Abstract
Hydrocephalus is one of the most prevalent form of developmental central nervous system (CNS) malformations. Cerebrospinal fluid (CSF) flow depends on both heartbeat and body movement. Furthermore, it has been shown that CSF flow within and across brain ventricles depends on cilia motility of the ependymal cells lining the brain ventricles, which play a crucial role to maintain patency of the narrow sites of CSF passage during brain formation in mice. Using whole-exome and whole-genome sequencing, we identified an autosomal-dominant cause of a distinct motile ciliopathy related to defective ciliogenesis of the ependymal cilia in six individuals. Heterozygous de novo mutations in FOXJ1, which encodes a well-known member of the forkhead transcription factors important for ciliogenesis of motile cilia, cause a motile ciliopathy that is characterized by hydrocephalus internus, chronic destructive airway disease, and randomization of left/right body asymmetry. Mutant respiratory epithelial cells are unable to generate a fluid flow and exhibit a reduced number of cilia per cell, as documented by high-speed video microscopy (HVMA), transmission electron microscopy (TEM), and immunofluorescence analysis (IF). TEM and IF demonstrate mislocalized basal bodies. In line with this finding, the focal adhesion protein PTK2 displays aberrant localization in the cytoplasm of the mutant respiratory epithelial cells.
Keywords: hydrocephalus, lung disease, cilia, ciliogenesis, ependyma, FOXJ1
Main Text
Hydrocephalus remains the most prevalent form of developmental central nervous system (CNS) malformation treated by pediatric neurosurgeons.1 While trauma, intraventricular hemorrhages, and CNS infections account for the majority of cases, heritable genetic mutations in human hydrocephalus are relatively rare.1 Here, we identify a genetic cause for this condition related to dysfunction of the CNS ependymal cilia.
Multiple motile cilia in the respiratory tract, the ependyma, or the female fallopian tubes as well as motile monocilia in the embryonic left/right organizer generate the mechanical force to drive extracellular fluid flow in a continuous and coordinated fashion. While formation of a single cilium is a complex process depending on several hundreds of different factors, ciliogenesis in multiciliated cells additionally requires development of a network of oriented cilia within a short period of time.2 Defects in cilia generation or motility lead to a mucociliary clearance disorder associated with laterality defects, male infertility, and very rarely hydrocephalus, which is referred to as primary ciliary dyskinesia (PCD [MIM: 244400]). So far, recessive mutations in 44 genes have been identified to cause PCD.
Here we present an autosomal-dominant cause of a distinct motile ciliopathy. Heterozygous de novo mutations in FOXJ1, which encodes a well-known member of the forkhead/winged-helix transcription factor DNA binding proteins, cause a motile ciliopathy that is characterized by hydrocephalus internus, chronic destructive airway disease, and randomization of left/right body asymmetry.
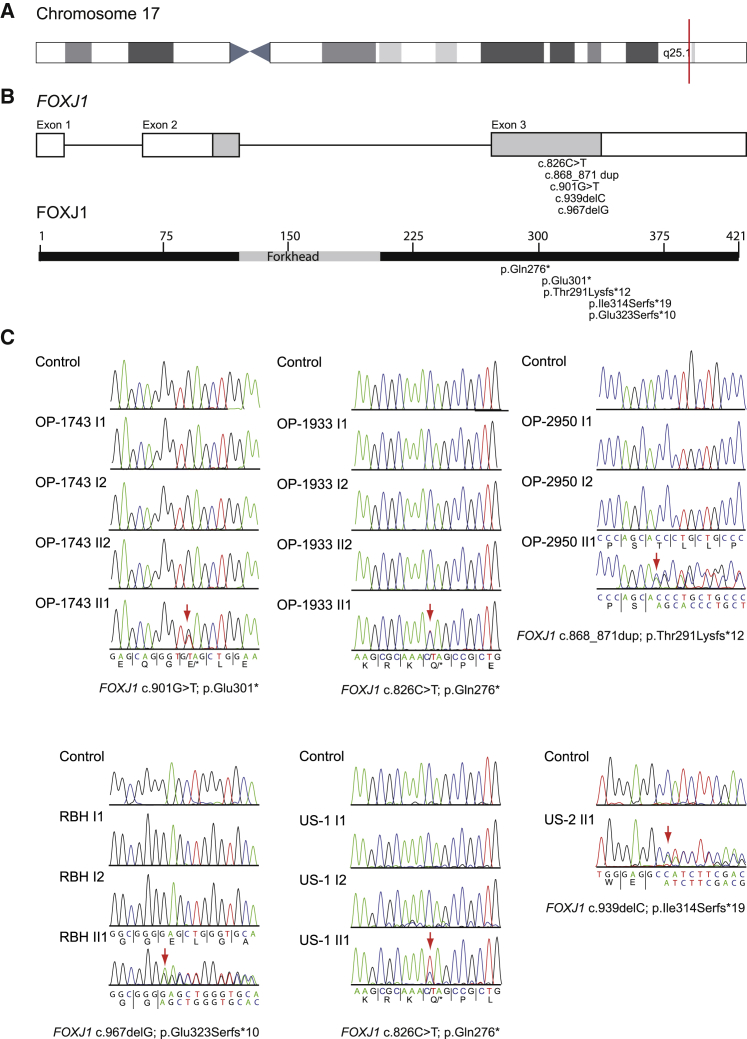
The study was approved by the Institutional Ethics Review boards and signed and informed consent was obtained. Whole-exome sequencing performed as previously described3 in an initial cohort of six individuals with both hydrocephalus and an associated mucociliary clearance disorder identified heterozygous loss-of-function mutations in FOXJ1 in three unrelated individuals (OP-1743 II1, c.901G>T [p.Glu301∗]; OP-2950 II1, c.868_871dup [p.Thr291Lysfs∗12]; OP-1933 II1, c.826C>T [p.Gln276∗]). Genome sequencing as part of the UK 100,000 Genomes Project4 revealed a further heterozygous FOXJ1 mutation (RBH II1, c.967delG [p.Glu323Serfs∗10]). In an additional US cohort of five individuals with mucociliary clearance disorder and hydrocephalus, whole-exome sequencing identified two further affected individuals with heterozygous variants in FOXJ1 (US-1 II1, c.826C>T [p.Gln276∗]; US-2 II1, c.939delC [p.Ile314Serfs∗19]) (Figures 1 and S1). These FOXJ1 variants were not identified in any of the parents (parentage was confirmed) or the unaffected siblings in the families and were therefore considered to have arisen de novo. For affected individual US-2 II1, no parental DNA was available. No mutations in other motile ciliopathy-related genes were identified. Thus, systematic genetic screening of a total of 468 individuals (n = 354 Germany; n = 114 USA) with mucociliary clearance disorders but without co-occurrence of hydrocephalus did not identify any FOXJ1 mutations (0%). Sequence analyses in a cohort of 11 individuals (n = 6 Germany; n = 5 USA) with hydrocephalus and associated mucociliary clearance disorder revealed in 45.5% (n = 5) heterozygous FOXJ1 mutations.
Figure 1.
