hARuCw-N_YADvh2NYZhP8yrj8N2D4ThfwV5hUv7Eva (study C, ilaprazole, 10%;) and fibrinogen (study A, ilaprazole, 12.5%; esomeprazole, 25%). Ilaprazole displayed a similar safety profile in healthy subjects and patients, and incidence were not related to dosing regimen and treatment. The AEs generally began on day 1 in the majority of subjects, and most AEs were associated with coagulation function. All the AEs were mild and transient in nature with no pattern of treatment‐related, and were recovered without any medication. No serious AEs or deaths occurred in the study. Except for abnormities mentioned above, there were no clinically significant changes in the physical examination, laboratory test, or electrocardiography reports before and after the administration of study drugs.
4. DISCUSSION
Ilaprazole is a novel PPI that provides effective and long‐lasting inhibition of intragastric acid secretion. The present studies compared the PK, PD and safety of intravenous ilaprazole with esomeprazole in healthy subjects and patients with duodenal ulcers, and thus recommended the optimum dose regimen for Phase 2b/3 studies.
Previous study indicated that ilaprazole infusion exhibited linear PK over the dose of 5–20 mg and the effect of pH control was closely related with dose.16 In the present study (study A), the dose‐proportionality was still concluded when the dose was expanded to 30 mg; however, PD effect did not increase accordingly (Figure 6). Regarding the mean 24‐hour pH, nocturnal pH, percentages of time intragastric pH > 6, as well as the time to first reach pH > 6 (Table 4), a saturated ceiling effect was observed when dose was increased up to 30 mg. There was no statistical difference between the 20‐mg and 30‐mg cohorts (P > .05). The saturated ceiling effect is associated with the mechanism of action of PPIs. It is known that PPIs reduce gastric acid secretion by inhibiting the H+‐K+‐ATPase of gastric parietal cells, and the irreversible inhibition will be saturated when the given dose is high enough to block all the released H+‐K+‐ATPase.
Figure 6.
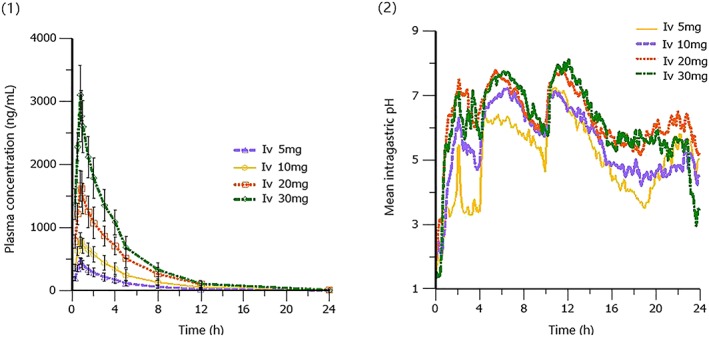
Mean plasma concentration–time and 24‐hour intragastric pH–time profiles of ilaprazole in healthy subjects following intravenous (Iv) infusion of 5, 10, 20 and 30 mg: (1) pharmacokinetics; (2) pharmacodynamics. Data for 5, 10 and 20 mg are from Ref. 13
Table 4.
Mean pharmacokinetic (PK) and pharmacodynamic (PD) parameters of ilaprazole in healthy subjects after infusion of 5, 10, 20 or 30 mg (mean ± standard deviation)
| Parameter | 5 mg (n = 15) | 10 mg (n = 15) | 20 mg (n = 15) | 30 mg (n = 10) | |
|---|---|---|---|---|---|
| PK | t1/2 (h) | 3.3 ± 0.90 | 3.4 ± 0.9 | 3.3 ± 0.8 | 3.0 ± 0.5 |
| tmax (h) a | 0.77 (0.75–0.83) | 0.75 (0.75–1.00) | 0.75 (0.75–0.83) | 0.75 (0.75–0.75) | |
| Cmax (ng m−1) | 482.2 ± 65.8 | 834.3 ± 101.2 | 1718.9 ± 255.1 | 347.9 ± 176.3 | |
| AUC0–t (μg mL h−1) | 1736.3 ± 501.7 | 3520.9 ± 915.3 | 6988.4 ± 1782.5 | 1970.2 ± 834.7 | |
| AUC0–∞ (μg mL h−1) | 1754.8 ± 511.3 | 3562.6 ± 947.8 | 7056.6 ± 1833.2 | 1984.2 ± 836.6 | |
| Vz (L) | 13.7 ± 2.6 | 14.0 ± 2.2 | 13.6 ± 1.8 | 29.1 ± 12.2 | |
| CL (L h−1) | 3.1 ± 0.9 | 3.0 ± 0.9 | 3.0 ± 0.8 | 5.9 ± 2.5 | |
| PD | Mean 24‐h pH | 5.1 ± 1.5 | 5.5 ± 1.1 | 6.3 ± 1.0 | 6.2 ± 0.6 |
| pH >6 (%) | 47.3 ± 29.5 | 52.8 ± 25.3 | 68.2 ± 25.1 | 63.6 ± 12.3 | |
| First time to pH >6 (h) | 4.3 (0.4–10.9) | 1.7 (0.3–6.5) | 1.0 (0.4–4.3) | 0.8 (0.5–1.9) | |
| Mean nocturnal pH | 5.0 ± 1.9 | 5.2 ± 1.4 | 6.1 ± 1.3 | 5.9 ± 0.7 | |
Data are represented as mean ± standard deviation, and data for 5, 10 and 20 mg are from Ref. 13.
