Abstract
Objective:
There are still contradictory opinions on the success rates of uterine artery embolization (UAE) for the treatment of myomas. In this scenario, our study aims to assess the effect of UAE on myoma shrinkage.
Materials and Methods:
The study included 337 women in reproductive age affected by a single symptomatic intramural myoma and declined surgery, undergoing UAE. The uterus and myoma diameters and volumes were determined on ultrasonographic scans before and 3, 6, and 12 months after the procedure.
Results:
The mean uterine volume before intervention was 226.46 ± 307.67 mm3, whereas myoma volume was 51.53 ± 65.53 mm3. Further myoma progression was registered in only four patients. In remaining women, uterus volume in average decreased for 149.99 ± 156.63 mm3, whereas myomas decreased for 36.57 ± 47.96 mm3. The mean volume reduction rate of the uterus was 49.54 ± 35.62 and for myoma was 57.58 ± 30.71. A significant decrease in both uterine and myoma volume was registered in every stage of the follow-up. The highest average decrease in uterine volume was in the first 3 months and myoma volume between 3 and 6 months following UAE. After 12 months follow-up, successful outcome (volume regression >50% respect to the baseline) was registered for uterus in 97.4% and for myoma in 67.9% of investigated patients.
Conclusion:
UAE was proven to allow a good success rate and can be considered as an effective alternative procedure for myoma treatment.
Keywords: Myoma, outcome, uterine artery embolization, volume reduction
INTRODUCTION
Uterine fibroids are the most common benign tumors in females with the incidence ranging from 20% to 25%.[1,2,3] The clinical manifestations of uterine fibroids mainly include menorrhagia and/or metrorrhagia which can lead to anemia, pain (dysmenorrhea, dyspareunia, and low back pain), issues with urinary function (frequency or obstruction), and constipation due to pressure on surrounding structures.[4,5] Uterine fibroids have also been associated with infertility even in asymptomatic women,[6] and their enlargement is dependent, at least in part, by hormonal influence.[7,8]
Till date, surgery represents the gold standard treatments for several benign[9,10,11] and oncological[12,13,14] conditions. In this scenario, symptomatic uterine fibroids larger than 5 cm in diameter are mainly treated with surgical therapy (myomectomy or even hysterectomy).[15] Usually, the hysteroscopic approach is recommended for submucosal myomas.[16,17,18] However, these invasive procedures carry a potential risk of complications and side effects, such as postoperative bleeding or infection. In addition, the recovery period, even after uncomplicated surgery, is measured in weeks.[19]
Uterine artery embolization (UAE) is a relatively new minimally invasive uterine preserving procedure that offers an alternative to the traditional surgical removal of the uterine fibroids.[15,20] The procedure is based on fibroids blood supply reduction, which consequently causes the myoma shrinkage. However, there are still contradictory opinions on the success rates of UAE, particularly regarding long-term outcomes.[19,20] This can be explained by the fact that in different studies outcome measures are unstandardized and/or subjective such as symptoms and quality of life.
Therefore, the study aim was to assess the UAE outcome (success rate and time needed for myoma shrinkage). For the purpose of this study, only objective ultrasonographic measurements of myoma and uterus size and volume were evaluated as the UAE outcome.
MATERIALS AND METHODS
All women in reproductive age who came to our clinic due to symptomatic uterine myomas in 5 years (2012–2016) and met inclusion criteria were consecutively enrolled in the study. The study was approved by the Ethics Committee of National Research Center of Mother and Child Health (approved on August 17, 2009, number 0111RK-00326). All patients signed written informed consent, after being extensively counseled on the potential risks and benefits of the procedure, and on possible alternative treatments.
On admission to the clinic, all patients had detailed history taken and thorough clinical gynecological examination. Uterine fibroids were diagnosed through bimanual, transvaginal and abdominal ultrasound (US), and other auxiliary imaging techniques (color Doppler; magnetic resonance imaging [MRI]), if needed. The localization of investigated myomas was precisely determined ultrasonographically. Finally, both uterus and myoma dimensions were measured on US/magnetic resonance (MR) scan, and these findings were used to calculate the volume (V) of the uterus and the myoma (volume = 0.5233 × longitudinal dimension D1 × anteroposterior dimension D2 × transverse dimension D3).
The study inclusion criteria were reproductive age (18–50 years), presence of a single intramural (FIGO PLAM-COEIN Types 3 and 4) symptomatic uterine myoma, and refuse of hysterectomy or myomectomy. Women were not included in the study if they were currently pregnant, had symptoms/signs of pelvic inflammatory disease, abnormal coagulation status, abnormal endometrial biopsy findings, abnormal Pap test, any malignancy, history of severe chronic disease or unstable cardiac status, severe anemia and presence of cervical, and broad ligament, pedunculated subserosal or submucosal myomas.
