Abstract
Lissencephaly comprises a spectrum of malformations of cortical development. This spectrum includes agyria, pachygyria, and subcortical band heterotopia; each represents anatomical malformations of brain cortical development caused by neuronal migration defects. The molecular etiologies of neuronal migration anomalies are highly enriched for genes encoding microtubules and microtubule-associated proteins, and this enrichment highlights the critical role for these genes in cortical growth and gyrification. Using exome sequencing and family based rare variant analyses, we identified a homozygous variant (c.997C>T [p.Arg333Cys]) in TUBGCP2, encoding gamma-tubulin complex protein 2 (GCP2), in two individuals from a consanguineous family; both individuals presented with microcephaly and developmental delay. GCP2 forms the multiprotein γ-tubulin ring complex (γ-TuRC) together with γ-tubulin and other GCPs to regulate the assembly of microtubules. By querying clinical exome sequencing cases and through GeneMatcher-facilitated collaborations, we found three additional families with bi-allelic variation and similarly affected phenotypes including a homozygous variant (c.1843G>C [p.Ala615Pro]) in two families and compound heterozygous variants consisting of one missense variant (c.889C>T [p.Arg297Cys]) and one splice variant (c.2025-2A>G) in another family. Brain imaging from all five affected individuals revealed varying degrees of cortical malformations including pachygyria and subcortical band heterotopia, presumably caused by disruption of neuronal migration. Our data demonstrate that pathogenic variants in TUBGCP2 cause an autosomal recessive neurodevelopmental trait consisting of a neuronal migration disorder, and our data implicate GCP2 as a core component of γ-TuRC in neuronal migrating cells.
Keywords: Lissencephaly, cortical malformation, TUBGCP2, GCP2, γ-TuRC, multilocus pathogenic variation
Main Text
Human cortical development is a complex and tightly regulated process that includes neurogenesis (proliferation of progenitor cells), neuronal migration, post-migrational cortical organization, and connectivity.1 Disruption of any of these steps may lead to malformations of cortical development (MCDs) that are common causes of developmental delay (DD), intellectual disability (ID), and epilepsy.2
Lissencephaly (LIS) is a subtype of MCDs and is caused by defects in neuronal migration that can manifest over a range of clinical severity, including agyria-pachygyria-subcortical band heterotopia spectrum. Rare variants in PAFAH1B1 (also referred to as LIS1 [MIM: 601545]) and DCX (MIM:300121) represent the most common causes of LIS-spectrum phenotypes. With the recent evolution of massively parallel DNA sequencing technologies and brain imaging, a growing number of LIS-associated genes have been identified.3 Interestingly, the majority of these genes (including PAFAH1B1 and DCX) encode tubulins and microtubule-associated proteins (MAPs). These findings provide insights into disease biology and a systematic understanding of the pathogenesis of LIS-spectrum phenotypes based on the cellular processes that are critical for migrating neuronal cells.
Microtubules are polymers of tubulin heterodimers assembled from various α- and β-tubulin isotypes. They are major constituents of the cytoskeleton and are essential for chromosome separation, intracellular transport, organelle positioning, and cell motility.4 Microtubule nucleation is spatially and temporally controlled by conserved machinery comprised of a multiprotein γ-tubulin ring complex (γ-TuRC) at a microtubule organizing center (MTOC). The basic subunit of γ-TuRC is γ-tubulin small complex (γ-TuSC), which consists of two molecules of γ-tubulin and one each of gamma-tubulin complex proteins 2 and 3 (GCP2 and GCP3). GCP2 and GCP3 are encoded by TUBGCP2 (tubulin-gamma-complex-associated protein 2 [MIM: 617817]) and TUBGCP3 (tubulin-gamma complex-associated protein 3 [MIM: 617818]), respectively. In budding yeast, seven γ-TuSC proteins form a helical complex, γ-TuRC, that serves as a critical template for microtubule formation. Higher eukaryotes have three additional GCP proteins, GCP4, GCP5, and GCP6 (encoded by TUBGCP4 [MIM: 609610], TUBGCP5 [MIM: 608147], and TUBGCP6 [MIM: 610053], respectively), which are thought to replace GCP2 and/or GCP3 molecules to form γ-TuSC-like complexes. Eukaryotic γ tubulin complex proteins GCP4, GCP5, and GCP6 have been postulated to play important roles in γ-TuRC assembly and spatiotemporal regulation of γ-TuRC activity, although their precise function remains unclear (Figure S1).4 TUBGCP2–6 all have relatively similar expression levels in the brain and other tissues as shown in Figure S2.
Human phenotypes with disruptions in either the tubulin isotypes or γ-TuRC components have provided valuable insight into their physiological functions in brain development. Complex cortical malformations caused by disruption of tubulin isotypes are referred to as tubulinopathies.5 Among them, monoallelic pathogenic variants in TUBA1A (α-tubulin), TUBB2B, TUBB, TUBB3 (β-tubulin), and TUBG1 (γ-tubulin) have been shown to encompass LIS-spectrum phenotypes.6, 7, 8, 9, 10, 11 These tubulin genes (TUBA1A, TUBB2B, TUBB, TUBB3, and TUBG1) are highly expressed in the brain and have varying degrees of expression in all other tissues (Figure S2). Rare bi-allelic variants in genes encoding these γ tubulin complex proteins cause microcephaly with or without chorioretinopathy (TUBGCP4, MCCRP3 [MIM: 616335]; TUBGCP5; and TUBGCP6, MCCRP1 [MIM: 251270]).12, 13, 14, 15 Notably, brain imaging in most of the individuals with those phenotypes usually did not show apparent structural brain abnormalities except for a simplified gyral pattern or pachygyria observed in some cases. These reports highlight critical functions of GCP4, GCP5, and GCP6 each in neuronal progenitor proliferation (Figure S1). The human phenotypes caused by the variants in TUBGCP2 and TUBGCP3 have not been elucidated.
Using exome sequencing (ES) and family based rare variant analyses approaches, we identified five individuals from four unrelated families with potential pathogenic variants in TUBGCP2. All affected individuals presented with microcephaly and showed LIS-spectrum phenotypes on brain imaging; these results emphasize the role of GCP2 as an indispensable component of microtubule nucleation with critical functions in neuronal migration in humans.
