Abstract
Background
Hypoxic preconditioning (HPC) may protect multiple organs from various injuries. We hypothesized that HPC would reduce lung injury in patients undergoing thoracoscopic lobectomy.
Methods
In a prospective randomized controlled trial, 70 patients undergoing elective thoracoscopic lobectomy were randomly allocated to the HPC group or the control group. Three cycles of 5-min hypoxia and 3-min ventilation applied to the nondependent lung served as the HPC intervention. The primary outcome was the PaO2/FiO2 ratio. Secondary outcomes included postoperative pulmonary complications, pulmonary function, and duration of hospital stay.
Results
HPC significantly increased the PaO2/FiO2 ratio compared with the control at 30 min after one-lung ventilation and 7 days after operation. Compared with the control, it also significantly improved postoperative pulmonary function and markedly reduced the postoperative hospital stay duration. No significant differences between groups were observed in the incidence of pulmonary complications or overall postoperative morbidity.
Conclusions
HPC improves postoperative oxygenation, enhances the recovery of pulmonary function, and reduces the duration of hospital stay in patients undergoing thoracoscopic lobectomy.
Trial registration
This study was registered in the Chinese Clinical Trial Registry (ChiCTR-IPR-17011249) on April 27, 2017.
Keywords: Hypoxic preconditioning, One-lung ventilation, PaO2/FiO2 ratio, Pulmonary complications, Thoracoscopic lobectomy, Non-small cell lung cancer
Background
Surgical resections of lobar and mediastinal lymphadenectomy remain the standard for treatment of early stage non-small cell lung cancer (NSCLC). One-lung ventilation (OLV) techniques are required to facilitate the surgical procedure in patients undergoing lung cancer surgery. However, this technique is associated with hypoxia and ischemia in the nondependent lung tissue [1–3]. Moreover, the nondependent lung is reventilated and reoxygenated when the surgical procedure is completed, which triggers a well-characterized hypoxia-reoxygenation injury similar to ischemia-reperfusion injury [4]. Lung injury is associated with significantly prolonged hospital stay and increased rates of postoperative morbidity and mortality. Therefore, prevention and mitigation of OLV-related lung injury would be expected to improve outcomes in patients undergoing lung surgery [5].
Hypoxic preconditioning (HPC) or ischemic preconditioning (IPC) can improve the tolerance of cells, tissues, entire organs, and even the organism itself to subsequent severe hypoxia or ischemia [6–8]. The phenomenon of preconditioning has been observed in various studies [9, 10]. To date, it has been clear that HPC serves as a protective mechanism for multiple organs against various kinds of injuries [6, 11–13]. For example, HPC induced neuroprotection against transient global cerebral ischemia in adult rats [10]. Furthermore, a recent clinical trial found that a 10-min HPC could protect the kidneys from ischemic injury and postsurgical dysfunction in patients undergoing coronary artery bypass surgery [14].
In our study, we hypothesized that HPC performed before surgery would improve oxygenation, reduce postoperative pulmonary complications and enhance recovery after thoracoscopic lobectomy in patients with lung cancer.
Methods
Ethics approval and consent to participate
This study was approved by the Institutional Ethics Committee of Zhoushan Hospital (Ref. 2017–008) and was registered in the Chinese Clinical Trial Registry (ChiCTR-IPR-17011249) on April 27, 2017. Our study adhered to CONSORT guidelines. Written consents to participate were obtained from all participants after enrollment.
Clinical trial course
From April to August 2017, we screened all patients scheduled for thoracoscopic lobectomy for clinical stage I or II NSCLC. The inclusion criteria for participants of this study were aged 18 to 75 years, with an American Society of Anesthesiologists physical status of I or II. The exclusion criteria of this study were severe pulmonary dysfunction or difficulty intubation of double-lumen tube (DLT).
Patients were randomly allocated to two groups (1:1) based on computer-generated random numbers. Allocation details were sealed in sequentially numbered opaque envelopes. All patients, surgeons and research staff performing follow-up were fully blinded to group allocation. The anesthetists were not blinded and were not involved in study follow-up.