UAE procedure was performed by two physicians 5–7 days after menstruation to reduce the risk of extensive hemorrhage. Before UAE, prophylactic antibiotic and local anesthesia were administered to all women. Then, the patients were placed in the lithotomic position and a small angiographic catheter was introduced into the femoral artery and guided into the uterine artery. This procedure was done either unilaterally (in case that myoma was laterally positioned on the myoma side of the uterus) or bilaterally. Superselective arteriography was performed with injection of 5–10 ml on contrast 1.0–1.5 ml/second. After secure placement of the catheter in the uterine arteries (under the beginning of the vaginal arteries), the embolizing drug was injected into the uterine artery from which it was taken by the blood flow to small arteries supplying fibroids, blocking them, and causing ischemic necrosis. The injection of embolizing drug was stopped after achieving stasis of contrast medium in the proximal parts of the uterine arteries, confirmed by the control superselective subtractional arteriography. In this study, both embolizing drugs currently in commercial use were randomly applied (Trisacryl gelatin microspheres 500–700 mkn in 1/3 of cases and polyvinyl alcohol – PVA 355–100 mkp in 2/3 of women). After the uterine, arteries were embolized, the catheter was removed, aseptic pressure bandage over the wound was placed, and the patient underwent standard postarteriographic monitoring and recovery.
US scans of the uterus and myoma were performed just before the UAE (baseline) and 3, 6, and 12 months after the procedure. Considering that, myoma volume is much more accurate for tumor overall mass evaluation than its largest diameter, the main outcome measure in this study was the change in myoma volume. The uterus and myoma volumes were determined every 3 months follow-up, and the volume change was calculated as the exact number by deducting the obtained volume from initial as well as volume on the previous examination (3 months before). Subsequently, volume change was classified as decreased (good outcome) versus no change or increase (adverse outcome). Moreover, uterus and myoma shrinkage was categorized as not adequate or adequate (successuful outcome) in consideration of the decrease percentage (less or more than 50%). Finally, we calculated the uterine and myoma volume reduction rates according to the formula: (initial volume – final volume)/initial volume × 100.
Data obtained before UAE and in all follow-up periods were compared and statistically analyzed by descriptive (minimum, maximum, mean, standard deviation, number, and percentage) and analytical statistics, using the IBM SPSS Statistics for Windows, Version 20.0. (IBM Corp., Armonk, NY). Chi-square test was used to investigate the differences in patients' outcome after a 1-year follow-up period. T-test and repeated measure ANOVA modeling were applied to assess the significance of the change in uterine and myoma volumes over follow-up periods.
RESULTS
The study included 337 patients with myomas, with a mean age of 42.71 ± 5.76 years. MRI scan was performed in 45 (13.3%) of these women, to have a better definition of location and size of the myomas. Investigated women had no previous pregnancies in 16.3%, while more than one quarter (28%) of women desired future pregnancy. Their main symptoms before UAE were menorrhagia (67.3%), bulk-like symptoms (25.4%), and pelvic pain (12.5%). Descriptive data of investigated myomas are reported in Table 1, whereas the outcome after 1-year follow-up is reported in Table 2. Figures 1 and 2 show the change of uterine and myoma volume, respectively.
Table 1.
Descriptive characteristics of investigated patients and myomas
| Parameters | Minimum | Maximum | Mean±SD |
|---|---|---|---|
| UV before UAE | 24.50 | 3780.00 | 226.46±307.67 |
| UV 3 months | 10.23 | 673.90 | 119.33±114.91 |
| UV 6 months | 8.52 | 294.90 | 123.69±65.71 |
| UV 12 months | 18.91 | 253.40 | 110.69±57.92 |
| UV 12 months change | +44.51 | -680.70 | -149.99±156.63 |
| UV difference 0-3 months | 23.31 | 543.91 | 92.63±113.74 |
| UV difference 3-6 months | 10.17 | 82.31 | 21.38±34.68 |
| UV difference 6-12 months | 9.55 | 91.21 | 17.06±32.72 |
| UV reduction rate | −132.69 | 85.64 | 49.54±35.62 |
| MV before UAE | 15.81 | 398.02 | 51.53±65.53 |
| MV 3 months | 10.21 | 382.10 | 46.41±63.93 |
| MV 6 months | 3.01 | 243.70 | 34.95±45.08 |
| MV 12 months | 0.88 | 128.70 | 24.89±27.98 |
| MV 12 months change | +10.76 | -283.42 | -36.57±47.96 |
| MV reduction rate | -103.76 | 100.00 | 57.58±30.71 |
| MV difference 0-3 months | 3.40 | 28.61 | 5.13±3.25 |
| MV difference 3-6 months | 5.13 | 138.41 | 16.81±27.77 |
| MV difference 6-12 months | 2.89 | 129.11 | 12.02±21.89 |
Difference: In (mm3) regardless of change direction. UV: Uterine volume (mm3), MV: Myoma volume (mm3), UAE: Uterine artery embolization, SD: Standard deviation
Table 2.