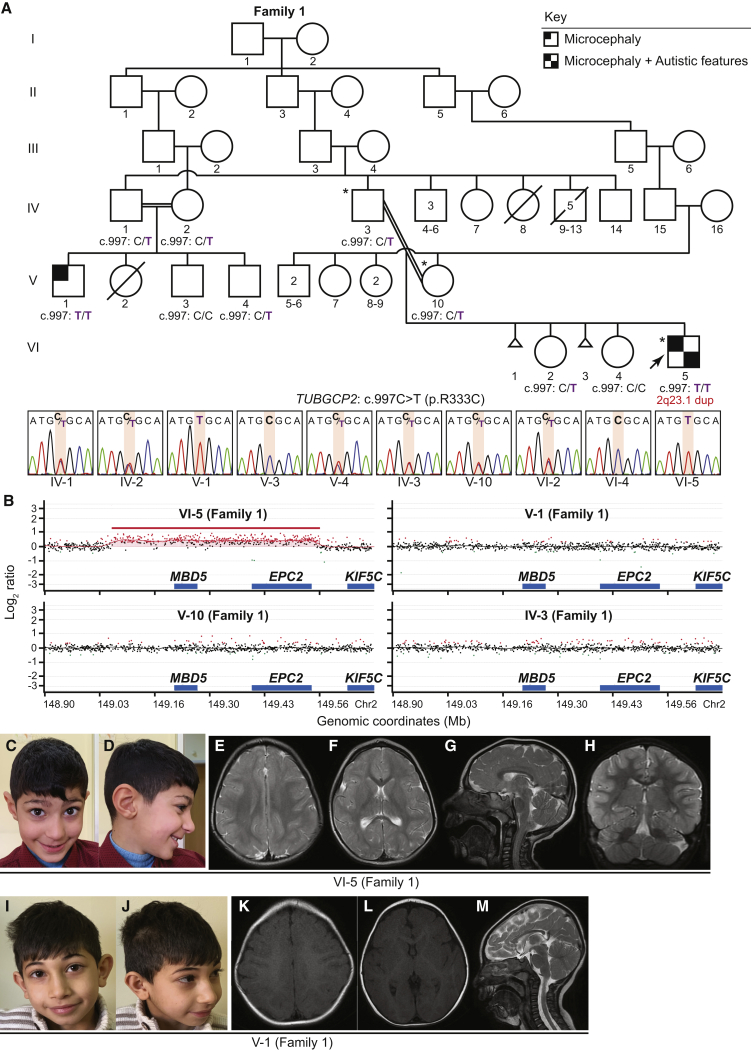
This study was performed with approval by the Institutional Review Board at Baylor College of Medicine. The database of Genotypes and Phenotypes (dbGaP) accession number for exome sequences reported in this paper and for which informed consent for data sharing in controlled-access databases has been provided is phs000711.v5.p1; identified variants have been deposited in ClinVar. Family 1 (Figure 1A) was enrolled in this study to uncover the underlying molecular cause of microcephaly, DD, and pachygyria in the proband. Informed consent was obtained according to the Baylor-Hopkins Center for Mendelian Genomics (BHCMG) research protocol. Trio ES (ES of the proband, mother, and father) was performed at the Baylor College of Medicine Human Genome Sequencing Center (BCM-HGSC) according to the methods described previously.16 Validation of the candidate variants identified by ES and confirmation of the segregation of these variants were performed using Sanger sequencing; primers are provided in Table S1. The copy number variant (CNV) detected by ES was orthogonally validated by a custom-designed Agilent oligonucleotide microarray (Figure 1B and see Supplemental Data for more information). After we identified the candidate homozygous variant, an affected paternal cousin with a similar phenotype (V-1, Figure 1A) and his close family members were enrolled for segregation studies. Family 2 was identified by querying clinical ES cases at Baylor Genetics. The proband of Family 2 (II-3, Figure 2A) underwent clinical ES at Baylor Genetics according to previously described methods.17 After we obtained written informed consent from the parents of the proband for our BHCMG research protocol, we used conventional Sanger sequencing in the parents’ blood-derived DNA to validate the TUBGCP2 variant detected by clinical ES. Families 3 and 4 (Figures 2J and 2P) were identified through GeneMatcher.18, 19 After written informed consent was obtained, trio ES was performed on Family 3 (II-2 and parents, Figure 2J) and Family 4 (II-1 and parents, Figure 2P). Details of ES and cDNA analysis are described in the Supplemental Data.
Figure 1.
Bi-allelic TUBGCP2 Variants, 2q23.1 Duplication, Facial Features, and Brain MRI Sections in a Consanguineous Family with Microcephaly and Developmental Delay
(A) Pedigree and Sanger sequencing showing segregation of the variants in TUBGCP2 in Family 1, a consanguineous family from Turkey. The proband is indicated with an arrow and all individuals who underwent exome sequencing are indicated with asterisks on the pedigrees. Note that the proband (individual VI-5 [Family 1]) has both the homozygous variant in TUBGCP2 and a de novo 2q23.1 duplication.
(B) Chromosomal microarray for individuals VI-5, V-1, V-10, and IV-3 (Family 1) in chromosome 2q23.1 region.
(C and D) Individual VI-5 (Family 1) at 6 years old, showing microcephaly, narrow forehead, upslanting palpebral fissures, bulbous nose, and prominent ear.
(E–H) Brain MRI of individual VI-5 (Family 1) at 1 year and 9 months old. T2-weighted images of axial sections demonstrate pachygyria (E and F). Deep gray matter was preserved. Sagittal section showed thin corpus callosum, especially in posterior region (G). Cerebellar hemispheres showed mild atrophy (H).
(I and J) Individual V-1 (Family 1) at 7 years old. Note that individuals VI-5 and V-1 (Family 1) show similar facial features.
(K–M) Brain MRI of individual V-1 (Family 1) at 6 months old, showing posterior dominant pachygyria (K). Deep gray matter (L) and brainstem and cerebellum (M) were preserved.
