Abstract
Aims
The aim of this systematic review and meta‐analysis was to synthesise the evidence relating to medication non‐adherence and its association with health outcomes in people aged ≥50 years.
Methods
Seven databases were searched up to February 2019 for observational studies that measured medication (non‐)adherence as a predictor of the following health outcomes in adults aged ≥50 years: healthcare utilisation (hospitalisation, emergency department visits, outpatient visits and general practitioner visits), mortality, adverse clinical events and quality of life. Screening and quality assessment using validated criteria were completed by 2 reviewers independently. Random effects models were used to generate pooled estimates of association using adjusted study results. The full methodological approach was published on PROSPERO (ID: CRD42017077264).
Results
Sixty‐six studies were identified for qualitative synthesis, with 11 of these studies eligible for meta‐analyses. A meta‐analysis including 3 studies measuring medication non‐adherence in adults aged ≥55 years showed a significant association with all‐cause hospitalisation (adjusted odds ratio 1.17, 95% confidence interval [CI] 1.12, 1.21). A meta‐analysis including 2 studies showed that medication non‐adherence was not significantly associated with an emergency department visit (adjusted odds ratio 1.05, 95% CI 0.90, 1.22). Good adherence was associated with a 21% reduction in long‐term mortality risk in comparison to medication non‐adherence (adjusted hazard ratio 0.79, 95% CI 0.63, 0.98).
Conclusion
Medication non‐adherence may be significantly associated with all‐cause hospitalisation and mortality in older people. Medication adherence should be monitored and addressed in this cohort to minimise hospitalisation, improve clinical outcomes and reduce healthcare costs.
Keywords: ageing population, hospitalisation, medication adherence, mortality
1. INTRODUCTION
Medication adherence is defined as the process by which patients take their medication as prescribed, consisting of 3 main components: initiation, implementation and discontinuation.1 Medication non‐adherence includes non‐initiation of treatment, suboptimal implementation of the regimen, or early discontinuation of treatment.
Medication non‐adherence may represent a greater risk in older people, resulting in poorer health outcomes compared to younger cohorts.2, 3, 4, 5 Drug‐related factors, such as dosing regimen, side effects and polypharmacy, and patient‐related factors, such as cognitive function, health literacy and multimorbidity, are barriers to medication adherence in this group.6 Non‐adherence may also be a product of age‐related functional decline.7 Middle‐aged participants (50–69 years) have been reported to be more adherent to their medication than both younger (<50 years) and older cohorts (≥70 years).8, 9
In general, research to date on medication non‐adherence has been disease‐specific, assessing the impact of suboptimal adherence on surrogate health outcomes such as blood pressure, cholesterol levels and biological response.10, 11, 12 As multimorbidity increases with age, a disease‐specific perspective may not be appropriate for older cohorts.13, 14
In 2002, a meta‐analysis of the influence of adherence on health outcomes concluded that adherent people were nearly 3 times more likely to experience a positive treatment outcome than non‐adherent people.15 However, this meta‐analysis was not limited to medication adherence, included surrogate outcomes, and did not provide an estimate specific to older people. A review of the barriers to good medication adherence in older people noted a lack of well‐designed studies of adherence in this population and called for future research to assess clinical outcomes associated with non‐adherence.6
The aim of this systematic review and meta‐analysis was to synthesise the evidence relating to medication non‐adherence in people aged ≥50 years, and its association with health outcomes including healthcare utilisation, quality of life (QoL), mortality and clinical events.
2. METHODS
This study was conducted and reported in accordance with the Preferred reporting items for systematic reviews and meta‐analyses (PRISMA) statement (Table S1).16 The protocol for this systematic review and meta‐analysis was published on PROSPERO on 11 December 2017 (ID: CRD42017077264).
2.1. Search strategy
Seven databases; PubMed, Embase, PsychoInfo, CinahlPlus, Web of Science, Scopus and the Cochrane library, were searched from inception through to February 2019 to identify studies meeting the inclusion criteria. Search strings were constructed using Boolean operators (AND, OR) and Medical Subject Headings and contained terms relating to medication adherence (such as medication adherence, compliance, persistence, treatment compliance etc.) combined with adherence measurement methods (electronic monitor*, medication event monitoring system*, MEMS, self‐report, morisky medication adherence scale, mmas, medication adherence rating scale, MARS, pharmacy refill*, dispens*, pharmacy record*, pharmacy claim* OR MPR OR PDC) combined with terms relating to older people (aged, frail elderly, aged 80 and over, aging, elderly, geriatric*, veteran*, older, senior*). Apart from MESH headings, the title/abstract field function was used for search terms. In addition, the reference lists of included studies were hand‐searched for relevant articles. The full search strategy for PubMed is provided in Table S2.
2.2. Inclusion criteria
We included observational studies that measured the association between medication (non‐) adherence and health outcomes in adults aged ≥50 years including:
Healthcare utilisation (hospitalisations, emergency department (ED) visits, physician office visits, outpatient visits).
QoL.
Mortality.
Adverse clinical events (any clinically significant events that may impact on healthcare utilisation, mortality and QoL such as falls, fractures and myocardial infarction).
Depression.
Implementation and/or initiation medication (non‐)adherence was measured objectively or through self‐report in the included studies. Objective measures such as the medication possession ratio (MPR) and proportion of days covered (PDC) with pharmacy refill claims data, electronic monitoring devices (MEMs) or pill count were included. Self‐report measures included validated self‐reported questionnaires such as Morisky Medication Adherence Scale.17, 18 Studies that measured the initiation and/or implementation phases of medication adherence were considered eligible. Studies published in a peer reviewed journal before February 2019 and report data from a primary study (not a review, or commentary) were included.
2.3. Exclusion criteria
We excluded intervention‐based studies with a control arm that received adherence support. Studies were excluded if the study population was aged <50 years. If the age range of the study population was not explicit, the author was contacted and a request was made for age stratified analyses, if feasible. Studies that reported the association between medication (non‐)adherence and surrogate outcomes e.g. blood pressure, or cholesterol were excluded. Studies that solely measured the discontinuation phase of adherence were excluded as discontinuation is a time‐to event phenomenon and outside the scope of this review. Non‐English studies were excluded.
2.4. Study selection
C.W. reviewed all abstracts retrieved from database searching for eligibility. A second reviewer (C.B. and S.T.) reviewed a 50% random sample of abstracts. Conflicts were resolved by a third reviewer (C.C.). C.W. screened all full texts and a second review (C.B., S.T., C.C. or M.C.) was carried out independently. Discussions were held until consensus on inclusion was reached.
2.5. Data extraction
A data extraction form was developed by adapting the Cochrane good practice data extraction form.19 The form was piloted by 3 reviewers (C.W., C.B. and M.C.) and included author, publication year, country, study design, participant characteristics, eligibility criteria, time period, type of medication, measures of adherence, measures of health outcomes, covariates, results, statistical methods, strengths and limitations, and conclusions. Data were extracted independently by C.W. Authors were contacted and asked to provide additional data where necessary.
2.6. Quality assessment
The Downs and Black scale was used to critically assess all eligible studies in a standardised way, including the measurement of adherence, study methodology and statistical analysis.20 Checklist items that were too specific to intervention studies were removed (Table S3). C.W. reviewed all included studies and a second reviewer (C.B., S.T., C.C. or G.C.) completed an independent quality appraisal and the score was averaged. Studies were categorised into 3 groups: low (<50%), medium (50%–79%) and high (≥80%) quality.
2.7. Synthesis of results
Studies were grouped together by: (i) the health outcome reported (e.g. hospitalisations, ED visits etc.); (ii) the source of adherence measurement (pharmacy refill claims, self‐report), and (iii) the statistical measure of association per the data analysis (regression β coefficients, odds ratios [ORs] etc.). Adjusted effect estimates were combined in meta‐analyses, where appropriate.
Random effects models were used to calculate pooled effect estimates for the association between medication non‐adherence and the various outcome measures.19 As the studies included in the analyses assessed adherence to different medications in varying populations and not all study results could be pooled, random effect models were constructed, using the Mantel–Haenszel approach.19, 21 Overall estimates of the association between medication non‐adherence and health outcomes are presented in forest plots. Funnel plot asymmetry was tested for using Egger's test for continuous outcomes and Harbord's test for dichotomous outcomes, if applicable.19 Data were analysed using STATA v.14 (College Station, TX, USA).
3. RESULTS
3.1. Study selection
Database searches identified 8188 unique results, of which 308 articles were selected for full‐text review. Ninety‐seven study authors were contacted for further details required for eligibility assessment and 41 replied. An additional 34 articles were identified from reference searching of articles included in review from data base searching (Table S4), resulting in a total of 342 eligible full‐texts. Sixty‐six full text articles were selected for data extraction (Figure 1).
Figure 1.