Uterine artery embolization outcome after a 1-year follow-up period
| Parameters | Frequency (%) | χ2 | P |
|---|---|---|---|
| Outcome UV 12 months (%) | |||
| Bad - <50 | 9 (2.7) | 34.105 | 0.001 |
| Good - >50 | 328 (97.3) | ||
| Outcome MV 12 months (%) | |||
| Bad - <50 | 108 (32.0) | 6.811 | 0.001 |
| Good - >50 | 229 (68.0) | ||
| Uterine decrease | |||
| No | 2 (0.56) | 85.683 | 0.001 |
| Yes | 335 (99.4) | ||
| Myoma decrease | |||
| No | 4 (1.2) | 67.213 | 0.001 |
| Yes | 333 (98.8) |
UV: Uterine volume (mm3), MV: Myoma volume (mm3)
Figure 1.
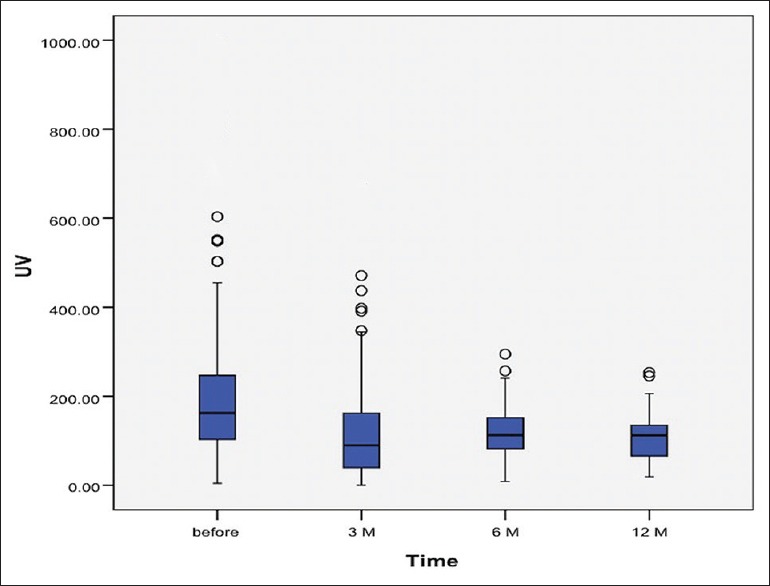
Change of uterine volume over follow-up period
Figure 2.
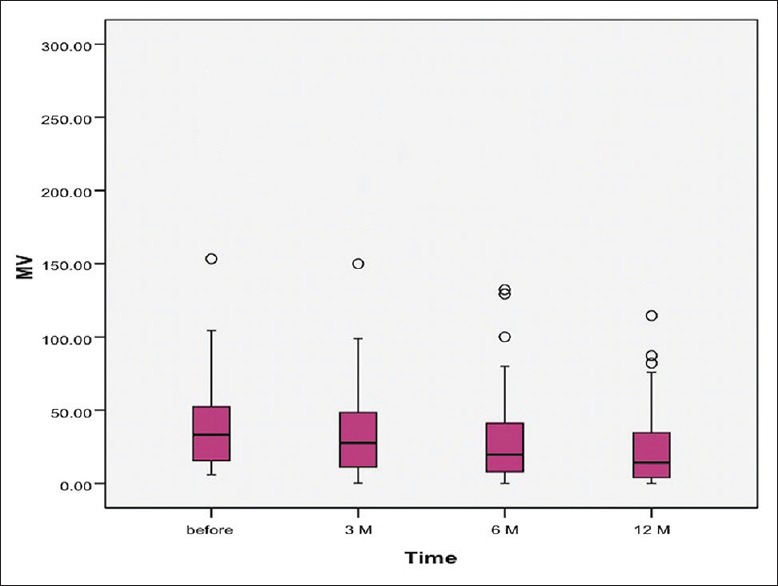
Change of myoma volume over follow-up period
The mean uterine volume before intervention was 226.46 ± 307.67 mm3, whereas myoma volume was 51.53 ± 65.53 mm3. There were just four cases of the totally noneffective procedure and further myoma progression. In addition, uterine volume increased in two patients regardless of the procedure. In remaining women, uterus in average decreased in volume for 149.99 ± 156.63 mm3, while myomas decreased for 36.57 ± 47.96 mm3. The mean uterine volume reduction rate was 49.54 ± 35.62, while myoma volume reduction rate was 57.58 ± 30.71 [Table 1]. A significant decrease in both uterine and myoma volume was registered in every stage of the follow-up (each 3 months) [Table 3]. Moreover, the highest average decrease in myoma volume was noted in the second follow-up period (between 3 and 6 months) after UAE. Conversely, the best reduction of uterine volume was registered in the first 3 months following UAE. In addition, a repeated measure ANOVA model was obtained for both uterine (Wilks Lambda = 0.528; F = 8.644; P = 0.001; Partial Eta2= 0.472; Observed power = 0.987) and myoma (Wilks Lambda = 0.338; F = 32.256; P = 0.001; Partial Eta2= 0.662; Observed power = 1.000) volumes. This multivariate analysis once again proved that differences in uterine and myoma volume between all follow-up periods were significant [Table 4]. Finally, after 12 months follow-up, successful outcome (volume regression >50% of initial) was registered for uterus in 97.4% and for myoma in 67,9% of investigated patients [Table 2].
