Highlights
-
•
The methodological quality of the studies ranged from “poor” to “good”.
-
•
Most results of the quality of the measurement properties were considered doubtful.
-
•
The Frenchay activities index and the Human activity profile showed the highest clinical utility scores.
-
•
The content validity needs to be better investigated.
-
•
Further studies with good methodological quality are required.
Keywords: Stroke, Physical activity, Self-report, Measurement properties, Outcome measures
Abstract
Background
Individuals with stroke demonstrate low levels of physical activity. Self-report measures of physical activity are frequently used and the choice of the best one to use for each purpose and context should take into account the measurement properties of these instruments.
Objective
To summarize the measurement properties and clinical utility of self-report measures of physical activity of post-stroke subjects and to evaluate both the methodological quality of the studies and the quality of the measurement properties.
Methods
Searches were made in MEDLINE, EMBASE, PEDro, LILACS, and SCIELO. Two reviewers independently screened studies that investigated measurement properties or clinical utility of self-report measures of physical activity in post-stroke subjects. The studies’ methodological quality, quality of the measurement properties, and clinical utility were evaluated.
Results
From the 11,826 identified studies, 19 were included. Six self-report tools were evaluated: The Activity card sort, Coded activity diary, Frenchay activities index (FAI), Human activity profile (HAP), Multimedia activity recall for children and adults, and the Nottingham leisure questionnaire. The methodological quality of the studies ranged from “poor” to “good”. Most of the results regarding the quality of the measurement properties were doubtful. None of the self-report tools had their content validity investigated. The FAI and HAP showed the highest clinical utility scores.
Conclusions
Content validity needs to be better investigated to determine if the instruments actually measure the physical activity domain. Further studies with good methodological quality are required to assist clinicians and researchers in selecting the best instrument to measure physical activity levels.
Introduction
Physical inactivity is globally recognized as a major cause of morbidity and is the fourth leading risk factor for mortality.1 Individuals with stroke demonstrate low levels of physical activity, which increase the risks for further cardiovascular diseases and stroke-related disabilities.2 The use of appropriate instruments to measure physical activity levels is important for determining trends over time, the effects of interventions, and the health benefits of physical activity.3
Physical activity is defined as any bodily movement produced by skeletal muscle contractions, which increases energy expenditure.4 Physical activity can be measured by self-report (e.g., questionnaires, diaries/logs, surveys, and interviews) or direct assessment tools (e.g., pedometers, accelerometers, and activity monitors).5 Self-report measures are frequently used due to their practicality, since most of them are easy to administer, have low cost, provide information regarding various types and intensities of activities, and may be used within a variety of contexts.6
A large number of questionnaires of self-report physical activity assessment tools for the adult population have been developed.3, 7 The choice of the best instrument to use for each purpose and context should take into account the characteristics of the instruments, especially their measurement properties (validity, reliability, and responsiveness) and clinical utility (the practicalities of using the measurement tools).8
Two systematic reviews3, 7 assessed the measurement properties of self-report physical activity tools in healthy adults. The authors highlighted limited investigated measurement properties of the evaluated tools and the low methodological quality of the included studies. Despite the important contribution of these systematic reviews,3, 7 they did not included people with chronic diseases, such as stroke patients. The results from studies aimed at evaluating the measurement properties of a tool in one population cannot be systematically generalized to others.8
A systematic review9 with chronically ill patients and elderly subjects assessed the development process and initial validation of self-report tools for the measurement of physical activity levels. Although some studies with post-stroke individuals were included, the search strategy was not specific for this population and the results regarding the measurement properties of the identified tools, specifically for subjects with stroke, were not reported. There was found only one systematic review,10 which investigated the measurement properties of physical activity assessment tools specifically for post-stroke subjects, but only direct assessment tools were assessed.
Therefore, the objectives of this systematic review were to summarize both the measurement properties and clinical utility of self-report measures of physical activity levels of subjects with stroke and to evaluate both the methodological quality of the studies on measurement properties and the quality of the measurement properties.
Methods
This study was reported using the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement guidelines.11 The protocol of this systematic review has been registered on the International Prospective Register of Systematic Reviews (#CRD42016037146; http://www.crd.york.ac.uk/PROSPERO/) and was recently published.12
Data sources and searches
The following electronic databases were searched: Medical Literature Analysis and Retrieval System Online, Excerpta Medica Database, Physiotherapy Evidence Database, Literatura Latino-Americana e do Caribe em Ciências da Saúde, and Scientific Eletronic Library Online. Databases were searched from their inception to December 2018. The reference lists of the included studies were also screened, to identify further studies. The search strategy was previous published,12 and included words related to four components: (1) health condition (stroke), (2) outcome measure (physical activity), (3) measurement properties, and (4) self-report measures.
Study selection
Details regarding the eligible studies were described in the previous published protocol.12 All full-text papers which investigated the measurement properties and/or clinical utility of self-report measures of physical activity levels in individuals with stroke were included. To be considered as self-report measures of physical activity levels, the authors should clearly state that the instrument provided a measure of physical activity or a measure of one of the dimensions of the physical activity (i.e. duration, frequency, or intensity). Studies published in English, Spanish, French, and Portuguese with adults (≥18 years of age), who had a stroke, were included, without further restrictions. Studies, which reported a specific activity, such as walking, exercise capacity, gait patterns, or the ability to perform activities of daily living, were excluded, as well as systematic reviews and studies with other neurological conditions, not related to stroke.
Two reviewers independently assessed the titles and abstracts of all identified records from the electronic searches. Full-text articles were screened for eligibility by the same reviewers. Disagreements were resolved by discussion and consensus. When, required, a third reviewer was consulted.
Data extraction and quality assessment
Relevant data from all the included studies were summarized in tables, as described in the published protocol.12 When the general characteristics of the self-report physical activity measures could not be extracted from the included studies, the original paper was consulted, to obtain the necessary information to be summarized.
The same reviewers independently assessed the methodological quality of the studies, the quality of the measurement properties, and the clinical utility. A third rater was available to solve any discrepancies.
