Abstract
Background
A three-drug regimen (macrolide, ethambutol, and rifampicin) is recommended for the treatment of Mycobacterium avium complex pulmonary disease (MAC-PD). Although macrolide has proven efficacy, the role of ethambutol and rifampicin in patients without acquired immune deficiency syndrome is not proven with clinical studies. We aimed to clarify the roles of ethambutol and rifampicin in the treatment of MAC-PD.
Methods
Patients treated for MAC-PD between March 1st, 2009 and October 31st, 2018 were reviewed retrospectively. Rates of culture conversion, microbiological cure, treatment failure, and recurrence were compared according to the maintenance (≥6 months) of ethambutol or rifampicin with macrolide.
Results
Among the 237 patients, 122 (51.5%) maintained ethambutol and rifampicin with macrolide, 58 (24.5%) maintained ethambutol and macrolide, 32 (13.5%) maintained rifampicin and macrolide, and 25 (10.6%) maintained macrolide only. Culture conversion was reached for 190/237 (80.2%) patients and microbiological cure was achieved for 129/177 (72.9%) who completed the treatment. Treatment failure despite ≥12 months of treatment was observed in 66/204 (32.4%), and recurrence was identified in 16/129 (12.4%) who achieved microbiological cure. Compared with maintenance of macrolide only, maintenance of ethambutol, rifampicin or both with macrolide were associated with higher odds of culture conversion [odds ratio (OR), 95% confidence interval (CI): 18.06, 3.67–88.92; 15.82, 2.38–105.33; and 17.12, 3.93–74.60, respectively]. Higher odds of microbiological cure were associated with maintenance of both ethambutol and rifampicin with macrolide (OR, 95% CI: 5.74, 1.54–21.42) and macrolide and ethambutol (OR, 95% CI: 5.12, 1.72–15.24) but not macrolide and rifampicin. Maintenance of both ethambutol and rifampicin with macrolide was associated with lower odds of treatment failure (OR, 95% CI: 0.09, 0.01–0.53) compared with macrolide only, while maintenance of one of these with macrolide was not. Maintenance of both ethambutol and rifampicin or one of these with macrolide did not decrease the probability of recurrence when compared with macrolide only.
Conclusions
Maintenance (≥6 months) of ethambutol and rifampicin with macrolide was associated with the most favorable treatment outcomes among patients with MAC-PD. Given the association between ongoing ethambutol use and microbiological cure, clinicians should maintain ethambutol unless definite adverse events develop.
Keywords: Mycobacterium avium complex pulmonary disease, Ethambutol, Rifampicin
Background
The prevalence of nontuberculous mycobacterial (NTM) pulmonary disease is increasing globally, with the most common pathogen being Mycobacterium avium complex (MAC) species [1–3]. MAC is a ubiquitous organism commonly found in water, dirt, and soil but can cause serious pulmonary diseases in some people. M. avium and M. intracellulare are the most well-known pathogens, but other MAC such as M. arosiense, M. bouchedurhonense, M. chimaera, M. colombiense, M. marseillense, M. timonense, M. vulneris, and M. yongonense can also cause pulmonary diseases [4].
Treatment for MAC pulmonary disease (MAC-PD) requires consideration of clinical and/or radiographical deterioration and usually comprises a multidrug regimen including macrolide, ethambutol, and rifampicin, with varying success [5]. A recent systematic review reported that the three-drug regimen (macrolide, ethambutol, and rifampicin) with occasional use of an injectable agent could cure MAC-PD in 57% of patients [6].
The recommendation of such a combination is based on previous studies about disseminated MAC disease in acquired immune deficiency syndrome (AIDS) patients [7]. The efficacy of macrolide among MAC-PD patients is well established, but macrolide monotherapy can induce microbiological resistance [8]. Therefore, companion drugs such as ethambutol or rifampicin are used. Nevertheless, the individual effectiveness of these two drugs in patients without AIDS is not proven with clinical studies. Considering the long-term use of medication and possible adverse events, recognizing the most efficacious accompanying drug is necessary.
The aim of this study was to elucidate the roles of ethambutol and rifampicin in the treatment of MAC-PD in adult patients.
Methods
Study population
Patients aged 19 years or older in whom treatment for MAC-PD was initiated between March 1st, 2009 and October 31st, 2018 at Seoul National University Hospital were included. Diagnosis of MAC-PD was based on the 2007 American Thoracic Society and the Infectious Diseases Society of America guideline [5] and the 2017 British Thoracic Society guideline [9]. Some patients were included in our previous reports [10, 11].
The study was conducted in accordance with the amended Declaration of Helsinki and was approved by the institutional review board of Seoul National University Hospital (protocol number: H-1705-017-851). Informed consent was waived because of the retrospective design of the study, and the information of each patient was anonymized prior to analyses.
Clinical, microbiological, and radiographic evaluation
Clinical, microbiological, and radiographic information were collected retrospectively. Cultures were grown in both solid Ogawa media and the BACTEC MGIT 960 system, and isolated NTM were identified into species [4]. Identification was performed based on sequence analysis of the 16S rRNA and rpoB gene using the algorithm described in the Clinical and Laboratory Standards Institute guidelines MM18-A [12]. Once the NTM was identified as MAC, a drug susceptibility test for clarithromycin was performed. The minimal inhibitory concentration (MIC) test was performed at the Korean Institute of Tuberculosis, and was determined using the broth microdilution method in accordance with the Clinical and Laboratory Standards Institute guidelines [12]. Isolates were considered susceptible if the MIC of clarithromycin was ≤8 μg/mL, resistant if ≥32 μg/mL, and intermediate if 16 μg/mL on Mueller–Hinton agar. Chest computed tomography findings were categorized as the nodular bronchiectatic form when bilateral bronchiectasis and cellular bronchiolitis were mainly present, and the upper lobe cavitary form when cavities in the upper lobes were observed [13].
Treatment and follow-up
The treatment regimen was mainly based on the 2007 American Thoracic Society and the Infectious Diseases Society of America guidelines, but was customized by the attending physician according to each patient’s condition [5]. Macrolide was included in every regimen, and ethambutol and rifampicin were considered as companion drugs. The companion drugs were omitted or stopped when they were considered clinically inappropriate; for example, ethambutol was omitted or stopped if a patient’s visual acuity deteriorated, and rifampicin was omitted or stopped when a patient had underlying liver diseases or newly developed hepatic dysfunction. Injectable drugs including streptomycin or amikacin were considered when the MAC-PD was extensive or refractory to other oral agents.