Table 3.
Significance of differences in uterine and myoma volumes over follow-up periods
| Paired samples test | Mean | 95% CI of the difference | t | P | ||
|---|---|---|---|---|---|---|
| Pair 1 | UV before - UV 3 months | 92.62 | 60.63 | 124.61 | 5.816 | 0.001 |
| Pair 2 | UV before - UV 6 months | 125.84 | 80.91 | 170.78 | 5.679 | 0.001 |
| Pair 3 | UV before - UV 12 months | 149.99 | 98.51 | 201.48 | 5.903 | 0.001 |
| Pair 4 | UV 3 months - UV 6 months | 21.38 | 9.27 | 33.48 | 3.595 | 0.001 |
| Pair 5 | UV 3 months - UV 12 months | 37.60 | 20.79 | 54.41 | 4.557 | 0.001 |
| Pair 6 | UV 6 months - UV 12 months | 17.05 | 5.45 | 28.65 | 2.995 | 0.005 |
| Pair 1 | MV before - MV 3 months | 5.12 | 4.37 | 5.88 | 13.592 | 0.001 |
| Pair 2 | MV before - MV 6 months | 21.98 | 14.18 | 29.79 | 5.640 | 0.001 |
| Pair 3 | MV before - MV 12 months | 36.56 | 23.34 | 49.78 | 5.551 | 0.001 |
| Pair 4 | MV 3 months - MV 6 months | 16.79 | 9.49 | 24.11 | 4.606 | 0.001 |
| Pair 5 | MV 3 months - MV 12 months | 31.41 | 18.77 | 44.05 | 4.988 | 0.001 |
| Pair 6 | MV 6 months - MV 12 months | 12.46 | 6.46 | 18.45 | 4.168 | 0.001 |
UV: Uterine volume (mm3), MV: Myoma volume (mm3), CI: Confidence interval
Table 4.
Multivariate pairwise comparisons of uterine and myoma mean volume change in time
| Time | Mean difference | P | 95% CI for difference |
|
|---|---|---|---|---|
| Upper bound | Lower bound | |||
| Uterine volume | ||||
| Before | ||||
| 3 months | 95.394 | 0.001 | 36.591 | 154.197 |
| 6 months | 116.583 | 0.001 | 48.294 | 184.872 |
| 12 months | 133.257 | 0.001 | 60.046 | 206.468 |
| 3 months | ||||
| Before | −95.394 | 0.001 | −154.197 | −36.591 |
| 6 months | 21.189 | 0.011 | 3.637 | 38.741 |
| 12 months | 37.863 | 0.001 | 13.877 | 61.848 |
| 6 months | ||||
| Before | −116.583 | 0.001 | −184.872 | −48.294 |
| 3 months | −21.189 | 0.011 | −38.741 | −3.637 |
| 12 months | 16.674 | 0.047 | 0.151 | 33.197 |
| 12 months | ||||
| Before | −133.257 | 0.001 | −206.468 | −60.046 |
| 3 months | −37.863 | 0.001 | −61.848 | −13.877 |
| 6 months | −16.674 | 0.047 | −33.197 | −0.151 |
| Myoma volume | ||||
| Before | ||||
| 3 months | 5.199 | 0.001 | 3.797 | 6.600 |
| 6 months | 23.534 | 0.001 | 12.420 | 34.647 |
| 12 months | 36.156 | 0.001 | 18.406 | 53.905 |
| 3 months | ||||
| Before | −5.199 | 0.001 | −6.600 | −3.797 |
| 6 months | 18.335 | 0.001 | 7.968 | 28.702 |
| 12 months | 30.957 | 0.001 | 13.971 | 47.943 |
| 6 months | ||||
| Before | −23.534 | 0.001 | −34.647 | −12.420 |
| 3 months | −18.335 | 0.001 | −28.702 | −7.968 |
| 12 months | 12.622 | 0.001 | 4.295 | 20.949 |
| 12 months | ||||
| Before | −36.156 | 0.001 | −53.905 | −18.406 |
| 3 months | −30.957 | 0.001 | −47.943 | −13.971 |
| 6 months | −12.622 | 0.001 | −20.949 | −4.295 |
CI: Confidence interval
DISCUSSION
Many benign[21,22] and malignant[23,24] gynecological diseases can be effectively managed by surgical treatment, although several other approaches are currently available for uterine myomas. UAE is a minimally invasive angiographic procedure targeting myomas and blocking their arterial blood supply with different embolic agents causing their infarction and regression.[25,26] UAE is a rather safe and successful substitute for hysterectomy or myomectomy for symptomatic myomas. Moreover, UAE is used as a high-quality preoperative myoma treatment, since it can both reduce the size of myomas as well as their vascularization and bleeding during surgery.[26]
Available literature data showed that UAE had similar results to hysterectomy in quality of life improvement and reduction in bleeding, pain, and bulk symptoms.[27] UAE is better than surgical myoma treatment due to uterus preservation, avoidance of general anesthesia or surgery complications and low risk of blood loss or transfusion.[28] Other advantages of UAE compared with surgical treatment are a lower risk of postoperative infection and shorter recovery time after the procedure.[29]