To avoid interference with the trial intervention, we used a standardized perioperative management protocol (including thoracic anesthesia, fluid management, surgical approach, and postoperative care). No patients received premedication. All patients were fasted 6 h to solids and 2 h to clear liquids. In the operating room, a radial arterial line was inserted for measuring the arterial pressure and sampling the arterial blood gas. For induction, patients received midazolam (0.05 mg/kg), sufentanil (5 μg/kg), propofol (1.5 mg/kg), and cisatracurium (0.2 mg/kg). After induction, an appropriate size of DLT was intubated, and the correct position of DLT was confirmed by fiberoptic bronchoscope in both supine and lateral positions. The fraction of inspired oxygen (FiO2) was initially set at 60%, and in cases of saturation of pulse oxygenation (SpO2) less than 92%, FiO2 was increased to 100%. Anesthesia was maintained with 1.0 to 2.5% sevoflurane, remifentanil, and cisatracurium. The protocol for ventilation was as follows: tidal volume of 8 ml/kg per ideal body weight during two-lung ventilation (TLV) and 6 ml/kg per ideal body weight during OLV, and respiratory rate of 12/min without positive end-expiratory pressure. All patients received 4 mL/kg/h Ringer’s lactate solution throughout the intraoperative period. In the postoperative period, maintenance fluids (2 mL/kg/h) were continued until patients were able to tolerate adequate oral intake. All operations were performed by an experienced thoracic surgical team specialized in video-assisted thoracoscopic surgery. At the end of the surgery, the tracheal tube was removed, and patients were transferred to the postanesthesia care unit. Ultrasound-guided unilateral thoracic paravertebral nerve block (T5–6 and T6–7) was used by injecting 10 mL of ropivacaine 0.25% for postoperative analgesia. All patients received additional analgesic treatment with paracetamol 1 g 4 times daily.
Intervention
In the HPC group (n = 38), HPC was applied after confirmation of the DLT position in the correct positions and before incision. HPC was applied on the nondependent lung by intermittent ventilation. Three cycles were performed, each consisting of 5-min of no ventilation and opening to atmosphere followed by 3-min of ventilation. The protocol for ventilation was as follows: tidal volume of 6 ml/kg per ideal body weight, respiratory rate of 12/min, FiO2 of 60%, sevoflurane of 1.5% and intermittent two-lung ventilation when SpO2 < 92%. The non-operated lung received continuous ventilation. In the control group (n = 38), patients received TLV after confirmation of the DLT position and before incision.
Outcome
The primary outcome was lung oxygenation expressed by the PaO2/FiO2 ratio. Secondary outcomes included the following: intraoperative adverse events, pulmonary function, postoperative pulmonary complications, and duration of hospital stay.
Arterial blood gases were evaluated at 30 min after OLV and at 7 day after surgery. The occurrence of intraoperative adverse events, such as tachycardia, bradycardia, hypotension, hypertension, and oxygen desaturation were recorded by the clinical anesthesiologists. Hypotension and bradycardia were defined as a decrease from baseline value by 20%. Hypertension and tachycardia were defined as an increase from baseline value by 20%. Oxygen desaturation was defined as a SpO2 value of < 92%. We used a portable spirometer (Spirobank, GTM, Medical International Research, Rome, Italy) to measure the pulmonary function of forced vital capacity (FVC) and forced expiratory volume in 1 s (FEV1) before surgery and repeated this measurement at 7 day after surgery. Every pulmonary function test was performed three times with the patient in a semirecumbent position, and the average value was recorded. Postoperative pulmonary complications were defined as pneumonia, atelectasis, pleural effusions, and prolonged air leak > 7 days. The duration of hospital stay was counted from the day of operation to the day of discharge.
Statistical analysis
Sample size calculation was based on the primary outcome of the PaO2/FiO2 ratio. A previous study showed that the means ± standard deviation (SD) value of PaO2/FiO2 during OLV was 192 ± 67 mmHg [15]. Our hypothesis was that the HPC would improve the PaO2/FiO2 ratio by 25%. Assuming a power of 80% and a level of significance of 0.05, it was estimated that 31 patients would be required for each group. To account for a 10% dropout rate, we included 35 patients in each group.