Assessment of the methodological quality of the included studies using the COSMIN taxonomy
The COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN) checklist was used to determine the methodological quality of the included studies.13, 14 In the COSMIN, nine measurement properties clustered within three domains, i.e. reliability, validity, and responsiveness, are considered relevant for the evaluation of outcome measurement instruments.14 In the reliability domain, internal consistency, test-retest, inter-and intra-rater reliability, as well as measurement errors, are assessed. In the validity domain, construct validity (structural validity and hypotheses testing), criterion-validity and content validity are assessed. By taking the lowest item rating, an overall quality score (‘poor,’ ‘fair,’ ‘good,’ ‘excellent’) was separately obtained for each evaluated measurement property.14
Quality of the measurement property using the Terwee's criteria
To assess the quality of the measurement properties, the criteria proposed by Terwee et al.15 was applied to the results of a study on measurement properties. Terwee et al.15 defined criteria for what constitutes good measurement properties. For example, for the assessment of validity, it is often recommended that hypotheses about expected results be tested, but no criteria have been defined about how many hypotheses should be confirmed to justify that a questionnaire has good validity. No criteria have been defined for what constitutes good agreement (acceptable measurement error), good responsiveness, neither for the required sample size of studies assessing measurement properties. Each measurement property is rated as positive (+), negative (−), or doubtful/indeterminate (?), depending upon the design, methods, and outcomes of the study.15 If a clear description of the design of the study is lacking, the evaluated measurement properties are rated as doubtful.15 In addition, if any important methodological weaknesses in the design or execution of the study are found, e.g., selection bias or an extremely heterogeneous study population, the evaluated measurement properties are also rated as doubtful.15 These criteria15 complement the evaluation of the measurement properties, since the COSMIN does not determine the cut-off values, which are considered adequate for the statistical analyses.16 In other words, the fact that a study used some statistics advocated by the COSMIN, does not guarantee the quality of the measurement property, as appropriated values may not have been reached.15, 16
Clinical utility
The clinical utility (feasibility) was assessed to quantify the practicalities of the identified tools. Previously developed criteria based upon factors that may influence whether clinicians would use a measurement tool in their practice17, 18 were used: application time, cost, need of specialized equipment/training, portability and accessibility. Both the item and the total scores (maximum of 12 points) were reported.18
Data synthesis
A systematic narrative synthesis was provided in text and table formats, to summarize and discuss the sample and methodological characteristics, as well as the findings regarding the measurement properties and clinical utility of the included studies on self-report measures of physical activity levels in individuals with stroke. Measures, which were investigated in more than one study, were grouped and the references of all studies were cited.
Results
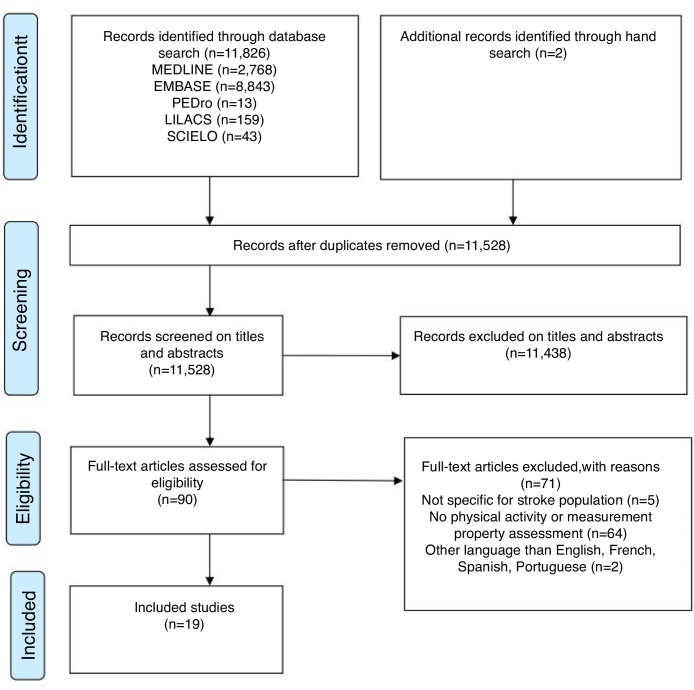
The electronic searches returned a total of 11,826 studies (300 were duplicates) and after the initial selection based upon the titles and abstracts, 90 were potentially eligible. Fig. 1 shows the flow of the studies through this review, including the reasons for exclusion. After screening the full-texts, 17 studies met the eligibility criteria. Two other relevant studies were found by screening the reference lists of the included articles. Therefore, 19 studies were included in this systematic review.19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37 The following six self-report measures of physical activity levels were identified, three of them in two different versions:19, 21, 24 The Activity Card Sort (ACS and ACS-Hong Kong version),19, 34 Coded Activity Diary,35 Frenchay Activities Index (FAI and FAI-chinese version),23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 36, 37 Human Activity Profile (HAP),33 Multimedia Activity Recall for Children and Adults (MARCA),22 and the Nottingham Leisure Questionnaire (original and short versions).20, 21 These measures are described in Table 1.
Figure 1.
Flow of studies through the review.
Table 1.
Description of the self-report physical activity assessment tools for subjects with stroke.