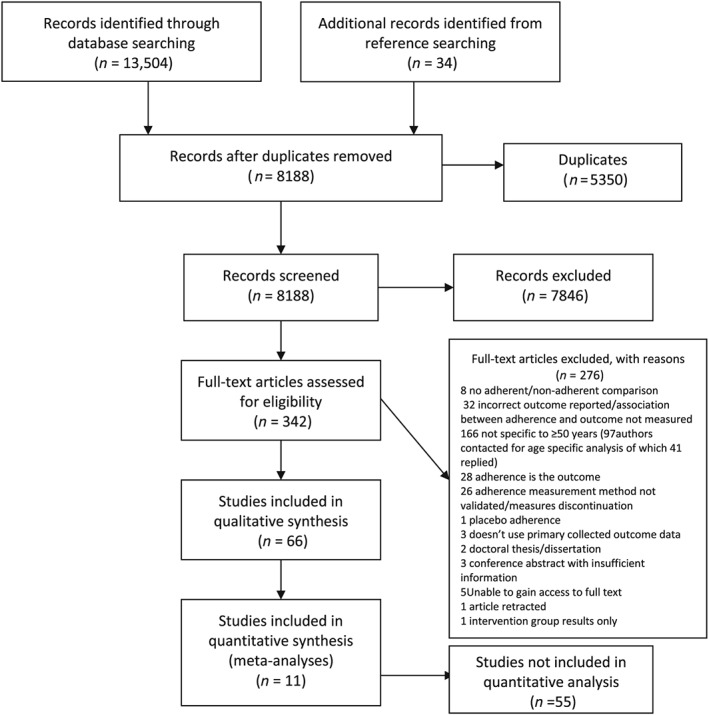
Study selection process for systematic review of the association between medication (non‐)adherence and health outcomes
3.2. Characteristics of included studies
The included studies focussed on a wide range of disease areas including pulmonary diseases,22, 23, 24 osteoporosis,25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46 depression,47, 48, 49 cardiovascular diseases,50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75 Parkinson's disease,76 epilepsy,77 chronic kidney disease,78 cancer79, 80 and diabetes mellitus.81, 82, 83, 84, 85 Two studies did not measure disease‐specific medication (non‐)adherence.4, 86 Thirty‐six studies were specific to, or provided age stratified analysis for people aged ≥64 years,4, 22, 30, 33, 34, 37, 41, 45, 46, 47, 49, 51, 54, 55, 58, 59, 61, 62, 65, 66, 67, 68, 69, 70, 71, 73, 76, 77, 78, 80, 81, 82, 83, 84, 85, 86 while the other 30 studies also included middle aged adults (≥50 years).23, 24, 25, 26, 27, 28, 29, 31, 32, 35, 36, 38, 39, 40, 42, 43, 44, 48, 50, 52, 53, 56, 57, 60, 63, 64, 72, 74, 75, 79 Thirteen authors of the included texts were contacted via e‐mail for further information, and 8 replied. In terms of adherence measurement, 54 studies measured medication (non‐)adherence using pharmacy claims data22, 23, 25, 26, 27, 28, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 49, 50, 51, 52, 55, 58, 59, 60, 61, 62, 64, 65, 66, 67, 68, 70, 71, 72, 73, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85 while 11 studies used self‐report methods, consisting of the 4‐item4, 29, 48, 54, 56, 69, 86 or 8‐item Morisky Medication Adherence Scale,24, 61, 74 or self‐reported PDC63 and MEMs was used in 4 studies.50, 52, 53, 57
Tables S5, S6 and S7 describe the characteristics of included studies.
3.3. Quality assessment
Overall, 48 studies were determined to be high quality,4, 22, 25, 27, 28, 30, 31, 32, 33, 34, 35, 36, 37, 38, 40, 41, 42, 43, 45, 47, 48, 49, 51, 55, 57, 58, 59, 60, 61, 62, 65, 66, 67, 68, 69, 71, 72, 73, 75, 77, 78, 79, 80, 81, 82, 83, 84, 86 17 were determined to be medium quality23, 26, 29, 39, 44, 46, 50, 52, 53, 54, 56, 63, 64, 70, 74, 76, 85 and 1 study was deemed to be low quality (Table S8).24 There was considerable variability with regards to external validity (generalisability), with 15 studies deemed medium quality23, 29, 41, 43, 44, 48, 54, 57, 61, 63, 67, 81, 82, 85, 86 and 11 studies achieving low quality.24, 46, 50, 52, 53, 56, 69, 70, 74, 75, 76 This was due to lack of detail on sampling or recruitment procedures or inclusion of specific populations only.
Most studies (n = 49) were rated as high quality with regards to confounding due to adequate adjustment, with 16 studies rated as medium quality23, 24, 26, 29, 34, 43, 46, 49, 50, 54, 63, 76, 77, 79, 80, 85 and 1 study as low quality due to a lack of sufficient adjustment.56 Exposure period was the same as the outcome measurement period in 9 studies measuring healthcare utilisation or adverse clinical outcomes (not censored at first incidence of event).22, 28, 35, 39, 44, 50, 52, 53, 76 Most included studies utilised large pharmacoepidemiological administrative data sources allowing for sufficiently powered analyses.25, 26, 27, 28, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 45, 46, 47, 51, 55, 58, 59, 60, 62, 64, 65, 66, 67, 68, 70, 71, 72, 73, 75, 77, 78, 79, 80, 81, 82, 84, 85
-
I
Healthcare utilisation
Results of individual studies measuring healthcare utilisation outcomes are presented in the supplementary material (Tables S9‐S15).
3.3.1. Hospitalisations
Twenty‐three studies described the association between medication (non‐)adherence and hospitalisations or length of hospital stay (LOS), either as an individual outcome,22, 23, 26, 27, 28, 36, 38, 39, 42, 44, 47, 52, 53, 58, 70, 77, 82, 83, 84, 85 or as part of a composite outcome.4, 61, 76 All‐cause hospitalisation was an outcome in 7 studies,4, 22, 42, 44, 53, 58, 77 whereas 13 studies reported disease‐specific hospitalisations.23, 38, 39, 47, 61, 70, 76, 82, 83, 84, 85 Five studies reported both outcomes.26, 27, 28, 36, 52 Overall, most (n = 17) showed an inverse relationship between optimal medication adherence and hospitalisations in adults ≥50 years (Table S9).27, 28, 36, 38, 44, 47, 52, 53, 58, 61, 70, 76, 77, 82, 83, 84, 85 Two studies reporting the association between adherence and LOS showed that good adherence was associated with reduced LOS.22, 77 However, in the study analysing inhaled corticosteroid adherence, this relationship was not significant.22
Two random effects meta‐analyses were constructed for the association of medication non‐adherence and hospitalisation. The first meta‐analysis pooled data from 3 studies reporting all‐cause hospitalisation as the outcome across 75,943 patients aged ≥55 years.27, 58, 77 Odds ratios for the association between non‐adherence (MPR < 0.80) to osteoporosis medications27 and antiepileptic medications,77 and intermediate adherence to statins (PDC 0.4–0.79)58 were combined. Medication non‐adherence was associated with a 17% increased risk of all‐cause hospitalisation (adjusted OR 1.17, 95% confidence interval [CI] 1.12,1.21, p ≤ 0.001). The second meta‐analysis pooled data from 3 studies reporting disease‐specific hospitalisation as the outcome across 659,436 patients aged ≥55 years.27, 82, 83 Bisphosphonate non‐adherence and association with osteoporosis–related hospitalisations was reported in 1 study,27 with antihypertensive nonadherence and association with diabetes–specific hospitalisations in the second study82 and statin nonadherence and association with hospitalisation for major coronary events reported in the third study.83 The pooled estimate for medication non‐adherence and disease‐specific hospitalisations did not reach statistical significance (adjusted OR 1.07, 95% CI 0.98,1.17, P = 0.14; Figure 2B).
Figure 2.
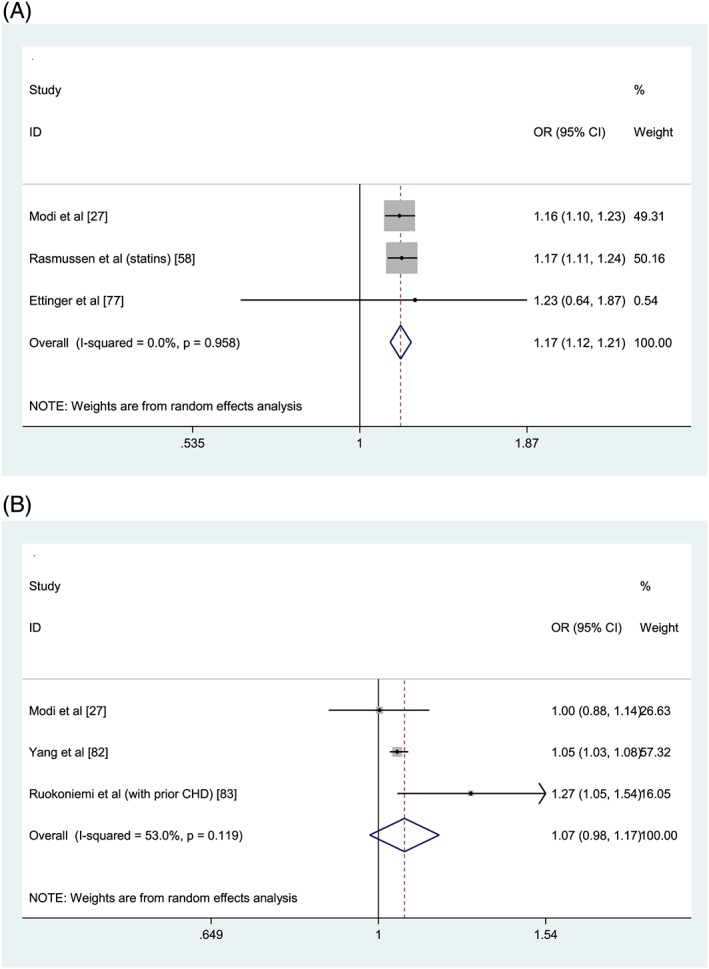
Forest plots of medication non‐adherence and association with all‐cause hospitalisations (A) and disease‐specific hospitalisations (B). OR = odds ratio; CI = confidence interval; PDC = proportion of days covered; MPR = medication possession ratio. Reference group: Adherent (PDC/MPR ≥80%). Intermediate adherence (PDC 40–79%) results are reported for Rasmussen et al. in (A). All‐cause hospitalisation (Figure 2A): Heterogeneity: χ2 = 0.08 (d.f. = 2), P = .958, I2 = 0.0% τ2 = 0.0000. Test for overall effect: Z= 7.65, P < .0001. Disease‐specific hospitalisation (Figure 2B): Heterogeneity: χ2 = 4.26 (d.f. = 2), P = .119, I2 = 53.0%, τ2= 0.0035. Test for overall effect: Z= 1.47, P =.143
3.3.2. ED visits
Eleven studies reported the association between medication (non‐)adherence and ED visits, either as an individual outcome,4, 22, 26, 27, 36, 42, 50, 52, 53, 77 or as part of a composite outcome (Table S10).76 Four studies reported disease‐specific ED visits.26, 27, 50, 76 Some studies reported no significant increase in the number of ED visits as a result of non‐adherence22, 27 but others reported a significant increase in ED visits using MEMs,50 or a significant decrease due to adherent behaviour.36, 52, 53 Non‐adherence to oral bisphosphonates was significantly associated with a reduced likelihood of osteoporosis‐specific ED visits.27 The number of all‐cause ED visits was significantly higher in patients adherent to their oral bisphosphonate therapy but this was not the case for osteoporosis–related ED visits.26 There was no statistically significant relationship between adherence and all‐cause ED visits in 3 studies.4, 27, 77
Data were pooled for a random effects meta‐analysis to estimate the association between medication non‐adherence, measured using pharmacy refill claims (MPR < 0.80), and likelihood of an ED visit (ORs) from 2 studies (Figure 3).27, 77 This meta‐analysis included 59,191 people aged ≥55 years prescribed bisphosphonate therapies or antiepileptic medications.27, 77 The pooled estimate was non‐significant (adjusted OR 1.05, 95% CI 0.90, 1.22, P = 0.56).