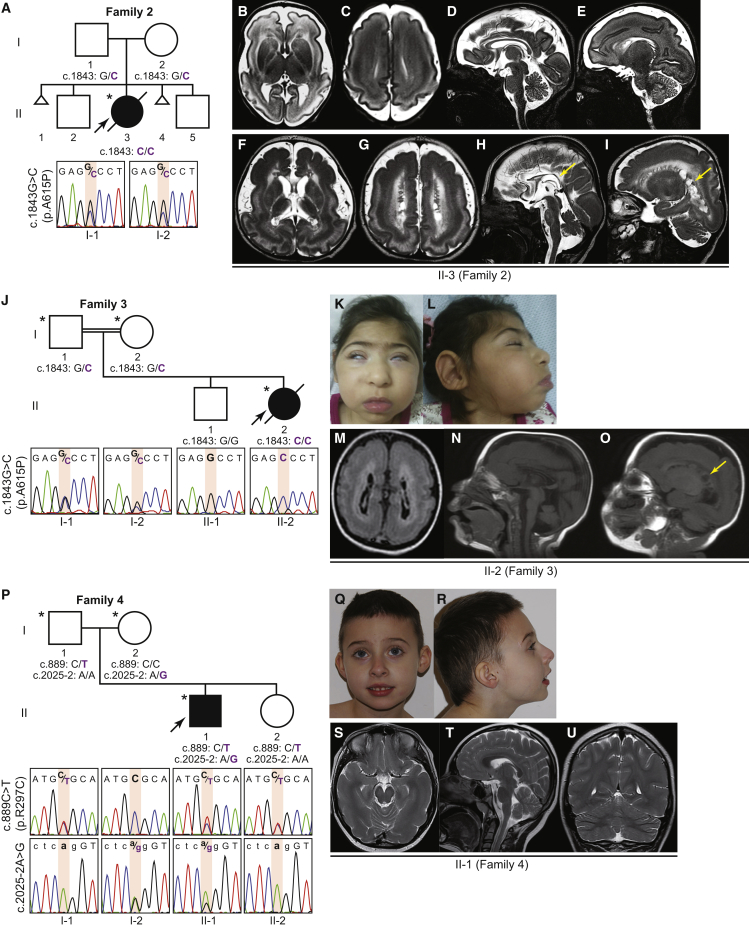
Figure 2.
Bi-allelic TUBGCP2 Variants in Families 2, 3, and 4
(A) Family 2 with an affected proband born to parents from India with no historical evidence for consanguinity. The genotype of the variant in TUBGCP2 in the proband (individual II-3 [Family 2]) was determined based on exome sequencing and parental genotypes confirmed by Sanger sequencing.
(B–I) T2 weighted brain MRI images in individual II-3 (Family 2) at 4 weeks (B–E) and at 5 months of age (F–I). Progression of the disease was shown by loss of white matter, thinning of corpus callosum (arrow in H), volume loss of pons, and exuberant subependymal cyst formation (arrow in I) at 5 months of age.
(J) Family 3 with an affected proband born to consanguineous parents from Iran. Note that the homozygous variant in TUBGCP2 identified in this family was the same variant identified in Family 2.
(K and L) Individual II-2 (Family 3) showing microcephaly, synophrys, micrognathia, and hypoplastic maxilla.
(M–O) Brain MRI of individual II-2 (Family 3) at 1 year of age showed pachygyria, hyperintense periventricular white matter, very thin corpus callosum, and subependymal cysts (arrow in O).
(P) Family 4 with an affected proband born to non-consanguineous parents from Poland. The DNA sequences shown in upper case denote coding sequences, whereas sequences in lower case denote the non-coding (intronic) region.
(Q and R) Individual II-1 (Family 4) showing microcephaly.
(S–U) Brain MRI of individual II-1 (Family 4) at 8 years of age shows pachygyria in the temporal lobes and partial thinning of the corpus callosum.
Clinical findings on the five affected individuals are summarized in Table 1. The pedigrees and photos depicting morphological features are shown in Figures 1 and 2. Detailed clinical descriptions of all individuals are provided in the Supplemental Data. Shared phenotypic features converged toward nervous system dysfunction that included microcephaly (4/4 individuals for which data were available), speech delay and dyslexia (5/5 individuals), truncal hypotonia (4/5 individuals), motor developmental delay (4/5 individuals), seizures (4/5 individuals), and micrognathia (3/5 individuals) (Table 1). The head circumferences for three individuals were: Individuals VI-5 and V-1 (Family 1) and individual II-1 (Family 4) were reported to be borderline normal or at just below normal percentiles at birth; however, they were all noted to have developed apparent progressive severe microcephaly (Z score −5) at follow up examinations. Individual II-3 (Family 2) and individual II-2 (Family 3) had more pronounced microcephaly at the time of their preterm births, and more severe microcephaly (Z score −9) was noted in the follow up examination of individual II-2 (Family 3); both individuals died around 3 years of age. Intrafamilial clinical variability was observed across the two affected male cousins of Family 1 (individuals VI-5 and V-1). Shared key clinical features among them included microcephaly, language impairment, and dysmorphic facial features (Figures 1C-D and I-J). Notably, individual VI-5 (Family 1) had more severe delayed motor and language development compared to his affected cousin (individual V-1). Moreover, individual VI-5 (Family 1) had additional clinical endophenotypic findings including autistic features and congenital malformations with widely spaced teeth, pectus excavatum, and broad first toes (Table 1 and Supplemental Note).
Table 1.
Summary of Neurological and Radiological Features of Individuals with Bi-allelic TUBGCP2 Variants
| Individuals | VI-5 (Family 1) | V-1 (Family 1) | II-3 (Family 2) | II-2 (Family 3) | II-1 (Family 4) |
|---|---|---|---|---|---|
| Basic Clinical Info | |||||
| gestation | term | term | preterm (31 weeks) | preterm (27 weeks) | term |
| OFC-birth/cm (Z score) | 32.5 (−1.8) | 32 (−2.1) | 25 (−2.0) | 21 (−2.3) | 35 (+0.4) |
| age at last examination | 6 years, 5 months | 7 years, 5 months | - | 1 year, 3 months | 4 years |
| OFC/cm (Z score) | 44.5 (−5.0) | 44 (−5.5) | NR | 34 (−9.0) | 46 (−4.0) |
| dysmorphic features | narrow forehead, upslanting palpebral fissures, thick eyebrows, bulbous nose, prominent ear, smooth philtrum, thin upper lip, widened and separated teeth | narrow forehead, bulbous nose, prominent ear, smooth philtrum, retrognathia | short and sloped forehead, thick eyebrows, puffy eyelids, full lips, retromicrognathia | bitemporal narrowing, upslanting palpebral fissure, synophrys, micrognathia, midfacial hypoplasia, prominent lower lip, prominent ears | smooth philtrum, prominent ears |
| Neurological Exam | |||||
| tone | truncal hypotonia | normal | truncal hypotonia | truncal hypotonia | normal |
| reflexes | NR | NR | brisk | NR | normal |
| spasticity | no | no | yes | No | No |
| ophthalmology | myopia | NR | cortical blindness | optic atrophy, retinal changes | myopia, astigmatism |
| auditory | normal | NR | normal | passed ABR test | normal |
| Psychomotor | |||||
| motor skills | delayed | normal | severely delayed | severely delayed | delayed |
| language | delayed | difficulty in reading | severely delayed | severely delayed | delayed |
| autistic features | yes | no | no | no | no |
| seizures; onset/type | 6 years, 9 months/generalized | no | 5 months/generalized | 7 months/generalized | NR/generalized |
| Tests | |||||
| EEG | continuous slow background activity and frequent multifocal epileptiform discharges (6.5 years) | NR | abnormal | frequent epileptiform discharges | paroxysmal epileptiform activity localized to central area |
| brain MRI | pachygyria, thin CC, mild cerebellar volume loss (21 months) | pachygyria (6 months) | pachygyria, subcortical band, subependymal heterotopia, multiple subependymal cysts, delayed myelination, thin CC and brainstem (5 months) | pachygyria, subcortical band, subependymal cysts, PVL, thin CC, thin brainstem (1 year) | pachygyria, delayed myelination, thin CC (8 years) |
Abbreviations: ABR, auditory brainstem response; dB, decibel; EEG, electroencephalogram; CC, corpus callosum; MRI, magnetic resonance imaging; nHL, normal hearing level; NR, not reported; OFC, occipital frontal circumference; PVL, periventricular leukomalacia
Brain magnetic resonance imaging (MRI) studies (Figures 1 and 2 and Figure S3) of all five individuals showed pachygyria (5/5), thin corpus callosum (4/5), cerebellar volume loss (3/5), subcortical band (2/5), subependymal cyst (2/5), delayed myelination (2/5), and thin brainstem (2/5). Retrospective analyses of head imaging studies by a board-certified neuroradiologist (author JVH) revealed remarkably consistent MRI findings. Polymicrogyria and dysplasia of the basal ganglia were not observed in this study.