| Instrument | Type/Time of Administration | N° questions/activities | Recall Period | Answer options | Scoring | Dimensions | Domains |
|---|---|---|---|---|---|---|---|
| Activity Card Sort (ACS)34, 43,* | Type: Sort photographs of activities Time: 20 min |
80 | Activities in which a person is currently involved, compared to those he/she was involved in the past | Sort photographs into one of five categories: never done, still doing, given up due to stroke, do less due to stroke, and started after stroke | Percentage of retained-activity level, calculated by dividing the total sum of current activities by the total sum of previous activities | Mode Frequency |
Instrumental, social, and high- and low-physical-demand leisure |
| Activity Card Sort - Hong Kong version (ACS-HK)19 | Type: Sort photographs of activities Time: 20 min |
65 | The same as above | Sort photographs into one of five categories: 1 = not done prior to current illness/injury; 2 = continued to do during illness/injury; 3 = given up due to illness/injury; 4 = beginning to do again; 5 = a new activity | The same as above | Mode Frequency |
The same as above |
| Coded activity diary35 | Type: Diary Time: NR |
List of 63 codes into categories in four columns: (1) time, (2) activity, (3) position, and (4) intensity of the activity | 30-min period | Choose the 63 codes into categories of activities and inform the position | Perceived intensity of each activity is rated on a 6–20 scale. | Intensity (MET.min and energy expenditure in kcal/min) | Selfcare, household tasks, work, therapy, leisure and home activities, and activities related to mobility and transport |
| Frenchay Activities Index (FAI)44, 45,* and Frenchay Activities Index – Chinese version (FAI-C)24 | Type: Interview or mail questionnaire Time: 5 min |
15 | First 10 items: past 3 months Last 5 items: past 6 months. |
4-point scale: 0 (never) to 3 (at least once a week) | Sum of items Total score ranges from 0 (no participation) to 45 (frequent participation) |
Frequency | Daily and social activities: domestic, work/leisure, and outdoors/other |
| Human Activity Profile (HAP)33, 46,* | Type: self-report questionnaire Time: 20 min |
94 | 3-point scale: still doing, have stopped doing’, or ‘never did the activity | Maximum activity score (MAS): sum of activities the subjects are still doing Adjusted activity score (AAS): subtract the number of activities that the respondent had discontinued performing from MAS |
Intensity (MET) | Self-care, transportation, home maintenance, entertainment, social, and physical exercises | |
| Multimedia Activity Recall for Children and Adults (MARCA)22, 47,* | Type: Computer delivered/time-diary format, self-administered or computer assisted personal/telephone interview Time: 15–20 min |
300 | 24 h period in time slots of 5 min or more | Choose one of the 300 activities and inform the duration | NA | Duration (min/d) Intensity (MET.min) |
Total sitting time, screen time, quiet time, sleep, social, self-care, work/study, chores (indoor and outdoor) |
| Nottingham Leisure Questionnaire20, 48,* | Type: Interview or self-report questionnaire Time: NR |
37 categories | Last year | Yes/no options. If the answer was yes, subjects were asked how often the activity was carried-out on a 5-point scale: never to very regularly |
NR | Frequency | Leisure activities |
| Nottingham Leisure Questionnaire (short version)21 | Type: Interview or self-report questionnaire Time: NR |
30 categories | Last few weeks | Yes/no options. If the answer was yes, subjects were asked how often the activity was carried out in 3-point scale: 0- never, 1-ocassionally, 2-regularly |
NR | Frequency | Leisure activities |
NA, not applicable; NR, not reported.
Supplementary reference consulted to obtain information from the instrument.
Characteristics of the studies
The 19 included studies19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37 involved 2411 participants with stroke, aged between 56 and 79 years and time since the onset of the stroke ranging from 6 days to 22 years. Table 2 presents the characteristics of the studies, as well as the investigated measurement properties and the results of the included studies.
Table 2.
Characteristics of the included studies and measurement properties of self-report physical activity assessment tools for subjects with stroke.
| Instrument | Reference | Study population | Measurement property | Results |
|---|---|---|---|---|
| Activity Card Sort - Hong Kong Version (ACS-HK) | Chan et al., 200619 | Hong Kong/China n = 60 Sex: 31(52%) men Group 1: Less active (n = 30), Age(y): 75(7), Time post-stroke(y): 1(0.2) Group 2: More active (n = 30), Age(y): 74(6), Time post-stroke(y): 1(1) |
Internal consistency | Cronbach's α = 0.89 |
| Test–retest reliability | Total group: ICC = 0.98, 95%CI = 0.97–0.99; Group 1: ICC = 0.91;Group 2: ICC = 0.92 | |||
| CV: Hypotheses testing | Difference between less active and more active groups: t = −1424, p = 0.001 Correlation between ACS-HK and ComQOL: r = 0.86, p = 0.001 |
|||
| Activity Card Sort (ACS) | Tucker et al., 201234 |
n = 29 Sex: 14(48%) men; Age(y): 61(13) Time post-stroke (y): 4(3) |
CV: Hypotheses testing | Correlation between Total ACS and RNL: r = 0.51, p = 0.01 Correlation between Total ACS and SIS Recovery r = 0.38; SIS Communication r = 0.46, p = 0.05; SIS Participation r = 0.41, p = 0.05; SIS Physical Domain r = 0.64, p = 0.01 Correlation between Total ACS and SF-36 Physical Function: r = 0.60, p = 0.01 |
| Coded activity diary | Vanroy et al., 201435 | Belgium n = 16 Sex: 9(56%) men; Age(y): 68(11) Time post-stroke(d): 78(53) Type of stroke: ischemic 9(56%) |
Criterion validity | Metabolic equivalent minutes (MET.min) between patient's diaries and observer's diaries: rs = 0.75, p = 0.001 Metabolic equivalent minutes (MET.min) between patient's diaries and Sensewear Pro2 armband (SWP2A): rs = 0.15, p = 0.59 Energy expenditure (kcal/12 h) between patient's diaries and observer's diaries: rs = 0.92, p = 0.0001 Energy expenditure (kcal/12 h) between patient's diaries and Sensewear Pro2 armband (SWP2A): rs = 0.29, p = 0.28 |
| Frenchay Activities Index (FAI) | Monteiro et al., 201727 | Salvador/Brazil n = 36 Sex: 13(36%) men; Age(y): 58(18) |
Inter-rater Reliability | Total FAI: ICC = 0.83, 95%CI = 0.69–0.91; p < 0.001; Total FAI: K = 0.66 (0.54–0.68); p < 0.001 |
|
n = 161 Sex: 50(31%) men; Age(y): 57(17) Time post-stroke(d): median 6(IQR4–12) Type of stroke: ischemic 98(61%) |
CV: Hypotheses testing | Correlation between FAI and NIHSS: rs = −0.23, p = 0.004 | ||
| Sarker et al., 201230 | London/United Kingdom n = 238 Sex: 124(52%) men; Age(y): 69(14) Time post-stroke(mo): 3 Type of stroke: ischemic 205(86%) |