For t max and time to first reach pH > 6 (h), median (range).
The general strategy of intravenous PPI treatment for peptic ulcer bleeding is giving a high dose followed by continuous infusions.13, 14, 15 Since the effect of ilaprazole 20 mg was comparable to that of 30 mg, the lower was selected as the loading dose. The following infusion of 10 mg once daily for 2 days was selected because: (i) a previous study indicated that ilaprazole 10 once daily for 5 days provided stable PK and PD, and was well tolerated in healthy subjects17; (ii) after receiving a bolus of 20 mg, ilaprazole 10 mg once daily for 2 days was assumed to produce comparable effect to that of 20 mg for 2 days based on a simulation using a mechanism‐based PK/PD model (not published). This was a modified indirect irreversible response model incorporating the circadian rhythm of gastric acid production as well as the effect of food intake.22, 23 Then this dose regimen (a loading dose of 20 mg followed by 10 mg for 2 days) was evaluated in healthy subjects (study B) and proved to provide significant and stable intragastric acid inhibition effect.
Since there is always a gap when extrapolating dose/exposure–response relationship from healthy subjects to patients, a patient study (study C) using esomeprazole as positive control was designed in order to: (i) evaluate the efficacy and safety of the proposed dose regimen in a limited sample size of patients; and (ii) to compare the potential differences between healthy subjects and patients. In study C, 20 patients received infusion of ilaprazole 20 mg followed by 10 mg once daily for 2 days or esomeprazole 40 mg twice daily for 3 days. There was significant difference between healthy subjects (study B) and patients (study C), in terms of AUC, Cmax and CL at the same dose (P < .05). As for the efficacy of ilaprazole (Table 4, day 1), the mean 24‐hour pH, percentages of time pH > 4, pH > 5 and pH > 6, as well as mean nocturnal pH of patients were higher than those of healthy subjects, indicating that patients might be more sensitive to the pH control effect of ilaprazole; however, there was no statistical difference (P > .05). Therefore, it was concluded that a loading dose of ilaprazole 20 mg followed by 10 mg once daily for 2 days provided similar intragastric acid inhibition effect between healthy subjects and patients although the PK property was different.
Esomeprazole is 1 of the most popular intravenous PPI currently used for peptic ulcer bleeding in China. In the present studies, the PD of ilaprazole infusion were compared with esomeprazole in healthy subjects (study A) and patients (study C). After single infusion of ilaprazole 30 mg or esomeprazole 80 mg (the maximum single investigated dose20), with lower dose, ilaprazole exhibited comparable intragastric acid inhibition effect in terms of onset of action and potency, and better effect during the night in healthy subjects (Figure 3). Likewise, following multiple infusions (3‐day treatment), with fewer times of administration, the pH control effect of ilaprazole (once daily for 3 days) was similar to that of esomeprazole (twice daily for 3 days) in patients, in terms of the dynamics of 24‐hour pH profile (Figure 3) and mean percentages of time pH > 6 (Figure 4). Although the sustained acid suppression of esomeprazole was better than that of ilaprazole after drug withdrawal (on day 4 in study B), it was associated with an 8‐fold higher dose of esomeprazole administered on days 2 and 3. Overall, it was concluded that, with lower dose and fewer administrations, ilaprazole was able to provide comparable intragastric acid inhibition to that of esomeprazole.