Trio ES for Family 1 identified a homozygous missense variant in TUBGCP2 (GenBank: NM_006659.1; c.997C>T [p.Arg333Cys]) (Figures 1A and 3A-B) in individual VI-5 (Family 1). Segregation of the variant by Sanger sequencing confirmed that affected individuals (individuals VI-5 and V-1 [Family 1]) are homozygous for variant alleles and healthy parents and siblings are carriers or homozygous wild type fulfilling Mendelian expectations for an autosomal recessive disease trait. The affected arginine residue in TUBGCP2 is well-conserved throughout species, and the variant is predicted to be likely damaging by mutation prediction tools with a Combined Annotation Dependent Depletion (CADD) score of 27.8 (Table 2). The altered amino acid lies in the conserved γ-tubulin ring protein (Grip) domain 1 common to all tubulin-gamma complex-associated proteins. Note that this region was not part of the original Grip1 motif determined by sequence similarity20 but was later found to be part of the conserved Grip1 domain by further characterization of the crystal structure of GCP421 (Figure 3B). Intrafamilial clinical variability observed across the two affected male cousins led us to explore the hypothesis that this observation might relate to pathogenic variation at a second locus.22 Of note, a de novo 2q23.1 duplication including MBD5 (MIM: 156200) was detected using XHMM23 on ES data and confirmed by chromosomal microarray in individual VI-5 (Family 1, Figure 1B). The minimum size of the duplication by chromosomal microarray was approximately 500 Kb (Chr2:149,067,292-149,567,481). Duplication of chromosome 2q23.1 was not found in other screened family members including the less severely affected cousin (individual V-1 [Family 1]) (Figure 1B and Figure S4).
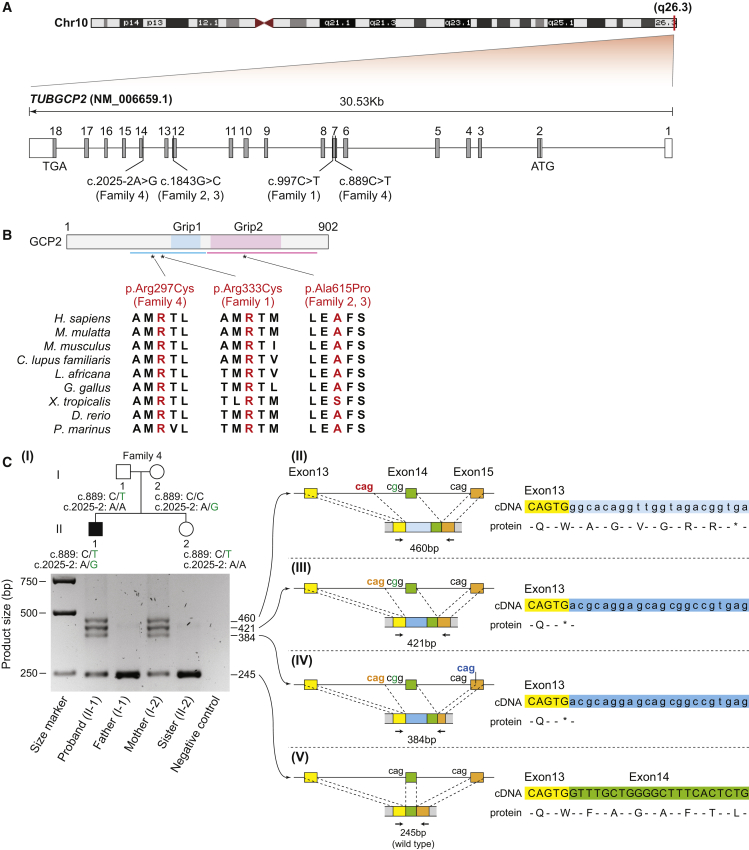
Figure 3.
Variant Locations and Their Effects on Amino Acid Changes and Splicing
(A) Schematic representation of TUBGCP2. The positions of the start codon (ATG), the stop codon (TGA), and the TUBGCP2 variants found in this study are shown.
(B) Structure of γ-tubulin complex protein 2 (GCP2) and the position of altered amino acid residues (297, 333, and 615). Light blue and pink boxes show the two highly conserved sequences among GCPs 2–6, which were designated as γ-tubulin ring protein (Grip) motif 1 and 2, respectively. Blue and pink bars represent the extended conserved domains revealed by the insight obtained from the GCP4 structure. Evolutionary conservation of the altered amino acid residues at positions 297, 333, and 615 are shown.
(C) TUBGCP2 cDNA analysis to investigate consequence of a splice site variant in members of Family 4. Gel electrophoresis of amplified cDNA products showed one PCR product corresponding to expected size from normal splicing (245bp, wild type) in all family members, whereas three additional PCR products (384, 421, and 460bp) were observed in the proband and the mother. Schematic representations of aberrant splicing caused by the splice site variant are shown. The three non-canonical alternative splice acceptor sites (CAG) are indicated with red, orange, and blue font colors. cDNA sequences and the corresponding amino acids sequences are shown. Asterisks indicate a stop codon.
Table 2.