Heterozygous De Novo Pathogenic Variants in FOXJ1 in Affected with Hydrocephalus and Chronic Destructive Airway Disease
(A) Schematic overview of chromosome 17. FOXJ1 (CCDS32739, GenBank: NM_001454.4) is located on chromosome 17q25.1 (red mark).
(B) FOXJ1 consists of two coding exons and one alternative first exon encoding a 2,641 bp transcript and 421 amino acid protein.
(C) Electropherograms of Sanger sequencing results for family OP-1933, OP-1743, OP-2950, RBH, and US-1. Consistent with de novo mutations, none of the variants were identified in either the parents or non-affected siblings. In US-2 no parental DNA was available.
FOXJ1 (CCDS32739; GenBank: NM_001454.4) located on chromosome 17q25.1 comprises two coding exons and one alternative first exon, encoding a 2,641 bp transcript and a predicted 421 amino acid protein (Figure 1). Consistent with a mutational hotspot in the FOXJ1 C-terminal region (Figure 1), all identified mutations localize within a small region of exon 3. Interestingly, the variant c.826C>T (p.Gln276∗) occurred de novo in two non-related individuals, OP-1933 II1 and US-1 II1, emphasizing that this gene region is especially susceptible to mutations.
All identified FOXJ1 variants are predicted to result in a premature termination codon (nonsense or frameshift type allele) consistent with haploinsufficiency being the disease cause. No LoF variants are reported in gnomAD, and gnomAD gene constraint scores (pLI = 0.97, o/e = 0) place FOXJ1 in the haploinsufficient gene category i.e., a high intolerance to loss-of-function alleles.5 Notably, haploinsufficiency with autosomal-dominant trait has been reported in other genes encoding forkhead transcription factors such as FOXC1 (FKHL7 [MIM: 601090]) (pLI = 0.95, o/e = 0) and FOXC2 (FKHL14 [MIM: 602402]) (pLI = 0.13 o/e = 0.3), which are associated with aberrant ocular development.6
We next performed 3′mRNA sequencing in FOXJ1 mutant respiratory cells (OP-1743 II1 and OP-2950 II1) to analyze effects of the detected de novo FOXJ1 mutations at a transcriptional level. Consistent with haploinsufficiency, FOXJ1 transcript levels are reduced compared to healthy control subjects (Figure S2) and FOXJ1 gene targets7 encoding ciliary axonemal proteins showing reduced transcript levels in FOXJ1 mutant cells as well.
FOXJ1, also referred to as hepatocyte nuclear factor-3/forkhead homolog 4 (HFH-4), has been studied in detail. Consistent with a distinct functional role for motile cilia, FOXJ1 expression has been detected in ciliated cells of the ependyma lining the brain ventricles, airways, oviduct, and the embryonic left/right organizer.8, 9, 10 Targeted mutation or knock-down of Foxj1 in zebrafish, Xenopus laevis, and mice9, 11, 12 resulted in a motile ciliopathy characterized by a reduced number of multiple motile cilia (MMC) and mislocalized basal bodies, which nucleate ciliary axonemes. These findings demonstrate the important functional role of FOXJ1 for the generation of motile cilia.
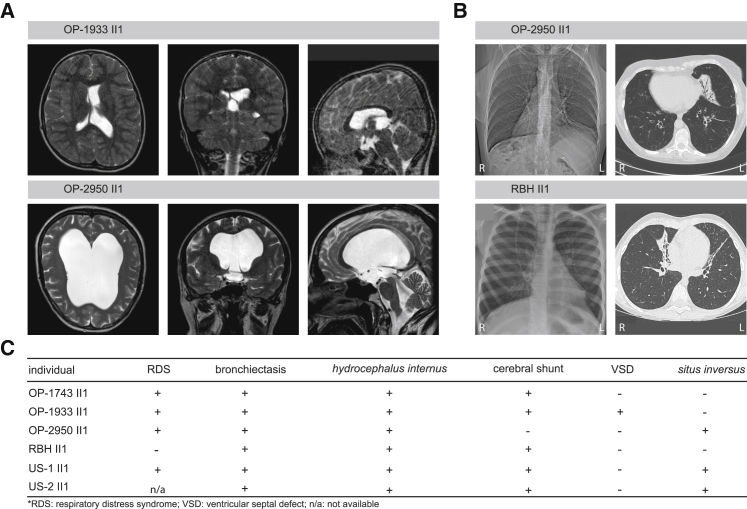
All six individuals with pathogenic FOXJ1 variants exhibited hydrocephalus internus (Figures 2 and S3). In five affected individuals (OP-1743 II1, OP-1933 II1, RBH II1, US-1 II1, and US-2 II2), obstructive hydrocephalus was detected within the first few weeks of life, which required immediate treatment by insertion of a ventriculo-peritoneal shunt system to relieve elevated intracranial pressure. Remarkably, hydrocephalus internus in OP-2950 II1 was detected at age 54 years, when clinical examination revealed macrocephaly, gait ataxia, and optic atrophy consistent with a long-standing, increased intracranial pressure. Cranial magnetic resonance imaging (MRI) confirmed obstructive hydrocephalus internus and placement of a ventriculo-peritoneal shunt system has been recommended.
Figure 2.
Affected with Pathogenic FOXJ1 Variants Display Obstructive Hydrocephalus, Randomization of Left/Right Body Asymmetry, and a Chronic Destructive Airway Disease
(A) Cranial magnetic resonance imaging of OP-1933 II1 was performed after shunt insertion (right lateral ventricle) to relieve raised intracranial pressure. The left lateral ventricle and the third ventricle are dilated. The lateral view documents stenosis of the aqueduct of Sylvius and a small fourth ventricle. OP-2950 II1 shows massively dilated brain ventricles. Lateral view indicates a patent aqueduct and a dilated fourth ventricle due to closure of the foramen of Magendii and the lateral apertures.
(B) Chest X-ray of OP-2950 II1 shows situs inversus totalis. The computed tomography scan of OP-2950 II1 and RBH II1 exhibit atelectasis and bronchiectasis of the middle lobe.
(C) Summary of clinical findings in the affected individuals.
The flow of cerebrospinal fluid (CSF) depends on heartbeat as well as body movement. In zebrafish, it has been proposed that the distribution of CSF within and across brain ventricles depends on ciliary motility.13 CSF is produced by the choroid plexus in both lateral ventricles and the choroid plexus of the third and the fourth ventricle. Before re-absorption, CSF traverses several narrow spaces such as the aqueduct of Sylvius (to enter the fourth ventricle), three openings including the foramen of Magendii, and the lateral apertures (to enter the subarachnoid spaces). We have previously reported that motile cilia of the ependymal cells lining the brain ventricles play a crucial role to maintain patency of the narrow sites of CSF passage during brain formation.14 Consistent with a role of FOXJ1 in generation of motile ependymal cilia, cranial MRI studies demonstrated stenosis of the cerebral aqueduct and/or foramina Magendii and Luschka responsible for the obstructive hydrocephalus internus in all six individuals (Figures 2 and S3). In agreement with this observation, Foxj1-deficient mice develop hydrocephalus.9, 15 Therefore, we assume that hydrocephalus is a characteristic clinical finding in FOXJ1 mutant individuals.