Adverse reactions to medications were recorded by the physician. Subjective events such as decreased visual acuity, deteriorating hearing, and nausea were either self-reported or enquired after by the attending physician. If patients had symptoms suggesting adverse drug reactions, they were referred to relevant specialists. The presumed offending drug was discontinued when clinically necessary.
Definitions
Maintenance of ethambutol or rifampicin was defined as more than 6 months of use as adopted in a previous study [14]. Clinical outcomes encompassing culture conversion, microbiological cure, treatment failure, and recurrence were defined among patients with ≥3 follow-up sputum culture studies. ‘Culture conversion’ was defined as three consecutive negative sputum culture results after treatment [15, 16]. The first date of culture-negative sputum specimen collection was defined as the day of culture conversion. ‘Microbiological cure’ was defined as the maintenance of negative culture conversion at the point of treatment completion [16]. ‘Treatment failure’ was defined as the persistence or re-emergence of multiple positive cultures for the causative species of NTM from respiratory samples after ≥12 months of antimycobacterial treatment while the patient was still on treatment [16]. ‘Recurrence’ was defined as the re-emergence of causative species of NTM with ≥2 positive cultures from sputum after achieving a microbiological cure [15, 16]. The first day of culture-positive sputum specimen collection was considered as the day of recurrence.
Statistical analysis
Values were organized as numbers (percentages) for dichotomous variables, and medians with interquartile range (IQR) for continuous variables. Logistic regression analysis was performed to seek potential predictors for culture conversion, microbiological cure, and treatment failure. Odds ratios (ORs) with 95% confidence intervals (CI) for each candidate predictor were calculated, and candidates with a p < 0.05 were included in the multivariable models. Kaplan-Meier curves were drawn with log-rank tests to compare probabilities of initial culture conversion and recurrence after treatment success, according to maintenance of the ethambutol and rifampicin. All statistical analyses and figure drawings were performed using Stata ver. 13.0 (Stata Corp., College Station, TX).
Results
Baseline characteristics
During the 9-year study period, 331 patients with MAC-PD initiated treatment. The following patients were excluded from analysis: 28 patients with short (< 6 months) duration of treatment at the time point of data collection, 26 patients who were previously treated for MAC-PD, 16 patients without ≥3 follow-up cultures, 16 patients with poor compliance to prescriptions (> 1-month omission of medication during the treatment period), and 8 patients with clarithromycin-resistant MAC infection. The remaining 237 patients were included for further analysis.
Of the 237 patients treated for MAC-PD, the median age was 64 years (IQR: 57–73) and 147 (62.0%) were female. All patients underwent baseline evaluation for the presence of human immunodeficiency virus antibody, all of which produced a negative result. M. avium (50.6%) and M. intracellulare (47.7%) were the main species detected. Among the 190 patients who underwent drug susceptibility testing for clarithromycin, the MIC were 8 μg/mL (2 patients), 4 μg/mL (18 patients), 2 μg/mL (69 patients), 1 μg/mL (78 patients), and ≤ 0.5 μg/mL (23 patients). Upon diagnosis, 81 (34.2%) patients had positive acid-fast bacilli smear results. The nodular bronchiectatic form was the dominant radiographic pattern (79.8%).
Among the 237 patients, 122 (51.5%) maintained treatment (≥6 months) with all three drugs, 58 (24.5%) maintained ethambutol and macrolide, 32 (13.5%) maintained rifampicin and macrolide, and 25 (10.6%) maintained macrolide only. Patients who maintained all three agents tended to be younger (p = 0.003) and had a higher proportion of infections with M. avium (p = 0.018) compared with the other groups of patients (Table 1).
Table 1.
Baseline characteristics patients according to the maintenance (≥6mo) of antimycobacterial agents
| Variables | Macrolide only n = 25 |
Macrolide and ethambutol n = 58 |
Macrolide and rifampicin n = 32 |
Macrolide, ethambutol, and rifampicin n = 122 |
P |
|---|---|---|---|---|---|
| Age (years) | 71 (65–80) | 66 (58–75) | 66 (56–73) | 62 (56–70) | 0.003 |
| BMI (kg/m2) | 20 (18–22) | 21 (18–22) | 20 (19–23) | 21 (19–22) | 0.649 |
| Sex, female | 11 (44.0%) | 34 (58.6%) | 18 (56.3%) | 84 (68.9%) | 0.086 |
| Smoking history | 0.444 | ||||
| Never smoker | 12 (48.0%) | 35 (60.3%) | 21 (65.6%) | 82 (67.2%) | |
| Former smoker | 9 (36.0%) | 14 (24.1%) | 5 (15.6%) | 21 (17.2%) | |
| Current smoker | 1 (4.0%) | 3 (5.2%) | 1 (3.1%) | 2 (1.6%) | |
| Comorbidities | |||||
| Asthma | 2 (8.0%) | 0 (0.0%) | 1 (3.1%) | 2 (1.6%) | 0.093 |
| COPD | 2 (8.0%) | 0 (0.0%) | 0 (0.0%) | 2 (1.6%) | 0.116 |
| History of tuberculosis | 11 (44.0%) | 17 (29.3%) | 7 (21.9%) | 26 (21.3%) | 0.100 |
| Drug use | |||||
| Immunomodulatory drugs | 2 (8.0%) | 0 (0.0%) | 0 (0.0%) | 6 (4.9%) | 0.112 |
| Steroid | 1 (4.0%) | 1 (1.7%) | 1 (3.1%) | 3 (2.5%) | 0.799 |
| MAC species | 0.018 | ||||
| M. avium | 9 (36.0%) | 24 (41.4%) | 12 (37.5%) | 75 (61.5%) | |
| M. intracellulare | 15 (60.0%) | 33 (56.9%) | 20 (62.5%) | 45 (36.9%) | |
| M. chimaera | 1 (4.0%) | 1 (1.7%) | 0 (0.0%) | 1 (0.8%) | |
| M. columbiense | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (0.8%) | |
| Smear positivity at diagnosis | 14 (56.0%) | 19 (32.8%) | 10 (31.3%) | 38 (31.2%) | 0.113 |
| Radiographic pattern | 0.847 | ||||
| Nodular bronchiectatic | 19 (76.0%) | 45 (77.6%) | 25 (78.1%) | 100 (82.0%) | |
| Upper lobe cavitary | 6 (24.0%) | 13 (22.4%) | 7 (21.9%) | 22 (18.0%) | |
| Pulmonary function tests | |||||
| FVC (% predicted) | 79 (63–86) | 88 (75–97) | 90 (74–96) | 89 (77–99) | 0.086 |
| FEV1 (% predicted) | 92 (64–99) | 94 (79–104) | 90 (80–108) | 95 (83–109) | 0.582 |
| FEV1/FVC (%) | 80 (64–89) | 78 (72–83) | 79 (70–87) | 77 (71–84) | 0.928 |
Values are presented as number (percentage) or median (interquartile range)
Abbreviations: BMI body mass index, COPD chronic obstructive pulmonary disease, MAC Mycobacterium avium complex, FVC forced vital capacity, FEV1 forced expiratory volume in 1 s
Treatment regimen
After initiation of treatment, patients were followed on 4- to 8-week intervals and sputum specimens were requested to be submitted for acid-fast bacilli smears and mycobacterial cultures on each visit. Median treatment duration was 18.6 months (IQR: 16.3–24.3). Azithromycin was prescribed in 183 patients (77.2%) and clarithromycin in 54 (22.8%). For 224 (94.5%) patients, ethambutol was received for a median of 16.3 (IQR: 7.7–21.0) months, and 180 (80.4%) maintained the drug for longer than 6 months. For 179 (75.9%) patients, rifampicin was received for a median of 18.2 (IQR: 11.1–23.8) months, and 154 (86.0%) maintained it for longer than 6 months (Table 2).