Statistical analyses were conducted using the SPSS 20.0 statistical software (IBM Corporation, Armonk, NY, USA). Quantitative data were expressed in the means ± SD or medians (interquartile range, IQR) and compared with independent t-test or Mann-Whitney U test, respectively. Categorical data were expressed as a frequency or percentage and compared with the Fisher’s exact test or the chi-square test. Variables with repeated measures were analyzed using a linear mixed model with a patient indicator as a random effect and group, time, and group-by-time as fixed effects. P values less than 0.05 were considered to be statistically significant.
Results
A flow diagram of this study is shown in Fig. 1. A total of 78 patients were screened for eligibility, and eight patients were excluded from analysis. Patients were randomized equally between the HPC and Control groups.
Fig. 1.
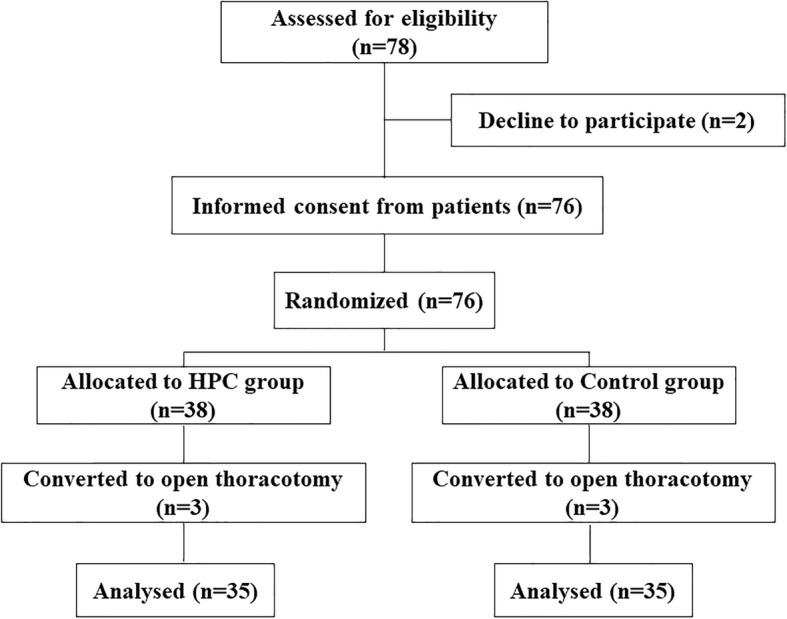
Participant flow of Hypoxic Preconditioning
Patient characteristics, and pulmonary function test results are shown in Table 1. No differences were observed between two groups regarding age, sex, weight, height, comorbidities, and preoperative pulmonary function.
Table 1.
Patient characteristics
| HPC group (n = 35) | Control group (n = 35) | |
|---|---|---|
| Age (y) | 63.1 ± 12.7 | 62.3 ± 13.5 |
| Sex (male/female) | 21/14 | 19/16 |
| Weight (kg) | 60.6 ± 10.9 | 58.7 ± 11.4 |
| Height (cm) | 165 ± 9 | 162 ± 8 |
| Comorbidities | ||
| Hypertension | 8 | 9 |
| CAD | 1 | 2 |
| Diabetes | 0 | 1 |
| Smoking | ||
| Non-smoker | 12 | 10 |
| Ex-smoker | 11 | 12 |
| Current smoker | 12 | 13 |
| % of predicted FVC | 92.3 ± 8.8 | 91.1 ± 9.2 |
| % of predicted FEV1 | 88.9 ± 10.2 | 86.2 ± 11.6 |
Values are expressed as the means ± standard deviation or number (n)
Abbreviations: CAD coronary artery disease, FVC forced vital capacity, FEV1 forced expiratory volume in 1 s, HPC hypoxic preconditioning
Intraoperative data is summarized in Table 2. The duration of surgery, types of resections, estimated blood loss, and incidence of adverse events were similar between the two groups.