While the prevalence of infant hydrocephalus occurs with a frequency of approximately 1 per 1,000 births,16 most cases are of a post-hemorrhagic nature due to prematurity (malabsorption of CSF). About 10% are related to primary causes but so far only a small number of associated genes have been identified.1 In PCD-affected individuals with severely impaired cilia beating due to altered axonemal motor protein composition of multiciliated cells (MCCs), the prevalence of hydrocephalus is only slightly increased (approximately 1/75; 1.3%).17 Individuals harboring bi-allelic mutations in MCIDAS and CCNO, which cause severe multiciliogenesis defects of MCCs, develop hydrocephalus much more often (10%).18 Nevertheless, hydrocephalus is not an obligatory finding in cases arising from these multiciliogenesis defects18, 20, 21 and hydrocephalus has been shown by Behan et al. not to be indicative for overall PCD, because hydrocephalus is very rarely present in PCD-affected individuals.17 Therefore, aberrant beating or reduced numbers of ependymal cilia alone does not fully explain the occurrence of human hydrocephalus.
Besides its function in motile ciliogenesis, FOXJ1 is known to be essential for ependymal cell maturation, which might contribute to the development of hydrocephalus in FOXJ1-mutant individuals.9, 15 During early postnatal periods, radial glial cells in various ventricular zones of the brain differentiate into ependymal cells and astrocytes. In mice, Foxj1 expression in the lateral ventricle is required for the differentiation of radial glial cells into ependymal cells and a small subset of Foxj1(+) astrocytes into a postnatal neural stem cell niche.15 A chemical screen for modulators of ependymal cell differentiation found that the mature multiciliated ependyma needs constant FOXJ1 expression to prevent cellular dedifferentiation back to a glial-like morphology.22 Interestingly, Abdi et al.22 reported that ependymal FOXJ1 has a short half-life, requiring non-canonical IκB kinase activity to prevent rapid degradation via the ubiquitin proteasome system. Thus, constant Foxj1 expression is crucial to maintain ependymal cell differentiation and prevent hydrocephalus. Autosomal-dominant mutations in FOXG1 (MIM: 613454), another member of the forkhead gene family, cause a neurodevelopmental disorder associated with brain malformations including corpus callosal dysgenesis, indicating that forkhead transcription factors play a crucial role in neurodevelopment.23
Consistent with a mucociliary clearance disorder, all individuals harboring FOXJ1 mutations suffered from recurrent infections of upper and lower airways, chronic productive cough, and bronchiectasis as well as chronic rhinitis and sinusitis (Figures 2 and S3). Postnatal respiratory distress syndrome was present in four individuals (OP-1743 II1, OP-1933 II1, OP-2950 II1, US-1 II1), a respiratory phenotype common to PCD. Nasal nitric oxide (NO) production rate is usually markedly reduced in PCD-affected individuals,24 but nasal NO production rates of the four tested FOXJ1-mutant individuals—OP-1933 II1 (141 nL/min), OP-2950 II1 (122 nL/min), RBH II1 (215 nL/min), and US-2 II1 (328 nL/min)—were within normal ranges. Nasal NO measurement was performed as previously reported in all affected individuals at an age >5 years (nasal cut-off value 77 nL/min).25 Thus, nasal NO measurement cannot be used to screen for individuals with FOXJ1 mutations, and affected individuals will not be identified by the nasal NO testing implemented early within the current diagnostic workup for PCD.26, 27
Affected individuals OP-2950 II1, US-1 II1, and US-2 II1 exhibited situs inversus, consistent with previous observations in mice, Xenopus laevis, and zebrafish, indicating that FOXJ1 has an important functional role in left/right body asymmetry determination during early embryogenesis.9, 11, 28, 29, 30 Of note, OP-1933 II1 presented with a ventricular septal cardiac defect, which might reflect the increased incidence of congenital heart defects in motile ciliopathies associated with randomization of left/right body asymmetry compared to the healthy population.31, 32
OP-2950 II1 was unable to become pregnant even after in vitro fertilization. Furthermore, one female individual (RBH II1) was diagnosed with hydrosalpinx, after she presented with abdominal pain at 15 years old (Figure S3). Together, these data suggest that FOXJ1 might also affect ciliogenesis in the female reproductive tract. MMCs line the fallopian tube and there are several reports of individuals with PCD where fallopian tube cilia dysmotility mirror those in the respiratory tract, possibly causing subfertility and ectopic pregnancy.33, 34, 35