Table 2.
Treatment regimen and outcomes of 237 patients with Mycobacterium avium complex lung disease
| Categories | Variables | Values |
|---|---|---|
| Drug use | Macrolide | 237 (100.0%) |
| Duration, months | 18.6 (16.3–24.3) | |
| Ethambutol | 224 (94.5%) | |
| Maintenance (≥6 months) | 180 (80.4%) | |
| Duration, for patients with maintenance, months | 18.0 (12.1–22.0) | |
| Duration, for patients without maintenance, months | 1.2 (0.0–3.2) | |
| Rifampicin | 179 (75.9%) | |
| Maintenance (≥6 months) | 154 (86.0%) | |
| Duration, for patients with maintenance, months | 18.7 (16.7–24.3) | |
| Duration, for patients without maintenance, months | 0.0 (0.0–0.5) | |
| Outcomes | Culture conversiona | 190/237 (80.2%) |
| Microbiological cureb | 129/177 (72.9%) | |
| Treatment failurec | 66/204 (32.4%) | |
| Recurrenced | 16/129 (12.4%) |
Values are presented as number (percentage) or median (interquartile range)
aAt least three consecutive negative results for sputum culture after the start of treatment. The first day of a negative result was considered the date of culture conversion
bMaintenance of negative culture conversion until the end of treatment. Assessed among patients who stopped taking antibiotics
cRe-emergence of multiple positive cultures or persistence with the causative species from respiratory samples after ≥12 months of antimycobacterial treatment, while the patient is still on treatment. Assessed among patients with ≥12 months of antimycobacterial treatment
dThe re-emergence of at least 2 positive cultures with the same species from sputum after cessation of antimycobacterial treatment. Assessed among patients with microbiological cure
Injectable agents were administered to 24 patients (10.1%) and these included streptomycin (18 patients) and amikacin (6 patient) (Table 3). A daily regimen was prescribed for 146 patients (61.6%), while 91 (38.4%) started a three-times-weekly regimen. The median dosage of ethambutol was 15.4 mg/kg for the daily regimen and 22.6 mg/kg for the three-times-weekly regimen. Other detailed dosage information is described in (Additional file 1: Table S1).
Table 3.
Detailed description of treatment regimens prescribed in 237 patients
| Treatment regimens | Values |
|---|---|
| Macrolide + Ethambutol + Rifampicin | 131 (55.3%) |
| Macrolide + Ethambutol | 43 (18.1%) |
| Macrolide + Ethambutol + Rifampicin + Clofazimine | 11 (4.6%) |
| Macrolide + Ethambutol + Rifampicin + Injection drug | 10 (4.2%) |
| Macrolide + Ethambutol + Rifampicin + Quinolone | 7 (3.0%) |
| Macrolide + Ethambutol + Clofazimine | 6 (2.5%) |
| Macrolide + Rifampicin | 6 (2.5%) |
| Macrolide | 4 (1.7%) |
| Macrolide + Ethambutol + Injection drug | 3 (1.3%) |
| Macrolide + Rifampicin + Injection drug | 2 (0.8%) |
| Macrolide + Ethambutol + Rifampicin + Quinolone + Injection drug | 2 (0.8%) |
| Macrolide + Rifampicin + Clofazimine | 1 (0.4%) |
| Macrolide + Ethambutol + Clofazimine + Quinolone | 1 (0.4%) |
| Macrolide + Ethambutol + Clofazimine + Injection drug | 1 (0.4%) |
| Macrolide + Ethambutol + Quinolone + Injection drug | 1 (0.4%) |
| Macrolide + Ethambutol + Rifampicin + Injection drug/Inhaled amikacin | 1 (0.4%) |
| Macrolide + Ethambutol + Rifampicin + Quinolone + Inhaled amikacin | 1 (0.4%) |
| Macrolide + Ethambutol + Rifampicin + Cycloserine + Pyrazinamide | 1 (0.4%) |
| Macrolide + Ethambutol + Rifampicin + Amoxicillin/clavulanate | 1 (0.4%) |
| Macrolide + Ethambutol + Rifampicin + Clofazimine + Injection drug/Inhaled amikacin | 1 (0.4%) |
| Macrolide + Ethambutol + Rifampicin + Clofazimine + Quinolone + Injection drug | 1 (0.4%) |
| Macrolide + Ethambutol + Rifampicin + Quinolone + Amoxicillin/clavulanate | 1 (0.4%) |
| Macrolide + Ethambutol + Rifampicin + Quinolone + Injection drug/Inhaled amikacin | 1 (0.4%) |
Values are presented as numbers (%). Includes drugs used after the diagnosis of Mycobacterium avium complex lung disease
Treatment outcomes
Culture conversion was achieved in 190 out of 237 (80.2%) patients after a median of 1.7 (IQR: 0.5–4.7) months of treatment, and a microbiological cure was achieved in 129 out of 177 (72.9%) patients who completed treatment. Of the 60 patients who did not complete treatment, 58 were still on active treatment, and 2 were lost to follow-up. The treatment failure rate was 32.4% (66 patients) among 204 patients with ≥12 months of treatment. Among the 129 patients who achieved a microbiological cure, 16 patients (12.4%) experienced recurrence with the same species of MAC (Table 2).