Conversely, the most important complications are ischemic uterine injury, sepsis, pulmonary embolism, and even death, but all of these major complications are quite rare.[29,30,31] Moreover, potential negative effect of UAE can be reduction in ovarian function, mostly in older patients in which menopause can occur earlier than expected, or in women who already had ovarian function impairment.[26] Still, ovarian failure after UAE seems to occur with the same incidence as after myomectomy[29] and so far, there is no diagnostic test to predict which patients are prone to UAE complications. Reintervention rates range from 9% to 28% in 1–5 year period, whereas the need for hysterectomy after UAE is from 1% to 10%.[27,28] Reintervention mostly occurs in the first 2 years after initial UAE.[29] Nevertheless, systematic reviews and meta-analyses showed that while UAE had fewer complications than myomectomy in long term, both interventions showed similar improvement in the quality of life and satisfaction.[1,26]
Primary uterus preservation after UAE is achieved in almost all women in all available studies.[32] However, in the follow-up, hysterectomy had to be performed in 5%–30% of cases due to UAE unresponsiveness or repeated symptoms such as pain and bleeding that could not be controlled by other means.[33,34] Several studies have shown convincing results of the UAE in terms of reducing both myoma and uterine size.[26,34] Effects of UAE seem not only to be permanent but also progresses with time.[35] The reported success rate of UAE assessed as the decrease in the largest myoma volume ranges from only 23 to even >90%.[19] Available literature data show that 3 months after UAE the median percentage reduction of the dominant myoma volume ranged from only 3 up to even 93%, but on an average, it was around 50%.[35,36] After 6-month follow-up, a 40%–60% fibroid volume reduction was observed in most studies. In some prospective studies, 3 years after UAE total uterine volume was reduced significantly, on an average up to 65%.[4] After 1-year follow-up period, a reduction of up to 70% of the myoma volume has been found.[26] Literature data indicate that more than after UAE 40% of myomas disappear completely at long-term follow-up while overall uterine restoration rate was around 37%.[36]
Nevertheless, great variability in fibroid volume reduction after UAE is reported in different studies, which might be due to confounding factors, yet to be identified.[35] Some authors have suggested that the potential development of collateral circulation in the uterus may contribute to unsuccessful UAE and even regrowth of the myomas.[28] Reperfusion is mostly registered for cases of adenomyosis, while none of the necrotized myomas showed reperfusion at long term.[36,37] Moreover, the initial dominant fibroid volume was associated with the percentage of volume reduction after UAE. Smaller fibroids had variable volume reduction rates, while larger fibroids responded better to UAE treatment.[35] This can be explained by differences in myoma vascularization where small myomas are less and large more perfused than surrounding uterine walls.[38] Therefore, some authors recommend measuring diffusion and perfusion indices on MRI for monitoring UAE response.[39]
In this study, the mean volume reduction rate of investigated myomas was 57.58%. Moreover, uterine volume in average decreased for around 150 mm3. Only 1.2% of cases had unsuccessful UAE with further myoma growth. Conversely, the general outcome for women in this study was adequate with the reduction of both myoma and the whole uterine volume. This reduction was significant in any of the follow-up 3 months periods, and it progressed over time.