Summary of TUBGCP2 Variant Alleles
| Individuals | Countries | Position (hg19) | Nucleotide (Protein) | Zygosity | Allele count / Zygosity (gnomAD) | CADD Score | Conservation (phylop) |
|---|---|---|---|---|---|---|---|
| Individual VI-5 (Family 1) | Turkey | Chr10: 135106570:C>T | c.997C>T (p.Arg333Cys) | Hmz | 148 Htz–0 Hmz | 27.8 | 2.351 |
| Individual V-1 (Family 1) | Turkey | Chr10: 135106570:C>T | c.997C>T (p.Arg333Cys) | Hmz | 148 Htz–0 Hmz | 27.8 | 2.351 |
| Individual II-3 (Family 2) | India | Chr10: 135099012:G>C | c.1843G>C (p.Ala615Pro) | Hmz | 1 Htz–0 Hmz | 29.6 | 2.839 |
| Individual II-2 (Family 3) | Iran | Chr10: 135099012:G>C | c.1843G>C (p.Ala615Pro) | Hmz | 1 Htz–0 Hmz | 29.6 | 2.839 |
| Individual II-1 (Family 4) | Poland | Chr10: 135106678:C>T | c.889C>T (p.Arg297Cys) | Comp Htz |
27 Htz–0 Hmz | 22.4 | −0.261 |
| Individual II-1 (Family 4) | Poland | Chr10: 135097508:A>G | c.2025-2A>G | Comp Htz |
- | ND | 2.014 |
Abbreviations: CADD, Combined Annotation Dependent Depletion; Comp Htz, compound heterozygous; Hmz, homozygous; Htz, heterozygous; ND, not determined
Independent analyses of clinical ES for the proband in Family 2 and trio ES for Family 3 yielded the same homozygous missense variant (c.1843G>C [p.Ala615Pro]) in two apparently unrelated families (Figures 2A, 2J, and 3A–3B; Table 1) with a CADD score of 29.6. The genotype of the proband in Family 2 (II-3) was determined based on her ES (variant reads/total reads = 72/72) along with confirmation by Sanger sequencing (because residual DNA from the proband was not available for validation) that the variant is heterozygous in both parents (Figure 2A). The altered amino acid resides in the Grip2 motif, which mediates interactions with γ-tubulin21 (Figure 3B).
Compound heterozygous variants were identified in individual II-1 (Family 4) (Figures 2P and 3A–3C); these variants included one heterozygous missense variant (c.889C>T [p.Arg297Cys]) with a CADD score of 22.4 and one splice acceptor site variant (c.2025-2A>G) in TUBGCP2. The altered amino acid lies in the same extended conserved Grip1 domain as does the variant identified in Family 1. To understand the consequence of the c.2025-2A>G variant to the canonical splice acceptor, we investigated the TUBGCP2 transcripts from peripheral blood samples via RT-PCR (Figure 3C). Gel electrophoresis of amplified cDNA products showed one PCR product corresponding to the expected size from normal splicing (245bp) in all family members, whereas three additional PCR products (384, 421, and 460bp) were observed in the proband and the mother, both of whom carried the c.2025-2A>G variant (Figure 3C, I). Sanger sequencing from these three cDNA-PCR products revealed that the splice site variant resulted in activation of three non-canonical alternative splice acceptor sites, two in intron 13 and one in exon 15 (Figure 3C, II–IV). Alternative splice acceptor sites in intron 13 resulted in retention of intron 13 segments, whereas the alternative splice acceptor site in exon 15 led to excision of an exon 15 segment. Both retentions of intron 13 segments caused frameshift variants that led to a premature termination codon (PTC) either immediately (Figure 3C, III and IV) or after six amino acid residues (Figure 3C, II).24 The transcripts with PTCs may be degraded by nonsense-mediated RNA decay (NMD) and/or translated into a truncated protein which lacks a part of the Grip2 domain; both of these results are expected to cause loss of function.24
In this study, we showed that unrelated affected individuals with distinct bi-allelic variants in TUBGCP2 shared clinical neurological phenotypic findings which included microcephaly, DD, and LIS-spectrum phenotypes. Taken together with the function of the TUBGCP2-encoded protein (GCP2) as a core component of γ-TuRC, which serves as a template for microtubule formation, we propose TUBGCP2 as a candidate tubulinopathy-associated gene. However, it is not obvious why the developing nervous system is specifically sensitive to pathogenic variants in TUBGCP2 given its global expression (Figure S2). This specific tissue and/or cell type vulnerability was also observed in other disorders, e.g., variants reported in centrosomal genes which cause primary microcephaly25 and in GARS, an aminoacyl-tRNA synthetase (MIM: 600287) in Charcot-Marie-Tooth disease type 2D (CMT2D [MIM: 601472]).26 We also observed that bi-allelic variants in TUBGCP2 are required to cause the LIS phenotype which segregates as a recessive trait, whereas the majority of other LIS-associated genes cause a dominant phenotype via monoallelic variants. Although the precise molecular mechanisms have not been determined, and it is unclear whether disease-associated alleles at this locus operate through a loss-of-function or gain-of-function mechanism, we hypothesize that γ-TuRC is probably functional even when GCP2 exists in a heterozygous state. In addition, growing evidence has shown that both bi-allelic and monoallelic variants in the same gene have the potential to cause the same or distinct phenotypes.27, 28, 29 When viewed from a gene dosage perspective, deviation from biological homeostasis may reflect a mutational burden at the locus, and dominant or recessive traits reflect this.
Based on anatomical findings documented by brain imaging, we observed a spectrum of clinical severity from mild to severe pachygyria (individual IV-1 [Family 4], individuals V-1 and VI-5 [Family 1], and individual II-3 [Family 2] and individual II-2 [Family 3] in order of severity). With limited data, it is challenging to assign a specific genotype-to-phenotype correlation or to generate a potential allelic series related to mutant variant allele strength and degree of potential perturbation of biological function. The variant identified in Family 2 and Family 3 resides within the Grip2 motif, which is required to interact with γ-tubulin,21 whereas variants identified in Family 1 and Family 4 lie within the extended conserved sequences of the Grip1 domain which mediate interactions between GCPs.21 These limited data and results indicate that variants in the Grip2 motif are presumably more damaging to the function of γ-TuRC.