A limitation of the studies was that the effect of H. pylori infection on the pH control of ilaprazole was only investigated in 10 patients. The finding that H. pylori‐positive patients appeared more sensitive to ilaprazole should be verified in future studies with large sample size. In addition, future studies assessing the potential drug–drug interaction of ilaprazole are needed.
In conclusion, ilaprazole was able to provide comparable intragastric acid inhibition to that of esomeprazole, with lower dose and fewer times of administration. There was no significant PD difference of ilaprazole between healthy subjects and patients and the effect was saturated when dose was increased to 30 mg. Finally, a loading dose of ilaprazole 20 mg followed by 10 mg once daily for 2 days was recommended for Phase 2b/3 studies.
COMPETING INTERESTS
H.W., X.L., W.X. and J.J. are employees of Peking Union Medical College Hospital. F.S. and N.O. are employees of Jiangsu Province Hospital. X.Q., H.H. and X.H. are employees of Livzon Pharmaceutical Group Inc.
CONTRIBUTORS
H.W., F.S., N.O., H.H. and J.J. designed the study. X.Q., F.L., H.H. and X.H. provided supporting data for the study. X.L. and W.X. performed the measurement of plasma concentrations. H.W., X.L., W.X. and F.S. performed the study and analysed the data.
Supporting information
Data S1 Supporting information
Data S2 Supporting information
ACKNOWLEDGEMENTS
The authors thank all the subjects who participated in this clinical trial, the personnel of the Hospital Clinic in Beijing and Jiangsu. The studies were supported by “13th Five‐Year” National Science and Technology Major Project for New Drugs (No.2017ZX09304031‐001 and No. 2018ZX09734006‐001), CAMS Innovation Fund for Medical Sciences (No. 2016‐12 M‐1‐010) and by Livzon Pharmaceutical Group Inc. (Zhuhai, Guangdong, China).
Wang H, Shao F, Liu X, et al. Efficacy, safety and pharmacokinetics of ilaprazole infusion in healthy subjects and patients with esomeprazole as positive control. Br J Clin Pharmacol. 2019;85:2547–2558. 10.1111/bcp.14076
Principal investigator: The authors confirm that the PI for this paper is Ji Jiang and that he had direct clinical responsibility for patients.
Contributor Information
Haitang Hu, Email: gzhht@126.com.
Ji Jiang, Email: jiangjipumch@sohu.com.
REFERENCES
- 1. Gyawali CP, Fass R. Management of Gastroesophageal Reflux Disease. Gastroenterology. 2018;154(2):302‐318. [DOI] [PubMed] [Google Scholar]
- 2. Scarpignato C, Gatta L, Zullo A, Blandizzi C, SIF‐AIGO‐FIMMG Group , Italian Society of Pharmacology, the Italian Association of Hospital Gastroenterologists, and the Italian Federation of General Practitioners . Effective and safe proton pump inhibitor therapy in acid‐related diseases ‐ a position paper addressing benefits and potential harms of acid suppression. BMC Med. 2016;14(1):179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carmelo S, Richard HH. Proton pump inhibitors: the beginning of the end or the end of the beginning? Curr Opin Pharmacol. 2008;8:677‐684. [DOI] [PubMed] [Google Scholar]
- 4. Kim EJ, Lee RK, Lee SM, Kim DY. General pharmacology of IY‐81149, a new proton pump inhibitor. Arzneimittelforschung. 2001;51(1):51‐59. [DOI] [PubMed] [Google Scholar]
- 5. Seo KA, Lee SJ, Kim KB, et al. Ilaprazole, a new proton pump inhibitor, is primarily metabolized to ilaprazole sulfone by CYP3A4 and 3A5. Xenobiotica. 2012;42(3):278‐284. [DOI] [PubMed] [Google Scholar]
- 6. Li Y, Zhang W, Guo D, Zhou G, Zhou H, Xiao Z. Pharmacokinetics of the new proton pump inhibitor ilaprazole in Chinese healthy subjects in relation to CYP3A5 and CYP2C19 genotypes. Clin ChimActa. 2008;391(1‐2):60‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cho H, Choi MK, Cho DY, et al. Effect of CYP2C19 genetic polymorphism on pharmacokinetics and pharmacodynamics of a new proton pump inhibitor, ilaprazole. J Clin Pharmacol. 2012;52(7):976‐984. [DOI] [PubMed] [Google Scholar]