Patients who maintained (≥6 months) treatment with either ethambutol, rifampicin or both with macrolide had a higher probability of culture conversion compared with maintenance of macrolide only (log-rank p < 0.001) (Fig. 1). Maintenance of ethambutol and rifampicin with macrolide (adjusted OR 17.12 with 95% CI 3.93–74.60), ethambutol and macrolide (adjusted OR 18.06 with 95% CI 3.67–88.92), and rifampicin and macrolide (adjusted OR 15.82 with 95% CI 2.38–105.33) showed higher odds for culture conversion compared with the macrolide only group. (Table 4).
Fig. 1.
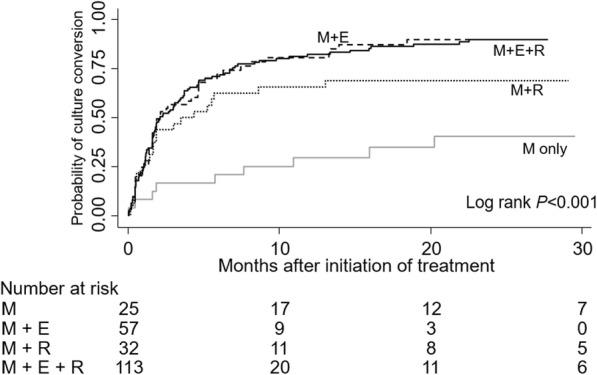
Probability of culture conversion according to the maintenance (≥6 months) of ethambutol and rifampicin. Abbreviations: M, macrolide; E, ethambutol; R, rifampicin
Table 4.
Predictors for culture conversion among treated Mycobacterium avium complex pulmonary disease patients
| Variables | Unadjusted OR (95% CI) | P | Adjusted OR (95% CI) | P |
|---|---|---|---|---|
| Age (years) | 0.95 (0.92–0.98) | 0.003 | 1.01 (0.96–1.06) | 0.680 |
| BMI (kg/m2) | 1.23 (1.06–1.41) | 0.005 | 1.21 (1.02–1.44) | 0.031 |
| Sex, female | 2.72 (1.42–5.23) | 0.003 | 1.43 (0.55–3.74) | 0.468 |
| History of tuberculosis | 0.53 (0.27–1.05) | 0.070 | ||
| MAC species | ||||
| M. avium | Reference | Reference | ||
| M. intracellulare | 0.26 (0.13–0.53) | < 0.001 | 0.29 (0.10–0.84) | 0.022 |
| Smear positivity at diagnosis | 0.18 (0.09–0.37) | < 0.001 | 0.10 (0.04–0.29) | < 0.001 |
| Radiographic pattern | ||||
| Nodular bronchiectatic form | Reference | Reference | ||
| Upper lobe cavitary form | 0.45 (0.22–0.92) | 0.029 | 0.39 (0.13–1.12) | 0.079 |
| Pulmonary function tests | ||||
| FVC (% predicted) | 1.01 (0.99–1.03) | 0.293 | ||
| FEV1 (% predicted) | 1.00 (0.98–1.01) | 0.742 | ||
| FEV1/FVC (%) | 0.97 (0.94–1.01) | 0.101 | ||
| Maintenance of antibiotics (≥6mo) | ||||
| M only | Reference | Reference | ||
| M + E | 9.68 (3.28–28.58) | < 0.001 | 18.06 (3.67–88.92) | < 0.001 |
| M + R | 4.54 (1.48–13.96) | 0.008 | 15.82 (2.38–105.33) | 0.004 |
| M + E + R | 14.91 (5.49–40.47) | < 0.001 | 17.12 (3.93–74.60) | < 0.001 |
Culture conversion was defined as at least three consecutive negative results for sputum culture the start of treatment
Abbreviations: OR odds ratio, CI confidence interval, BMI body mass index, MAC Mycobacterium avium complex, FVC forced vital capacity, FEV1 forced expiratory volume in 1 s, M macrolide, E ethambutol, R rifampicin
Predictors for a microbiological cure as well as treatment failure were also evaluated. Maintenance of both ethambutol and rifampicin (adjusted OR 5.12 with 95% CI 1.72–15.24), or maintenance of ethambutol (adjusted OR 5.74 with 95% CI 1.54–21.42) with macrolide was associated with higher rates of microbiological cure, while maintenance of rifampicin with macrolide was not (adjusted OR 2.43 with 95% CI 0.69–8.58) (Table 5). Compared with the macrolide only group, odds of treatment failure decreased when ethambutol and rifampicin were maintained with macrolide (adjusted OR 0.09 with 95% CI 0.01–0.53). However, maintenance of ethambutol (adjusted OR 0.17 with 95% CI 0.03–1.09) or rifampicin (adjusted OR 0.13 with 95% CI 0.01–1.13) with macrolide did not have this effect (Table 6).
Table 5.
Predictors for microbiological cure among patients who completed treatment
| Variables | Unadjusted OR (95% CI) | P | Adjusted OR (95% CI) | P |
|---|---|---|---|---|
| Age (years) | 0.96 (0.93–1.00) | 0.029 | 0.99 (0.96–1.03) | 0.615 |
| BMI (kg/m2) | 1.13 (0.97–1.32) | 0.112 | ||
| Sex, female | 1.50 (0.76–2.96) | 0.238 | ||
| History of tuberculosis | 0.51 (0.24–1.04) | 0.065 | ||
| MAC species | ||||
| M. avium | Reference | Reference | ||
| M. intracellulare | 0.32 (0.16–0.64) | 0.001 | 0.33 (0.15–0.73) | 0.006 |
| Smear positivity at diagnosis | 0.39 (0.19–0.79) | 0.008 | 0.46 (0.21–1.02) | 0.056 |
| Radiographic pattern | ||||
| Nodular bronchiectatic form | Reference | |||
| Upper lobe cavitary form | 0.87 (0.38–1.98) | 0.738 | ||
| Pulmonary function tests | ||||
| FVC (% predicted) | 1.01 (0.99–1.03) | 0.229 | ||
| FEV1 (% predicted) | 1.00 (0.99–1.02) | 0.801 | ||
| FEV1/FVC (%) | 0.97 (0.94–1.01) | 0.126 | ||
| Maintenance of antibiotics (≥6mo) | ||||
| M only | Reference | Reference | ||
| M + E | 7.58 (2.19–26.26) | 0.001 | 5.74 (1.54–21.42) | 0.009 |
| M + R | 2.98 (0.93–9.57) | 0.067 | 2.43 (0.69–8.58) | 0.168 |
| M + E + R | 7.58 (2.77–20.79) | < 0.001 | 5.12 (1.72–15.24) | 0.003 |
Microbiological cure was defined as maintenance of negative culture conversion at the end of treatment. Assessed among 177 patients who completed treatment
Abbreviations: OR odds ratio, BMI body mass index, MAC Mycobacterium avium complex, FVC forced vital capacity, FEV1 forced expiratory volume in 1 s, M macrolide, E ethambutol, R rifampicin
Table 6.