Criterion validity | Correlation between FAI and BI: rs = 0.80, 95%CI = 0.74–0.84 Correlation between FAI and NEADL: rs = 0.90, 95%CI = 0.88–0.92 |
|
| Lu et al., 201226 | Taiwan/China n = 52 Sex: 37(71%) men; Age(y): 59(12) Time post-stroke(mo): >6 |
Test–retest reliability | t = 0.0(3.5), p = 0.94; ICC = 0.89, 95%CI = 0.81–0.93; LoA = 6.9 | |
| Measurement error | SEM = 2.4; SRD(SRD%) = 6.7(14.9) | |||
| Lin et al., 201225 | Taiwan/China n = 127 Sex: 93(73%) men; Age(y): 55(12) Time post-stroke(mo): 17(16) Type of stroke: infarction 50(39%) |
Internal consistency | Cronbach's α = 0.73–0.81 MNSQ infit = 0.63–1.49; t = −4.9–4.9 MNSQ outfit = 0.76–1.37; t = −3.20–3.20 |
|
| Wu et al., 201137 | Taiwan/China n = 70 Sex: 46(66%) men; Age(y): 56(12) Time post-stroke (mo): 20(13) |
Criterion validity | Correlation between FAI and NEADL: rs = 0.80, 95%CI = 0.70–0.90, p < 0.01 Correlation between FAI and SIS/ADL: rs = 0.40, 95%CI = 0.20–0.60, p < 0.01; SIS/Total: rs = 0.40, 95%CI = 0.20–0.60, p < 0.01; Correlation between FAI and MAL/amout of use: rs = 0.30, 95%CI = 0.10–0.50, p < 0.01; MAL/quality of movement: rs = 0.30, 95%CI = 0.10–0.50, p < 0.01 |
|
| Responsiveness | Responsiveness of FAI to detect change from before and after treatments of constraint-induced theray, bilateral arm training and control treatment. SRM (variant of effect size) is the mean change in score divided by the standard deviation of the changed scores. SRM = 0.5, 95% CI = 0.3–0.7 indicate a moderate change |
|||
| Schepers et al., 200631 | Dutch/German n = 163 Sex: 102(63%) men; Age(y): 56(11) Time post-stroke(d): median 41 Type of stroke: ischemic 121(74%) |
Responsiveness | Responsiveness of FAI to detect change from six months and one year post stroke. Effect size were calculated dividing the mean absolute change score by the standard deviation of the baseline score. Effect size = 0.59 indicate a moderate change |
|
| Post and de Witte, 200329 | Dutch/German n = 45 Sex: 26(58%) men; Age(y): 56(11) Time post-stroke(w):31(32) Type of stroke: ischemic 31(69%) |
Inter-rater reliability | ICC = 0.90, 95%CI = 0.82–0.94; K = 0.41–0.90 | |
| Green et al., 200123 |
n = 22 Sex: 16(73%) men; Age(y): 72(7) Time post-stroke(mo): 15(0.5) |
Test–retest reliability | K = 0.25–1.0; Bland Altman: difference of −0.60(3.5), 95% limits of agreement −2.21–0.93. | |
| Piercy et al., 200028 | Oxfordshire/England n = 68 (n = 33 stroke, n = 35 carers) Sex: 27(40%) men; Age (y): 71(15) |
Inter-rater reliability | rs = 0.93, p < 0.001; K = 0.27–0.80; Bland Altman: difference 0.76(5), median –1(IRQ −4–2), 95% limits of agreement −9.9–8.4 | |
| Schuling et al., 199332 | Netherland n = 188 (n = 92 pre-stroke, n = 96 post-stroke group) Sex: 77(41%) men; Age(y): median 76(IQR10) Time post-stroke(w): 26 pre-stroke group Time post-stroke(mo): 6 post-stroke group |
Internal consistency | Cronbach's α = 0.78 prestroke group Cronbach's α = 0.87 poststroke group |
|
| CV: Hypotheses testing | Correlation between FAI and BI: r = 0.66 Correlation between FAI and subscales of SIP: r = −0.14 − (−0.73) |
|||
| Wade et al., 198536 | Frenchay/England n = 14 Time post-stroke(w): 1 |
Inter-rater reliability | rs = 0.80, p < 0.001 | |
|
n = 581 Age(y): 72(10); Time post-stroke(w): 3 |
CV: Structural validity | Factor analysis (varimax rotation): factor 1–30% variance, factor 2–17% variance, factor 3–7% variance | ||
|
n = 935 (n = 491 6mo, n = 444 1 y) Age(y): 71(10); Time post-stroke: 6 mo/1 y |
CV: Hypotheses testing | Correlation between FAI and BI: r = 0.60–0.65, p < 0.01 Correlation between FAI and Wakefield Depression: r = −0.35 − (−0.37), p < 0.01 |
||
|
n = 383 Sex: 200 (52%) men Age(y):71(10); Time post-stroke: 1 y |
Responsiveness | Responsiveness of FAI to detect change from six months and one year post stroke. The average (SD) increase in FAI between the two-time points was 1.26(6.1) | ||
| Frenchay Activities Index - Chinese version (FAI-C) | Imam and Miller, 201224 | Chinese community in Vancouver/Canada n = 66 Sex: 19(29%) men; Age(y): 79(9) Time post-stroke(y): 22(10) |
Test–retest reliability | ICC = 0.86, 95% CI = 0.79–0.92 |
| CV: Hypotheses testing | Correlation between FAI-C and RNL: r = 0.61, p < 0.01 Correlation between FAI-C and ABC: r = 0.55, p < 0.01 Correlation between FAI-C and TUG: r = −0.68, p < 0.001 |
|||
| Human Activity Profile (HAP) | Teixeira-Salmela et al., 200733 |
n = 24 Sex: 13(54%) men Age(y): 64(12) Time post-stroke(y): 2(2) |
Criterion validity | MAS between subject HAP and observed performance: r = 0.95, p < 0.01 MAS between proxy HAP and observed performance: r = 0.80, p < 0.01 AAS between subject HAP and observed performance: r = 0.99, p < 0.01 AAS between proxy HAP and observed performance: r = 0.87, p < 0.01 |
| Multimedia Activity Recall for Children and Adults (MARCA) |
English, 201622 | Meulborne/Australia n = 40 (validity: n = 36, reliability: n = 30) Sex: 26(65%) men; Age(y): 67(11) Time post-stroke(y): 4(10) Type of stroke: ischemic 29(73%) Severity of stroke: mild 34(85%) |
Test–retest reliability | ICC = 0.83, 95%CI = 0.68–0.92 for total score ICC = 0.95, 95%CI = 0.89–0.97 for superdomains |
| CV: Hypotheses testing | Total sitting time (min/d) between MARCA and activPAL3 activity monitor: ICC = 0.67, 95%CI = 0.38–0.84 Total daily energy expenditure (Kj/d) between MARCA and Sensewear armband: ICC = 0.62, 95%CI = 0.32–0.80 |
|||
| Nottingham Leisure Questionnaire (short version) | Drummond et al., 200121 | Nottingham/United Kingdon n = 121 Time post-stroke(y): 1 |
Test–retest reliability | K = 0.44–0.94; Bland and Altman: difference −0.25(3.23), 95% limits of agreement 6.21 − (−6.71) |
| Nottingham Leisure Questionnaire |
Drummond and Walker, 199420 | Nottingham/United Kingdon n = 20 Sex: 11(55%) men; Age(y): 73(9) Time post-stroke(d): 654(178) |
Inter-rater reliability | K = 0.65–1.0 |
|
n = 21 Sex: 12(57%) men; Age(y): 73(9) Time post-stroke(d): 477(50) |
Test–retest reliability | K = 0.23–1.0 |
CV, construct validity; BI, Barthel Index; NEADL, Nottingham Extended Activities of Daily Living; SIS, Stroke Impact Scale; MAL, Motor Activity Log; RNL, Reintegration to Normal Living Scale; ABC, Activities-specific Balance Confidence Scale; TUG, Timed Up and Go; SIP, Sckiness Impact Profile; NIHSS, National Institutes of Health Stroke Scale; ComQOL, Comprehensive Quality of Life Scale; SF-36, 36-item Short-Form Medical Outcomes Study; MAS, Maximum Activity Score; AAS, Ajusted Activity Score; SEM, Standard Error of Measurement; SRD, Smallest Real Difference; MNSQ, Mean Squares; SRM, Standardized Response Mean.