Table 2.
Intraoperative characteristics
| HPC group (n = 35) | Control group (n = 35) | P value | |
|---|---|---|---|
| Left/right lobectomy | 18/17 | 19/16 | 0.81 |
| Anesthesia time (min) | 213 ± 41 | 220 ± 45 | 0.50 |
| Operation time (min) | 164 ± 39 | 171 ± 43 | 0.48 |
| OLV time (min) | 145 ± 27 | 142 ± 31 | 0.67 |
| FiO2 (%) | 97.7 ± 9.4 | 100 | 0.16 |
| Crystalloid (ml) | 812 ± 65 | 843 ± 72 | 0.33 |
| Colloid (ml) | 215 ± 46 | 201 ± 52 | 0.53 |
| Estimated blood loss (ml) | 150 (50–200) | 160 (50–250) | 0.58 |
| Adverse events (%) | 14 (40%) | 12 (34.3%) | 0.62 |
| Tachycardia | 3 | 1 | 0.61 |
| Bradycardia | 2 | 5 | 0.43 |
| Hypotension | 4 | 2 | 0.67 |
| Hypertension | 5 | 3 | 0.71 |
| Oxygen desaturation | 0 | 1 | 0.31 |
Values are expressed as the means ± standard deviation, median (interquartile range), number or percentage of patients
As shown in Table 3, the PaO2/FiO2 ratio in the HPC group was significantly higher than that in the control group at 30 min after OLV and 7 day after operation. In addition, postoperative values of FVC and FEV1 were significantly greater in the HPC group than in the control group.
Table 3.
Changes in the PaO2/FiO2 ratio and in pulmonary function
| Time | HPC | Control | |
|---|---|---|---|
| PaO2/FiO2 | OLV 30 min | 2.01 ± 0.53* | 1.83 ± 0.64 |
| 7d after surgery | 1.95 ± 0.38* | 1.74 ± 0.32 | |
| FVC | Baseline | 2.84 ± 0.66 | 2.72 ± 0.97 |
| 7d after surgery | 2.77 ± 0.93* | 2.54 ± 0.86 | |
| FEV1 | Baseline | 2.32 ± 0.50 | 2.28 ± 0.80 |
| 7d after surgery | 2.27 ± 0.38* | 2.06 ± 0.57 |
Values are expressed as the means ± standard deviation. *P < 0.05 compared with control group
Abbreviations: FVC forced vital capacity, FEV1 forced expiratory volume in 1 s, HPC hypoxic preconditioning; OLV, one-lung ventilation
Postoperative complications, mortality, and duration of hospital stay are shown in Table 4. No significant differences were observed between groups in the incidence of pulmonary complications or overall postoperative morbidity. HPC was associated with a statistically significant reduction in postoperative hospital stay.
Table 4.
Postoperative complications, mortality, and duration of hospital stay
| HPC group (n = 35) | Control group (n = 35) | |
|---|---|---|
| Pulmonary complications | ||
| Atelectasis | 3 | 1 |
| Pneumonia | 3 | 4 |
| Prolonged air leak | 0 | 0 |
| Pleural effusion | 0 | 1 |
| Others | ||
| Urinary tract infection | 1 | 0 |
| Neurologic complications | 0 | 0 |
| Overall morbidity | 7 | 6 |
| Mortality | 0 | 0 |
| Postoperative hospital stay (d) | 8.32 ± 3.77* | 10.87 ± 6.58 |
Values are expressed as the means ± standard deviation, number of patients. *P < 0.05 compared with control group
Abbreviations: HPC hypoxic preconditioning
Discussion
This randomized controlled trial demonstrated that HPC performed before surgery improves postoperative oxygenation, enhances recovery of pulmonary function, and reduces the duration of hospital stay in patients who underwent thoracoscopic lobectomy. These results support our hypothesis that the application of HPC on the nondependent lung protects lungs from injury. In addition, no differences in intraoperative adverse events were observed with and without HPC.