Figure 3.
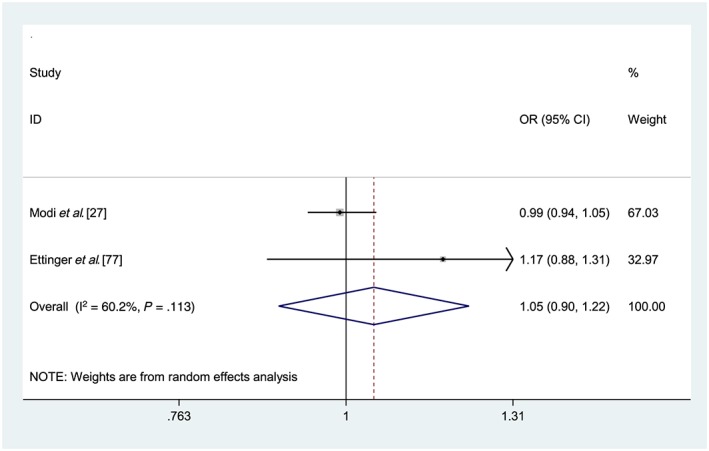
Forest plot of medication non‐adherence and association with emergency department visits. OR = odds ratio; CI = confidence interval; PDC = proportion of days covered; MPR = medication possession ratio. Reference group: Adherent (PDC/MPR ≥ 80%). Heterogeneity: χ2 = 2.51 (d.f. = 1), P = .113, I2 = 60.2%, τ2= 0.0084. Test for overall effect: Z= 0.57, P = .566
Similarly, the effect estimates of 2 studies measuring the association of medication non‐adherence with the number of ED visits, using adjusted regression coefficients, were pooled in a random effects model, but again the result was non‐significant (adjusted β 0.07, 95% CI –0.29, 0.49).22, 77
3.3.3. Physician visits
Four studies described the relationship between medication (non‐)adherence and physician office visits (Table S11).22, 26, 42, 77 Non‐adherence to inhaled corticosteroid therapy was associated with an increased number of physician office visits but this relationship was not significant.22 Non‐adherence to antiepileptic medication in adults aged ≥65 years was also associated with a significantly increased number of physician visits.77 Non‐adherence to bisphosphonate therapy was associated with significantly less disease‐specific, but not all‐cause physician office visits.26 Conversely, adherence to bisphosphonate therapy in another osteoporosis study was associated with an increased likelihood of experiencing at least 1 osteoporosis‐related physician office visit.42
3.3.4. Outpatient services
Six studies reported the association between medication (non‐)adherence and outpatient service utilisation; 5 as an individual outcome26, 27, 36, 42, 77 and 1 as part of a composite outcome (Table S12).76 There was no clear relationship between adherence and outpatient utilisation across the studies. A random effects meta‐analysis including 2 studies27, 36 of osteoporosis medication nonadherence indicated no significant relationship with all‐cause outpatient utilisation (adjusted incidence rate ratio 1.09, 0.87–1.36, P = 0.46) and osteoporosis‐specific outpatient utilisation (adjusted incidence rate ratio 1.08, 0.66–1.75, P = 0.12), respectively.
-
II
Quality of life
Six studies reported the association between medication (non‐)adherence and QoL (Table S13),24, 29, 56, 57, 69, 74 and 5 of these studies specifically measured health‐related QoL (HRQoL).24, 29, 56, 69, 74 A significant relationship was observed in 2 studies involving older hypertensive patients.69, 74 Raloxifene adherence was significantly correlated with HRQoL after 3 months of starting treatment for osteoporosis, but this relationship was not significant at 1 year.29 Medication adherence was not significantly associated with respiratory specific HRQoL in a study of chronic obstructive pulmonary disease (COPD) patients, but this may have been due to the small sample size (n = 62).24 Two studies compared the average HRQoL score across adherent and non‐adherent patient groups.56, 57 In the first study, atrial fibrillation patients adherent to rivaroxaban reporting significantly higher QoL scores overall and across all individual domains than non‐adherent patients,57 while, in the second study, good adherence to antihypertensive treatment was associated with higher HRQoL scores across all domains using the WHOQOL‐BREF, but not with the death and intimacy sections of the WHOQOL‐OLD.56
-
III
Mortality
Sixteen studies measured the association between medication (non‐)adherence and mortality (Table S14).38, 49, 51, 55, 58, 59, 60, 62, 64, 66, 75, 78, 79, 80, 81, 85 Suboptimal adherence was associated with a significantly increased risk of mortality in most studies evaluating mortality independently. However, 1 study found an increased risk of mortality associated with good adherence to ACEI/ARB therapy in dialysis patients.75 Three studies followed‐up patients for ≤1 year and found adherence to have a protective effect on mortality.51, 59, 62 A study examining statin adherence, which had an average follow up ≥4 years, showed that adherence to statins demonstrated an inverse dose–response effect regarding mortality risk.60 Across 2 studies that measured the association between cardiovascular medication adherence and cardiovascular mortality, 1 found a significant relationship64 while a non‐significant association was observed in the other study.66 Good adherence to metformin had a significant protective mortality effect when follow up was ≥4 years, but this relationship was not significant when participants were only followed up for <4 years.81 In an Australian cohort, adherence to cardiovascular medications was not significantly associated with the composite outcome of death and cardiovascular events.54
Two meta‐analyses were conducted for the analysis of the association between adherence and mortality. The first meta‐analysis combined results from 2 studies of patients aged ≥65 years on cardiovascular medications (statin group in Rasmussen et al.).58, 78 Low medication adherence (measured categorically) was significantly associated with an increased risk of mortality in comparison to high adherence (PDC ≥ 80%; adjusted hazard ratio [HR] 1.24, 95% CI 1.14–1.35, P < .001; Figure 4A). The second meta‐analysis pooled adjusted hazard ratios in a random effects meta‐analysis for the association between good medication adherence (dichotomously measured) from 5 studies including 246 168 patients aged ≥50 years.38, 49, 75, 79, 80 The included studies analysed the relationship between antihypertensive,75 endocrine,79, 80 antidepressant49 and osteoporosis38 medications and all set the adherence threshold at 80%. Medication non‐adherence (MPR/PDC ≥ 80%) was significantly associated with a 21% reduction in the mortality risk (adjusted HR 0.79, 95% CI 0.63–0.98, P = .03; Figure 4B).
-
IV
Adverse clinical events
Figure 4.