Predictors for treatment failure among patients with ≥12 months of antimycobacterial treatment
| Variables | Unadjusted OR (95% CI) | P | Adjusted OR (95% CI) | P |
|---|---|---|---|---|
| Age (years) | 1.03 (1.00–1.06) | 0.029 | 0.99 (0.93–1.04) | 0.628 |
| BMI (kg/m2) | 0.86 (0.75–0.99) | 0.037 | 0.88 (0.72–1.07) | 0.194 |
| Sex, female | 0.55 (0.30–1.00) | 0.048 | 0.76 (0.27–2.14) | 0.602 |
| History of tuberculosis | 1.70 (0.90–3.23) | 0.103 | ||
| MAC species | ||||
| M. avium | Reference | Reference | ||
| M. intracellulare | 2.91 (1.58–5.39) | 0.001 | 1.46 (0.51–4.17) | 0.484 |
| Smear positivity at diagnosis | 3.32 (1.79–6.16) | < 0.001 | 7.42 (2.68–20.56) | < 0.001 |
| Radiographic pattern | ||||
| Nodular bronchiectatic form | Reference | |||
| Upper lobe cavitary form | 1.78 (0.89–3.58) | 0.105 | ||
| Pulmonary function tests | ||||
| FVC (% predicted) | 0.98 (0.97–1.00) | 0.093 | ||
| FEV1 (% predicted) | 1.00 (0.98–1.01) | 0.760 | ||
| FEV1/FVC (%) | 1.03 (1.00–1.07) | 0.043 | 1.04 (0.99–1.09) | 0.099 |
| Maintenance of antibiotics (≥6mo) | ||||
| M only | Reference | Reference | ||
| M + E | 0.28 (0.09–0.82) | 0.021 | 0.17 (0.03–1.09) | 0.061 |
| M + R | 0.57 (0.18–1.82) | 0.344 | 0.13 (0.01–1.13) | 0.064 |
| M + E + R | 0.19 (0.07–0.50) | 0.001 | 0.09 (0.01–0.53) | 0.008 |
Treatment failure was defined as re-emergence of multiple positive cultures or persistence with the causative species from respiratory samples after ≥12 months of antimycobacterial treatment, while the patient is still on treatment. Assessed among 204 patients with ≥12 months of antimycobacterial treatment
Abbreviations: OR odds ratio, BMI body mass index, MAC Mycobacterium avium complex, FVC forced vital capacity, FEV1 forced expiratory volume in 1 s, M macrolide, E ethambutol, R rifampicin
Maintenance of both ethambutol and rifampicin or one of these with macrolide did not decrease the probability of recurrence (log-rank p = 0.511).
Acquisition of macrolide resistance
Drug susceptibility testing was repeated for 47 of 66 patients for whom treatment had failed, and for 12 of 16 patients in whom MAC-PD recurred. Macrolide resistance (MIC ≥32 μg/mL) was acquired by five patients who experienced treatment failure but these did not include any with recurrence of MAC-PD. The patients who acquired macrolide resistance included three males and two females, with a median age of 59 (IQR: 58–59). M. intracellulare was the dominant species (four patients), and an upper lobe cavitary pattern (three patients) and positive smear results at the initiation of treatment (three patients) were also common. None could maintain the three-drug regimen: two maintained macrolide only, two maintained macrolide with ethambutol, and one maintained macrolide with rifampicin. Common causes for omitting or stopping medication included decreased visual acuity (four patients), drug-drug interactions (two patients), and severe nausea (two patients). Detailed information about each patient is provided in (Additional file 1: Table S2).
Adverse events
During treatment, 116 (49.0%) patients experienced adverse events. Deterioration of visual acuity was the most common event reported (31.6%). Other events included elevated hepatic transaminase (5.9%), general weakness (5.5%), anorexia (5.1%), nausea (4.6%), rash (4.2%), and worsened hearing loss (3.4%).
Among the 224 patients who received ethambutol, 92 (41.1%) of them stopped taking this drug because of adverse events including deteriorated visual acuity (72 patients), anorexia (8 patients), rash (8 patients), and hepatic dysfunction (8 patients). Early cessation (< 6 months) of ethambutol was observed in 44 patients (19.6%), mainly because of deteriorated visual acuity (35 patients), rash (6 patients), and anorexia (5 patients). Of the 179 patients who received rifampicin, 47 (26.3%) of them had to stop administration due to general weakness (10 patients), nausea (9 patients), anorexia (8 patients), hepatic dysfunction (7 patients), and abdominal pain (6 patients). Early cessation (< 6 months) of rifampicin was observed in 25 patients (14.0%), mostly as a result of anorexia (6 patients), general weakness (6 patients), nausea (5 patients), hepatic dysfunction (5 patients), and rash (4 patients). Patient characteristics regarding the usage of ethambutol and rifampicin and the reasons for withholding these drugs are provided in (Additional file 1: Table S3).
Discussion
In this study, among the 237 patients who started treatment for MAC-PD, 190 (80.2%) reached culture conversion, and 129 of the 177 patients who completed treatment (72.9%) achieved microbiological cure. Of the 204 patients who underwent ≥12 months of treatment, 66 (32.4%) were classified as having treatment failure. Sixteen of the 129 patients with microbiological cure (12.4%) experienced recurrence of MAC-PD. Most patients started out using macrolide (100.0%), ethambutol (94.5%), and rifampicin (75.9%) as a multidrug combination treatment. Maintenance (≥6 months) of both ethambutol and rifampicin with macrolide was associated with the most favorable outcomes: higher odds of culture conversion as well as microbiological cure, and lower odds of treatment failure.