Assessment of the methodological quality using the COSMIN taxonomy
The methodological quality of the included studies ranged from “poor” to “good”, based upon the COSMIN scores (Table 3). Three studies investigated internal consistency: two showed “poor”, ACS19 and FAI,32 and one “good”, FAI,25 methodological quality. Reliability was analyzed in 11 studies: three showed “poor”, FAI23, 36 and Nottingham Leisure Questionnaire,20 five “fair”, FAI,27, 28, 29 MARCA22 and Nottingham Leisure Questionnaire,21 and three “good”, ACS19 and FAI,24, 26 methodological quality. Measurement error was described in one study with “good”, FAI,26 methodological quality. Validity was evaluated in 12 studies and methodological quality was rated as “poor” in six, ACS,34 Coded Activity Diary,35 FAI,27, 36 HAP,33 and Nottingham Leisure Questionnaire,21 “fair” in five, ACS,19 FAI,29, 35, 36 and MARCA,22 and “good” in two studies, FAI.24, 31 The most investigated type was construct validity (hypotheses testing) in eight studies, ACS,19, 34 FAI,24, 27, 32, 36 MARCA,22 and Nottingham Leisure Questionnaire.21 Responsiveness was analyzed in three studies and showed “fair” methodological quality, FAI.31, 36, 37 Content validity was never investigated by any of the included studies (Table 3).
Table 3.
Methodological quality of the included studies using the COSMIN checklist14 (poor, fair, good, excellent) and quality rating of the results on measurement properties, based upon the Terwee's criteria15 (+, −, ?).
| Tool | Reference | Measurement properties |
||||||
|---|---|---|---|---|---|---|---|---|
| Reliability |
Validity |
Responsiveness | ||||||
| Internal consistency | Reliability |
Measurement error |
Construct Validity |
Criterion validity | ||||
| Structural validity | Hypotheseses testing | |||||||
| Activity Card Sort - Hong Kong Version (ACS-HK) | Chan et al., 200619 | Poor/? | Good/+ | NT | NT | Fair/? | NT | NT |
| Activity Card Sort (ACS) | Tucker et al., 201234 | NT | NT | NT | NT | Poor/+ | NT | NT |
| Coded activity diary | Vanroy et al., 201435 | NT | NT | NT | NT | NT | Poor/? | NT |
| Frenchay Activities Index (FAI) | Monteiro et al., 201727 | NT | Fair/+ | NT | NT | Poor/? | NT | NT |
| Sarker et al., 201230 | NT | NT | NT | NT | NT | Fair/? | NT | |
| Lu et al., 201226 | NT | Good/+ | Good/? | NT | NT | NT | NT | |
| Lin et al., 201225 | Good/+ | NT | NT | NT | NT | NT | NT | |
| Wu et al., 201137 | NT | NT | NT | NT | NT | Fair/? | Fair/? | |
| Schepers et al., 200631 | NT | NT | NT | NT | NT | NT | Fair/? | |
| Post et al., 200329 | NT | Fair/+ | NT | NT | NT | NT | NT | |
| Green et al., 200123 | NT | Poor/- | NT | NT | NT | NT | NT | |
| Piercy et al., 200028 | NT | Fair/- | NT | NT | NT | NT | NT | |
| Schuling et al., 199332 | Poor/? | NT | NT | NT | Good/+ | NT | NT | |
| Wade et al., 198536 | NT | Poor/- | NT | Fair/? | Poor/? | NT | Fair/? | |
| Frenchay Activities Index – Chinese version (FAI-C) | Imam and Miller, 201224 | NT | Good/+ | NT | NT | Good/+ | NT | NT |
| Human Activity Profile (HAP) | Teixeira-Salmela et al., 200733 | NT | NT | NT | NT | NT | Poor/+ | NT |
| Multimedia Activity Recall for Children and Adults (MARCA) | English, 201622 | NT | Fair/+ | NT | NT | Fair/? | NT | NT |
| Nottingham Leisure Questionnaire (short version) | Drummond et al., 200121 | NT | Fair/− | NT | NT | Poor/? | NT | NT |
| Nottingham leisure questionnaire | Drummond and Walker, 199420 | NT | Poor/− | NT | NT | NT | NT | NT |
NT, not tested; (+), positive; (−), negative; (?), doubtful.
Quality of the measurement property using the Terwee's criteria
Based upon the Terwee et al.15 criteria, 15 studies were classified as doubtful/indeterminate (Table 3): two studies on internal consistency, ACS19 and FAI,32 one on measurement error, FAI,26 eight on validity, ACS,19 Coded activity diary,35 FAI,27, 30, 36, 37 MARCA,22 and Nottingham Leisure Questionnaire,21 and three on responsiveness, FAI.31, 36, 37 Positive ratings were reported by the following 11 studies: one on internal consistency, FAI25 (Cronbach's α = 0.73–0.81); six on reliability, ACS,19 FAI,24, 26, 27, 29 and MARCA22 (intra-class correlation coefficients (ICCs) ranging from 0.83 to 0.98); and four on validity, ACS,34 FAI,24, 32 and HAP33 (at least 75% of the results were in accordance with the established hypotheses or Pearson correlation coefficients (r) ranged from 0.80 to 0.99 (criterion validity). Only five studies were classified as negative on reliability, FAI23, 28, 36 and Nottingham Leisure Questionnaire20, 21 and the reason for this was that the Kappa coefficients were below 0.7 for some items of the evaluated tools (Table 3).