Consistent with a possible spermatozoon flagellar defect, the affected male US-2 II1 did not father a child. Fertility evaluation revealed an adequate sperm count but with reduced motility and abnormal morphology (severe oligoasthenoteratospermia). However, future studies are required to determine whether male infertility is a constant finding in FOXJ1-mutant males and indeed is related to altered sperm flagella movement.
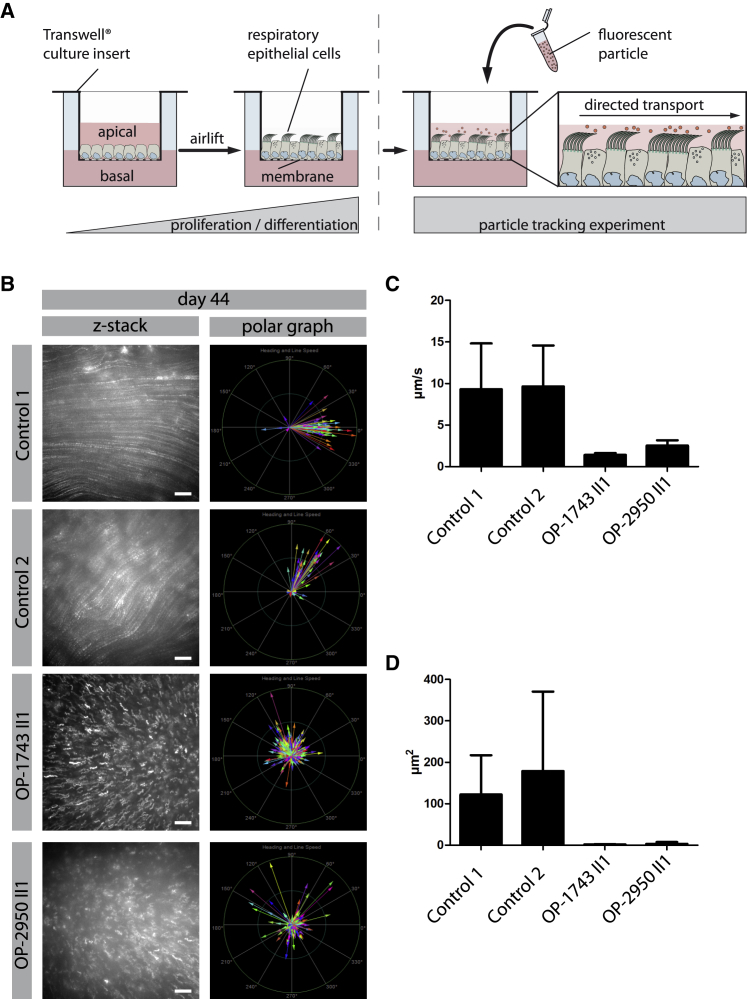
To study the effects of FOXJ1 haploinsufficiency at the cellular level, we analyzed respiratory epithelial cells from FOXJ1-mutant individuals and healthy control subjects by high-speed video microscopy analysis (HVMA). HVMA in OP-1933 II1, OP-2950 II1, and RBH II1 showed a reduced number of motile cilia per MCC. The number of cilia per MCC varied between zero to almost normal numbers, but most cells were lined with very few cilia per cell (Figure S4). To corroborate these findings we investigated the amount of cells with (1) normal (>100 cilia per MCC), (2) slightly reduced (4–100 cilia per MCC), and (3) severely reduced (0–4 cilia per MCC) numbers of cilia in FOXJ1-mutant individuals OP-1743 II1, OP-1933 II1, and OP-2950 II1 and healthy control subjects by IF utilizing anti-acetylated α-tubulin as a cilia marker. In all FOXJ1-mutant individuals, the number of cells with normal amounts of cilia was reduced compared to healthy control subjects (Figure S4). The residual cilia of their MCCs exhibited a stiff beating pattern with reduced beating amplitude (Videos S1, S2, S3, and S4). To distinguish between reduced generation and secondary loss of MMCs, we cultured primary respiratory epithelial cells from OP-1743 II1, OP-1933 II1, and OP-2950 II1 and performed in vitro ciliogenesis experiments in spheroid and air-liquid interface (ALI) cultures. HVMA after in vitro ciliogenesis of spheroid cultures confirmed reduced numbers of cilia per MCC, as well as the abnormal beating pattern in samples from OP-1933 II1 and OP-2950 II1. This finding is consistent with a primary defect of ciliogenesis, as previously reported in various FOXJ1-deficient model organisms9, 11, 30 (Videos S5, S6, and S7). The variable cellular phenotype might result from haploinsufficiency. Thus, in some cells FOXJ1 expression might be still sufficient to generate some or normal amounts of cilia whereas in other cells the process of ciliogenesis is more severely hampered because FOXJ1 expression is too low to drive normal ciliogenesis. Next, we tested whether the residual motile cilia of FOXJ1-mutant cells are still able to generate a directed fluid flow. To mimic the process of particle lung clearance in vitro, we added fluorescent particles to the apical compartment of the ALI-Transwell inserts from OP-1743 II1 and OP-2950 II1 as well as healthy control subjects and performed particle-tracking experiments with the cultivated respiratory epithelial cells. Consistent with aberrant mucociliary clearance, FOXJ1-mutant cilia were not able to propel mucous along the surface of the differentiated epithelium (Figure 3, Videos S8, S9, S10, S11, S12, and S13).
Figure 3.
Air Liquid Interface (ALI) Cultures of FOXJ1-Mutant Respiratory Epithelial Cells Are Unable to Generate a Directed Fluid Flow
(A) Schematic depicts the experimental set-up of particle tracking analyses performed on ALI-cultured respiratory epithelial cells. Respiratory epithelial cells from FOXJ1-mutants (OP-1743 II1, OP-2950 II1) as well as healthy control subjects were cultured under ALI conditions. After complete differentiation (30 days, 37 days, and 44 days after airlift), 0.5 μm fluorescent particles were added to the apical compartments of the cells.
(B–D) Tracking videos are represented as z stack projections, while the transport direction of each particle is summarized in polar graphs (B). Under healthy conditions the fluorescent particles were transported in a linear direction along the cell layer, whereas the particle transport in FOXJ1 mutant cells (OP-1743 II1, OP-2950 II1) was non-oriented, highly reduced in speed (μm/s) (C) and in mean squared displacement (μm2) (D). For statistical evaluation, 15 videos per person were analyzed. Thereby, 253 particles were tracked per video on average. Scale bars represent 20 μm.
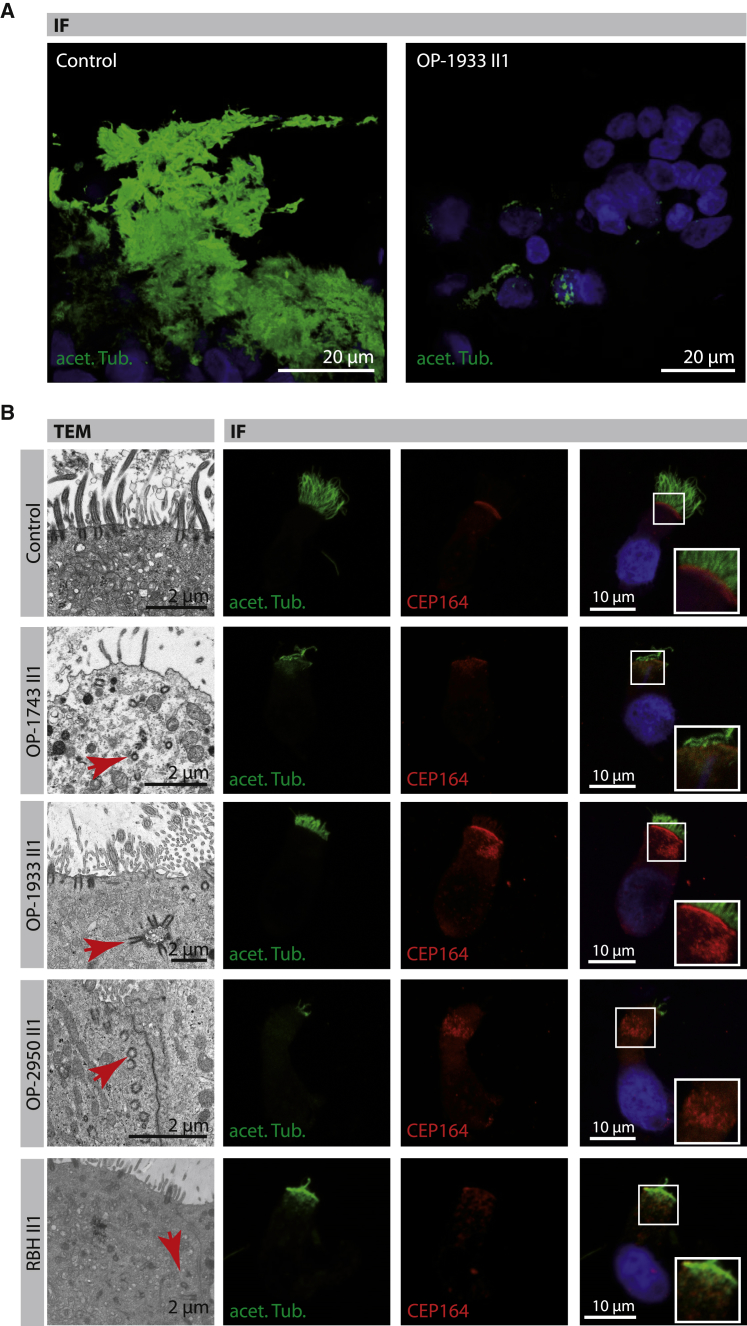
To further characterize the ciliogenesis defect at the cellular level, we performed transmission electron microscopy (TEM) as previously described21 on native respiratory epithelial cells after nasal brushing (OP-1743 II1, OP-1933 II1, OP-2950 II1, RBH II1, US-1II1, US-2 II1) as well as after spheroid cultures (OP-1933 II1, OP-2950 II1). Consistent with a defect in ciliogenesis, the number of cilia per MCC was markedly reduced in most cells across all different samples (Figure 4). The apical cell regions showed a severe decrease of basal bodies. Whereas the overall number of basal bodies per MCC was apparently not altered, basal bodies were mislocalized within the cytoplasm, indicating a defect in apical docking that is consistent with previous reports on MCC in Foxj1-deficient mice9, 36 (Figure 4).
