Abstract
Introduction
Previous in vivo optical coherence tomography studies have proposed the retinal choroid as a potential oculovascular biomarker for Alzheimer's disease (AD). However, the clinical use of the choroid as a purported surrogate marker remains poorly understood. We pursued a histopathological approach to assess choroidal thickness and vascular morphology in severe disease.
Methods
Human postmortem tissues from 8 patients with AD (mean age: 80.1 ± 12.7 years) and from 11 age-matched controls (mean age: 78.4 ± 16.57 years) were analyzed. Thickness, area, and vascularity of the retinal choroid and its sublayers were measured from the nasal and temporal quadrants of the superior retina.
Results
Nasally, the choroid was thinner in the patients with AD than in the controls (22% thickness reduction; P < .001), but to our surprise, the choroid was thicker in the patients with AD than in the controls (~60% increase; P < .03) within the macula, temporally. The choroidal area was also significantly greater in the patients with AD than in the controls (~60% increase; P < .03). Choroidal thickening in AD was strongly correlated with the stromal vessel number (R2 = 0.96, P < .001).
Discussion
We found significant differences in the retinal choroid by layer and by region, nasally and temporally with respect to the optic nerve. Intriguingly, the choroid was markedly thicker in the central macular region and was strongly associated with vessel number in the stromal vascular layer. These quantified histological findings in severe disease expand our understanding of vascular pathology in AD and suggest vascularity as a potential biomarker supplementary to thickness when evaluating the retinal choroid in AD.
Keywords: Alzheimer's disease, Choroid, Retina, Vascular biomarkers, Histopathology, Morphometric analysis, Biomarker
1. Background
Alzheimer's disease (AD) is the leading cause of dementia, affecting more than 5.8 million Americans, and the most expensive disease in the United States at a total cost of $290 billion in 2019 [1]. As the field of medicine prolongs life, the number of people suffering from AD rises together with aging and is estimated to quadruple by 2050 [1]. Timely and effective diagnostic approaches become critical, as therapies are more likely to be successful in early disease stages rather than in the later stages [2].
Ophthalmologic impairments including loss of visual acuity, color recognition, and motion perception have all been reported in patients with AD. Notably, these visual changes have been documented not only in the early stages of disease, but also even before the onset of dementia [[2], [3], [4], [5], [6], [7]]. Cortical deficits alone do not explain these visual deficits. In fact, these visual abnormalities in AD have been associated with degeneration of the anterior visual pathways, as first demonstrated in our laboratory [[8], [9], [10], [11], [12]]. In addition, recent studies have demonstrated similar mechanisms of cortical neurodegeneration involving amyloid deposition occurring in the optic nerve and retina [[13], [14], [15]].
Recent studies have suggested that vascular factors affecting cerebral microcirculation may contribute to AD pathogenesis. Specifically, the onset of clinical dementia is likely preceded by decreased cerebral blood flow followed by decreased amyloid β (Aβ) peptide clearance and consequent neurotoxicity [[16], [17], [18], [19]]. Following the advent of optical coherence tomography (OCT), in vivo studies have revealed significant thickness changes of the retinal choroid in patients with AD. In turn, the choroid has been proposed as a potential early and noninvasive vascular biomarker in the eye for neurodegeneration in the brain [[19], [20], [21], [22], [23]]. However, the clinical use of the choroid as a purported surrogate marker for AD remains inconclusive given inconsistent findings between groups and various limitations associated with live-patient, OCT-based studies [19]. These limitations include the difficulty for cognitively compromised patients to cooperate with study protocols and the persistent challenge of unequivocally diagnosing AD clinically [24,25]. Deviations in the reported results of retinal nerve fiber layer thickness in patients with AD have been shown to arise from intrinsic OCT apparatus variability including eye tracking system, length of examination time, and resolution for adequately visualizing the retina [[26], [27]]. These challenges with using OCT to evaluate the retina in patients with AD are similarly applicable to measuring the retinal choroid. Furthermore, previous studies have focused predominantly on total choroidal thickness with less attention to the choroidal sublayers or vascular morphology. Given these vicissitudes of using an in vivo approach, we pursued an ex vivo approach using human postmortem tissue to perform a precise, quantitative assessment of the retinal choroid and to provide a more comprehensive understanding of this potential oculovascular biomarker for AD diagnosis.
2. Methods
2.1. Neuropathologic assessment and AD diagnosis
The diagnosis of AD was determined neuropathologically in accordance with modified Consortium to Establish a Registry for Alzheimer's Disease (CERAD) or National Institute on Aging (NIA)-Reagan criteria and Braak-Braak Alzheimer classification [[28], [29], [30]]. Multiple brain areas were assessed for Aβ burden and neurofibrillary pathology (NFT). Thioflavin-S fluorescent and Gallyas silver stains were used to evaluate amyloid plaques and tangles in formalin-fixed, paraffin-embedded tissues. To determine the severity of disease, tissues were independently reviewed and scored by three different neuropathologists. Clinical charts of controls and patients with AD were reviewed. The inclusion criteria for AD cases included a neuropathological diagnosis of high-likelihood AD in accordance with the NIA-Reagan Institute criteria [29,30], frequent neuritic plaques in accordance with modified CERAD criteria [29], and Braak stage V or VI [30] based on the neuropathological reports (Dr. C. Miller, ADRC at USC). Eyes from age-matched patients without an AD diagnosis served as control specimens in the study.