Clinical utility
Table 4 reports the clinical utility (feasibility) of the measures included in this review. Most of the tools are simple ‘paper and pencil’ tests, which are freely available and, thus, scored high on the clinical utility criteria for cost, portability, and need of specialized equipment.18 When information was not found (unknown), the item scored zero, as previously adopted.38 The FAI and the HAP showed the highest clinical utility scores (Table 4).
Table 4.
Clinical utility of self-report physical activity assessment tools for subjects with stroke.
| Instrument | Application time | Cost | Specialized equipment/training | Portability | Accessibility | Total Score |
|---|---|---|---|---|---|---|
| Activity Card Sort (ACS): original and Hong-Kong versions | 2 | 2 | 1 | 2 | 2 | 9 |
| Coded activity diary | 0 | 3 | 2 | 2 | 2 | 9 |
| Frenchay Activities Index (FAI): original and Chinese versions | 3 | 3 | 2 | 2 | 2 | 12 |
| Human Activity Profile (HAP) | 2 | 3 | 2 | 2 | 1 | 10 |
| Multimedia Activity Recall for Children and Adults (MARCA) | 2 | 0 | 1 | 1 | 0 | 4 |
| Nottingham Leisure Questionnaire: original and short versions | 0 | 3 | 2 | 2 | 2 | 9 |
Discussion
This review is the first to systematically appraise and summarize the evidence on the measurement properties and clinical utility of self-report physical activity assessment tools for individuals with stroke, taking the methodological quality of the included studies into account. Six self-report physical activity assessment tools were evaluated and their methodological quality ranged from “poor” to “good”. The majority of the results regarding the quality of the measurement properties were considered doubtful. The most investigated properties were reliability and construct validity. Content validity was never investigated by any of the studies included in this review. The FAI and the HAP showed the highest clinical utility scores.
Two systematic reviews3, 7 with healthy adults described the International Physical Activity Questionnaire (IPAQ) as the most often used and validated self-report physical activity assessment tool. However, in the present review, none of the included studies investigated the measurement properties of the IPAQ in subjects with stroke. Only one self-report physical activity assessment tool included in the previous reviews3, 7 was assessed in this present review: the HAP. This indicates that despite the high number of available self-report physical activity tools, only few had their measurement properties investigated for the stroke population.
Methodological flaws were identified in the majority of the studies which investigated internal consistency. It is recommended that internal consistency be assessed in two ways: through the classic approach (Cronbach's alpha coefficients) or by the item response theory (Rasch mathematical model).13, 14, 15 The majority of the studies that investigated internal consistency did not apply factor analysis to assess unidimensionality, which is the most recommended method to verify the number of dimensions into which the items are distributed.13, 14, 15
Reliability was the most investigated measurement property and six studies20, 21, 23, 27, 28, 36showed negative results regarding the quality of this measurement property (ICC or weighted Kappa <0.70). The methodological quality of all these studies ranged from “poor” to “fair”, because of the small sample size (<50 participants). One study, published in 1985,36 was rated as “poor” because it employed Pearson correlation coefficients for analysis. Nowadays, there is a consensus that the recommended statistical tests for reliability are ICC and Kappa.13, 14
The main deficiency regarding the analysis of the reliability domain was the lack of examination of measurement error, which was only reported in one study.26 Measurement error method offer an approach to quantitatively estimate the magnitude of the various sources of errors, which may influence the results.13, 14 The low quality rating on the measurement error of the study26 was due to the lack of information regarding the smallest detectable change or minimal important change.
Construct validity was mostly investigated by hypothesis testing. For assessing this domain, it is important to formulate specific hypotheses.15 However, only two studies24, 32 formulated such hypotheses. The methodological quality of these studies was rated as “good” and positive results on the measurement properties were found.24, 32
The methodological quality of the four studies30, 33, 35, 37 which investigated criterion-related validity ranged from “poor” to “fair” and doubtful results were found in three.30, 35, 37 These results were justified by the small sample (<50 participants) and the difficulty to establish an adequate gold standard tool for the assessment of physical activity levels. Only one study33 showed convincing arguments regarding the gold-standard measure, but had insufficient sample.
There were not found any studies which investigated the content validity of self-report physical activity tools for stroke subjects. When a measurement tool has adequate content validity, it means that its items cover the entire universe of interest, reflect the relative importance of each part of this universe, and is free from factors that are irrelevant to the purpose of the measure.8, 15 Content validity assessment is an important step in the process of development and investigation of measurement properties of a tool. The confusion between content and face validities, as well as the lack of knowledge regarding the systematic methods already available for content validity investigation, have been pointed-out as the main factors related to the absence of information on content validity for the majority of the instruments used in the rehabilitation field.39, 40, 41
Only if the content validity of a questionnaire is adequate, one will consider using it and the investigation of other measurement properties is useful.39, 40, 41 Faced with this, the question is whether the instruments included in this review are actually considered measures of physical activity. The FAI, for example, is an instrument that contains some items related to physical activity and others related to the performance of activities of daily living. This demonstrates a certain conflict with the physical activity terminology, which was already pointed out by previous reviews.3, 7 This demonstrates that the scientific literature needs to establish clearer criteria on what is a measure of physical activity, thus avoiding conflicts with the use of the terminology and facilitating the use of appropriate instruments.
Only three studies31, 36, 37 assessed the responsiveness of self-report physical activity tools in subjects with stroke. These studies had “fair” methodological quality and doubtful results on the measurement properties, due to the lack of reports on the smallest detectable change or minimal important change.15 Treatment effects cannot be detected if a self-report tool shows poor responsiveness.7, 9 A systematic review42 which investigated the efficacy of interventions to increase physical activity levels after stroke reported the lack of self-report tools that had their responsiveness investigated for subjects with stroke.
The majority of the tools included in the present review did not meet all the criteria to be feasible for use in clinical practice. These results were due to lack of information on the duration20, 21, 35 and cost22 or because some of the tools are not freely accessible for the clinicians,19, 34 or require specialized and not portable equipment.22 Both clinical utility and measurement properties should be considered when selecting the most appropriate instrument for use in clinical practice.