For women with myomas who plan further pregnancies, it is beneficial to minimize the number of incisions in the uterus, which is enabled by the UAE.[26,40] Literature data show that after UAE childbearing may not be impaired, which is proven by registered pregnancies in 14%–69% of infertility patients treated with UAE.[27,41] On the other hand, higher miscarriage rates, as well as abnormal placentation, have been reported after UAE making UAE the last therapeutically option in infertility patients.[30,42] Still, some investigations showed that after UAE pregnancy rates, as well as pregnancy complications, were comparable with age-adjusted rates for women with myomas who had no treatment for their condition.[39,42] In this scenario, further studies of fertility after UAE are still needed.[27]
CONCLUSION
The results of this study have proven that UAE has quite a good success rate (myoma 50% shrinkage in 68% of patients) and few complications (<4%). Therefore, it can be considered as a rather safe and effective alternative procedure for the treatment of uterine myomas. Furthermore, based on our results that the highest average decrease in myoma volume was noted between 3 and 6 months after UAE, it could be advised that patients should have a regular ultrasonographic control in 6 months from the procedure to adequately evaluate the UAE outcome.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Gupta JK, Sinha A, Lumsden MA, Hickey M. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev. 2014;12:CD005073. doi: 10.1002/14651858.CD005073.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Terzić M. Focused ultrasound for treatment of uterine myoma: From experimental model to clinical practice. Srp Arh Celok Lek. 2008;136:193–5. doi: 10.2298/sarh0804193t. [DOI] [PubMed] [Google Scholar]
- 3.Laganà AS, Vergara D, Favilli A, La Rosa VL, Tinelli A, Gerli S, et al. Epigenetic and genetic landscape of uterine leiomyomas: A current view over a common gynecological disease. Arch Gynecol Obstet. 2017;296:855–67. doi: 10.1007/s00404-017-4515-5. [DOI] [PubMed] [Google Scholar]
- 4.Narayan A, Lee AS, Kuo GP, Powe N, Kim HS. Uterine artery embolization versus abdominal myomectomy: A long-term clinical outcome comparison. J Vasc Interv Radiol. 2010;21:1011–7. doi: 10.1016/j.jvir.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Terzic M, Maricic S, Dotlic J. Vaginal removal of very large nascent uterine myoma – Case report and literature review. Geburtshilfe Frauenheilkd. 2013;73:724–6. doi: 10.1055/s-0032-1328724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terzic MM, Dotlic JR, Maricic SB, Babovic IR. Hydatidiform mole mimicking an enlarged uterine fibromyoma four months after ART. Centr Eur J Med. 2011;6:201–4. [Google Scholar]
- 7.Vitagliano A, Noventa M, Di Spiezio Sardo A, Saccone G, Gizzo S, Borgato S, et al. Uterine fibroid size modifications during pregnancy and puerperium: Evidence from the first systematic review of literature. Arch Gynecol Obstet. 2018;297:823–35. doi: 10.1007/s00404-017-4621-4. [DOI] [PubMed] [Google Scholar]
- 8.Vitale SG, Padula F, Gulino FA. Management of uterine fibroids in pregnancy: Recent trends. Curr Opin Obstet Gynecol. 2015;27:432–7. doi: 10.1097/GCO.0000000000000220. [DOI] [PubMed] [Google Scholar]
- 9.Wortman M, Daggett A, Ball C. Operative hysteroscopy in an office-based surgical setting: Review of patient safety and satisfaction in 414 cases. J Minim Invasive Gynecol. 2013;20:56–63. doi: 10.1016/j.jmig.2012.08.778. [DOI] [PubMed] [Google Scholar]
- 10.Abdusattarova K, Mettler L, Alkatout I, Dempfle A. Endoscopic treatment of symptomatic fibroids at reproductive age and beyond. Minim Invasive Ther Allied Technol. 2017;26:355–61. doi: 10.1080/13645706.2017.1312457. [DOI] [PubMed] [Google Scholar]
- 11.Vitale SG, Laganà AS, Noventa M, Giampaolino P, Zizolfi B, Butticè S, et al. Transvaginal bilateral sacrospinous fixation after second recurrence of vaginal vault prolapse: Efficacy and impact on quality of life and sexuality. Biomed Res Int. 2018;2018:5727165. doi: 10.1155/2018/5727165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bristow RE, Puri I, Chi DS. Cytoreductive surgery for recurrent ovarian cancer: A meta-analysis. Gynecol Oncol. 2009;112:265–74. doi: 10.1016/j.ygyno.2008.08.033. [DOI] [PubMed] [Google Scholar]