Figure 4.
FOXJ1 Mutant Respiratory Epithelial Cells Show a Reduced Number of Cilia and Mislocalized Basal Bodies by Transmission Electron Microscopy (TEM) and Immunofluorescence Microscopy Analysis (IF)
(A) Respiratory epithelial cells cultured as spheroids from a healthy control subject and OP-1933 II1. Cilia are stained with antibodies targeting acetylated α-tubulin (acet. Tub.; green) after complete differentiation. Cells of OP-1933 II1 demonstrate a variable reduction of cilia in comparison to the control. Nuclei are stained with Hoechst33342 (blue).
(B) Transmission electron microscopy (TEM) photographs of multiciliated cells (MCC; first row) from a healthy control subject show basal bodies attached to the apical membrane and nucleating multiple motile cilia. Respiratory epithelial cells from mutant individuals with pathogenic FOXJ1 variants (OP-1743 II1, OP-1933 II1, OP-2950 II1, RBH II1) exhibit mislocalized basal bodies (representative examples shown by red arrows) within the cytoplasm, consistent with a basal body docking defect. Respiratory epithelial cells are stained with antibodies targeting acetylated α-tubulin (acet. Tub.; green) and antibodies targeting mother centrioles (CEP164, red). In control cells, basal bodies (red) are aligned at the apical cell region, whereas in FOXJ1 mutant cells they are mainly mislocalized within the cytoplasm, consistent with TEM findings. Right row shows higher magnification images of regions of CEP164-positive basal bodies. Nuclei were stained with Hoechst33342 (blue).
To corroborate these findings, we performed high-resolution immunofluorescence microscopy analyses (IF) with antibodies targeting acetylated α-tubulin (marker of the ciliary axonemes) and the centrosomal protein 164 (CEP164, basal body marker) as previously described.21 As expected, the number of cilia per MCC in samples of FOXJ1 mutant individuals varied but was severely reduced in most cells, consistent with our findings obtained by HVMA and TEM. The number of CEP164-positive basal bodies per MCC was not altered but basal bodies were mislocalized within the cytoplasm, consistent with a basal body docking defect documented by TEM (Figure 4).
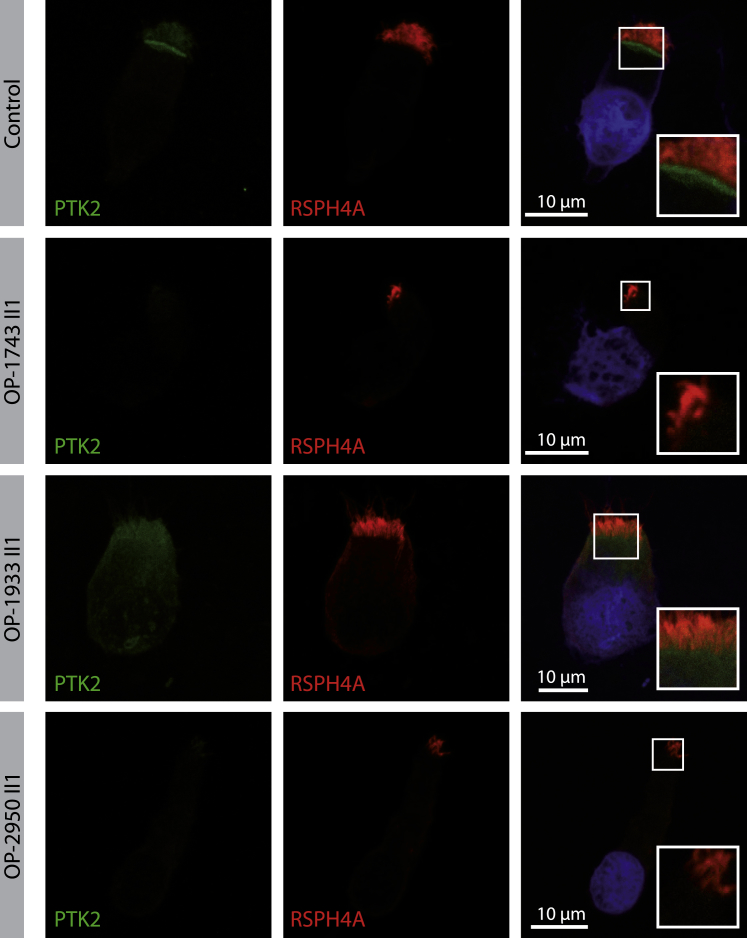
Since focal adhesion components have been shown to be responsible for anchoring basal bodies to the actin network of multiciliated cells,37 we analyzed protein tyrosine kinase 2 (PTK2) also known as focal adhesion kinase, localization in respiratory epithelial utilizing anti-PTK2 antibodies. Consistent with previous findings in Xenopus laevis,37 PTK2 localizes in the basal body area in respiratory epithelial cells of healthy control subjects (Figures 5 and S5) and consistent with a basal body docking defect, PTK2 localization was markedly reduced in FOXJ1 mutant respiratory epithelial cells (Figure 5).
Figure 5.
PTK2, a Member of the Subapical Protein Network, Shows Abnormal Localization in FOXJ1 Mutant Cells by Immunofluorescence Microscopy Analysis (IF)
Respiratory epithelial cells from control and FOXJ1 mutant individuals (OP-1743 II1, OP-1933 II1, OP-2950 II1) are stained with antibodies targeting PTK2 (green). PTK2, which forms complexes named ciliary adhesions that are associated with basal bodies and striated rootlets, shows reduced localization in FOXJ1 mutant cells compared to the control. Regions around the subapical cell membrane showing PTK2 at higher magnification (right row). Nuclei are stained with Hoechst33342 (blue).
Because FOXJ1 is known not only to be involved in ciliogenesis in multiciliated cells but also for expression of axonemal proteins related to ciliary motility,11, 12 we thoroughly examined the axonemal structure and composition of FOXJ1 mutant respiratory cilia by TEM and IF. Surprisingly, ciliary cross sections often exhibited variable ultrastructural abnormalities. Whereas some cross sections did not show any abnormalities of the 9+2 axonemal architecture, all affected individuals exhibited defects of microtubular organization or missing central tubules (Figure S6). Thus, we found heterogeneous ultrastructural defects in FOXJ1 mutant cilia indicating that TEM is not a sufficient test to diagnose FOXJ1 mutant individuals.