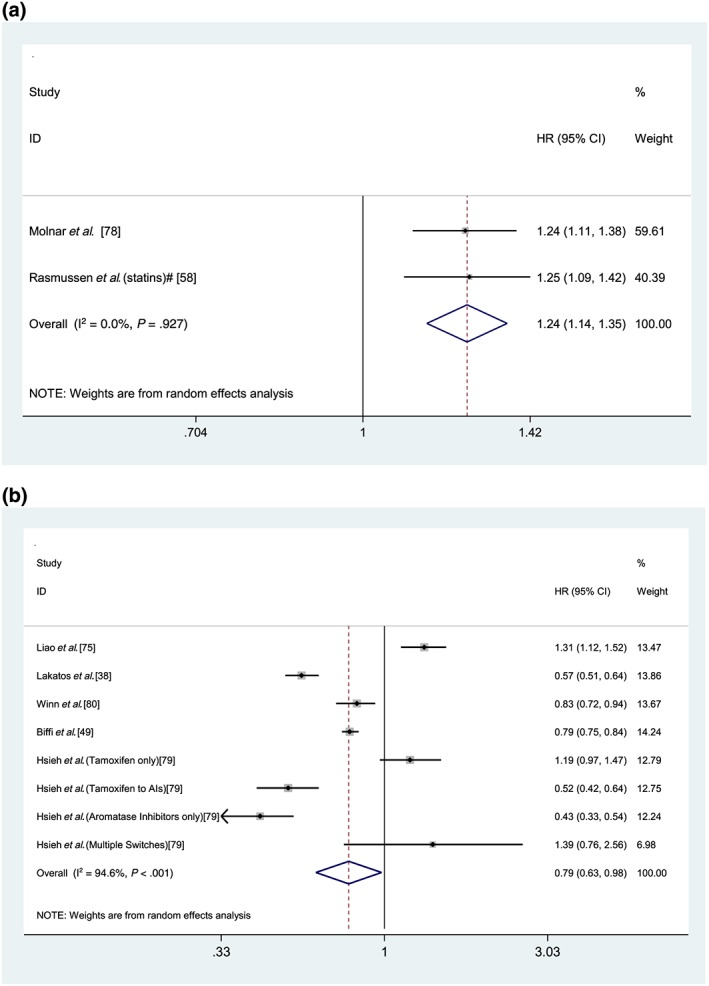
Forest plot of low medication adherence (A) and association with mortality (follow up≥1 year) and good medication adherence (B) and association with mortality (follow up≥1 year). HR = hazard ratio; CI = confidence interval; PDC = proportion of days covered. (Figure 2A) All studies measure adherence categorically. Reference group: Adherent (PDC ≥ 80%); *low medication adherence: PDC ≤ 60%; #low medication adherence: PDC < 40%; Heterogeneity: χ2 = 0.01 (d.f. = 3), P = .927, I2 = 0.0%, τ2= 0.0000. Test for overall effect: Z = 5.09, P < .001. (Figure 2B) All studies measured adherence dichotomously. Reference group for all studies: non‐adherent (PDC ≤ 80%). Heterogeneity: χ2 = 130.72 (d.f. = 7), P < .001, I2 = 94.6%, τ2 = 0.0906. Test for overall effect: Z = 2.12, P = .034
Twenty‐eight studies reported on the association between medication (non‐)adherence and adverse clinical events (Table S15).25, 26, 27, 30, 31, 32, 33, 34, 35, 37, 38, 40, 41, 42, 43, 45, 46, 54, 63, 65, 66, 67, 68, 71, 72, 73, 85, 86 Seventeen studies specified the number of fractures or fracture risk as the outcome,25, 26, 27, 30, 31, 32, 33, 34, 37, 38, 40, 41, 42, 43, 45, 46, 67 whereas falls risk,86 osteonecrosis of the jaw (with alendronate therapy)35 and cardiovascular events54, 63, 65, 66, 68, 71, 72, 73, 85 were reported in the other studies. Good adherence was associated with reduced fracture risk or number of fractures in 88% of the studies reporting fracture as an outcome.25, 26, 27, 31, 32, 33, 34, 37, 38, 40, 41, 43, 45, 46, 67 However, fracture site analysed34, 45, 46 affected the significance of this association, as did the type of antiosteoporotic therapy.30, 34 High adherence to oral bisphosphonates used for secondary prevention was significantly associated with reduced risk of fractures in 1 study, but this relationship was not significant for primary prevention.30
Older people who self‐reported low adherence to their medications showed a 50% increased likelihood of experiencing a fall in comparison to peers reporting good adherence.86 Higher adherence to alendronate (MPR > 50%) was associated with a significantly increased risk of osteonecrosis of the jaw in long term users.35 Good adherence to cardiovascular medications was associated with a significantly lower risk of experiencing adverse cardiovascular outcomes in eight studies,54, 65, 66, 68, 71, 72, 73, 85 with 1 study reporting insignificant results, possibly due to the low event rate.63
-
V
Depression
One study reported depression score as an outcome. Self‐reported non‐adherence to antidepressant medication was not significantly associated with depression severity score at 12 months, using the Montgomery–Åsberg Depression Rating Scale.48
3.4. Risk of publication bias across studies
Due to each meta‐analysis containing <10 studies, it was not possible to assess for publication bias by visual inspection of funnel plots.19
4. DISCUSSION
This systematic review is the first to specifically examine the association between medication (non‐)adherence and health outcomes in adults aged ≥50 years. The main findings of this review indicate that medication non‐adherence has a significantly negative association with a range of important health outcomes in older people, specifically hospitalisation and mortality.
The results showed that non‐adherent individuals aged ≥50 years have a 17% higher risk of having a hospitalisation due to any cause compared to those considered adherent, consistent with other reviews not specific to older people.87, 88, 89, 90, 91 The previous disease‐specific reviews only conducted qualitative synthesis of the evidence regarding adherence and hospitalisation risk. A COPD‐specific review included 2 studies that reported an increase in hospitalisations with medication non‐adherence.87 One of the studies conducted a cross‐sectional analysis of the association between COPD implementation adherence (PDC ≥ 0.80) on hospitalisation rate and found adherent patients had a 10% lower rate of hospitalisations than their non‐adherent counterparts.92 Another study in the COPD review was a secondary analysis of a randomised controlled trial that monitored adherence to inhaled corticosteroid therapy and placebo using MEMs, with adherers having a 12% lower rate of hospitalisation annually in comparison to non‐adherers.93 A review of non‐adherence to antidepressants,89 reported on 3 studies that assessed the association between antidepressant non‐adherence and hospitalisation, 2 of which were not specific to adults aged ≥50 years.94, 95 Duloxetine adherence was associated with a reduced hospitalisation risk of 14% in comparison to non‐adherence.94 The second study did not show a significant difference in the average number of hospital visits between adherent and non‐adherent patients initiated on antidepressants.95 However, the follow‐up period for outcome measurement was relatively short (6 months) after antidepressant initiation.95 A review of the economic impact of antipsychotic medication non‐adherence found that reduced hospitalisation rates in adherent patients were the main driver of overall reduced psychiatric care costs in 3 of the 8 studies reviewed.91 However, unlike the studies included in this current review, treatment persistence was measured. The meta‐analysis for the association between medication non‐adherence and disease‐specific hospitalisations did not reach statistical significance, mainly driven by a non–significant result for osteoporosis‐specific hospitalisations in 1 large study.27 However, this may have been due to discharge coding errors as only primary or secondary ICD‐9 diagnosis codes for inpatient admissions were counted as an osteoporosis–related hospitalisation.
Alternatively, the lack of statistical significance observed with the disease‐specific meta‐analysis estimate may indicate the presence of healthy adherer bias in the all‐cause hospitalisation result. The healthy adherer phenomenon occurs when people who are adherent to their medications are healthier overall and exhibit health seeking behaviours such as being physically active, participating in preventative screening services and not smoking.96 A previous meta‐analysis found that good adherence to pharmacological therapy for a variety of diseases, was associated with a 44% reduced mortality risk in comparison to poor adherence.97 However, the authors highlighted the positive association between good placebo adherence and lower mortality risk, highlighting the healthy adherer hypothesis. Studies may be subject to unmeasured confounding bias from this healthy adherer effect, due to lack of information in administrative databases, such as smoking status, body mass index etc.97 It has previously been suggested that use of a new user study design would assist in reducing this confounding, along with adjustment for adherence to medications unrelated to clinical outcome.96 While the majority of included studies in this review had a new user design, the time period for determining prior medication use varied from 3 months in 1 study,59 to many years in others.32, 65, 84 Few were able to adjust for indicators of healthy adherer bias (smoking status, vaccine receipt, exercise)30, 31, 41, 60, 69 or conduct sensitivity analyses to estimate the potential effect of unmeasured confounding on effect estimates.33, 65 Future cohort studies should record and adjust for lifestyle behaviours or participation in health screening services when analysing the relationship between medication (non‐)adherence and health outcomes. Negative control analysis, using outcomes unrelated to therapeutic endpoints such as car accidents, may also strengthen robustness of observational adherence studies.
Medication non‐adherence had no significant association with the likelihood of experiencing an ED visit in the meta‐analysis. The association between non‐adherence and ED visits is not clear in the literature. In fact, a recent Korean study found that after 2 years of being adherent, high‐grade COPD patients had an increased likelihood of having ED visits in comparison to non‐adherent patients.98 This is in contrast to the significant association the authors found between adherence and lower intensive care unit use and healthcare costs within the same population.98 Given the acute nature of ED visits, there is evidence that a wide range of clinical, psychological and social factors are associated with ED visits in adults,99 and studies assessing adherence need to adjust for a range of confounding variables. These confounders can also be time‐varying, and only 1 of the studies included in our ED meta‐analysis (Figure 3) separated the time period for outcome measurement from the exposure assessment period.27 Healthcare utilisation has been reported to be a predictor of medication non‐adherence as well as an outcome, due to regimen complexity increasing following service use.100, 101 In cross‐sectional analyses, contamination bias may be present102; therefore, adherence should be measured in the period preceding outcome measurement when determining the influence of (non‐)adherence on healthcare utilisation.