- 13.Rossetti D, Vitale SG, Tropea A, Biondi A, Laganà AS. New procedures for the identification of sentinel lymph node: Shaping the horizon of future management in early stage uterine cervical cancer. Updates Surg. 2017;69:383–8. doi: 10.1007/s13304-017-0456-6. [DOI] [PubMed] [Google Scholar]
- 14.Vitale SG, Capriglione S, Zito G, Lopez S, Gulino FA, Di Guardo F, et al. Management of endometrial, ovarian and cervical cancer in the elderly: Current approach to a challenging condition. Arch Gynecol Obstet. 2019;299:299–315. doi: 10.1007/s00404-018-5006-z. [DOI] [PubMed] [Google Scholar]
- 15.Silberzweig JE, Powell DK, Matsumoto AH, Spies JB. Management of uterine fibroids: A focus on uterine-sparing interventional techniques. Radiology. 2016;280:675–92. doi: 10.1148/radiol.2016141693. [DOI] [PubMed] [Google Scholar]
- 16.Vitale SG, Sapia F, Rapisarda AM, Valenti G, Santangelo F, Rossetti D, et al. Hysteroscopic morcellation of submucous myomas: A systematic review. Biomed Res Int. 2017;2017:6848250. doi: 10.1155/2017/6848250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salazar CA, Isaacson KB. Office operative hysteroscopy: An update. J Minim Invasive Gynecol. 2018;25:199–208. doi: 10.1016/j.jmig.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 18.Laganà AS, Vitale SG, Muscia V, Rossetti P, Buscema M, Triolo O, et al. Endometrial preparation with dienogest before hysteroscopic surgery: A systematic review. Arch Gynecol Obstet. 2017;295:661–7. doi: 10.1007/s00404-016-4244-1. [DOI] [PubMed] [Google Scholar]
- 19.van der Kooij SM, Hehenkamp WJ, Volkers NA, Birnie E, Ankum WM, Reekers JA, et al. Uterine artery embolization vs. hysterectomy in the treatment of symptomatic uterine fibroids: 5-year outcome from the randomized EMMY trial. Am J Obstet Gynecol. 2010;203:105.e1–13. doi: 10.1016/j.ajog.2010.01.049. [DOI] [PubMed] [Google Scholar]
- 20.Gingold JA, Gueye NA, Falcone T. Minimally invasive approaches to myoma management. J Minim Invasive Gynecol. 2018;25:237–50. doi: 10.1016/j.jmig.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Vercellini P, Carmignani L, Rubino T, Barbara G, Abbiati A, Fedele L, et al. Surgery for deep endometriosis: A pathogenesis-oriented approach. Gynecol Obstet Invest. 2009;68:88–103. doi: 10.1159/000219946. [DOI] [PubMed] [Google Scholar]
- 22.Laganà AS, Vitale SG, Trovato MA, Palmara VI, Rapisarda AM, Granese R, et al. Full-thickness excision versus shaving by laparoscopy for intestinal deep infiltrating endometriosis: Rationale and potential treatment options. Biomed Res Int. 2016;2016:3617179. doi: 10.1155/2016/3617179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho JE, Nezhat FR. Robotics and gynecologic oncology: Review of the literature. J Minim Invasive Gynecol. 2009;16:669–81. doi: 10.1016/j.jmig.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 24.Bellia A, Vitale SG, Laganà AS, Cannone F, Houvenaeghel G, Rua S, et al. Feasibility and surgical outcomes of conventional and robot-assisted laparoscopy for early-stage ovarian cancer: A retrospective, multicenter analysis. Arch Gynecol Obstet. 2016;294:615–22. doi: 10.1007/s00404-016-4087-9. [DOI] [PubMed] [Google Scholar]
- 25.Edwards RD, Moss JG, Lumsden MA, Wu O, Murray LS, Twaddle S, et al. Uterine-artery embolization versus surgery for symptomatic uterine fibroids. N Engl J Med. 2007;356:360–70. doi: 10.1056/NEJMoa062003. [DOI] [PubMed] [Google Scholar]
- 26.Ghiaroni J, Lopez GE, Coutinho Junior AC, Schanaider A. Uterine artery embolization with spherical PVA-PVAc particles as preparation for surgical resection of myomas. Rev Col Bras Cir. 2013;40:386–91. doi: 10.1590/s0100-69912013000500007. [DOI] [PubMed] [Google Scholar]
- 27.Laughlin-Tommaso SK. Non-surgical management of myomas. J Minim Invasive Gynecol. 2018;25:229–36. doi: 10.1016/j.jmig.2017.08.642. [DOI] [PubMed] [Google Scholar]