In PCD, ciliary defects are typically restricted to specific structures depending on the underlying genetic defect. However, cilia in FOXJ1 mutant cells showed various abnormalities. To further elaborate these findings, we next studied respiratory cells from FOXJ1-affected individuals using antibodies targeting distinct axonemal structural components such as (1) the ODA intermediate chain DNAI2 and heavy chain DNAH5 (absent in PCD-affected subjects with ODA defects),38, 39 (2) the nexin-dynein regulatory complex protein GAS8 (absent in PCD-affected subjects with defects of microtubular organization, e.g., due to pathogenic CCDC40 or CCDC39 variants),40, 41 and (3) the radial spoke head protein RSPH4A (absent in PCD-affected subjects with pathogenic RSPH4A variants).42 IF analyses of residual cilia did not show abnormalities, but it is important to note that subtle reductions of protein content cannot be detected by IF. Thus, normal IF analyses of residual cilia are consistent with absence of gross axonemal abnormalities but did not exclude more subtle axonemal defects detected using TEM (Figure S7).
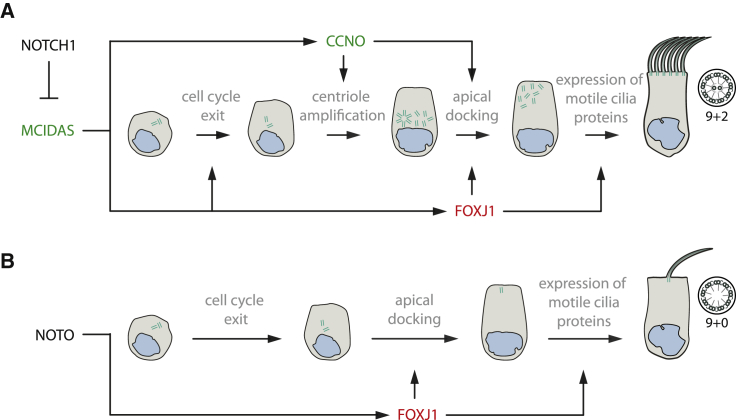
We previously reported mutations in CCNO or MCIDAS causing a mucociliary clearance disorder referred to as reduced generation of multiple motile cilia (RGMC), due to a defect of mother centriole generation and migration at the late stage of MMC generation.20, 21 FOXJ1 probably acts downstream of MCIDAS and in parallel to CCNO in the NOTCH1-dependent pathway of multiciliogenesis20 (Figure 6). While FOXJ1 deficiency can be classified as RGMC, the cellular disease phenotype is distinct from CCNO or MCIDAS defects. FOXJ1 haploinsufficient MCCs in the respiratory tract exhibit a normal number of basal bodies, which are mislocalized within the cytoplasm due to a basal body docking defect. OP-2950 II1, US-1 II1, and US-2 II1 exhibit situs inversus, which is consistent with previous reports in mice, zebrafish, and Xenopus laevis that FOXJ1 deficiency causes randomization of the left/right body asymmetry.9, 11, 12 This observation implies that determination of left/right body asymmetry is independent of the NOTCH1-dependent multiciliogenesis pathway. NOTO encodes for a homeodomain transcription factor specifically expressed at the left/right organizer of mouse and other vertebrate embryos; NOTO transcriptionally activates FOXJ1 expression and thus also regulates ciliogenesis.44 This NOTO-dependent activation of FOXJ1 at the left/right organizer is crucial for proper determination of the left/right asymmetry, probably explaining situs anomalies in some FOXJ1 mutant individuals (Figure 6).
Figure 6.
FOXJ1 Is an Essential Component in Signaling Pathways for the Generation of Motile Cilia
Schematics illustrating the function of FOXJ1 in the generation of motile cilia in the NOTCH1- and NOTO-dependent pathway in (A) multiciliated cells and (B) the ciliated cells of the embryonic node,44 respectively. Pathogenic variants in MCIDAS and CCNO (marked in green) are known to cause a ciliogenesis defect in multiple motile cilia causing a mucociliary clearance disorder referred to as reduced generation of multiple motile cilia20, 21 (RGMC).
This study emphasizes the pathophysiological link between the development of hydrocephalus and a severe mucociliary clearance disorder, which should be considered in clinical care of affected individuals with hydrocephalus and respiratory symptoms. Early clinical and genetic diagnosis will aid implementation of appropriate neurological as well as respiratory care in FOXJ1 mutant individuals.
Declaration of Interests
The authors declare no competing interests.
Acknowledgments
We thank the PCD-affected individuals and their families for their participation and acknowledge the German PCD support group Kartagener Syndrom und Primaere Ciliaere Dyskinesie e.V, UK PCD Family Support Group, and North American PCD Foundation. The University Children’s Hospital Muenster and the Royal Brompton and Harefield NHS Trust are part of ERN-Lung, the European Reference Network for Rare Diseases. We would like to thank K.-P. Schlingmann for the discussion and A. Borgscheiper, A. Dorißen, D. Ernst, S. Helms, M. Herting, J. Quante, A. Robbers, L. Schwiddessen, F.J. Seesing, S. Sivalingam, M. Tekaat, K. Wohlgemuth, C. Westermann, and S. Wilkinson for excellent clinical and technical work. We thank the investigators and coordinators of Genetics Disorders of Mucociliary Clearance Consortium (GDMCC); Drs. J. Stonebraker and H. Dang of UNC for technical and bioinformatics assistance, respectively; Ms. Kimberly Burns of UNC for electron microscopy support; McDonnell Genome Institute (Washington University); and Drs. S. Mane, F. Lopez-Giraldez, and W. Dong from Yale Center for Mendelian Genomics (UM1 HG006504) for providing whole-exome sequencing and bioinformatics support. We thank Sayyid Hasan (RBHT) as WL GMC Validation Coordinator of results from the UK 100K Project, Dr. Anne Schmidt for the clinical workup of subject RBH II-1 as well as Mitali P. Patel for the IF stainings. This work was funded by the Deutsche Forschungsgemeinschaft (DFG), the National Institutes of Health (US), 100,000 Genomes Project National Institute for Health Research and NHS England (UK), IZKF Muenster, “Innovative Medical Research” of the University of Muenster Medical School, and Great Ormond Street Children’s Charity. The authors participate in the COST action BEAT-PCD. The findings in subject RBH II1 were made possible through access to the data and findings generated by the 100,000 Genomes Project. Please find detailed funding information in the Supplemental Data.
Published: October 17, 2019
Footnotes
Supplemental Data can be found online at https://doi.org/10.1016/j.ajhg.2019.09.022.