2.2. Human tissues
Human postmortem control eyes were received from the Lions VisionGift eye bank in Portland, Oregon, and the AD eyes were acquired through the Alzheimer's Disease Research Center (ADRC) Neuropathology Core at the University of Southern California (USC), funded by the NIA. The Institutional Review Board (IRB) at USC approved the acquisition of donor tissue through the ADRC at USC. All experiments were performed in accordance with the relevant guidelines and regulations as detailed by the IRB at USC. The need to obtain informed consent was waived by the IRB. We analyzed 1 eye from each postmortem subject, which comprised 8 patients with AD (mean age of 80 ± 12.7 years) and 11 age-matched control subjects (mean age of 78 ± 16.57 years), derived at autopsy (Supplementary Material, Table 1 and 2). The groups did not differ significantly in terms of age or years of education. Exclusion criteria were co-occurring and/or confounding ophthalmologic diseases. Tissues with significant posterior pole ocular pathology such as age-related macular degeneration and optic neuropathies such as glaucoma, ischemic optic neuropathy, and diabetic retinopathy were not included in the study. Moreover, exclusion criteria for controls provided by the eye tissue banks were the presence of neurological and psychiatric disorders, treatment with chemotherapeutic agents before death, human immunodeficiency virus infection, alcohol abuse, and diabetes.
2.3. Histology
Postmortem eyes were immersion-fixed in 10% neutral buffered formalin. The eyes were dissected horizontally through the upper region of the optic nerve extending circumferentially through the superior nasal and temporal retinas. This single ribbon of tissue was processed and embedded into paraffin blocks, sectioned at 5 μm on a retracting microtome, and stained with hematoxylin and eosin. All postmortem tissue samples from patients with AD were histologically confirmed for retinal evidence of Aβ plaque accumulation using our previously established protocol [14]. Controls were processed using the same protocol with the omission of the primary antibody to assess nonspecific labeling.
2.4. Morphometric analysis
Digitized histopathology images were acquired using the Aperio ScanScope-model CS2 (Leica Biosystems, Buffalo Grove, Illinois) equipped with a five-slide capacity and high-resolution magnification capabilities using a line scanning method. The 40x lens on the digital pathology scanner produced a rapid and seamless image of the choroid including the entire superior-nasal and superior-temporal regions in a single section. The system was calibrated before each measurement session at 0.25 μM/pixel linked with the 40x scanning lens protocol [31,32].
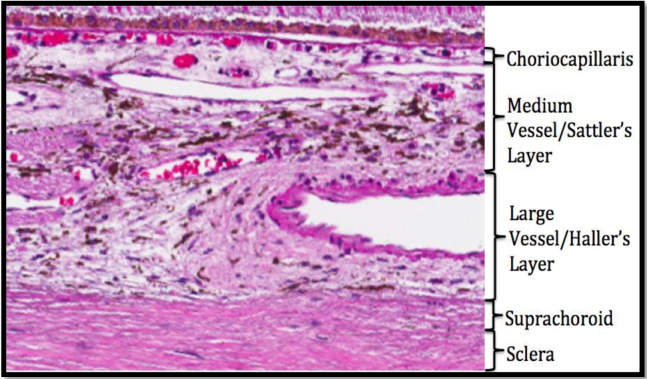
Fig. 1 depicts the boundaries of the choroid and its vascular sublayers on histopathology. Measurements of choroidal thickness were performed using ImageScope v12.1.0.5029 software (Leica Biosystems) and obtained at approximately every 500 microns spanning the total distance beginning from the middle of the optic nerve to the center of the macula within the superior ribbon of the tissue. This amounted to a total of 8 measuring points for the choroid, superotemporally. In addition, the same total distance was projected superonasally relative to the optic nerve with measurements performed again at approximately every 500 microns for a total of 8 measuring points for the choroid. Full choroidal thickness (Full CT) was defined as the distance between Bruch's membrane (the lower boundary of retinal pigmented epithelium [RPE]) and the choroid-sclera interface formally known as the suprachoroid on histopathology.
Fig. 1.
Illustration of the choroid and its stratified sublayers in a representative control micrograph stained with hematoxylin and eosin on postmortem histopathology.
We also performed a subanalysis of the central macular region, spanning a length of 1.5 mm within the ribbon of the tissue. Choroidal sublayers, namely the choriocapillaris and the stroma, were included in the analysis. Choriocapillaris thickness (CC Th) was defined as the distance between Bruch's membrane and the upper boundary of the stroma. Stromal thickness (Stroma Th), which encompasses both the medium and large vascular layers, was defined as the distance between the lower boundary of the choriocapillaris and the choroid-sclera interface also known as the suprachoroid. Additional parameters including the vessel area and vessel number were also recorded. The total choroidal area (TCA) was measured as the product of Full choroidal thickness (CT) and the length of the region (i.e. 1.5 mm). The total choriocapillaris area (TCCA) was measured as the product of CC Th and 1.5 mm. The total stromal area (TSA) was measured as the product of Stroma Th and 1.5 mm. The luminal area of the choroidal vessels was manually measured using an Aperio ScanScope-model CS2 (Leica Biosystems, Buffalo Grove, Illinois) area tool, providing an automated value for the enclosed vessel luminal areas. Specifically, the choriocapillaris luminal area (CCLA) was measured as the summed total of the vessel lumen area within the choriocapillaris layer. The stroma luminal area (SLA) was measured as the summed total of the vessel lumen area within the stroma layer. To assess choroidal vascularity, we manually counted the number of vessels within the choriocapillaris (CC vessel number) and stromal (Stromal vessel number) layers spanning the 1.5 mm length of the central macular region. The validities of the performed measurements were subsequently assessed by a masked histopathologist.