Treatment outcomes in this study were comparable with those of previous studies. In two retrospective analyses and one randomized controlled trial, rates of culture conversion for MAC-PD using a macrolide-containing regimen was reported as 40–95% [14, 15, 17]. In addition, two meta-analyses reported culture conversion rates of 55–57% without recurrence [6, 8].
Our study showed that maintenance of ethambutol was more strongly associated with microbiological cure than rifampicin. Ethambutol inhibits arabinosyltransferase and blocks arabinogalactan synthesis, which forms part of the mycobacterial wall [18–20]. With such mechanism, the synergistic effect of ethambutol is expected when used with other antimycobacterial agents. The effects of ethambutol on MAC was reported in animal studies, which reduced MAC growth in the blood, liver, and spleen [21]. In a human study, ethambutol reduced the mycobacteremia level in AIDS patients [22]. Another study reported that more than 5 months of ethambutol use was associated with improvement in MAC culture [23]; however, that study used the reduction in colony count as an outcome rather than the strict criteria of culture conversion or treatment success [16, 24]. Given the favorable effects of maintenance of ethambutol in our study, cessation of ethambutol early during the course of treatment based on uncertain adverse events should be avoided [25, 26].
Although our study raised concerns regarding the effectiveness of rifampicin and macrolide without ethambutol in terms of microbiological cure, the importance of a rifamycin in the treatment of MAC was noted in earlier studies: rifabutin was effective in reducing mycobacterial titers among AIDS patients with disseminated MAC infection [27], and synergistic effects of rifampicin and ethambutol on MAC were shown in vitro in liquid media [28]. In addition, maintenance of a single drug (rifampicin or ethambutol) supplemented with macrolide could result in the emergence of clarithromycin resistance, as was shown in the present study. However, the effect of rifampicin has been questioned in other studies: in an open-label randomized controlled trial comparing a two-drug regimen (clarithromycin, ethambutol) with a three-drug regimen (clarithromycin, ethambutol, and rifampicin), the 12-month sputum-negative conversion rates between the two groups were equivalent [17]. Another study demonstrated a good treatment success rate among MAC-PD patients treated with a three-drug regimen of macrolide, ethambutol, and clofazimine without rifampicin [29]. The weaker effect of rifampicin for the treatment of MAC-PD could be explained based on a lowered serum level of macrolide through induction of cytochrome p450 by rifampicin, although one previous report suggested that the serum concentration does not affect treatment outcomes [30, 31]. It can also be explained that rifampicin has antimycobacterial activity by binding to the β subunit of RNA polymerase, thereby inhibiting RNA synthesis [32]. In slowly growing mycobacteria such as MAC species, such a mechanism may result in a bacteriostatic effect rather than a bactericidal effect [33]. In fact, clinical significance of synergistic activity of rifampicin and ethambutol against slow-growing NTM was doubted in a previous study [34].
Although maintenance of ethambutol appears to be very important in the treatment of MAC-PD, the possibility of optic neuropathy should be considered with prolonged use of ethambutol. Among patients taking ethambutol for pulmonary TB, ethambutol-induced optic neuropathy develops in 1–2% in a dose-dependent manner [35]. In addition, about 0.2–0.3% of patients may experience irreversible visual function loss. In our study, about one-third of patients experienced worsened visual acuity and it was major cause of earlier cessation of ethambutol. Given its effectiveness as well as risk of optic neuropathy, the best strategy could be long-term use of ethambutol with active surveillance of visual acuity and rapid objective assessment in the setting of subjective deterioration.
This study had some limitations. First, it was a retrospective study based on a single center. Patients receiving treatment at the time of data retrieval could not be included in several of the analyses. Potential confounders that were not considered in our study (e.g. concomitant medications or the radiographic extent of the disease) may exist between the study groups. Future well-designed, randomized controlled clinical trials will be necessary to fully understand the roles of ethambutol and rifampicin in MAC-PD. Second, the adverse events of medication were self-reported, and the visual acuity loss was not evaluated objectively. This may have led to a high prevalence of ethambutol-induced ocular toxicity. However, there are currently no standardized protocols for detecting ethambutol-induced ocular toxicity, and self-reported visual loss is a reliable means to determine optic neuritis from ethambutol [36]. Third, re-infection and relapse of MAC could not be differentiated between among patients with recurrence because genetic comparisons of the causative strains were not performed in our study. The lack of such analyses might have resulted in the insignificant associations between maintenance of ethambutol or rifampicin and the risk of recurrence after microbiological cure.
Conclusions
Maintenance (≥6 months) of ethambutol and rifampicin treatment with macrolide was associated with the most favorable treatment outcomes among patients with MAC-PD. Given the impact of ethambutol on microbiological cure, clinicians should maintain ethambutol unless definite and serious adverse events develop from ethambutol treatment.
Supplementary information
Additional file 1: Table S1. Detailed dosage information about the antimycobacterial agents used in the treatment of Mycobacterium avium complex lung disease. Table S2. Detailed description of 5 patients who developed clarithromycin resistance during treatment failure. Table S3. Patient characteristics according to the use of ethambutol and rifampicin.
Acknowledgements
We thank the Medical Research Collaborating Centre (Seoul National University Hospital, Seoul, South Korea) for the statistical analyses and Alexander Pishief, LLB, BBmedSc, from Edanz Group (www.edanzediting.com/ac) for English editing a draft of this manuscript.
Abbreviations
- AIDS
Acquired immune deficiency syndrome
- CI
Confidence interval
- IQR
Interquartile range
- MAC
Mycobacterium avium complex
- MIC
Minimal inhibitory concentration
- NTM
Nontuberculous mycobacteria
- OR
Odds ratio
- PD
Pulmonary disease
Authors’ contributions
HJK, JSL, NK, SKH and JJY contributed to the conception and design of the study. HJK, JSL and JJY contributed to the acquisition of the data. HJK, JC, and CHL contributed to the analysis of the data. HJK and JJY contributed to the interpretation of the data. HJK drafted the article. HJK and JJY critically revised the article for important intellectual content. HJK, JSL, NK, JC, CHL, SKH and JJY had access to the final version of the manuscript and approved the version to be published. HJK, JSL, NK, JC, CHL, SKH and JJY made significant contributions to the work. JJY had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
None.
Availability of data and materials
Data are available from the corresponding author upon a reasonable request.