Limitations
It is possible that some relevant papers were not retrieved by the electronic literature search, because of the variability in terminology regarding physical activity. However, a manual search in all of the references cited by the retrieved studies was performed in an attempt to avoid loss of information. The pre-established criteria to select self-report measures of physical activity levels based upon the authors statements, may have included instruments that do not provide real measures of physical activity. Therefore, it is necessary that future studies investigate the content validity of these instruments, based upon a clear definition of what physical activity is and follow systematical and rigorous process to correctly investigate this important measurement property.
Conclusion
The present systematic review highlights the paucity of studies that investigated the measurement properties of self-report physical activity assessment tools in subjects with stroke. Important measurement properties, such as content validity, need to be further addressed by well-designed studies, to determine if the instruments actually measure physical activity. The majority of the tools did not meet all the criteria to be feasible for use in clinical practice. Further high-methodological quality studies on self-report physical activity assessment tools in post-stroke subjects are required to assist clinicians and researchers in choosing the best instrument to measure physical activity levels.
Study organization and funding
Financial support for this research was provided by national funding agencies: Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Pró-reitoria de Pesquisa da Universidade Federal de Minas Gerais (PRPq/UFMG). This financial support includes scholarships and research grants. These agencies are not involved in any other aspect of this study.
Authors’ contributions
All authors contributed to the conception/design of the study and provided final approval of the version to be published.
Funding sources
This work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Pró-reitoria de Pesquisa da Universidade Federal de Minas Gerais (PRPq/UFMG).
Conflicts of interest
The authors declare that they have no competing interests.
References
- 1.WHO Guidelines Approved by the Guidelines Review Committee . World Health Organization; Geneva: 2010. Global Recommendations on Physical Activity for Health. [PubMed] [Google Scholar]
- 2.Gallanagh S., Quinn T.J., Alexander J. Physical activity in the prevention and treatment of stroke. ISRN Neurol. 2011;2011:953818. doi: 10.5402/2011/953818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Poppel M.N., Chinapaw M.J., Mokkink L.B. Physical activity questionnaires for adults: a systematic review of measurement properties. Sports Med. 2010;40:565–600. doi: 10.2165/11531930-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 4.Caspersen C.J., Powell K.E., Christenson G.M. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100:126–131. [PMC free article] [PubMed] [Google Scholar]
- 5.Ainsworth B., Cahalin L., Buman M. The current state of physical activity assessment tools. Prog Cardiovas Dis. 2015;57:387–395. doi: 10.1016/j.pcad.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Prince S.A., Adamo K.B., Hamel M.E. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5:56. doi: 10.1186/1479-5868-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silsbury Z., Goldsmith R., Rushton A. Systematic review of the measurement properties of self-report physical activity questionnaires in healthy adult populations. BMJ Open. 2015;5:e008430. doi: 10.1136/bmjopen-2015-008430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mokkin L.B., Prinsen C.A., Bouter L.M. The COnsensus-bases Standards fot the selection of health Measurement INstruments (COSMIN) and how to select an outcome measurement instrument. Braz J Phys Ther. 2016;20:105–113. doi: 10.1590/bjpt-rbf.2014.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frei A., Williams K., Vetsch A. A comprehensive systematic review of the development process of 104 patient-reported outcomes (PROs) for physical activity in chronically ill and elderly people. Health Qual Life Outcomes. 2011;9:116. doi: 10.1186/1477-7525-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fini N.A., Holland A.E., Keating J. How is physical activity monitored in people following stroke? Disabil Rehabil. 2015;37:1717–1731. doi: 10.3109/09638288.2014.978508. [DOI] [PubMed] [Google Scholar]
- 11.Moher D., Liberati A., Tetzlaff J. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martins J.C., Aguiar L.T., Nadeau S. Measurement properties of self-report physical activity assessment tools in stroke: a protocol for a systematic review. BMJ Open. 2017;7:e012655. doi: 10.1136/bmjopen-2016-012655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mokkink L.B., Terwee C.B., Patrick D.L. The COSMIN study reached international consensus on taxonomy, terminology, and definitions of measurement properties for health-related patient-reported outcomes. J Clin Epidemiol. 2010;63:737–745. doi: 10.1016/j.jclinepi.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Terwee C.B., Mokkink L.B., Knol D.L. Rating the methodological quality in systematic reviews of studies on measurement properties: a scoring system for the COSMIN checklist. Qual Life Res. 2011;21:651–657. doi: 10.1007/s11136-011-9960-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terwee C.B., Bot S.D., de Boer M.R. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007;60:34–42. doi: 10.1016/j.jclinepi.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Lima E., Teixeira-Salmela L.F., Simões L. Assessment of the measurement properties of the post-stroke motor function instruments available in Brazil: a systematic review. Braz J Phys Ther. 2016;20:114–125. doi: 10.1590/bjpt-rbf.2014.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tyson S.F., Watson A., Moss S. Development of an evidence based framework for the physiotherapy assessment of neurological conditions. Disabil Rehabil. 2007;30:142–144. doi: 10.1080/09638280701216847. [DOI] [PubMed] [Google Scholar]
- 18.Tyson S.F., Brown P. How to measure fatigue in neurological conditions? A systematic review of psychometric properties and clinical utility of measures used so far. Clin Rehabil. 2014;28:804–816. doi: 10.1177/0269215514521043. [DOI] [PubMed] [Google Scholar]