2.5. Statistical methods
Statistical analysis was performed using SPSS V.20 package software. To compare the groups, independent t-test and the Mann-Whitney U test were applied. The normality assumption for the independent variables was checked with the Shapiro-Wilk test. The variables that were compliant with the normality assumption were subjected to the independent t-test, whereas those that did not meet the normality assumption were subjected to the Mann-Whitney U test. For the analysis of correlation, Pearson correlation coefficient and Spearman correlation coefficient were used. The statistical significance was established at P < .05.
3. Results
3.1. Superonasal versus superotemporal
As seen in Fig. 2, the choroid was significantly thinner in patients with AD than in controls, superonasally. Superotemporally, however, the choroid was thicker in patients with AD than in controls, especially within the central region of the macula (Fig. 3). Fig. 4 depicts quantitative analyses of these qualitative findings.
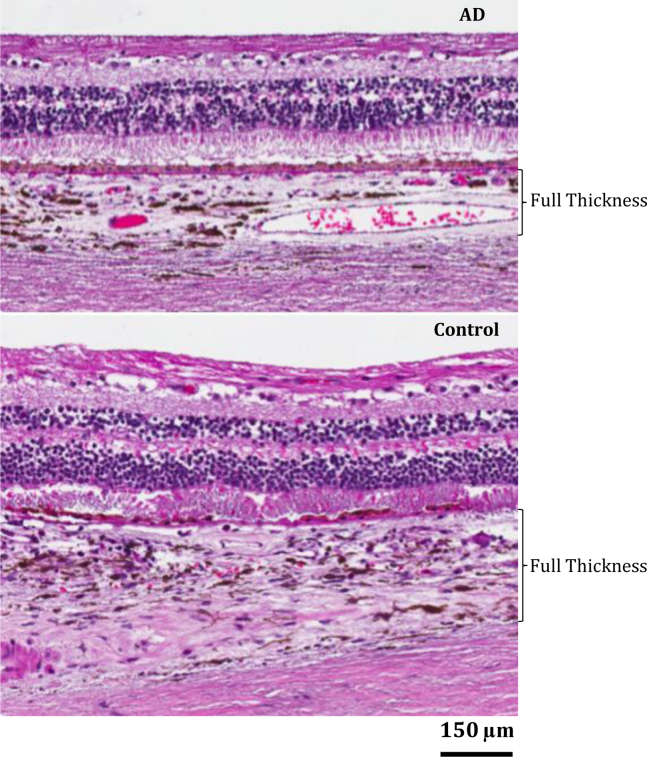
Fig. 2.
Qualitative assessment of the superonasal choroid in representative AD (Top) and control (Bottom) micrographs on light microscopy revealed superonasal choroidal thinning in patients with AD relative controls. Abbreviation: AD, Alzheimer's disease.
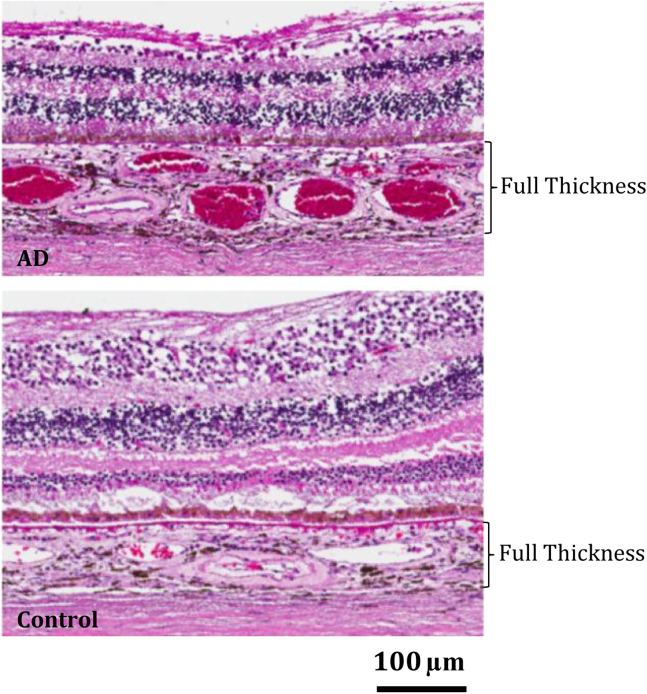
Fig. 3.
Qualitative assessment of the superotemporal choroid in representative AD (Top) and control (Bottom) micrographs on light microscopy revealed superotemporal choroidal thickening with increased vascularity in patients with AD relative to controls, most pronounced in the macular region. Abbreviation: AD, Alzheimer's disease.