Web Resources
gnomAD Browser, https://gnomad.broadinstitute.org/
OMIM, https://www.omim.org/
Supplemental Data
References
- 1.Kahle K.T., Kulkarni A.V., Limbrick D.D., Jr., Warf B.C. Hydrocephalus in children. Lancet. 2016;387:788–799. doi: 10.1016/S0140-6736(15)60694-8. [DOI] [PubMed] [Google Scholar]
- 2.Fliegauf M., Benzing T., Omran H. When cilia go bad: cilia defects and ciliopathies. Nat. Rev. Mol. Cell Biol. 2007;8:880–893. doi: 10.1038/nrm2278. [DOI] [PubMed] [Google Scholar]
- 3.Altmüller J., Motameny S., Becker C., Thiele H., Chatterjee S., Wollnik B., Nürnberg P. A systematic comparison of two new releases of exome sequencing products: the aim of use determines the choice of product. Biol. Chem. 2016;397:791–801. doi: 10.1515/hsz-2015-0300. [DOI] [PubMed] [Google Scholar]
- 4.Wheway G., Mitchison H.M., Genomics England Research Consortium Opportunities and Challenges for Molecular Understanding of Ciliopathies-The 100,000 Genomes Project. Front. Genet. 2019;10:127. doi: 10.3389/fgene.2019.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lek M., Karczewski K.J., Minikel E.V., Samocha K.E., Banks E., Fennell T., O’Donnell-Luria A.H., Ware J.S., Hill A.J., Cummings B.B., Exome Aggregation Consortium Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith R.S., Zabaleta A., Kume T., Savinova O.V., Kidson S.H., Martin J.E., Nishimura D.Y., Alward W.L., Hogan B.L., John S.W. Haploinsufficiency of the transcription factors FOXC1 and FOXC2 results in aberrant ocular development. Hum. Mol. Genet. 2000;9:1021–1032. doi: 10.1093/hmg/9.7.1021. [DOI] [PubMed] [Google Scholar]
- 7.Mukherjee I., Roy S., Chakrabarti S. Identification of Important Effector Proteins in the FOXJ1 Transcriptional Network Associated With Ciliogenesis and Ciliary Function. Front. Genet. 2019;10:23. doi: 10.3389/fgene.2019.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blatt E.N., Yan X.H., Wuerffel M.K., Hamilos D.L., Brody S.L. Forkhead transcription factor HFH-4 expression is temporally related to ciliogenesis. Am. J. Respir. Cell Mol. Biol. 1999;21:168–176. doi: 10.1165/ajrcmb.21.2.3691. [DOI] [PubMed] [Google Scholar]
- 9.Brody S.L., Yan X.H., Wuerffel M.K., Song S.K., Shapiro S.D. Ciliogenesis and left-right axis defects in forkhead factor HFH-4-null mice. Am. J. Respir. Cell Mol. Biol. 2000;23:45–51. doi: 10.1165/ajrcmb.23.1.4070. [DOI] [PubMed] [Google Scholar]
- 10.Hackett B.P., Brody S.L., Liang M., Zeitz I.D., Bruns L.A., Gitlin J.D. Primary structure of hepatocyte nuclear factor/forkhead homologue 4 and characterization of gene expression in the developing respiratory and reproductive epithelium. Proc. Natl. Acad. Sci. USA. 1995;92:4249–4253. doi: 10.1073/pnas.92.10.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stubbs J.L., Oishi I., Izpisúa Belmonte J.C., Kintner C. The forkhead protein Foxj1 specifies node-like cilia in Xenopus and zebrafish embryos. Nat. Genet. 2008;40:1454–1460. doi: 10.1038/ng.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choksi S.P., Lauter G., Swoboda P., Roy S. Switching on cilia: transcriptional networks regulating ciliogenesis. Development. 2014;141:1427–1441. doi: 10.1242/dev.074666. [DOI] [PubMed] [Google Scholar]
- 13.Olstad E.W., Ringers C., Hansen J.N., Wens A., Brandt C., Wachten D., Yaksi E., Jurisch-Yaksi N. Ciliary Beating Compartmentalizes Cerebrospinal Fluid Flow in the Brain and Regulates Ventricular Development. Curr. Biol. 2019;29:229–241.e6. doi: 10.1016/j.cub.2018.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ibañez-Tallon I., Pagenstecher A., Fliegauf M., Olbrich H., Kispert A., Ketelsen U.-P., North A., Heintz N., Omran H. Dysfunction of axonemal dynein heavy chain Mdnah5 inhibits ependymal flow and reveals a novel mechanism for hydrocephalus formation. Hum. Mol. Genet. 2004;13:2133–2141. doi: 10.1093/hmg/ddh219. [DOI] [PubMed] [Google Scholar]
- 15.Jacquet B.V., Salinas-Mondragon R., Liang H., Therit B., Buie J.D., Dykstra M., Campbell K., Ostrowski L.E., Brody S.L., Ghashghaei H.T. FoxJ1-dependent gene expression is required for differentiation of radial glia into ependymal cells and a subset of astrocytes in the postnatal brain. Development. 2009;136:4021–4031. doi: 10.1242/dev.041129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munch T.N., Rostgaard K., Rasmussen M.-L.H., Wohlfahrt J., Juhler M., Melbye M. Familial aggregation of congenital hydrocephalus in a nationwide cohort. Brain. 2012;135:2409–2415. doi: 10.1093/brain/aws158. [DOI] [PubMed] [Google Scholar]
- 17.Behan L., Dimitrov B.D., Kuehni C.E., Hogg C., Carroll M., Evans H.J., Goutaki M., Harris A., Packham S., Walker W.T., Lucas J.S. PICADAR: a diagnostic predictive tool for primary ciliary dyskinesia. Eur. Respir. J. 2016;47:1103–1112. doi: 10.1183/13993003.01551-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amirav I., Wallmeier J., Loges N.T., Menchen T., Pennekamp P., Mussaffi H., Abitbul R., Avital A., Bentur L., Dougherty G.W., Israeli PCD Consortium Investigators Systematic Analysis of CCNO Variants in a Defined Population: Implications for Clinical Phenotype and Differential Diagnosis. Hum. Mutat. 2016;37:396–405. doi: 10.1002/humu.22957. [DOI] [PubMed] [Google Scholar]
- 20.Boon M., Wallmeier J., Ma L., Loges N.T.N.T., Jaspers M., Olbrich H., Dougherty G.W.G.W., Raidt J., Werner C., Amirav I. MCIDAS mutations result in a mucociliary clearance disorder with reduced generation of multiple motile cilia. Nat. Commun. 2014;5:4418. doi: 10.1038/ncomms5418. [DOI] [PubMed] [Google Scholar]
- 21.Wallmeier J., Al-Mutairi D.A.D.A., Chen C.-T.C.-T., Loges N.T.N.T., Pennekamp P., Menchen T., Ma L., Shamseldin H.E.H.E., Olbrich H., Dougherty G.W.G.W. Mutations in CCNO result in congenital mucociliary clearance disorder with reduced generation of multiple motile cilia. Nat. Genet. 2014;46:646–651. doi: 10.1038/ng.2961. [DOI] [PubMed] [Google Scholar]