A significantly increased mortality risk was associated with medication non‐adherence (<80%) and low adherence, respectively. However, there was significant statistical heterogeneity observed across the pooled studies for the dichotomous adherence exposure, possibly due to the variety of medication classes included. Data obtained from Liao et al. indicated that only 8% of ACEI/ARBs users were determined to be adherent (n = 410),75 whereas 80% of all participants aged ≥50 years were considered adherent to endocrine therapy,79 with 47% of participants considered adherent to osteoporotic therapy.38 In addition, 3 studies included results for participants aged 50–64 years,38, 75, 79 while the other the other studies were specific to those aged ≥65 years.49, 80 In a previous meta‐analysis, poor adherence to any cardiovascular medication resulted in a 38% increased risk of death in comparison to good adherence.103 This review was not age‐specific, allowing for a greater number of studies to be included in the meta‐analysis (n = 23). In addition, the meta‐analysis was based on pooled unadjusted risk ratio estimates, while the current meta‐analyses included adjusted HRs. As cause of death was not available for the observational studies included in the meta‐analyses, caution should be exercised in interpreting a causal relationship between adherence and mortality.
Most studies of adherence to oral bisphosphonates included in this review showed a significant association between poor adherence and non‐vertebral fracture risk, which was established in a previous review.104 Only 1 of the included studies focussing on adherence to osteoporosis therapies adjusted for adherence to calcium and vitamin D supplementation,33 while all participants in another received such supplementation as part of randomised controlled trial protocols.43 In most countries, calcium and vitamin D preparations are available over the counter and, as such, are not recorded in pharmacoepidemiological databases, resulting in possible residual confounding. High non‐adherence and discontinuation rates with oral bisphosphonates treatment have been frequently highlighted in the literature.104, 105, 106 As such, alternative treatment options, such as the 6‐monthly denosumab injection, might be considered the preferred choice in osteoporosis treatment for patients who have low adherence levels.106
Consistent with previous reviews, we found no clear association between non‐adherence and QoL.107, 108 In a review specific to COPD medications,107 only 1 of the included studies measured the impact of adherence to nebulizers over a 4‐week period, finding that adherence was negatively correlated with disease‐specific HRQoL, measured at the end of the study.109 The relationship between adherence and QoL can be influenced by many factors such as disease severity, cognitive functioning and the patient's understanding of their medication regimen.107, 108 Therefore, to truly understand the complex relationship between adherence and QoL, multivariable regression analyses should be conducted, which were absent in most of the QoL studies included in this review. Antidepressant medication adherence was not significantly associated with depression severity, which may be explained by the significance of depression severity as a predictor of non‐adherence.110, 111, 112
4.1. Strengths and limitations
This is the first systematic review and meta‐analysis to focus on the association between medication (non‐)adherence and health outcomes in middle‐ and older‐aged adults. We searched a large range of databases, hand searched reference lists and attempted additional contact with authors to ensure inclusion of all relevant papers. We also allowed for inclusion of author‐provided, unpublished, age‐stratified estimates to undertake meta‐analysis.
A further strength of this review, unlike previous research, was that it was not disease specific. A range of medication classes and medical conditions were included, as multimorbidity is common in this cohort. Beneficial health outcomes accrued as being adherent to 1 therapeutic medication class may not be disease–specific, particularly in multimorbidity. An overall estimation of the association between medication non‐adherence and generic healthcare utilisation may be more appropriate for patients on complex medication regimens and generalisable to older populations.
There are some limitations to this systematic review and meta‐analysis. Firstly, conference abstracts and unpublished grey literature were not included and, as such, the review may be subject to some publication bias. However, this bias is expected to be low since a large amount of published literature on medication adherence is available.
Secondly, we only included studies that were specific to middle‐ and older‐aged people (≥50 years) as published in the article text or if the author was contacted and responded with the required age‐specific data. The main focus of the review was medication‐taking behaviours in middle‐ and older‐aged populations as they migrate through the ageing process, and the association with subsequent health outcomes. As there is no standardised age cut‐off for older people in the literature, we aimed to be as inclusive as possible of studies that mainly included older populations thus, an age cut‐off of ≥50 years was designated. We did not contact study authors where the median/mean age of the population was <50 years or the age range of the study population specified <65 years only (e.g. 18–64 years). Further, over half the authors emailed did not respond or were not available at the email addresses listed. Therefore, we may have omitted relevant data from the meta‐analyses.
Thirdly, adherence measurements employed using methods such as serum concentrations/viral loads, diary accounts or self‐reported methods that had not been validated were excluded. Serum concentrations may not accurately reflect medicine‐taking behaviour and may be subject to pharmacodynamic variability, particularly in elderly people.113 Patient diaries are not always reliable113 and asking a single question may not be specific enough to capture non‐adherence. Therefore, we included adherence measurement methods that we knew to be reliable and valid for use in this cohort.
In addition, we wished to estimate the clinical burden of medication non‐adherence using real‐world data, where patients are not receiving interventional adherence support. As such, this systematic review included mainly observational studies, which are not subject to the same randomisation processes as interventional trials. Therefore, residual confounding is likely to be present. Some included studies addressed the potential influence that such unmeasured confounding may have had on estimates by conducting sensitivity analyses. As with all research, healthy volunteer bias may be present in some studies. In addition, there is variation in the covariates controlled for within individual studies included in the qualitative synthesis. However, studies included in meta‐analyses controlled for important demographic, clinical and medication regimen variables.
Finally, studies that focussed only on medication discontinuation or persistence were not included in the review. Discontinuation of a medication may be appropriate in certain clinical situations. However, this can be difficult to determine in administrative database studies largely due to a lack of clinical information. Future research should assess the impact of medication discontinuation, where not clinically indicated, on health outcomes.
4.2. Implications
This review has provided a comprehensive and systematic assessment of the evidence on the association between medication non‐adherence and adverse health outcomes in older populations. It has highlighted the critical need for further research in this area. Despite a relatively large evidence base, meta‐analysis was only feasible for 3 of the specified health outcomes and included only a small number of studies. A recent review on the economic burden of medication non‐adherence cited the wide variability in adherence measurement and study methodologies as a barrier to meta‐analysis.114 This is evident in the current review. More concerted action is needed to establish and standardise methods of measuring adherence in older patients with multimorbidity, which are comparable across studies. There is an urgent need for the development of innovative approaches to both detect and monitor medication non‐adherence in community‐dwelling older people with multimorbidity.115 Study methodologies also need to be strengthened and adjust for different exposure periods, allowing for sufficient follow‐up to establish association with health outcomes. Further evaluation of the effectiveness of adherence‐enhancing interventions, such as pharmacist‐led medication review on minimising preventable healthcare utilisation should be explored.116
5. CONCLUSION
Medication non‐adherence may be significantly associated with all‐cause hospitalisation and mortality in older people. Hospitalisation in older people is a major driver of high medical costs,102 and medication adherence should be monitored and non‐adherence addressed in this cohort to help minimise hospitalisation, improve clinical outcomes and reduce healthcare costs.
COMPETING INTERESTS
There are no competing interests to declare.
CONTRIBUTORS
C.W., C.C. and K.B. were involved in the concept, design, analysis and interpretation of the results. C.W., C.B. and S.T. were involved in abstract screening. C.W., C.B., S.T., C.C. and M.C. were involved in full‐text selection and quality appraisal of included studies. C.W., C.C. and K.B. were involved in preparation of the manuscript and all authors made suggestions and approved the final manuscript.
Supporting information
TABLE S1
PRISMA checklist
TABLE S2
Search Strategy PubMed
TABLE S3
Quality appraisal checklist
TABLE S4
List of citations located by hand searching
TABLE S5
Characteristics of included studies measuring healthcare utilisation and adverse events
TABLE S6
Characteristics of included studies measuring mortality
TABLE S7
Characteristics of included studies measuring quality of life or depression severity
TABLE S8
Quality appraisal rating of included studies
TABLE S9
Results of individual studies into the association between medication adherence and hospitalisation
TABLE S10
Results of individual studies into the association between medication adherence and emergency department visits
TABLE S11
Results of individual studies into the association between medication adherence and physician visits
TABLE S12
Results of individual studies into the association between medication adherence and outpatient visits
TABLE S13
Results of individual studies into the association between medication adherence and quality of life
TABLE S14
Results of individual studies into the association between medication adherence and mortality
TABLE S15
Results of individual studies into the association between medication adherence and adverse events
ACKNOWLEDGEMENTS
C.W., C.C., S.T., C.B. and K.B. were funded by the Health Research Board (HRB), Research Leaders Award (HRB RL‐2015‐1579). M.C. was funded by a HRB Summer Student Scholarship (HRB SS‐2018‐043). This work was conducted as part of the SPHeRE Programme under Grant No. SPHeRE/2013/1. The funding body had no part in the concept, design, screening, analysis, interpretation or manuscript preparation.