- 28.Choi HJ, Jeon GS, Kim MD, Lee JT, Yoon JH. Is uterine artery embolization for patients with large myomas safe and effective? A retrospective comparative study in 323 patients. J Vasc Interv Radiol. 2013;24:772–8. doi: 10.1016/j.jvir.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Fonseca MC, Castro R, Machado M, Conte T, Girao MJ. Uterine artery embolization and surgical methods for the treatment of symptomatic uterine leiomyomas: A systemic review and meta-analysis followed by indirect treatment comparison. Clin Ther. 2017;39:1438–5500. doi: 10.1016/j.clinthera.2017.05.346. [DOI] [PubMed] [Google Scholar]
- 30.Kröncke T, David M. Uterine artery embolization (UAE) for fibroid treatment: Results of the 6th radiological gynecological expert meeting. Geburtshilfe Frauenheilkd. 2017;77:689–92. doi: 10.1055/s-0043-106259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Imankulova B, Terzic M, Ukybassova T, Bapayeva G, Lesbekov T, Mustafinova G, et al. Repeated pulmonary embolism with cardiac arrest after uterine artery embolization for uterine arteriovenous malformation: A case report and literature review. Taiwan J Obstet Gynecol. 2018;57:890–3. doi: 10.1016/j.tjog.2018.10.021. [DOI] [PubMed] [Google Scholar]
- 32.Schnapauff D, Russ M, Kröncke T, David M. Analysis of Presurgical Uterine Artery Embolization (PUAE) for very large uterus myomatosus; patient's desire to preserve the uterus; case series and literature review. Rofo. 2018;190:616–22. doi: 10.1055/s-0044-101555. [DOI] [PubMed] [Google Scholar]
- 33.de Bruijn AM, Ankum WM, Reekers JA, Birnie E, van der Kooij SM, Volkers NA, et al. Uterine artery embolization vs. hysterectomy in the treatment of symptomatic uterine fibroids: 10-year outcomes from the randomized MMY trial. Am J Obstet Gynecol. 2016;215:745.e1–12. doi: 10.1016/j.ajog.2016.06.051. [DOI] [PubMed] [Google Scholar]
- 34.Poulsen B, Munk T, Ravn P. Long-term follow up after uterine artery embolization for symptomatic uterine leiomyomas. Acta Obstet Gynecol Scand. 2011;90:1281–3. doi: 10.1111/j.1600-0412.2011.01229.x. [DOI] [PubMed] [Google Scholar]
- 35.Czuczwar P, Woźniak S, Szkodziak P, Woźniakowska E, Paszkowski M, Wrona W, et al. Predicting the results of uterine artery embolization: Correlation between initial intramural fibroid volume and percentage volume decrease. Prz Menopauzalny. 2014;13:247–52. doi: 10.5114/pm.2014.45001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim MD, Lee HS, Lee MH, Kim HJ, Cho JH, Cha SH, et al. Long-term results of symptomatic fibroids treated with uterine artery embolization: In conjunction with MR evaluation. Eur J Radiol. 2010;73:339–44. doi: 10.1016/j.ejrad.2008.10.040. [DOI] [PubMed] [Google Scholar]
- 37.Kohi MP, Spies JB. Updates on uterine artery embolization. Semin Intervent Radiol. 2018;35:48–55. doi: 10.1055/s-0038-1636521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harman M, Zeteroǧlu S, Sengül M, Etlik O, Arslan H. Uterine leiomyoma embolization: Role of power Doppler ultrasonography. Tani Girisim Radyol. 2003;9:240–5. [PubMed] [Google Scholar]
- 39.Cao M, Qian L, Zhang X, Suo X, Lu Q, Zhao H, et al. Monitoring leiomyoma response to uterine artery embolization using diffusion and perfusion indices from diffusion-weighted imaging. Biomed Res Int. 2017;2017:3805073. doi: 10.1155/2017/3805073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mohan PP, Hamblin MH, Vogelzang RL. Uterine artery embolization and its effect on fertility. J Vasc Interv Radiol. 2013;24:925–30. doi: 10.1016/j.jvir.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 41.Torre A, Fauconnier A, Kahn V, Limot O, Bussierres L, Pelage JP, et al. Fertility after uterine artery embolization for symptomatic multiple fibroids with no other infertility factors. Eur Radiol. 2017;27:2850–9. doi: 10.1007/s00330-016-4681-z. [DOI] [PubMed] [Google Scholar]
- 42.Mara M, Maskova J, Fucikova Z, Kuzel D, Belsan T, Sosna O, et al. Midterm clinical and first reproductive results of a randomized controlled trial comparing uterine fibroid embolization and myomectomy. Cardiovasc Intervent Radiol. 2008;31:73–85. doi: 10.1007/s00270-007-9195-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