We found that individual VI-5 (Family 1) had both the homozygous variant in TUBGCP2 and a de novo 2q23.1 duplication (Figures 1A–B and Figure S4). Duplication of the 2q23.1 region, which includes the dosage-dependent gene, MBD5, is linked to ID, dysmorphism, language impairments, infantile hypotonia, gross motor delay, and autistic features (MRD1 [MIM: 156200]).30, 31 Detailed phenotyping showed that individual VI-5 (Family 1) shows a blended phenotype resulting from dual molecular diagnoses of TUBGCP2 and duplication of the 2q23.1 region. Phenotypic features of DD, ID, microcephaly, facial dysmorphia, hypotonia, and pachygyria were consistent with those observed among other individuals with variants in TUBGCP2, and additional unique features of autistic behavior, widely spaced teeth, and broad toes could be explained by duplication of the 2q23.1 region. This case highlights intrafamilial clinical heterogeneity in Family 1 and suggests that shared phenotypic features of DD, ID, and hypotonia among the dual diagnoses probably exacerbated the symptoms in individual VI-5 (Family 1) so that the clinical course should be classified as more severe (Figure S5 and Supplemental Data).
Severe or atypical phenotypes are not uncommon in clinical medicine and can perplex the physician and families. Our approach of detailed phenotyping and family based extensive molecular analyses will contribute to the understanding of how a combinations of rare variants can lead to different phenotypes in individuals.22, 32, 33
Brain imaging patterns have helped us interpret the phenotypic range caused by disruption of LIS-associated genes. For instance, brain imaging of individuals with rare variants in the α- and β-tubulin genes is characterized by cortical malformations of LIS, polymicrogyria, and tubulinopathy-associated dysgyria, as well as non-cortical malformations of basal ganglia dysplasia, brainstem hypoplasia, and cerebellar hypoplasia. In contrast, pathogenic variants in TUBG1 and LIS1 cause posterior-dominant thick LIS, and non-cortical malformation is generally not seen.3, 11, 34
In this study, brain imaging of the five individuals with variants in TUBGCP2 showed shared LIS-spectrum phenotypes, i.e., varying severity of temporal or posterior dominant pachygyria and subcortical band. Serial brain MRI images were available for individual II-3 (Family 2), and they showed progressive volume loss in the corpus callosum, cerebellum, and pons, possibly indicating a neurodegenerative process (Figure 2B-I). Additionally, large subependymal cysts were observed in individual II-3 (Family 2) and individual III-2 (Family 3), who were both born prematurely and carry the same homozygous variant in TUBGCP2. We hypothesize that both the shared variant and periventricular leukomalacia related to prematurity contributed to the severity and extent of cystic formation in these individuals. In individual II-1 (Family 4), hyperintense periventricular white matter of both cerebral hemispheres was seen in FLAIR MRI images (Figure S3). However, we could not determine the etiology of this finding. Taken together, the imaging phenotype associated with variants in TUBGCP2 is more in line with the phenotypes resulting from variants in LIS1 and TUBG1.
Our study also demonstrated that variants in TUBGCP2 cause neuronal migration defects, as opposed to the main feature of microcephaly observed in individuals with bi-allelic variants in TUBGCP4, TUBGCP5, and TUBGCP6. Clinical observation demonstrated that even the individual with milder phenotypic features in this study (individual IV-1 [Family 4]) had both microcephaly and pachygyria. In contrast, the most prominent feature caused by mutations in TUBGCP6 is microcephaly, presumably caused by defects in centriole biogenesis. In one study, even the most severely affected individual (individual P9: head circumference Z score −10 at 9 years old) showed no apparent structural brain abnormalities in brain imaging.15 We hypothesize from these observations that γ-TuRC assembly (including composition of GCPs), recruitment, and activation are differentially regulated in proliferating neuronal progenitor cells and migrating post-mitotic neuronal cells.
Recently, other γ-TuRC components (e.g., MOZART1, MOZART2A, MOZART2B, NME7, and NEDD1), CM1-domain γ-TuRC tethering factors (e.g., CDK5RAP2 and Pericentrin), and γ-TuRC-independent microtubule nucleators (e.g., CKAP5 and TPX2) have been discovered and studied in detail.4, 35, 36 Human phenotypes caused by defects in most of these proteins have not been described. Moreover, recent advances in genetic tools and imaging technologies to probe microtubules in situ have enabled investigation of microtubule nucleation, dynamics, and functions in vivo.37 Human phenotypic annotation of genes involved in microtubule nucleation and assembly, together with basic cellular and molecular biology that addresses diversity in the regulation of these processes in vivo, will likely foment knowledge of the mechanisms of microtubule regulation in brain development and perhaps provide a better understanding of the pathophysiology of the disease, and thus pathways and thoughts stimulating therapeutic development.33
Declaration of Interests
J.R.L. has stock ownership in 23andMe, is a paid consultant for Regeneron Pharmaceuticals, and is a co-inventor on multiple United States and European patents related to molecular diagnostics for inherited neuropathies, eye diseases, and bacterial genomic fingerprinting. The Department of Molecular and Human Genetics at Baylor College of Medicine receives revenue from clinical genetic testing conducted at Baylor Genetics Laboratories. Other authors have no potential conflicts to report.
Acknowledgments
We thank the individuals and their families for participation in this study. This study was supported in part by a grant from the National Human Genome Research Institute (NHGRI) and National Heart, Lung, and Blood Institute (NHBLI) to the Baylor-Hopkins Center for Mendelian Genomics (BHCMG, UM1 HG006542); an NHGRI grant to the Baylor College of Medicine Human Genome Sequencing Center (U54HG003273 to R.A.G.); and grants from the National Institute of Neurological Disorders and Stroke (NINDS) (R35NS105078 to J.R.L.) and the Muscular Dystrophy Association (MDA) (512848 to J.R.L.). J.E.P. was supported by NHGRI grant K08 HG008986. T.M. is supported by the Uehara Memorial Foundation. D.P. is supported by the Clinical Research Training Scholarship in Neuromuscular Disease partnered by the American Academy of Neurology (AAN), the American Brain Foundation (ABF), and the Muscle Study Group (MSG); and by the National Institutes of Health (NIH) Brain Disorders and Development Training Grant (T32 NS043124-17). D.M. is supported by a Medical Genetics Research Fellowship Program through the United States National Institute of Health (T32 GM007526-42). H.N. and K.K. were supported by the Iran National Science Foundation (INSF) (950022 to H.N. and 96011200 to K.K.), and the National Institute for Medical Research Development (NIMAD) (958715 to H.N. and 957060 to KK). W.W., P.G., M.B-F., and M.D. were supported by a grant from National Science Centre, Poland (2015/19/B/NZ2/01824). The Genotype-Tissue Expression (GTEx) Project was supported by the Common Fund of the Office of the Director of the National Institutes of Health, and by the National Cancer Institute (NCI), NHGRI, NHLBI, National Institute on Drug Abuse (NIDA), National Institute of Mental Health (NIMH), and NINDS. The data used for the analyses described in this manuscript were obtained from the GTEx Portal on 07/10/2019 and dbGaP accession number phs000424.v7.p2 on 07/10/2019.