Ethics approval and consent to participate
The study was conducted in accordance with the amended Declaration of Helsinki and was approved by the institutional review board of Seoul National University Hospital (protocol number: H-1705-017-851). Informed consent was waived because of the retrospective design of the study, and the information of each patient was anonymized prior to analyses.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hyung-Jun Kim, Email: hyung405@gmail.com.
Jong Sik Lee, Email: riboom@naver.com.
Nakwon Kwak, Email: kwaknakwon@naver.com.
Jaeyoung Cho, Email: apricot6@snu.ac.kr.
Chang-Hoon Lee, Email: kauri670@empal.com.
Sung Koo Han, Email: hansk@snu.ac.kr.
Jae-Joon Yim, Email: yimjj@snu.ac.kr.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12890-019-0982-8.
References
- 1.Shiau MY, Lee MS, Huang TL, Tsai JN, Chang YH. Mycobacterial prevalence and antibiotic resistance frequency trends in Taiwan of mycobacterial clinical isolates from 2002 to 2014. Medicine (Baltimore) 2016;95(12):e2942. doi: 10.1097/MD.0000000000002942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McShane PJ, Glassroth J. Pulmonary disease due to nontuberculous mycobacteria: current state and new insights. Chest. 2015;148(6):1517–1527. doi: 10.1378/chest.15-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marras TK, Chedore P, Ying AM, Jamieson F. Isolation prevalence of pulmonary non-tuberculous mycobacteria in Ontario, 1997 2003. Thorax. 2007;62(8):661–666. doi: 10.1136/thx.2006.070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daley CL. Mycobacterium avium complex disease. Microbiol Spectr. 2017;5(2):1–36. doi: 10.1128/microbiolspec.TNMI7-0045-2017. [DOI] [PubMed] [Google Scholar]
- 5.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 6.Kwak Nakwon, Park Jimyung, Kim Eunyoung, Lee Chang-Hoon, Han Sung Koo, Yim Jae-Joon. Treatment Outcomes of Mycobacterium avium Complex Lung Disease: A Systematic Review and Meta-analysis. Clinical Infectious Diseases. 2017;65(7):1077–1084. doi: 10.1093/cid/cix517. [DOI] [PubMed] [Google Scholar]
- 7.Shafran SD, Singer J, Zarowny DP, Phillips P, Salit I, Walmsley SL, et al. A comparison of two regimens for the treatment of Mycobacterium avium complex bacteremia in AIDS: rifabutin, ethambutol, and clarithromycin versus rifampin, ethambutol, clofazimine, and ciprofloxacin. Canadian HIV Trials Network Protocol 010 Study Group. N Engl J Med. 1996;335(6):377–383. doi: 10.1056/NEJM199608083350602. [DOI] [PubMed] [Google Scholar]
- 8.Field SK, Fisher D, Cowie RL. Mycobacterium avium complex pulmonary disease in patients without hiv infection. Chest. 2004;126(2):566–581. doi: 10.1378/chest.126.2.566. [DOI] [PubMed] [Google Scholar]
- 9.Haworth CS, Banks J, Capstick T, Fisher AJ, Gorsuch T, Laurenson IF, et al. British Thoracic Society guidelines for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD) Thorax. 2017;72(Suppl 2):ii1–ii64. doi: 10.1136/thoraxjnl-2017-210927. [DOI] [PubMed] [Google Scholar]
- 10.Kim SJ, Yoon SH, Choi SM, Lee J, Lee CH, Han SK, et al. Characteristics associated with progression in patients with of nontuberculous mycobacterial lung disease: a prospective cohort study. BMC Pulm Med. 2017;17(1):5. doi: 10.1186/s12890-016-0349-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee AR, Lee J, Choi SM, Seong MW, Kim SA, Kim M, et al. Phenotypic, immunologic, and clinical characteristics of patients with nontuberculous mycobacterial lung disease in Korea. BMC Infect Dis. 2013;13:558. doi: 10.1186/1471-2334-13-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clinical and Laboratory Standards Institute . Interpretive criteria for identification of bacteria and fungi by DNA target sequencing; approved guideline. CLSI document MM18-A. Wayne: Clinical and Laboratory Standards Institute; 2008. [Google Scholar]
- 13.Kim HS, Lee KS, Koh WJ, Jeon K, Lee EJ, Kang H, et al. Serial CT findings of Mycobacterium massiliense pulmonary disease compared with Mycobacterium abscessus disease after treatment with antibiotic therapy. Radiology. 2012;263(1):260–270. doi: 10.1148/radiol.12111374. [DOI] [PubMed] [Google Scholar]
- 14.Jarand J, Davis JP, Cowie RL, Field SK, Fisher DA. Long-term follow-up of Mycobacterium avium complex lung disease in patients treated with regimens including clofazimine and/or rifampin. Chest. 2016;149(5):1285–1293. doi: 10.1378/chest.15-0543. [DOI] [PubMed] [Google Scholar]
- 15.Jeong BH, Jeon K, Park HY, Kim SY, Lee KS, Huh HJ, et al. Intermittent antibiotic therapy for nodular bronchiectatic Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2015;191(1):96–103. doi: 10.1164/rccm.201408-1545OC. [DOI] [PubMed] [Google Scholar]
- 16.van Ingen Jakko, Aksamit Timothy, Andrejak Claire, Böttger Erik C., Cambau Emmanuelle, Daley Charles L., Griffith David E., Guglielmetti Lorenzo, Holland Steven M., Huitt Gwen A., Koh Won-Jung, Lange Christoph, Leitman Philip, Marras Theodore K., Morimoto Kozo, Olivier Kenneth N., Santin Miguel, Stout Jason E., Thomson Rachel, Tortoli Enrico, Wallace Richard J., Winthrop Kevin L., Wagner Dirk. Treatment outcome definitions in nontuberculous mycobacterial pulmonary disease: an NTM-NET consensus statement. European Respiratory Journal. 2018;51(3):1800170. doi: 10.1183/13993003.00170-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miwa S, Shirai M, Toyoshima M, Shirai T, Yasuda K, Yokomura K, et al. Efficacy of clarithromycin and ethambutol for Mycobacterium avium complex pulmonary disease. A preliminary study. Ann Am Thorac Soc. 2014;11(1):23–29. doi: 10.1513/AnnalsATS.201308-266OC. [DOI] [PubMed] [Google Scholar]