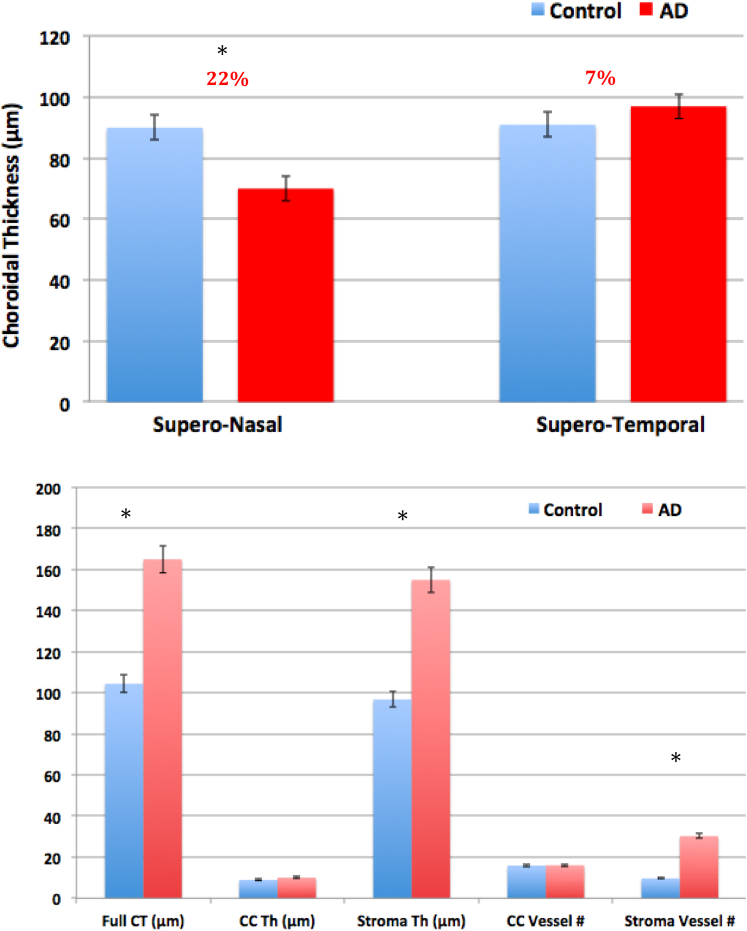
Figure 4.
(Top) Illustrates average choroidal thickness comparison between controls (blue) and AD (red) tissue samples for the superonasal and superotemporal regions (∗P < .05). Error bars denote standard deviation. (Bottom) Illustrates comparison of choroidal parameters between AD and control tissue samples within the macular region of the choroid including full choroidal thickness (Full CT); choriocapillaris thickness (CC Th); stromal thickness (Stroma Th) and vessel number for the respective layers (∗P < .05). Abbreviation: AD, Alzheimer's disease.
3.2. Macular choroid subanalysis: Thickness and area
As seen in Fig. 4, the macular stroma was markedly thicker in patients with AD than in controls. The macular choriocapillaris was also thicker in patients with AD, although not statistically significant.
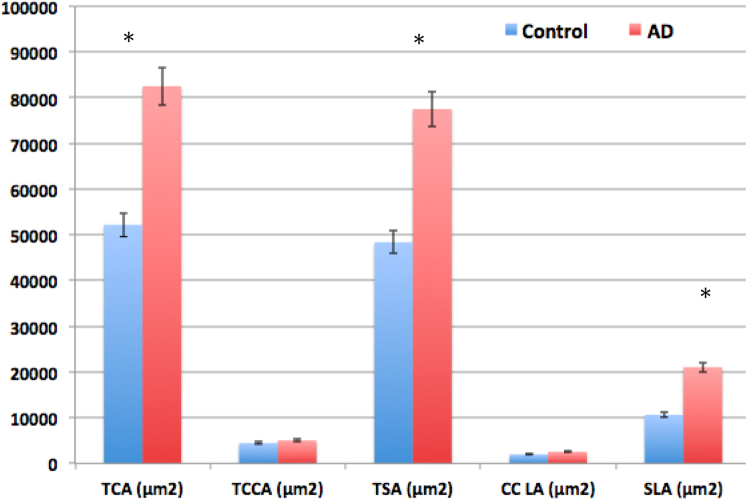
Fig. 5 displays the quantitative findings for the choroidal area. The TCA was significantly greater in patients with AD than in controls. With respect to the choroidal sublayers, the TSA was markedly larger in patients with AD than in controls. TCCA was similarly larger in patients with AD, although not statistically significant (Fig. 5). With respect to the luminal area, SLA was markedly greater in patients with AD than in controls. The CCLA was similarly greater in patients with AD, although not statistically significant.
Fig. 5.
Illustrates comparison of choroidal parameters between AD and control tissue samples within the macular region of the choroid including total choroidal area (TCA); total choriocapillaris area (TCCA); total stroma area (TSA); choriocapillaris luminal area (CC LA); stroma luminal area (SLA) (∗P < .05). Abbreviation: AD, Alzheimer's disease.
3.3. Macular choroid subanalysis: Vascularity
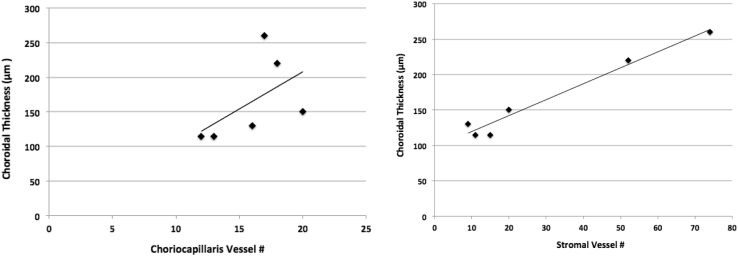
As seen in Fig. 4, the stromal vessel number was significantly greater in patients with AD than in controls. In contrast, the CC vessel number was not significantly different between patients with AD and controls (Fig. 4). Pearson correlation testing (Fig. 6) revealed that choroidal thickening in patients with AD was strongly correlated with stromal vessel number (R2 = 0.96, P < .001) and not with CC vessel number (R2 = 0.28, P = .2).
Fig. 6.
Correlation testing for choroidal thickness as a function of vessel number for the stroma (Right) and choriocapillaris (Left).
4. Discussion
We provide, for the first time, a histomorphometric analysis of choroidal thickness and vascularity in severe disease. These tissues were histopathologically confirmed for retinal amyloid deposition and were derived from patients, who were neuropathologically confirmed for severe AD. We found significant morphological changes in the choroid by layer and by region, superonasally and superotemporally, with respect to the optic nerve. Taken together, these findings expand our understanding of vascular pathology in AD and suggest that choroidal vascularity in addition to thickness may together be useful as markers for assessing disease.