Walsh CA, Cahir C, Tecklenborg S, Byrne C, Culbertson MA, Bennett KE. The association between medication non‐adherence and adverse health outcomes in ageing populations: A systematic review and meta‐analysis. Br J Clin Pharmacol. 2019;85:2464–2478. 10.1111/bcp.14075
REFERENCES
- 1. Vrijens B, De Geest S, Hughes DA, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. 2012;73(5):691‐705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hughes CM. Medication non‐adherence in the elderly. Drugs Aging. 2004;21(12):793‐811. [DOI] [PubMed] [Google Scholar]
- 3. Cahir C, Fahey T, Teljeur C, Bennett K. Medication adherence and adverse health outcomes in community dwelling older patients. Value Health. 2013;16(7):A335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vik SA, Hogan DB, Patten SB, Johnson JA, Romonko‐Slack L, Maxwell CJ. Medication nonadherence and subsequent risk of hospitalisation and mortality among older adults. Drugs Aging. 2006;23(4):345‐356. [DOI] [PubMed] [Google Scholar]
- 5. Banning M. Older people and adherence with medication: a review of the literature. Int J Nurs Stud. 2008;45(10):1550‐1561. [DOI] [PubMed] [Google Scholar]
- 6. Gellad WF, Grenard JL, Marcum ZA. A systematic review of barriers to medication adherence in the elderly: looking beyond cost and regimen complexity. Am J Geriatr Pharmacother. 2011;9(1):11‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shruthi R, Jyothi R, Pundarikaksha HP, Nagesh GN, Tushar TJ. A study of medication compliance in geriatric patients with chronic illnesses at a tertiary care hospital. J Clin Diagn Res. 2016;10(12):FC40‐FC43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mann DM, Woodward M, Muntner P, Falzon L, Kronish I. Predictors of nonadherence to statins: a systematic review and meta‐analysis. Ann Pharmacother. 2010;44(9):1410‐1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Doggrell SA. Adherence to medicines in the older‐aged with chronic conditions: does intervention by an allied health professional help? Drugs Aging. 2010;27(3):239‐254. [DOI] [PubMed] [Google Scholar]
- 10. Fung V, Huang J, Brand R, Newhouse JP, Hsu J. Hypertension treatment in a medicare population: adherence and systolic blood pressure control. Clin Ther. 2007;29(5):972‐984. [DOI] [PubMed] [Google Scholar]
- 11. Chi MD, Vansomphone SS, Liu ILA, et al. Adherence to statins and LDL‐cholesterol goal attainment. Am J Manag Care. 2014;20(4):e105‐e112. [PubMed] [Google Scholar]
- 12. Boussari O, Subtil F, Genolini C, et al. Impact of variability in adherence to HIV antiretroviral therapy on the immunovirological response and mortality. BMC Med Res Methodol. 2015;15(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross‐sectional study. Lancet. 2012;380(9836):37‐43. [DOI] [PubMed] [Google Scholar]
- 14. Marengoni A, Angleman S, Melis R, et al. Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev. 2011;10(4):430‐439. [DOI] [PubMed] [Google Scholar]
- 15. DiMatteo MR, Giordani PJ, Lepper HS, Croghan TW. Patient adherence and medical treatment outcomes: a meta‐analysis. Med Care. 2002;40(9):794‐811. [DOI] [PubMed] [Google Scholar]
- 16. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264‐269. [DOI] [PubMed] [Google Scholar]
- 17. Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self‐reported measure of medication adherence. Med Care. 1986;24(1):67‐74. [DOI] [PubMed] [Google Scholar]
- 18. Morisky DE, Ang A, Krousel‐Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich). 2008;10(5):348‐354. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19. Deeks J, Higgins J, Altman D, Green S. Cochrane handbook for systematic reviews of interventions version 5.1. 0 (updated March 2011). 2011.
- 20. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non‐randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tufanaru C, Munn Z, Stephenson M, Aromataris E. Fixed or random effects meta‐analysis? Common methodological issues in systematic reviews of effectiveness. Int J Evid Based Healthc. 2015;13(3):196‐207. [DOI] [PubMed] [Google Scholar]
- 22. Balkrishnan R, Christensen DB. Inhaled corticosteroid use and associated outcomes in elderly patients with moderate to severe chronic pulmonary disease. Clin Ther. 2000;22(4):452‐469. [DOI] [PubMed] [Google Scholar]
- 23. Matuszewski K, Velayudhan P, Flint N, Pierpaoli P. Noncompliance with drug therapy for chronic obstructive pulmonary disease: a risk factor for hospitalization? Value Health. 1999;2(6):446‐451. [DOI] [PubMed] [Google Scholar]
- 24. Horvat N, Locatelli I, Kos M, Janezic A. Medication adherence and health‐related quality of life among patients with chronic obstructive pulmonary disease. Acta Pharm. 2018;68(1):117‐125. [DOI] [PubMed] [Google Scholar]
- 25. Chan D‐C, Chang C‐C, Lim L‐C, et al. Association between teriparatide treatment persistence and adherence, and fracture incidence in Taiwan: analysis using the National Health Insurance Research Database. Osteoporosis Int. 2016;27(9):2855‐2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Eisenberg DF, Placzek H, Gu T, Krishna A, Tulsi BB. Cost and consequences of noncompliance to oral bisphosphonate treatment. J Manag Care Spec Pharm. 2015;21(1):56‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Modi A, Siris ES, Tang J, Sen S. Cost and consequences of noncompliance with osteoporosis treatment among women initiating therapy. Curr Med Res Opin. 2015;31(4):757‐765. [DOI] [PubMed] [Google Scholar]
- 28. Zhao Y, Johnston S, Smith D, McMorrow D, Krohn K, Krege J. Association between teriparatide adherence and healthcare utilization and costs in real‐world US kyphoplasty/vertebroplasty patients. Osteoporosis Int. 2013;24(9):2525‐2533. [DOI] [PubMed] [Google Scholar]
- 29. Guilera M, Fuentes M, Grifols M, Ferrer J, Badia X, Investigators OS. Does an educational leaflet improve self‐reported adherence to therapy in osteoporosis? The OPTIMA study. Osteoporosis Int. 2006;17(5):664‐671. [DOI] [PubMed] [Google Scholar]
- 30. Cadarette SM, Solomon DH, Katz JN, Patrick AR, Brookhart M. Adherence to osteoporosis drugs and fracture prevention: no evidence of healthy adherer bias in a frail cohort of seniors. Osteoporosis Int. 2011;22(3):943‐954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cotté FE, Mercier F, De Pouvourville G. Relationship between compliance and persistence with osteoporosis medications and fracture risk in primary health care in France: a retrospective case‐control analysis. Clin Ther. 2008;30(12):2410‐2422. [DOI] [PubMed] [Google Scholar]
- 32. Abrahamsen B, Eiken P, Prieto‐Alhambra D, Eastell R. Risk of hip, subtrochanteric, and femoral shaft fractures among mid and long term users of alendronate: nationwide cohort and nested case‐control study. BMJ. 2016;353:i3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Blouin J, Dragomir A, Moride Y, Ste‐Marie LG, Fernandes JC, Perreault S. Impact of noncompliance with alendronate and risedronate on the incidence of nonvertebral osteoporotic fractures in elderly women. Br J Clin Pharmacol. 2008;66(1):117‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ferrari S, Nakamura T, Hagino H, Fujiwara S, Lange JL, Watts NB. Longitudinal change in hip fracture incidence after starting risedronate or raloxifene: an observational study. J Bone Miner Metab. 2011;29(5):561‐570. [DOI] [PubMed] [Google Scholar]
- 35. Eiken PA, Prieto‐Alhambra D, Eastell R, Abrahamsen B. Surgically treated osteonecrosis and osteomyelitis of the jaw and oral cavity in patients highly adherent to alendronate treatment: a nationwide user‐only cohort study including over 60,000 alendronate users. Osteoporosis Int. 2017;28(10):2921‐2928. [DOI] [PubMed] [Google Scholar]
- 36. Kjellberg J, Jorgensen AD, Vestergaard P, Ibsen R, Gerstoft F, Modi A. Cost and health care resource use associated with noncompliance with oral bisphosphonate therapy: an analysis using Danish health registries. Osteoporosis Int. 2016;27(12):3535‐3541. [DOI] [PubMed] [Google Scholar]
- 37. Keshishian A, Boytsov N, Burge R, et al. Examining the effect of medication adherence on risk of subsequent fracture among women with a fragility fracture in the U.S. Medicare population. J Manag Care Spec Pharm. 2017;23(11):1178‐1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lakatos P, Takacs I, Marton I, et al. A retrospective longitudinal database study of persistence and compliance with treatment of osteoporosis in Hungary. Calcif Tissue Int. 2016;98(3):215‐225. [DOI] [PubMed] [Google Scholar]
- 39. Landfeldt E, Strom O, Robbins S, Borgstrom F. Adherence to treatment of primary osteoporosis and its association to fractures‐‐the Swedish adherence register analysis (SARA). Osteoporosis Int. 2012;23(2):433‐443. [DOI] [PubMed] [Google Scholar]
- 40. Lin TC, Yang CY, Yang YH, Lin SJ. Alendronate adherence and its impact on hip‐fracture risk in patients with established osteoporosis in Taiwan. Clinical Pharmacol Ther. 2011;90(1):109‐116. [DOI] [PubMed] [Google Scholar]
- 41. Patrick AR, Brookhart MA, Losina E, et al. The complex relation between bisphosphonate adherence and fracture reduction. J Clin Endocrinol Metab. 2010;95(7):3251‐3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sharman Moser S, Yu J, Goldshtein I, et al. Cost and consequences of nonadherence with Oral bisphosphonate therapy: findings from a real‐world data analysis. Ann Pharmacother. 2016;50(4):262‐269. [DOI] [PubMed] [Google Scholar]