- 18.Deng L, Mikusova K, Robuck KG, Scherman M, Brennan PJ, McNeil MR. Recognition of multiple effects of ethambutol on metabolism of mycobacterial cell envelope. Antimicrob Agents Chemother. 1995;39(3):694–701. doi: 10.1128/AAC.39.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takayama K, Kilburn JO. Inhibition of synthesis of arabinogalactan by ethambutol in Mycobacterium smegmatis. Antimicrob Agents Chemother. 1989;33(9):1493–1499. doi: 10.1128/AAC.33.9.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belanger AE, Besra GS, Ford ME, Mikusova K, Belisle JT, Brennan PJ, et al. The embAB genes of Mycobacterium avium encode an arabinosyl transferase involved in cell wall arabinan biosynthesis that is the target for the antimycobacterial drug ethambutol. Proc Natl Acad Sci U S A. 1996;93(21):11919–11924. doi: 10.1073/pnas.93.21.11919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bermudez LE, Kolonoski P, Petrofsky M, Wu M, Inderlied CB, Young LS. Mefloquine, moxifloxacin, and ethambutol are a triple-drug alternative to macrolide-containing regimens for treatment of Mycobacterium avium disease. J Infect Dis. 2003;187(12):1977–1980. doi: 10.1086/375352. [DOI] [PubMed] [Google Scholar]
- 22.Kemper CA, Havlir D, Haghighat D, Dube M, Bartok AE, Sison JP, et al. The individual microbiologic effect of three antimycobacterial agents, clofazimine, ethambutol, and rifampin, on Mycobacterium avium complex bacteremia in patients with AIDS. J Infect Dis. 1994;170(1):157–164. doi: 10.1093/infdis/170.1.157. [DOI] [PubMed] [Google Scholar]
- 23.Lam PK, Griffith DE, Aksamit TR, Ruoss SJ, Garay SM, Daley CL, et al. Factors related to response to intermittent treatment of Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2006;173(11):1283–1289. doi: 10.1164/rccm.200509-1531OC. [DOI] [PubMed] [Google Scholar]
- 24.Griffith DE, Brown BA, Murphy DT, Girard WM, Couch L, Wallace RJ., Jr Initial (6-month) results of three-times-weekly azithromycin in treatment regimens for Mycobacterium avium complex lung disease in human immunodeficiency virus-negative patients. J Infect Dis. 1998;178(1):121–126. doi: 10.1086/515597. [DOI] [PubMed] [Google Scholar]
- 25.Wallace RJ, Jr, Brown-Elliott BA, McNulty S, Philley JV, Killingley J, Wilson RW, et al. Macrolide/Azalide therapy for nodular/bronchiectatic mycobacterium avium complex lung disease. Chest. 2014;146(2):276–282. doi: 10.1378/chest.13-2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koh WJ, Jeong BH, Jeon K, Lee NY, Lee KS, Woo SY, et al. Clinical significance of the differentiation between Mycobacterium avium and Mycobacterium intracellulare in M avium complex lung disease. Chest. 2012;142(6):1482–1488. doi: 10.1378/chest.12-0494. [DOI] [PubMed] [Google Scholar]
- 27.Sullam PM, Gordin FM, Wynne BA. Efficacy of rifabutin in the treatment of disseminated infection due to Mycobacterium avium complex. The Rifabutin Treatment Group. Clin Infect Dis. 1994;19(1):84–86. doi: 10.1093/clinids/19.1.84. [DOI] [PubMed] [Google Scholar]
- 28.Hoffner SE, Svenson SB, Kallenius G. Synergistic effects of antimycobacterial drug combinations on Mycobacterium avium complex determined radiometrically in liquid medium. Eur J Clin Microbiol. 1987;6(5):530–535. doi: 10.1007/BF02014241. [DOI] [PubMed] [Google Scholar]
- 29.Field SK, Cowie RL. Treatment of Mycobacterium avium-intracellulare complex lung disease with a macrolide, ethambutol, and clofazimine. Chest. 2003;124(4):1482–1486. doi: 10.1378/chest.124.4.1482. [DOI] [PubMed] [Google Scholar]
- 30.Wallace RJ, Jr, Brown BA, Griffith DE, Girard W, Tanaka K. Reduced serum levels of clarithromycin in patients treated with multidrug regimens including rifampin or rifabutin for Mycobacterium avium-M. intracellulare infection. J Infect Dis. 1995;171(3):747–750. doi: 10.1093/infdis/171.3.747. [DOI] [PubMed] [Google Scholar]
- 31.Koh WJ, Jeong BH, Jeon K, Lee SY, Shin SJ. Therapeutic drug monitoring in the treatment of Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2012;186(8):797–802. doi: 10.1164/rccm.201206-1088OC. [DOI] [PubMed] [Google Scholar]
- 32.Wehrli W. Rifampin: mechanisms of action and resistance. Rev Infect Dis. 1983;5(Suppl 3):S407–S411. doi: 10.1093/clinids/5.Supplement_3.S407. [DOI] [PubMed] [Google Scholar]
- 33.Prince DS, Peterson DD, Steiner RM, Gottlieb JE, Scott R, Israel HL, et al. Infection with Mycobacterium avium complex in patients without predisposing conditions. N Engl J Med. 1989;321(13):863–868. doi: 10.1056/NEJM198909283211304. [DOI] [PubMed] [Google Scholar]
- 34.van Ingen J, Hoefsloot W, Mouton JW, Boeree MJ, van Soolingen D. Synergistic activity of rifampicin and ethambutol against slow-growing nontuberculous mycobacteria is currently of questionable clinical significance. Int J Antimicrob Agents. 2013;42(1):80–82. doi: 10.1016/j.ijantimicag.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 35.Chamberlain PD, Sadaka A, Berry S, Lee AG. Ethambutol optic neuropathy. Curr Opin Ophthalmol. 2017;28(6):545–551. doi: 10.1097/ICU.0000000000000416. [DOI] [PubMed] [Google Scholar]
- 36.Kim KL, Park SP. Visual function test for early detection of ethambutol induced ocular toxicity at the subclinical level. Cutan Ocul Toxicol. 2016;35(3):228–232. doi: 10.3109/15569527.2015.1079784. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Detailed dosage information about the antimycobacterial agents used in the treatment of Mycobacterium avium complex lung disease. Table S2. Detailed description of 5 patients who developed clarithromycin resistance during treatment failure. Table S3. Patient characteristics according to the use of ethambutol and rifampicin.
Data Availability Statement
Data are available from the corresponding author upon a reasonable request.