Histological examination of the superior-nasal choroid in patients with AD revealed significant thinning. Previous studies have similarly shown choroidal thinning in patients with AD, although predominantly in the macular choroid [[20], [21], [22], [23]]. We expand upon these findings by illustrating choroidal thickness changes beyond the macula. Choroidal thinning in patients with AD within the ocular vasculature might involve mechanisms of thinning similar to those of the choroid plexus within the cerebral vasculature. Pathological inflammatory cascades have been postulated as the cause of progressive neurodegeneration and vasoregression of the neurovascular choroid [[33], [34], [35]]. A previous study using a rat model similarly identified microglia recruitment and complement protein activation in the ocular choroid, suggesting an inflammatory response to local Aβ deposition in the retina [36]. Taken together, these findings suggest that the changes exhibited in the retinal choroid may be related to Aβ deposition in the retina.
The superior-temporal choroid was thicker in patients with AD than in controls, especially the stromal layer of the macular choroid. Further analysis revealed that the stroma was significantly more vascular in AD and choroidal thickness in the macular region was strongly correlated with stromal vessel number. Previous studies have demonstrated the shared vascular abnormalities between AD and aging with respect to lipid accumulation and atherosclerotic narrowing of the cerebral vessels [37]. In fact, given this shared vascular pathology, AD is often characterized as a disease of aging, albeit accelerated by impaired Aβ clearance [17,18,34,35,37,38]. Notably, these vascular changes seen with aging in the neurovascular choroid have similarly been shown in the oculovascular choroid. In age-related macular degeneration (AMD), lipid accumulation and atherosclerosis create an ischemic environment, which disturbs the normal paracrine relationship between trophic factors and the choroid [[39], [40], [41]]. In response to this hyperlipaemic state, the choroid may undergo structural changes including smooth-muscle cell proliferation, increased synthesis of extracellular matrix, and vascular proliferation from downstream upregulation of vascular endothelial growth factor (VEGF) and other trophic factors, ultimately leading to retinal dysfunction [[40], [41], [42], [43]]. Our choroidal findings in AD resemble those of AMD and may be explained by a shared vascular pathology between aging and AD in the oculovasculature similar to that of the neurovasculature.
The thickness profiles of the superior-nasal and superior-temporal choroid matched the distribution of retinal Aβ deposits in the mid- and far-periphery of these tissues as demonstrated by our group's previous study [14]. Our laboratory recently showed that the superior-nasal retina was markedly thinner than the superior-temporal retina of these tissues in AD [44]. Histologically, the macular choroid houses an abundant vascular supply in the stroma to nourish the large density of photoreceptors in the macula [45]. Consistent with this, the macula was the most atrophic area of the retina in severe AD as shown in our previous study [44]. Hence, thickening of the macular choroid in the temporal quadrant of the retina may represent a compensatory vascular-proliferative response in severe disease stages. Vessels in the choroid may simply be more numerous or, more likely, distended in response to angiogenic factors such as VEGF.
Our findings of choroidal thickening in patients with AD were unexpected, as most in vivo OCT studies have reported choroidal thinning in patients with AD. Notably, however, these prior live-patient studies have been variable in their results. Bulut et al. [22] found the choroid to be thinner temporally than nasally in AD, whereas Cunha et al. [19] found the choroid to be thinner nasally than temporally. In addition, choroidal thickness changes in AD as measured in vivo have not correlated with patient psychometric scores [20,22,23]. In turn, the association between choroidal thickness and dementia is controversial and the use of the choroid as a biomarker for disease remains inconclusive. These discrepancies in thickness findings may be attributed to not only the challenges of unequivocally diagnosing AD clinically, but also the limitations of OCT in measuring the true thickness of the choroid. In particular, structural domain enhanced depth imaging (EDI)-OCT reportedly obscures choroidal boundaries, reducing the reliability of choroidal measurements in vivo [46,47]. Our histopathological approach enabled precise measurements of choroidal thickness in AD.
Aside from live-patient OCT studies, a previous study conducted by Tsai et al. [36] showed thinning of the macular choroid in postmortem human tissues. Although we similarly used a histological approach, our study differs from that conducted by Tsai et al. in several ways. First, our study of choroidal thickness in patients with AD was specific to the severe, late stages of disease. Second, tissues were specifically derived from the superior retina, which has been shown to have the highest amyloid burden [14,15]. Third, choroidal thickness was defined as the distance between Bruch's membrane and the choroid-sclera interface (i.e. suprachoroid). These histological boundaries measure the choroid extending as far as the vessel walls closest to the sclera. In contradistinction, the anatomical boundaries used by Tsai et al. for measuring choroidal thickness are not clearly defined. Furthermore, our study evaluated thickness and vascularity of the choroidal sublayers, which corroborated our choroidal thickness findings in AD.
Our measurements were subject to postmortem tissue swelling and tissue shrinkage as induced by fixation. However, both tissue groups prepared under identical protocols and deviations would equally affect both patients with AD and controls, mitigating measurement bias. Despite the limited sample size, the present study represents the largest histological analysis of choroidal thickness and vascularity in AD. Including only one eye for each patient is a particular strength of our study as it avoids artificial sample size enhancement that would have arisen from including both eyes. In addition, we used 16 measurements per layer to account for case-specific differences in choroidal structure, further strengthening the significance of our findings.