Published: October 17, 2019
Footnotes
Supplemental Data can be found online at https://doi.org/10.1016/j.ajhg.2019.09.017.
Contributor Information
Tadahiro Mitani, Email: tadahiro.mitani@bcm.edu.
Pawel Gawlinski, Email: pawel.gawlinski@imid.med.pl.
Accession Numbers
The accession number for the reported case is dbGaP: phs000711.v5.p1, which can be found at https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000711.v5.p1.
Web Resource
Database of Genotypes and Phenotypes (dbGaP), https://www.ncbi.nlm.nih.gov/gap/
GeneMatcher Browser, https://genematcher.org/
Genotype-Tissue Expression-GTEx Portal, https://gtexportal.org/home/
gnomAD Browser, https://gnomad.broadinstitute.org/
Online Mendelian Inheritance in Man, https://www.omim.org/
UCSC Genome Browser, https://genome.ucsc.edu
Supplemental Data
References
- 1.Guerrini R., Dobyns W.B. Malformations of cortical development: clinical features and genetic causes. Lancet Neurol. 2014;13:710–726. doi: 10.1016/S1474-4422(14)70040-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barkovich A.J., Guerrini R., Kuzniecky R.I., Jackson G.D., Dobyns W.B. A developmental and genetic classification for malformations of cortical development: update 2012. Brain. 2012;135:1348–1369. doi: 10.1093/brain/aws019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan A.P., Chong W.K., Mankad K. Comprehensive genotype-phenotype correlation in lissencephaly. Quant. Imaging Med. Surg. 2018;8:673–693. doi: 10.21037/qims.2018.08.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tovey C.A., Conduit P.T. Microtubule nucleation by γ-tubulin complexes and beyond. Essays Biochem. 2018;62:765–780. doi: 10.1042/EBC20180028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bahi-Buisson N., Cavallin M. University of Washington; Seattle: 1993. Tubulinopathies Overview. [PubMed] [Google Scholar]
- 6.Keays D.A., Tian G., Poirier K., Huang G.-J., Siebold C., Cleak J., Oliver P.L., Fray M., Harvey R.J., Molnár Z. Mutations in α-tubulin cause abnormal neuronal migration in mice and lissencephaly in humans. Cell. 2007;128:45–57. doi: 10.1016/j.cell.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaglin X.H., Poirier K., Saillour Y., Buhler E., Tian G., Bahi-Buisson N., Fallet-Bianco C., Phan-Dinh-Tuy F., Kong X.P., Bomont P. Mutations in the beta-tubulin gene TUBB2B result in asymmetrical polymicrogyria. Nat. Genet. 2009;41:746–752. doi: 10.1038/ng.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breuss M., Heng J.I.-T., Poirier K., Tian G., Jaglin X.H., Qu Z., Braun A., Gstrein T., Ngo L., Haas M. Mutations in the β-tubulin gene TUBB5 cause microcephaly with structural brain abnormalities. Cell Rep. 2012;2:1554–1562. doi: 10.1016/j.celrep.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tischfield M.A., Baris H.N., Wu C., Rudolph G., Van Maldergem L., He W., Chan W.-M., Andrews C., Demer J.L., Robertson R.L. Human TUBB3 mutations perturb microtubule dynamics, kinesin interactions, and axon guidance. Cell. 2010;140:74–87. doi: 10.1016/j.cell.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poirier K., Lebrun N., Broix L., Tian G., Saillour Y., Boscheron C., Parrini E., Valence S., Pierre B.S., Oger M. Mutations in TUBG1, DYNC1H1, KIF5C and KIF2A cause malformations of cortical development and microcephaly. Nat. Genet. 2013;45:639–647. doi: 10.1038/ng.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bahi-Buisson N., Poirier K., Fourniol F., Saillour Y., Valence S., Lebrun N., Hully M., Bianco C.F., Boddaert N., Elie C., LIS-Tubulinopathies Consortium The wide spectrum of tubulinopathies: what are the key features for the diagnosis? Brain. 2014;137:1676–1700. doi: 10.1093/brain/awu082. [DOI] [PubMed] [Google Scholar]
- 12.Scheidecker S., Etard C., Haren L., Stoetzel C., Hull S., Arno G., Plagnol V., Drunat S., Passemard S., Toutain A. Mutations in TUBGCP4 alter microtubule organization via the γ-tubulin ring complex in autosomal-recessive microcephaly with chorioretinopathy. Am. J. Hum. Genet. 2015;96:666–674. doi: 10.1016/j.ajhg.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maver A., Čuturilo G., Kovanda A., Miletić A., Peterlin B. Rare missense TUBGCP5 gene variant in a patient with primary microcephaly. Eur. J. Med. Genet. 2018 doi: 10.1016/j.ejmg.2018.12.003. S1769-7212(18)30274-X. [DOI] [PubMed] [Google Scholar]
- 14.Puffenberger E.G., Jinks R.N., Sougnez C., Cibulskis K., Willert R.A., Achilly N.P., Cassidy R.P., Fiorentini C.J., Heiken K.F., Lawrence J.J. Genetic mapping and exome sequencing identify variants associated with five novel diseases. PLoS ONE. 2012;7:e28936. doi: 10.1371/journal.pone.0028936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin C.-A., Ahmad I., Klingseisen A., Hussain M.S., Bicknell L.S., Leitch A., Nürnberg G., Toliat M.R., Murray J.E., Hunt D. Mutations in PLK4, encoding a master regulator of centriole biogenesis, cause microcephaly, growth failure and retinopathy. Nat. Genet. 2014;46:1283–1292. doi: 10.1038/ng.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karaca E., Harel T., Pehlivan D., Jhangiani S.N., Gambin T., Coban Akdemir Z., Gonzaga-Jauregui C., Erdin S., Bayram Y., Campbell I.M. Genes that Affect Brain Structure and Function Identified by Rare Variant Analyses of Mendelian Neurologic Disease. Neuron. 2015;88:499–513. doi: 10.1016/j.neuron.2015.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White J., Beck C.R., Harel T., Posey J.E., Jhangiani S.N., Tang S., Farwell K.D., Powis Z., Mendelsohn N.J., Baker J.A. POGZ truncating alleles cause syndromic intellectual disability. Genome Med. 2016;8:3. doi: 10.1186/s13073-015-0253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sobreira N., Schiettecatte F., Valle D., Hamosh A. GeneMatcher: a matching tool for connecting investigators with an interest in the same gene. Hum. Mutat. 