- 19.Chan V.W.K., Chung J.C.C., Packer T.L. Validity and reliability of the activity card sort-Hong Kong version. OTJR (Thorofane N J) 2006;26:152–158. [Google Scholar]
- 20.Drummond A.E.R., Walker M.F. The Nottingham leisure questionnaire for stroke patients. Br J Occup Ther. 1994;57:414–418. [Google Scholar]
- 21.Drummond A.E.R., Parker C.J., Gladman J.R. Development and validation of the Nottingham Leisure Questionnaire (NLQ) Clin Rehabil. 2001;15:647–656. doi: 10.1191/0269215501cr438oa. [DOI] [PubMed] [Google Scholar]
- 22.English C., Healy G.N., Coates A. Sitting and activity time in people with stroke. Phys Ther. 2016;96:193–201. doi: 10.2522/ptj.20140522. [DOI] [PubMed] [Google Scholar]
- 23.Green J., Forster A., Young J. A test–retest reliability study of the Barthel index, the rivermead mobility index, the Nottingham extended activities of daily living scale and the Frenchay activities index in stroke patients. Disabil Rehabil. 2001;23:670–676. doi: 10.1080/09638280110045382. [DOI] [PubMed] [Google Scholar]
- 24.Imam B., Miller W.C. Reliability and validity of scores of a Chinese version of the Frenchay Activities Index. Arch Phys Med Rehabil. 2012;93:520–526. doi: 10.1016/j.apmr.2011.07.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin K.C., Chen H.F., Wu C.Y. Multidimensional Rasch validation of the Frenchay Activities Index in stroke patients receiving rehabilitation. J Rehabil Med. 2012;44:58–64. doi: 10.2340/16501977-0911. [DOI] [PubMed] [Google Scholar]
- 26.Lu W.S., Chen C.C., Huang S.L. Smallest real difference of 2 instrumental activities of daily living measures in patients with chronic stroke. Arch Phys Med Rehabil. 2012;93:1097–1100. doi: 10.1016/j.apmr.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 27.Monteiro M., Maso I., Sasaki A.C., Barreto-Neto N., Oliveira-Filho J., Pinto E.B. Validation of the Frenchay activity index on stroke victims. Arq Neuropsiquiatr. 2017;75:167–171. doi: 10.1590/0004-282X20170014. [DOI] [PubMed] [Google Scholar]
- 28.Piercy M., Carter J., Mant J. Inter-rater reliability of the Frenchay activities index in patients with stroke and their careers. Clin Rehabil. 2000;14:433–440. doi: 10.1191/0269215500cr327oa. [DOI] [PubMed] [Google Scholar]
- 29.Post M.W.M., de Witte L.P. Good inter-rater reliability of the Frenchay Activities Index in stroke patients. Clin Rehabil. 2003;17(5):548–552. doi: 10.1191/0269215503cr648oa. [DOI] [PubMed] [Google Scholar]
- 30.Sarker S.J., Rudd A.G., Douiri A. Comparison of 2 extended activities of daily living scales with the Barthel Index and predictors of their outcomes: cohort study within the South London Stroke Register (SLSR) Stroke. 2012;43:1362–1369. doi: 10.1161/STROKEAHA.111.645234. [DOI] [PubMed] [Google Scholar]
- 31.Schepers V.P., Ketelaar M., Visser-Meily J.M. Responsiveness of functional health status measures frequently used in stroke research. Disabil Rehabil. 2006;28:1035–1040. doi: 10.1080/09638280500494694. [DOI] [PubMed] [Google Scholar]
- 32.Schuling J., de Haan R., Limburg M. The Frenchay activities index. Assessment of functional status in stroke patients. Stroke. 1993;24:1173–1177. doi: 10.1161/01.str.24.8.1173. [DOI] [PubMed] [Google Scholar]
- 33.Teixeira-Salmela L.F., Devaraj R., Olney S.J. Validation of the human activity profile in stroke: a comparison of observed, proxy and self-reported scores. Disabil Rehabil. 2007;29:1518–1524. doi: 10.1080/09638280601055733. [DOI] [PubMed] [Google Scholar]
- 34.Tucker F.M., Edwards D.F., Mathews L.K. Modifying health outcome measures for people with aphasia. Am J Occup Ther. 2012;66:42–50. doi: 10.5014/ajot.2012.001255. [DOI] [PubMed] [Google Scholar]
- 35.Vanroy C., Vanlandewijck Y., Cras P. Is a coded physical activity diary valid for assessing physical activity level and energy expenditure in stroke patients? PLOS ONE. 2014;9:e98735. doi: 10.1371/journal.pone.0098735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wade D.T., Legh-Smith J., Langton H.R. Social activities after stroke: measurement and natural history using the Frenchay Activities Index. Int Rehabil Med. 1985;7:176–181. doi: 10.3109/03790798509165991. [DOI] [PubMed] [Google Scholar]
- 37.Wu C.Y., Chuang L.L., Lin K.C. Responsiveness and validity of two outcome measures of instrumental activities of daily living in stroke survivors receiving rehabilitative therapies. Clin Rehabil. 2011;25:175–183. doi: 10.1177/0269215510385482. [DOI] [PubMed] [Google Scholar]
- 38.Conell L.A., Tyson S.F. Clinical reality of measuring upper-limb ability in neurologic conditions: a systematic review. Arch Phys Med Rehabil. 2012;93:221–228. doi: 10.1016/j.apmr.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 39.Benson J., Clark F. A guide for instrument development and validation. Am J Occup Ther. 1982;36:789–800. doi: 10.5014/ajot.36.12.789. [DOI] [PubMed] [Google Scholar]
- 40.Lynn M.R. Determination and quantification of content validity. Nurs Res. 1986;35:382–385. [PubMed] [Google Scholar]
- 41.Polit D.F., Beck C.T., Owen S.V. Is the CVI an acceptable indicator of content validity? Appraisal and recommendations. Res Nurs Health. 2007;30:459–467. doi: 10.1002/nur.20199. [DOI] [PubMed] [Google Scholar]
- 42.Aguiar L.T., Nadeau S., Martins J.C., Teixeira-Salmela L.F., Britto R.R., Faria C.D.C.M. Efficacy of interventions aimed at improving physical activity in individuals with stroke: a systematic review. Disabil Rehabil. 2018;19:1–16. doi: 10.1080/09638288.2018.1511755. [DOI] [PubMed] [Google Scholar]
- 43.Katz N., Karpin H., Lak A. Participation in occupational performance: reliability and validity of the Activity Card Sort. OTJR. 2003;23:10–17. [Google Scholar]
- 44.Holbrook M., Skilbeck C.E. An activities index for use with stroke patients. Age Ageing. 1983;12:166–170. doi: 10.1093/ageing/12.2.166. [DOI] [PubMed] [Google Scholar]
- 45.Martinsson L., Eksborg S. Activity Index – a complementary ADL scale to the Barthel Index in the acute stage in patients with severe stroke. Cerebrovasc Dis. 2006;22:231–239. doi: 10.1159/000094009. [DOI] [PubMed] [Google Scholar]
- 46.Fix A.J., Daughton D.M. 1988. Human Activity Profile: Professional Manual. Odessa. [Google Scholar]
- 47.Gomersall S.R., Olds T.S., Ridley K. Development and evaluation of an adult use-of-time instrument with an energy expenditure focus. J Sci Med Sport. 2011;14:143–148. doi: 10.1016/j.jsams.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 48.Drummond A.E.R. Leisure activity after stroke. Inter Disabil Stud. 1990;12:157–160. doi: 10.3109/03790799009166608. [DOI] [PubMed] [Google Scholar]