5. Conclusions and future directions
We provide, for the first time, a histomorphometric analysis of both choroidal thickness and vascularity in severe AD. These postmortem tissues were histologically confirmed for Aβ and were derived from patients, who were neuropathologically confirmed for severe disease. Different patterns of thinning were exhibited in the superior nasal and temporal regions of the retinal choroid relative to the optic nerve. Thickening of the temporal choroid was especially apparent in the macular region, where the retina has the highest energy demand [48] and is reportedly most atrophic in AD [38,44]. Increased choroidal thickness in AD was significantly correlated with increased vessel number. We postulate that a compensatory vascular proliferation in response to metabolic dysfunction in AD may explain the thickening of the macular choroid in severe disease. These oculovascular findings in AD suggest that choroidal vascularity may serve as a useful marker in addition to thickness for assessing disease severity.
Overall, a comprehensive understanding of the retinal choroid as measured ex vivo can be useful for assessing the clinical validity and use of the choroid as an ocular vascular biomarker in vivo using OCT. Such an in vivo quantitative means of assessing disease may allow monitoring of AD and enable the assessment of purported treatments. Therefore, future studies are suggested. Since our study focused on AD tissues derived from patients with severe AD (Braak stages V and VI), histological comparisons from earlier disease stages would be worthwhile to assess thickness changes as a function of earlier disease progression. Given the geographical differences in choroidal thickness shown by our ex vivo study, additional in vivo studies assessing thickness changes in these regions beyond the macula may be warranted. Recent studies conducted by Koronyo et al. [15] have demonstrated the highest amyloid burden specifically in the superior retina. With this in mind, the current morphometric analysis focused on choroidal thickness changes in the superior retina. This is a particular strength of our study as these tissues were similarly derived and histopathologically confirmed for Aβ deposits in the superior quadrants, as previously demonstrated in our laboratory [14,44]. Nevertheless, future studies evaluating choroidal thickness in alternative retinal quadrants may be worthwhile. Finally, our choroidal findings are observational in nature and additional studies are necessary to elucidate the precise mechanism of thickness and vascular changes in advanced disease stages.
Research in Context.
-
1.
Systematic review: For the literature review, commonly available medical databases and important review papers in the field were searched.
-
2.
Interpretation: The present study provides an improved understanding of the retinal choroid as a purported biomarker for a profound neurodegenerative disease on the histological level and proposes an objectively precise and quantifiable means of assessing this disease in ways that may ultimately facilitate our pursuits towards live-patient imaging, non-invasively.
-
3.
Future directions: Future studies should explore histological comparisons from earlier disease stages to assess thickness changes as a function of earlier disease progression. In addition, the mechanisms underlying choroidal thickness and vascular changes need to be elucidated.
Acknowledgments
The authors thank the Lions Vision Gift eye bank and the Alzheimer's Disease Research Center (ADRC) Neuropathology Core at the University of Southern California (USC) for providing the tissue samples for this study. They also thank funding sources including Research to Prevent Blindness (RPB), National Institute on Aging (NIA), and International Foundation for Optic Nerve Disease (IFOND).
Footnotes
Disclosure: S. Asanad has unrestricted funding from Research to Prevent Blindness Inc.; F.N. Ross-Cisneros, None; E. Barron, None; M. Nassisi, None; W. Sultan, None; R. Karanjia, None; A.A. Sadun has unrestricted funding from Research to Prevent Blindness Inc., and clinical trials in LHON (GenSight and Stealth), but has no proprietary interest or receipt of consultant compensation.
Funding: This work was supported by the National Institutes of Health, National Institute on Aging grant #P50-AG05142 (USCADRC Neuropathology Core Grant); and the Research to Prevent Blindness Inc., unrestricted grant.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.dadm.2019.08.005.
Supplementary data
References
- 1.2019 Alzheimer's disease facts and figures. Alzheimers Dement. 2019;15:321–387. [Google Scholar]
- 2.Pillai J.A., Cummings J.L. Clinical trials in predementia stages of Alzheimer disease. Med Clin North Am. 2013;97:439–457. doi: 10.1016/j.mcna.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Katz B., Rimmer S. Ophthalmologic manifestations of Alzheimer's disease. Surv Ophthalmol. 1989;34:31–43. doi: 10.1016/0039-6257(89)90127-6. [DOI] [PubMed] [Google Scholar]
- 4.Uhlmann R.F., Larson E.B., Koepsell T.D., Rees T.S., Duckert L.G. Visual impairment and cognitive dysfunction in Alzheimer's disease. J Gen Intern Med. 1991;6:126–132. doi: 10.1007/BF02598307. [DOI] [PubMed] [Google Scholar]