The HPC intervention used in this study can also be referred to as intermittent ventilation or intermittent hypoxia. A previous study has demonstrated that intermittent hypoxia increased hypoxic pulmonary vasoconstriction of the nondependent lung [16]. Another previous study also demonstrated that intermittent ventilation had a beneficial effect on oxygenation during OLV for thoracic surgery [17]. However, previous studies only assessed the effect of HPC on intraoperative oxygenation.
In the current study, we assessed the protective effect of HPC on lung injury in patients undergoing thoracoscopic lobectomy. The PaO2/FiO2 ratio was chosen as the primary outcome because it is a useful indicator for detecting impaired gas exchange and oxygenation among patients receiving a wide range of FiO2 [18]. The current study demonstrated a positive effect of HPC that not only mitigated the decrease of the PaO2/FiO2 ratio during OLV but also improved postoperative oxygenation.
Lung injury following thoracic surgery has been recognized as a major cause of postoperative complication, mortality, and delayed discharge [19–21]. The exact mechanism of lung injury is still unclear. Previous studies have found that lung re-expansion provoked severe oxidative stress, which was associated with major adverse effects after lung resection [22–24]. Moreover, HPC performed before OLV was shown to alleviate systematic inflammatory response and oxidative stress in patients with lung cancer [25]. Thus, we postulated that the protective effect of HPC on lung injury was partly due to the attenuation of oxidative stress in this study. Although a trend toward a reduced incidence of postoperative pulmonary complications was observed in patients who received HPC, this did not reach statistical significance. A possible explanation is that the small sample size of this study may be insufficient to detect this difference between the two groups.
Pulmonary function after thoracic surgery reduced in both groups. The current study found that lung function was better preserved in patients who received HPC than in those who did not receive HPC. These results suggest that the use of HPC is beneficial to the recovery of pulmonary function. In a previous study of an animal model, intermittent hypoxia was shown to be an effective approach for improving respiratory function after cervical spinal cord injury [26].
The primary limitation of our preliminary trial was the relatively small size of the study population. Given the intrinsic biases of a small sample, the findings of the present study should be confirmed with further studies.
Conclusions
In conclusion, our study suggests that HPC may be a promising prevention option for lung injury, especially in patients undergoing lung cancer surgery. HPC performed before OLV improves postoperative oxygenation, enhances recovery of pulmonary function, and reduces the duration of hospital stay.
Acknowledgements
We thank the thoracic surgeons of Zhoushan Hospital for their assistance.
Abbreviations
- DLT
Double-lumen tube
- FEV1
Forced expiratory volume in 1 s
- FiO2
Fraction of inspired oxygen
- FVC
Forced vital capacity
- HPC
Hypoxic preconditioning
- IPC
Ischemic preconditioning
- NSCLC
Non-small cell lung cancer
- OLV
One-lung ventilation
- SpO2
Saturation of pulse oxygenation
- TLV
Two-lung ventilation
Authors’ contributions
YZ and FW conceived and designed the study. WZ, MC, HL, JY and JL performed this study and analyzed the data. WZ and MC wrote the manuscript. YZ and FW reviewed and edited the manuscript. All authors read and approved the final manuscript.
Funding
This study was funded by Zhoushan Municipal Commission Fund of Health and Family Planning [2017A11] and Health Commission of Zhejiang Province [2018KY855]. The funding agents play no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was approved by the Institutional Ethics Committee of Zhoushan Hospital (Ref. 2017–008) and written informed consent for enrollment in this study was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wenjing Zhang and Mo Chen contributed equally to this work.
Contributor Information
Wenjing Zhang, Email: 503230729@qq.com.
Mo Chen, Email: chenmo2019@gmail.com.
Hongbin Li, Email: lmzz1016@126.com.
Jia Yuan, Email: 27683628@qq.com.
Jingjing Li, Email: 378908367@qq.com.
Feixiang Wu, Email: feixiangwu@hotmail.com.
Yan Zhang, Email: zy476291@163.com.