- 8. de Bortoli N, Martinucci I, Giacchino M, et al. The pharmacokinetics of ilaprazole for gastro‐esophageal reflux treatment. Expert Opin Drug Metab Toxicol. 2013;9(10):1361‐1369. [DOI] [PubMed] [Google Scholar]
- 9. Shin JS, Lee JY, Cho KH, et al. The pharmacokinetics, pharmacodynamics and safety of oral doses of ilaprazole 10, 20 and 40 mg and esomeprazole 40 mg in healthy subjects: a randomised, open‐label crossover stud. Aliment Pharmacol Ther. 2014;40(5):548‐561. [DOI] [PubMed] [Google Scholar]
- 10. Du YQ, Guo WY, Zou DW, et al. Acid inhibition effect of ilaprazole on helicobacter pylori‐negative healthy volunteers: an open randomized cross‐over study. J Dig Dis. 2012;13(2):113‐119. [DOI] [PubMed] [Google Scholar]
- 11. Periclou AP, Goldwater R, Lee SM, et al. A comparative pharmacodynamics study of IY‐81149 versus omeprazole in patients with gastroesophageal reflux disease. Clin Pharmacol Ther. 2000;68(3):304‐311. [DOI] [PubMed] [Google Scholar]
- 12. Ji XQ, Du JF, Chen G, Chen G, Yu B. Efficacy of ilaprazole in the treatment of duodenal ulcers: a meta‐analysis. World J Gastroenterol. 2014;20(17):5119‐5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tringali A, Manta R, Sica M, Bassotti G, Marmo R, Mutignani M. Comparing intravenous and oral proton pump inhibitor therapy for bleeding peptic ulcers following endoscopic management: a systematic review and meta‐analysis. Br J Clin Pharmacol. 2017;83(8):1619‐1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gralnek IM, Dumonceau JM, Kuipers EJ, et al. Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy. 2015;47:a1‐a46. [DOI] [PubMed] [Google Scholar]
- 15. Sung JJ, Chiu PW, Chan FKL, et al. Asia‐Pacific working group consensus on non‐variceal upper gastrointestinal bleeding: an update 2018. Gut. 2018;67(10):1757‐1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang HY, Ou N, Lang L, Shi R, Hu P, Jiang J. Pharmacokinetics and pharmacodynamics of intravenous ilaprazole in healthy subjects after single ascending doses. Xenobiotica. 2016;46:1133‐1141. [DOI] [PubMed] [Google Scholar]
- 17. Wang HY, Lang L, Ou N, et al. Pharmacokinetics, pharmacodynamics and safety of multiple‐infusion ilaprazole in healthy Chinese subjects. Clin Drug Investig. 2016;36(6):463‐470. [DOI] [PubMed] [Google Scholar]
- 18. Hummel J, McKendrick S, Brindley C, French R. Exploratory assessment of dose proportionality: review of current approaches and proposal for a practical criterion. Pharm Stat. 2009;8(1):38‐49. [DOI] [PubMed] [Google Scholar]
- 19. Saitoh T, Watanabe Y, Kubo Y, et al. Intragastric acidity and circadian rhythm. Biomed Pharmacother. 2001;55(Suppl 1):138s‐141s. [DOI] [PubMed] [Google Scholar]
- 20. Yang H, Li J, Zhao Q, et al. Pharmacokinetics, pharmacodynamics, and safety of esomeprazole injection/infusion in healthy Chinese volunteers: a five‐way crossover study. J Gastroenterol Hepatol. 2013;28(12):1823‐1828. [DOI] [PubMed] [Google Scholar]
- 21. Kirchheiner J, Glatt S, Fuhr U, et al. Relative potency of proton‐pump inhibitors‐comparison of effects on intragastric pH. Eur J Clin Pharmacol. 2009;65(1):19‐31. [DOI] [PubMed] [Google Scholar]
- 22. Puchalski TA, Krzyzanski W, Blum RA, Jusko WJ. Pharmacodynamic modeling of lansoprazole using an indirect irreversible response model. J Clin Pharmacol. 2001;41(3):251‐258. [DOI] [PubMed] [Google Scholar]
- 23. Liu D, Yang H, Jiang J, et al. Pharmacokinetic and Pharmacodynamic Modeling Analysis of Intravenous Esomeprazole in Healthy Volunteers. J Clin Pharmacol. 2016;56(7):816‐826. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 Supporting information
Data S2 Supporting information