- 5.Salamone G., Di lorenzo C., Mosti S., Lupo F., Cravello L., Palmer K. Color discrimination performance in patients with Alzheimer's disease. Dement Geriatr Cogn Disord. 2009;27:501–507. doi: 10.1159/000218366. [DOI] [PubMed] [Google Scholar]
- 6.Tzekov R., Mullan M. Vision function abnormalities in Alzheimer disease. Surv Ophthalmol. 2014;59:414–433. doi: 10.1016/j.survophthal.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Javaid F.Z., Brenton J., Guo L., Cordeiro M.F. Visual and Ocular Manifestations of AD and their use as biomarkers for diagnosis and progression. Front Neurol. 2016;7:55. doi: 10.3389/fneur.2016.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hinton D.R., Sadun A.A., Blanks J.C., Miller C.A. Optic-nerve degeneration in Alzheimer's disease. N Engl J Med. 1986;315:485–487. doi: 10.1056/NEJM198608213150804. [DOI] [PubMed] [Google Scholar]
- 9.Sadun A.A., Johnson B., Miao M. Proceedings of the Sixth Meeting of the International Neuro-Ophthalmology Society, Hakone Japan, 1986. Aeolus Press; Amsterdam, Holland: 1987. Axon caliber populations in the human optic nerve: change with age and disease; pp. 5–20. [Google Scholar]
- 10.Sadun A.A., Borchert M., DeVita E., Hinton D.R., Bassic C.J. Assessment of visual impairment in patients with Alzheimer's disease. Am J Ophthalmol. 1987;104:113–120. doi: 10.1016/0002-9394(87)90001-8. [DOI] [PubMed] [Google Scholar]
- 11.Blanks J.C., Hinton D.R., Sadun A.A. Retinal ganglion cell degeneration in Alzheimer's disease. Brain Res. 1989;501:364–372. doi: 10.1016/0006-8993(89)90653-7. [DOI] [PubMed] [Google Scholar]
- 12.Blanks J.C., Torigoe Y., Hinton D.R., Blanks R.H. Retinal pathology in Alzheimer's disease. I. Ganglion cell loss in foveal/parafoveal retina. Neurobiol Aging. 1996;17:377–384. doi: 10.1016/0197-4580(96)00010-3. [DOI] [PubMed] [Google Scholar]
- 13.Koronyo-Hamaoui M., Koronyo Y., Ljubimov A.V., Miller C.A., Ko M.K., Black K.L. Identification of amyloid plaques in retinas from Alzheimer's patients and noninvasive in vivo optical imaging of retinal plaques in a mouse model. Neuroimage. 2011;54:S204–S217. doi: 10.1016/j.neuroimage.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.La Morgia C., Ross-Cisneros F.N., Koronyo Y., Hannibal J., Gallassi R., Gallassi R. Melanopsin retinal ganglion cell loss in Alzheimer disease. Ann Neurol. 2016;79:90–109. doi: 10.1002/ana.24548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koronyo Y., Biggs D., Barron E., Boyer D.S., Pearlman J.A., Au W.J. Retinal amyloid pathology and proof-of-concept imaging trial in Alzheimer’s disease. JCI Insight. 2017;2:e93621. doi: 10.1172/jci.insight.93621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalaria R.N. The role of cerebral ischemia in Alzheimer's disease. Neurobiol Aging. 2000;21:321–330. doi: 10.1016/s0197-4580(00)00125-1. [DOI] [PubMed] [Google Scholar]
- 17.de la Torre J.C. Is Alzheimer's disease a neurodegenerative or a vascular disorder? Data, dogma, and dialectics. Lancet Neurol. 2004;3:184–190. doi: 10.1016/S1474-4422(04)00683-0. [DOI] [PubMed] [Google Scholar]
- 18.Kalaria R.N., Akinyemi R., Ihara M. Does vascular pathology contribute to Alzheimer changes? J Neurol Sci. 2012;322:141–147. doi: 10.1016/j.jns.2012.07.032. [DOI] [PubMed] [Google Scholar]
- 19.Cunha J.P., Proença R., Dias-santos A., Melancia D., Almeida R., Águas H. Choroidal thinning: Alzheimer's disease and aging. Alzheimers Dement (Amst) 2017;8:11–17. doi: 10.1016/j.dadm.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gharbiya M., Trebbastoni A., Parisi F., Manganiello S., Cruciani F., D'Antonio F. Choroidal thinning as a new finding in Alzheimer's disease: evidence from enhanced depth imaging spectral domain optical coherence tomography. J Alzheimers Dis. 2014;40:907–917. doi: 10.3233/JAD-132039. [DOI] [PubMed] [Google Scholar]
- 21.Bayhan H.A., Aslan bayhan S., Celikbilek A., Tanık N., Gürdal C. Evaluation of the chorioretinal thickness changes in Alzheimer's disease using spectral-domain optical coherence tomography. Clin Exp Ophthalmol. 2015;43:145–151. doi: 10.1111/ceo.12386. [DOI] [PubMed] [Google Scholar]
- 22.Bulut M., Yaman A., Erol M.K., Kurtulus F., Toslak D., Dogan B. Choroidal thickness in patients with mild cognitive impairment and Alzheimer's type dementia. J Ophthalmol. 2016;2016:7291257. doi: 10.1155/2016/7291257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trebbastoni A., Marcelli M., Mallone F., D'Antonio F., Imbriano L., Campanelli A. Attenuation of choroidal thickness in patients with Alzheimer disease: evidence from an Italian Prospective Study. Alzheimer Dis Assoc Disord. 2017;31:128–134. doi: 10.1097/WAD.0000000000000176. [DOI] [PubMed] [Google Scholar]
- 24.Dubois B., Padovani A., Scheltens P., Rossi A., Dell'agnello G. Timely diagnosis for Alzheimer's Disease: a literature review on benefits and challenges. J Alzheimers Dis. 2016;49:617–631. doi: 10.3233/JAD-150692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amieva H., Le Goff M., Millet X., Orgogozo J.M., Pérès K., Barberger-Gateau P. Prodromal Alzheimer's disease: successive emergence of the clinical symptoms. Ann Neurol. 2008;64:492–498. doi: 10.1002/ana.21509. [DOI] [PubMed] [Google Scholar]