- 43. Rabenda V, Reginster JY. Positive impact of compliance to strontium ranelate on the risk of nonvertebral osteoporotic fractures. Osteoporosis Int. 2010;21(12):1993‐2002. [DOI] [PubMed] [Google Scholar]
- 44. Zhao Y, Johnston SS, Smith DM, McMorrow D, Krege J, Krohn K. Association between teriparatide adherence and healthcare utilization and costs among hip fracture patients in the United States. Bone. 2014;60:221‐226. [DOI] [PubMed] [Google Scholar]
- 45. Wang Z, Ward MM, Chan L, Bhattacharyya T. Adherence to oral bisphosphonates and the risk of subtrochanteric and femoral shaft fractures among female medicare beneficiaries. Osteoporosis Int. 2014;25(8):2109‐2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Curtis JR, Westfall AO, Cheng H, Lyles K, Saag KG, Delzell E. Benefit of adherence with bisphosphonates depends on age and fracture type: results from an analysis of 101,038 new bisphosphonate users. J Bone Miner Res. 2008;23(9):1435‐1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cooper DC, Trivedi RB, Nelson KM, et al. Antidepressant adherence and risk of coronary artery disease hospitalizations in older and younger adults with depression. J am Geriatr Soc. 2014;62(7):1238‐1245. [DOI] [PubMed] [Google Scholar]
- 48. Bosworth HB, Voils CI, Potter GG, Steffens DC. The effects of antidepressant medication adherence as well as psychosocial and clinical factors on depression outcome among older adults. Int J Geriatr Psychiatry. 2008;23(2):129‐134. [DOI] [PubMed] [Google Scholar]
- 49. Biffi A, Scotti L, Rea F, et al. Adherence to antidepressants and mortality in elderly patients with cardiovascular disease. Clin Drug Investig. 2018;38(7):593‐602. [DOI] [PubMed] [Google Scholar]
- 50. Hope CJ, Wu J, Tu W, Young J, Murray MD. Association of medication adherence, knowledge, and skills with emergency department visits by adults 50 years or older with congestive heart failure. Am J Health Syst Pharm. 2004;61(19):2043‐2049. [DOI] [PubMed] [Google Scholar]
- 51. Murphy GK, McAlister FA, Eurich DT. Cardiovascular medication utilization and adherence among heart failure patients in rural and urban areas: a retrospective cohort study. Can J Cardiol. 2015;31(3):341‐347. [DOI] [PubMed] [Google Scholar]
- 52. Murray M, Tu W, Wu J, Morrow D, Smith F, Brater D. Factors associated with exacerbation of heart failure include treatment adherence and health literacy skills. Clin Pharmacol Ther. 2009;85(6):651‐658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tu W, Morris AB, Li J, et al. Association between adherence measurements of metoprolol and health care utilization in older patients with heart failure. Clin Pharmacol Ther. 2005;77(3):189‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nelson MR, Reid CM, Ryan P, Willson K, Yelland L. Self‐reported adherence with medication and cardiovascular disease outcomes in the second Australian National Blood Pressure Study (ANBP2). Med J Aust. 2006;185(9):487. [DOI] [PubMed] [Google Scholar]
- 55. Tang KL, Quan H, Rabi DM. Measuring medication adherence in patients with incident hypertension: a retrospective cohort study. BMC Health Serv Res. 2017;17(1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tavares DMS, Guimarães MO, Ferreira PCS, Dias FA, Martins NPF, Rodrigues LR. Quality of life and accession to the pharmacological treatment among elderly hypertensive. Rev Bras Enferm. 2016;69(1):134‐141. [DOI] [PubMed] [Google Scholar]
- 57. Márquez‐Contreras E, Martell‐Claros N, Gil‐Guillén V, et al. Quality of life with rivaroxaban in patients with non‐valvular atrial fibrilation by therapeutic compliance. Qual Life Res. 2017;26(3):647‐654. [DOI] [PubMed] [Google Scholar]
- 58. Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence‐based pharmacotherapy and long‐term mortality after acute myocardial infarction. JAMA. 2007;297(2):177‐186. [DOI] [PubMed] [Google Scholar]
- 59. Ko DT, Chiu M, Guo H, Austin PC, Marquis J‐F, Tu JV. Patterns of use of thienopyridine therapy after percutaneous coronary interventions with drug‐eluting stents and bare‐metal stents. Am Heart J. 2009;158(4):592‐598. e591. [DOI] [PubMed] [Google Scholar]
- 60. Shalev V, Chodick G, Silber H, Kokia E, Jan J, Heymann AD. Continuation of statin treatment and all‐cause mortality: a population‐based cohort study. Arch Intern Med. 2009;169(3):260‐268. [DOI] [PubMed] [Google Scholar]
- 61. Krousel‐Wood M, Holt E, Joyce C, et al. Differences in cardiovascular disease risk when antihypertensive medication adherence is assessed by pharmacy fill versus self‐report: the cohort study of medication adherence among older adults (CoSMO). J Hypertens. 2015;33(2):412‐420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Korhonen MJ, Robinson JG, Annis IE, et al. Adherence tradeoff to multiple preventive therapies and all‐cause mortality after acute myocardial infarction. J am Coll Cardiol. 2017;70(13):1543‐1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sun Y, Li C, Zhang L, et al. Poor adherence to P2Y12 antagonists increased cardiovascular risks in Chinese PCI‐treated patients. Front Med. 2017;11(1):53‐61. [DOI] [PubMed] [Google Scholar]
- 64. Lenzi J, Rucci P, Castaldini I, et al. Does age modify the relationship between adherence to secondary prevention medications and mortality after acute myocardial infarction? A nested case‐control study. Euro J Clin Pharmacol. 2015;71(2):243‐250. [DOI] [PubMed] [Google Scholar]
- 65. Rannanheimo PK, Tiittanen P, Hartikainen J, et al. Impact of statin adherence on cardiovascular morbidity and all‐cause mortality in the primary prevention of cardiovascular disease: a population‐based cohort study in Finland. Value Health. 2015;18(6):896‐905. [DOI] [PubMed] [Google Scholar]
- 66. Perreault S, Yu AY, Cote R, Dragomir A, White‐Guay B, Dumas S. Adherence to antihypertensive agents after ischemic stroke and risk of cardiovascular outcomes. Neurology. 2012;79(20):2037‐2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rea F, Bonassi S, Vitale C, et al. Exposure to statins is associated to fracture risk reduction in elderly people with cardiovascular disease: evidence from the AIFA‐I‐GrADE observational project. Pharmacoepidemiol Drug Saf. 2017;26(7):775‐784. [DOI] [PubMed] [Google Scholar]
- 68. Yang Q, Chang A, Ritchey MD, Loustalot F. Antihypertensive medication adherence and risk of cardiovascular disease among older adults: a population‐based cohort study. J am Heart Assoc. 2017;6(6):e006056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Park NH, Song MS, Shin SY, Jeong JH, Lee HY. The effects of medication adherence and health literacy on health‐related quality of life in older people with hypertension. Int J Older People Nurs. 2018;13(3):e12196. [DOI] [PubMed] [Google Scholar]
- 70. Corrao G, Rea F, Ghirardi A, Soranna D, Merlino L, Mancia G. Adherence with antihypertensive drug therapy and the risk of heart failure in clinical practice. Hypertension. 2015;66(4):742‐749. [DOI] [PubMed] [Google Scholar]
- 71. Kettani FZ, Dragomir A, Cote R, et al. Impact of a better adherence to antihypertensive agents on cerebrovascular disease for primary prevention. Stroke. 2009;40(1):213‐220. [DOI] [PubMed] [Google Scholar]
- 72. Perreault S, Dragomir A, Blais L, et al. Impact of better adherence to statin agents in the primary prevention of coronary artery disease. Euro J Clin Pharmacol. 2009;65(10):1013‐1024. [DOI] [PubMed] [Google Scholar]
- 73. Perreault S, Dragomir A, Blais L, Berard A, Lalonde L, White M. Impact of adherence to statins on chronic heart failure in primary prevention. Br J Clin Pharmacol. 2008;66(5):706‐716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Al‐Ruthia YS, Hong SH, Graff C, Kocak M, Solomon D, Nolly R. Examining the relationship between antihypertensive medication satisfaction and adherence in older patients. Res Social Adm Pharm. 2017;13(3):602‐613. [DOI] [PubMed] [Google Scholar]
- 75. Liao KM, Cheng HT, Lee YH, Chen CY. The effectiveness and safety of angiotensin‐converting enzyme inhibition or receptor blockade in vascular diseases in patients with hemodialysis. Medicine. 2017;96(13):e6525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kulkarni AS, Balkrishnan R, Anderson RT, Edin HM, Kirsch J, Stacy MA. Medication adherence and associated outcomes in medicare health maintenance organization‐enrolled older adults with Parkinson's disease. Mov Dis. 2008;23(3):359‐365. [DOI] [PubMed] [Google Scholar]
- 77. Ettinger AB, Manjunath R, Candrilli SD, Davis KL. Prevalence and cost of nonadherence to antiepileptic drugs in elderly patients with epilepsy. Epilepsy Behav. 2009;14(2):324‐329. [DOI] [PubMed] [Google Scholar]
- 78. Molnar MZ, Gosmanova EO, Sumida K, et al. Predialysis cardiovascular disease medication adherence and mortality after transition to dialysis. Am J Kid Dis. 2016;68(4):609‐618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hsieh KP, Chen LC, Cheung KL, Chang CS, Yang YH. Interruption and non‐adherence to long‐term adjuvant hormone therapy is associated with adverse survival outcome of breast cancer women‐‐an Asian population‐based study. PloS One. 2014;9(2):e87027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Winn AN, Dusetzina SB. The association between trajectories of endocrine therapy adherence and mortality among women with breast cancer. Pharmacoepidemiol Drug Saf. 2016;25(8):953‐959. [DOI] [PubMed] [Google Scholar]