- 22.Abdi K., Lai C.-H., Paez-Gonzalez P., Lay M., Pyun J., Kuo C.T. Uncovering inherent cellular plasticity of multiciliated ependyma leading to ventricular wall transformation and hydrocephalus. Nat. Commun. 2018;9:1655. doi: 10.1038/s41467-018-03812-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ariani F., Hayek G., Rondinella D., Artuso R., Mencarelli M.A., Spanhol-Rosseto A., Pollazzon M., Buoni S., Spiga O., Ricciardi S. FOXG1 is responsible for the congenital variant of Rett syndrome. Am. J. Hum. Genet. 2008;83:89–93. doi: 10.1016/j.ajhg.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beydon N., Chambellan A., Alberti C., de Blic J., Clément A., Escudier E., Le Bourgeois M. Technical and practical issues for tidal breathing measurements of nasal nitric oxide in children. Pediatr. Pulmonol. 2015;50:1374–1382. doi: 10.1002/ppul.23167. [DOI] [PubMed] [Google Scholar]
- 25.Leigh M.W., Hazucha M.J., Chawla K.K., Baker B.R., Shapiro A.J., Brown D.E., Lavange L.M., Horton B.J., Qaqish B., Carson J.L. Standardizing nasal nitric oxide measurement as a test for primary ciliary dyskinesia. Ann. Am. Thorac. Soc. 2013;10:574–581. doi: 10.1513/AnnalsATS.201305-110OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lucas J.S., Barbato A., Collins S.A., Goutaki M., Behan L., Caudri D., Dell S., Eber E., Escudier E., Hirst R.A. European Respiratory Society guidelines for the diagnosis of primary ciliary dyskinesia. Eur. Respir. J. 2017;49:1601090. doi: 10.1183/13993003.01090-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shapiro A.J., Zariwala M.A., Ferkol T., Davis S.D., Sagel S.D., Dell S.D., Rosenfeld M., Olivier K.N., Milla C., Daniel S.J., Genetic Disorders of Mucociliary Clearance Consortium Diagnosis, monitoring, and treatment of primary ciliary dyskinesia: PCD foundation consensus recommendations based on state of the art review. Pediatr. Pulmonol. 2016;51:115–132. doi: 10.1002/ppul.23304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen J., Knowles H.J., Hebert J.L., Hackett B.P. Mutation of the mouse hepatocyte nuclear factor/forkhead homologue 4 gene results in an absence of cilia and random left-right asymmetry. J. Clin. Invest. 1998;102:1077–1082. doi: 10.1172/JCI4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang M., Bolfing M.F., Knowles H.J., Karnes H., Hackett B.P. Foxj1 regulates asymmetric gene expression during left-right axis patterning in mice. Biochem. Biophys. Res. Commun. 2004;324:1413–1420. doi: 10.1016/j.bbrc.2004.09.207. [DOI] [PubMed] [Google Scholar]
- 30.Yu X., Ng C.P., Habacher H., Roy S. Foxj1 transcription factors are master regulators of the motile ciliogenic program. Nat. Genet. 2008;40:1445–1453. doi: 10.1038/ng.263. [DOI] [PubMed] [Google Scholar]
- 31.Kennedy M.P., Omran H., Leigh M.W., Dell S., Morgan L., Molina P.L., Robinson B.V., Minnix S.L., Olbrich H., Severin T. Congenital heart disease and other heterotaxic defects in a large cohort of patients with primary ciliary dyskinesia. Circulation. 2007;115:2814–2821. doi: 10.1161/CIRCULATIONAHA.106.649038. [DOI] [PubMed] [Google Scholar]
- 32.Harrison M.J., Shapiro A.J., Kennedy M.P. Congenital Heart Disease and Primary Ciliary Dyskinesia. Paediatr. Respir. Rev. 2016;18:25–32. doi: 10.1016/j.prrv.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 33.Vanaken G.J., Bassinet L., Boon M., Mani R., Honoré I., Papon J.-F., Cuppens H., Jaspers M., Lorent N., Coste A. Infertility in an adult cohort with primary ciliary dyskinesia: phenotype-gene association. Eur. Respir. J. 2017;50:1700314. doi: 10.1183/13993003.00314-2017. [DOI] [PubMed] [Google Scholar]
- 34.Raidt J., Werner C., Menchen T., Dougherty G.W., Olbrich H., Loges N.T., Schmitz R., Pennekamp P., Omran H. Ciliary function and motor protein composition of human fallopian tubes. Hum. Reprod. 2015;30:2871–2880. doi: 10.1093/humrep/dev227. [DOI] [PubMed] [Google Scholar]
- 35.Antony D., Becker-Heck A., Zariwala M.A., Schmidts M., Onoufriadis A., Forouhan M., Wilson R., Taylor-Cox T., Dewar A., Jackson C., Uk10k Mutations in CCDC39 and CCDC40 are the major cause of primary ciliary dyskinesia with axonemal disorganization and absent inner dynein arms. Hum. Mutat. 2013;34:462–472. doi: 10.1002/humu.22261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gomperts B.N., Gong-Cooper X., Hackett B.P. Foxj1 regulates basal body anchoring to the cytoskeleton of ciliated pulmonary epithelial cells. J. Cell Sci. 2004;117:1329–1337. doi: 10.1242/jcs.00978. [DOI] [PubMed] [Google Scholar]
- 37.Antoniades I., Stylianou P., Skourides P.A. Making the connection: ciliary adhesion complexes anchor basal bodies to the actin cytoskeleton. Dev. Cell. 2014;28:70–80. doi: 10.1016/j.devcel.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 38.Loges N.T., Olbrich H., Fenske L., Mussaffi H., Horvath J., Fliegauf M., Kuhl H., Baktai G., Peterffy E., Chodhari R. DNAI2 mutations cause primary ciliary dyskinesia with defects in the outer dynein arm. Am. J. Hum. Genet. 2008;83:547–558. doi: 10.1016/j.ajhg.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olbrich H., Häffner K., Kispert A., Völkel A., Volz A., Sasmaz G., Reinhardt R., Hennig S., Lehrach H., Konietzko N. Mutations in DNAH5 cause primary ciliary dyskinesia and randomization of left-right asymmetry. Nat. Genet. 2002;30:143–144. doi: 10.1038/ng817. [DOI] [PubMed] [Google Scholar]
- 40.Becker-Heck A., Zohn I.E., Okabe N., Pollock A., Lenhart K.B., Sullivan-Brown J., McSheene J., Loges N.T., Olbrich H., Haeffner K. The coiled-coil domain containing protein CCDC40 is essential for motile cilia function and left-right axis formation. Nat. Genet. 2011;43:79–84. doi: 10.1038/ng.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Merveille A.-C., Davis E.E., Becker-Heck A., Legendre M., Amirav I., Bataille G., Belmont J., Beydon N., Billen F., Clément A. CCDC39 is required for assembly of inner dynein arms and the dynein regulatory complex and for normal ciliary motility in humans and dogs. Nat. Genet. 2011;43:72–78. doi: 10.1038/ng.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frommer A., Hjeij R., Loges N.T.N.T., Edelbusch C., Jahnke C., Raidt J., Werner C., Wallmeier J., Große-Onnebrink J., Olbrich H. Immunofluorescence Analysis and Diagnosis of Primary Ciliary Dyskinesia with Radial Spoke Defects. Am. J. Respir. Cell Mol. Biol. 2015;53:563–573. doi: 10.1165/rcmb.2014-0483OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beckers A., Alten L., Viebahn C., Andre P., Gossler A. The mouse homeobox gene Noto regulates node morphogenesis, notochordal ciliogenesis, and left right patterning. Proc. Natl. Acad. Sci. USA. 2007;104:15765–15770. doi: 10.1073/pnas.0704344104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.