- 26.Thomson K., Yeo J.M., Waddell B., Cameron J.R., Pal S. A systematic review and meta- analysis of RNFL change in dementia, using OCT. Alzheimers Dement (Amst) 2015;1:136–143. doi: 10.1016/j.dadm.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hart N.J., Koronyo Y., Black K.L., Koronyo-Hamaoui M. Ocular indicators of Alzheimer's: exploring disease in the retina. Acta Neuropathol. 2016;132:767–787. doi: 10.1007/s00401-016-1613-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hyman B.T., Trojanowski J.Q. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol. 1997;56:1095–1097. doi: 10.1097/00005072-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Mirra S.S., Heyman A., McKeel D., Sumi M., Crain B.J., Brownlee L.M. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 30.Braak H., Braak E. Staging of Alzheimer's disease-related neurofibrillary changes. Neurobiol Aging. 1995;16:271–278. doi: 10.1016/0197-4580(95)00021-6. [DOI] [PubMed] [Google Scholar]
- 31.Webster J.D., Dunstan R.W. Whole-slide imaging and automated image analysis: considerations and opportunities in the practice of pathology. Vet Pathol. 2014;51:211–223. doi: 10.1177/0300985813503570. [DOI] [PubMed] [Google Scholar]
- 32.Daniel C., Rojo M.G., Bourquard K. Standards to support information systems integration in anatomic pathology. Arch Pathol Lab Med. 2009;133:1841–1849. doi: 10.5858/133.11.1841. [DOI] [PubMed] [Google Scholar]
- 33.Jellinger K.A. Alzheimer disease and cerebrovascular pathology: an update. J Neural Transm. 2002;109:813–836. doi: 10.1007/s007020200068. [DOI] [PubMed] [Google Scholar]
- 34.Miao J., Xu F., Davis J., Otte-Höller I., Verbeek M.M., Van Nostrand W.E. Cerebral microvascular amyloid beta protein deposition induces vascular degeneration and neuroinflammation in transgenic mice expressing human vasculotropic mutant amyloid beta precursor protein. Am J Pathol. 2005;167:505–515. doi: 10.1016/s0002-9440(10)62993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marchesi V.T. Alzheimer's dementia begins as a disease of small blood vessels, damaged by oxidative-induced inflammation and dysregulated amyloid metabolism: implications for early detection and therapy. FASEB J. 2011;25:5–13. doi: 10.1096/fj.11-0102ufm. [DOI] [PubMed] [Google Scholar]
- 36.Tsai Y., Lu B., Ljubimov A.V., Girman S., Ross-Cisneros F.N., Sadun A.A. Ocular changes in TgF344-AD Rat model of Alzheimer's disease. Invest Ophthalmol Vis Sci. 2014;55:523–534. doi: 10.1167/iovs.13-12888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wildsmith K.R., Holley M., Savage J.C., Skerrett R., Landreth G.E. Evidence for impaired amyloid β clearance in Alzheimer's disease. Alzheimers Res Ther. 2013;5:33. doi: 10.1186/alzrt187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sadun A.A., Asanad S. The eye in Alzheimer's disease. Ophthalmology. 2018;4:511–512. doi: 10.1016/j.ophtha.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 39.Schlingemann R.O. Role of growth factors and the wound healing response in age related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2004;242:91–101. doi: 10.1007/s00417-003-0828-0. [DOI] [PubMed] [Google Scholar]
- 40.Blaauwgeers H.G., Holtkamp G.M., Rutten H., Witmer A.N., Koolwijk P., Partanen T.A. Polarized vascular endothelial growth factor secretion by human retinal pigment epithelium and localization of vascular endothelial growth factor receptors on the inner choriocapillaris. Evidence for a trophic paracrine relation. Am J Pathol. 1999;155:421–428. doi: 10.1016/S0002-9440(10)65138-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pauleikhoff D., Harper A., Marshall J., Bird A.C. Aging changes in Bruch's membrane: a histochemical and morphological study. Ophthalmology. 1989;97:171–177. [PubMed] [Google Scholar]
- 42.Holz F.G., Sheraidah G., Pauleikhoff D., Bird A.C. Analysis of lipid deposits extracted from human macular and peripheral Bruch's membrane. Arch Ophthalmol. 1994;112:402–406. doi: 10.1001/archopht.1994.01090150132035. [DOI] [PubMed] [Google Scholar]
- 43.Kaarniranta K., Salminen A., Haapasalo A., Soininen H., Hiltunen M. Age-related macular degeneration (AMD): Alzheimer's disease in the eye? J Alzheimers Dis. 2011;24:615–631. doi: 10.3233/JAD-2011-101908. [DOI] [PubMed] [Google Scholar]
- 44.Asanad S., Ross-Cisneros F.N., Nassisi M., Barron E., Karanjia R., Sadun A.A. The retina in Alzheimer's disease: histomorphometric analysis of an ophthalmological biomarkers. Invest Ophthalmol Vis Sci. 2019;60:1491–1500. doi: 10.1167/iovs.18-25966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shimizu K., Ujiie K. Morphology of the submacular choroid: vascular structure. Ophthalmologica. 1981;183:5–10. doi: 10.1159/000309126. [DOI] [PubMed] [Google Scholar]
- 46.Shin J.W., Shin Y.U., Lee B.R. Choroidal thickness and volume mapping by a six radial scan protocol on spectral-domain optical coherence tomography. Ophthalmology. 2011;119:1017–1023. doi: 10.1016/j.ophtha.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 47.Mwanza J.C., Hochberg J.T., Banitt M.R., Feuer W.J., Budenz D.L. Lack of association between glaucoma and macular choroidal thickness measured with enhanced depth imaging optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52:3430–3435. doi: 10.1167/iovs.10-6600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wong-Riley M. Energy metabolism of the visual system. Eye Brain. 2010;2010:99–116. doi: 10.2147/EB.S9078. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.