2015;36:928–930. doi: 10.1002/humu.22844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sobreira N., Schiettecatte F., Boehm C., Valle D., Hamosh A. New tools for Mendelian disease gene identification: PhenoDB variant analysis module; and GeneMatcher, a web-based tool for linking investigators with an interest in the same gene. Hum. Mutat. 2015;36:425–431. doi: 10.1002/humu.22769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gunawardane R.N., Martin O.C., Cao K., Zhang L., Dej K., Iwamatsu A., Zheng Y. Characterization and reconstitution of Drosophila gamma-tubulin ring complex subunits. J. Cell Biol. 2000;151:1513–1524. doi: 10.1083/jcb.151.7.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guillet V., Knibiehler M., Gregory-Pauron L., Remy M.-H., Chemin C., Raynaud-Messina B., Bon C., Kollman J.M., Agard D.A., Merdes A., Mourey L. Crystal structure of γ-tubulin complex protein GCP4 provides insight into microtubule nucleation. Nat. Struct. Mol. Biol. 2011;18:915–919. doi: 10.1038/nsmb.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karaca E., Posey J.E., Coban Akdemir Z., Pehlivan D., Harel T., Jhangiani S.N., Bayram Y., Song X., Bahrambeigi V., Yuregir O.O. Phenotypic expansion illuminates multilocus pathogenic variation. Genet. Med. 2018;20:1528–1537. doi: 10.1038/gim.2018.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fromer M., Moran J.L., Chambert K., Banks E., Bergen S.E., Ruderfer D.M., Handsaker R.E., McCarroll S.A., O’Donovan M.C., Owen M.J. Discovery and statistical genotyping of copy-number variation from whole-exome sequencing depth. Am. J. Hum. Genet. 2012;91:597–607. doi: 10.1016/j.ajhg.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coban-Akdemir Z., White J.J., Song X., Jhangiani S.N., Fatih J.M., Gambin T., Bayram Y., Chinn I.K., Karaca E., Punetha J., Baylor-Hopkins Center for Mendelian Genomics Identifying Genes Whose Mutant Transcripts Cause Dominant Disease Traits by Potential Gain-of-Function Alleles. Am. J. Hum. Genet. 2018;103:171–187. doi: 10.1016/j.ajhg.2018.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jayaraman D., Bae B.-I., Walsh C.A. The Genetics of Primary Microcephaly. Annu. Rev. Genomics Hum. Genet. 2018;19:177–200. doi: 10.1146/annurev-genom-083117-021441. [DOI] [PubMed] [Google Scholar]
- 26.Antonellis A., Ellsworth R.E., Sambuughin N., Puls I., Abel A., Lee-Lin S.-Q., Jordanova A., Kremensky I., Christodoulou K., Middleton L.T. Glycyl tRNA synthetase mutations in Charcot-Marie-Tooth disease type 2D and distal spinal muscular atrophy type V. Am. J. Hum. Genet. 2003;72:1293–1299. doi: 10.1086/375039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harel T., Yoon W.H., Garone C., Gu S., Coban-Akdemir Z., Eldomery M.K., Posey J.E., Jhangiani S.N., Rosenfeld J.A., Cho M.T., Baylor-Hopkins Center for Mendelian Genomics. University of Washington Center for Mendelian Genomics Recurrent De Novo and Biallelic Variation of ATAD3A, Encoding a Mitochondrial Membrane Protein, Results in Distinct Neurological Syndromes. Am. J. Hum. Genet. 2016;99:831–845. doi: 10.1016/j.ajhg.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rainger J., Pehlivan D., Johansson S., Bengani H., Sanchez-Pulido L., Williamson K.A., Ture M., Barker H., Rosendahl K., Spranger J., UK10K. Baylor-Hopkins Center for Mendelian Genomics Monoallelic and biallelic mutations in MAB21L2 cause a spectrum of major eye malformations. Am. J. Hum. Genet. 2014;94:915–923. doi: 10.1016/j.ajhg.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pehlivan D., Bayram Y., Gunes N., Coban Akdemir Z., Shukla A., Bierhals T., Tabakci B., Sahin Y., Gezdirici A., Fatih J.M., Baylor-Hopkins Center for Mendelian Genomics The Genomics of Arthrogryposis, a Complex Trait: Candidate Genes and Further Evidence for Oligogenic Inheritance. Am. J. Hum. Genet. 2019;105:132–150. doi: 10.1016/j.ajhg.2019.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mullegama S.V., Elsea S.H. Clinical and Molecular Aspects of MBD5-Associated Neurodevelopmental Disorder (MAND) Eur. J. Hum. Genet. 2016;24:1235–1243. doi: 10.1038/ejhg.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mullegama S.V., Rosenfeld J.A., Orellana C., van Bon B.W.M., Halbach S., Repnikova E.A., Brick L., Li C., Dupuis L., Rosello M. Reciprocal deletion and duplication at 2q23.1 indicates a role for MBD5 in autism spectrum disorder. Eur. J. Hum. Genet. 2014;22:57–63. doi: 10.1038/ejhg.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Posey J.E., Harel T., Liu P., Rosenfeld J.A., James R.A., Coban Akdemir Z.H., Walkiewicz M., Bi W., Xiao R., Ding Y. Resolution of Disease Phenotypes Resulting from Multilocus Genomic Variation. N. Engl. J. Med. 2017;376:21–31. doi: 10.1056/NEJMoa1516767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Posey J.E., O’Donnell-Luria A.H., Chong J.X., Harel T., Jhangiani S.N., Coban Akdemir Z.H., Buyske S., Pehlivan D., Carvalho C.M.B., Baxter S., Centers for Mendelian Genomics Insights into genetics, human biology and disease gleaned from family based genomic studies. Genet. Med. 2019;21:798–812. doi: 10.1038/s41436-018-0408-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brock S., Stouffs K., Scalais E., D’Hooghe M., Keymolen K., Guerrini R., Dobyns W.B., Di Donato N., Jansen A.C. Tubulinopathies continued: refining the phenotypic spectrum associated with variants in TUBG1. Eur. J. Hum. Genet. 2018;26:1132–1142. doi: 10.1038/s41431-018-0146-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teixidó-Travesa N., Roig J., Lüders J. The where, when and how of microtubule nucleation - one ring to rule them all. J. Cell Sci. 2012;125:4445–4456. doi: 10.1242/jcs.106971. [DOI] [PubMed] [Google Scholar]
- 36.Roostalu J., Surrey T. Microtubule nucleation: beyond the template. Nat. Rev. Mol. Cell Biol. 2017;18:702–710. doi: 10.1038/nrm.2017.75. [DOI] [PubMed] [Google Scholar]
- 37.Muroyama A., Lechler T. Microtubule organization, dynamics and functions in differentiated cells. Development. 2017;144:3012–3021. doi: 10.1242/dev.153171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.