- 81. Simard P, Presse N, Roy L, et al. Association between metformin adherence and all‐cause mortality among new users of metformin: a nested case‐control study. Ann Pharmacother. 2018;52(4):305‐313. [DOI] [PubMed] [Google Scholar]
- 82. Yang Y, Thumula V, Pace PF, Banahan BF 3rd, Wilkin NE, Lobb WB. Nonadherence to angiotensin‐converting enzyme inhibitors and/or angiotensin II receptor blockers among high‐risk patients with diabetes in Medicare part D programs. J am Pharm Assoc. 2010;50(4):527‐531. [DOI] [PubMed] [Google Scholar]
- 83. Ruokoniemi P, Korhonen MJ, Helin‐Salmivaara A, et al. Statin adherence and the risk of major coronary events in patients with diabetes: a nested case‐control study. Br J Clin Pharmacol. 2011;71(5):766‐776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Korhonen MJ, Ruokoniemi P, Ilomaki J, Meretoja A, Helin‐Salmivaara A, Huupponen R. Adherence to statin therapy and the incidence of ischemic stroke in patients with diabetes. Pharmacoepidemiol Drug Saf. 2016;25(2):161‐169. [DOI] [PubMed] [Google Scholar]
- 85. Yashkin AP, Sloan F. Adherence to guidelines for screening and medication use: mortality and onset of major macrovascular complications in elderly persons with diabetes mellitus. J Aging Health. 2018;30(4):503‐520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Berry SD, Quach L, Procter‐Gray E, et al. Poor adherence to medications may be associated with falls. J Gerontol a Biol Sci Med Sci. 2010;65(5):553‐558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. van Boven JFM, Chavannes NH, van der Molen T, Rutten‐van Mölken MPMH, Postma MJ, Vegter S. Clinical and economic impact of non‐adherence in COPD: a systematic review. Respir Med. 2014;108(1):103‐113. [DOI] [PubMed] [Google Scholar]
- 88. Capoccia K, Odegard PS, Letassy N. Medication adherence with diabetes medication: a systematic review of the literature. Diabetes Educ. 2016;42(1):34‐71. [DOI] [PubMed] [Google Scholar]
- 89. Ho SC, Chong HY, Chaiyakunapruk N, Tangiisuran B, Jacob SA. Clinical and economic impact of non‐adherence to antidepressants in major depressive disorder: a systematic review. J Affect Dis. 2016;193:1‐10. [DOI] [PubMed] [Google Scholar]
- 90. Bårnes CB, Ulrik CS. Asthma and adherence to inhaled corticosteroids: current status and future perspectives. Respir Care. 2015;60(3):455‐468. [DOI] [PubMed] [Google Scholar]
- 91. Dilla T, Ciudad A, Álvarez M. Systematic review of the economic aspects of nonadherence to antipsychotic medication in patients with schizophrenia. Patient Prefer Adherence. 2013;7:275‐284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Simoni‐Wastila L, Wei Y‐J, Qian J, et al. Association of chronic obstructive pulmonary disease maintenance medication adherence with all‐cause hospitalization and spending in a Medicare population. Am J Geriatr Pharmacother. 2012;10(3):201‐210. [DOI] [PubMed] [Google Scholar]
- 93. Vestbo J, Anderson JA, Calverley PMA, et al. Adherence to inhaled therapy, mortality and hospital admission in COPD. Thorax. 2009;64(11):939‐943. [DOI] [PubMed] [Google Scholar]
- 94. Liu X, Tepper PG, Able SL. Adherence and persistence with duloxetine and hospital utilization in patients with major depressive disorder. Int Clin Psychopharmacol. 2011;26(3):173‐180. [DOI] [PubMed] [Google Scholar]
- 95. White TJ, Vanderplas A, Ory C, Dezii CM, Chang E. Economic impact of patient adherence with antidepressant therapy within a managed care organization. Dis Manage Health Outcomes. 2003;11(12):817‐822. [Google Scholar]
- 96. Shrank WH, Patrick AR, Brookhart MA. Healthy user and related biases in observational studies of preventive interventions: a primer for physicians. J Gen Intern Med. 2011;26(5):546‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Simpson SH, Eurich DT, Majumdar SR, et al. A meta‐analysis of the association between adherence to drug therapy and mortality. BMJ. 2006;333(7557):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kim J‐A, Lim MK, Kim K, Park J, Rhee CK. Adherence to inhaled medications and its effect on healthcare utilization and costs among high‐grade chronic obstructive pulmonary disease patients. Clin Drug Invest. 2018;38(4):333‐340. [DOI] [PubMed] [Google Scholar]
- 99. Wallace E, Stuart E, Vaughan N, Bennett K, Fahey T, Smith SM. Risk prediction models to predict emergency hospital admission in community‐dwelling adults: a systematic review. Med Care. 2014;52(8):751‐765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Elliot R. Non‐adherence to medicines: not solved but solvable. J Health Serv Res Policy. 2009;14:58‐61. [DOI] [PubMed] [Google Scholar]
- 101. Mixon AS, Neal E, Bell S, Powers JS, Kripalani S. Care transitions: a leverage point for safe and effective medication use in older adults‐‐a mini‐review. Gerontology. 2015;61(1):32‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005;43(6):521‐530. [DOI] [PubMed] [Google Scholar]
- 103. Chowdhury R, Khan H, Heydon E, et al. Adherence to cardiovascular therapy: a meta‐analysis of prevalence and clinical consequences. Eur Heart J. 2013;34(38):2940‐2948. [DOI] [PubMed] [Google Scholar]
- 104. Imaz I, Zegarra P, González‐Enríquez J, Rubio B, Alcazar R, Amate JM. Poor bisphosphonate adherence for treatment of osteoporosis increases fracture risk: systematic review and meta‐analysis. Osteoporosis Int. 2010;21(11):1943‐1951. [DOI] [PubMed] [Google Scholar]
- 105. Compston JE, Seeman E. Compliance with osteoporosis therapy is the weakest link. Lancet. 2006;368(9540):973‐974. [DOI] [PubMed] [Google Scholar]
- 106. Karlsson L, Lundkvist J, Psachoulia E, Intorcia M, Strom O. Persistence with denosumab and persistence with oral bisphosphonates for the treatment of postmenopausal osteoporosis: a retrospective, observational study, and a meta‐analysis. Osteoporosis Int. 2015;26(10):2401‐2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Agh T, Domotor P, Bartfai Z, Inotai A, Fujsz E, Meszaros A. Relationship between medication adherence and health‐related quality of life in subjects with COPD: a systematic review. Respir Care. 2015;60(2):297‐303. [DOI] [PubMed] [Google Scholar]
- 108. Cleemput I, Kesteloot K, DeGeest S. A review of the literature on the economics of noncompliance. Room for methodological improvement. Health Policy. 2002;59(1):65‐94. [DOI] [PubMed] [Google Scholar]
- 109. Bosley C, Corden Z, Rees P, Cochrane G. Psychological factors associated with use of home nebulized therapy for COPD. Eur Respir J. 1996;9(11):2346‐2350. [DOI] [PubMed] [Google Scholar]
- 110. Benner JS, Glynn RJ, Mogun H, Neumann PJ, Weinstein MC, Avorn J. Long‐term persistence in use of statin therapy in elderly patients. JAMA. 2002;288(4):455‐461. [DOI] [PubMed] [Google Scholar]
- 111. Qian J, Simoni‐Wastila L, Rattinger GB, et al. Association between depression and maintenance medication adherence among Medicare beneficiaries with chronic obstructive pulmonary disease. Int J Geriatr Psychiatry. 2014;29(1):49‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Krousel‐Wood M, Joyce C, Holt E, et al. Predictors of decline in medication adherence: results from the cohort study of medication adherence among older adults. Hypertension. 2011;58(5):804‐810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487‐497. [DOI] [PubMed] [Google Scholar]
- 114. Cutler RL, Fernandez‐Llimos F, Frommer M, Benrimoj C, Garcia‐Cardenas V. Economic impact of medication non‐adherence by disease groups: a systematic review. BMJ Open. 2018;8(1):e016982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Patton DE, Hughes CM, Cadogan CA, Ryan CA. Theory‐based interventions to improve medication adherence in older adults prescribed polypharmacy: a systematic review. Drugs Aging. 2017;34(2):97‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Renaudin P, Boyer L, Esteve MA, Bertault‐Peres P, Auquier P, Honore S. Do pharmacist‐led medication reviews in hospitals help reduce hospital readmissions? A systematic review and meta‐analysis. Br J Clin Pharmacol. 2016;82(6):1660‐1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1
PRISMA checklist
TABLE S2
Search Strategy PubMed
TABLE S3
Quality appraisal checklist
TABLE S4
List of citations located by hand searching
TABLE S5
Characteristics of included studies measuring healthcare utilisation and adverse events
TABLE S6
Characteristics of included studies measuring mortality
TABLE S7
Characteristics of included studies measuring quality of life or depression severity
TABLE S8
Quality appraisal rating of included studies
TABLE S9
Results of individual studies into the association between medication adherence and hospitalisation
TABLE S10
Results of individual studies into the association between medication adherence and emergency department visits
TABLE S11
Results of individual studies into the association between medication adherence and physician visits
TABLE S12
Results of individual studies into the association between medication adherence and outpatient visits
TABLE S13
Results of individual studies into the association between medication adherence and quality of life
TABLE S14
Results of individual studies into the association between medication adherence and mortality
TABLE S15
Results of individual studies into the association between medication adherence and adverse events