References
- 1.Gothard J. Lung injury after thoracic surgery and one-lung ventilation. Curr Opin Anaesthesiol. 2006;19(1):5–10. doi: 10.1097/01.aco.0000192783.40021.c1. [DOI] [PubMed] [Google Scholar]
- 2.Schloss B, Martin D, Beebe A, Klamar J, Tobias JD. Phenylephrine to treat hypoxemia during one-lung ventilation in a Pediatric patient. Thorac Cardiovasc Surg Rep. 2013;2(1):16–18. doi: 10.1055/s-0033-1343734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karzai W, Schwarzkopf K. Hypoxemia during one-lung ventilation: prediction, prevention, and treatment. Anesthesiology. 2009;110(6):1402–1411. doi: 10.1097/ALN.0b013e31819fb15d. [DOI] [PubMed] [Google Scholar]
- 4.Lohser J, Slinger P. Lung injury after one-lung ventilation: a review of the pathophysiologic mechanisms affecting the ventilated and the collapsed lung. Anesth Analg. 2015;121(2):302–318. doi: 10.1213/ANE.0000000000000808. [DOI] [PubMed] [Google Scholar]
- 5.Serpa Neto A, Hemmes SNT, Barbas CSV, Beiderlinden M, Fernandez-Bustamante A, Futier E, et al. Incidence of mortality and morbidity related to postoperative lung injury in patients who have undergone abdominal or thoracic surgery: a systematic review and meta-analysis. Lancet Respir Med. 2014;2(12):1007–1015. doi: 10.1016/S2213-2600(14)70228-0. [DOI] [PubMed] [Google Scholar]
- 6.Lu G-W, Yu S, Li R-H, Cui X-Y, Gao C-Y. Hypoxic preconditioning: a novel intrinsic cytoprotective strategy. Mol Neurobiol. 2005;31(1–3):255–271. doi: 10.1385/MN:31:1-3:255. [DOI] [PubMed] [Google Scholar]
- 7.Xiang X, Lin H, Liu J, Duan Z. Equivalent cardioprotection induced by ischemic and hypoxic preconditioning. Thorac Cardiovasc Surg. 2013;61(3):229–233. doi: 10.1055/s-0032-1322609. [DOI] [PubMed] [Google Scholar]
- 8.Serebrovska TV, Shatilo VB. Remote ischemic preconditioning versus intermittent hypoxia training: a comparative analysis for cardioprotection. Fiziolohichnyi Zhurnal Kiev Ukr. 2015;61(3):99–117. doi: 10.15407/fz61.03.099. [DOI] [PubMed] [Google Scholar]
- 9.Chen C-L, Zheng H, Xuan Y, Amat A, Chen L, Yu J, et al. The cardioprotective effect of hypoxic and ischemic preconditioning in dogs with myocardial ischemia-reperfusion injury using a double-bypass model. Life Sci. 2015;141:25–31. doi: 10.1016/j.lfs.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Zhan L, Wang T, Li W, Xu ZC, Sun W, Xu E. Activation of Akt/FoxO signaling pathway contributes to induction of neuroprotection against transient global cerebral ischemia by hypoxic pre-conditioning in adult rats. J Neurochem. 2010;114(3):897–908. doi: 10.1111/j.1471-4159.2010.06816.x. [DOI] [PubMed] [Google Scholar]
- 11.Choukèr A, Ohta A, Martignoni A, Lukashev D, Zacharia LC, Jackson EK, et al. In vivo hypoxic preconditioning protects from warm liver ischemic/reperfusion injury through the adenosine A2B receptor. Transplantation. 2012;94(9):894–902. doi: 10.1097/TP.0b013e31826a9a46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z, Fang B, Tan Z, Zhang D, Ma H. Hypoxic preconditioning increases the protective effect of bone marrow mesenchymal stem cells on spinal cord ischemia/reperfusion injury. Mol Med Rep. 2016;13(3):1953–1960. doi: 10.3892/mmr.2016.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharp FR, Ran R, Lu A, Tang Y, Strauss KI, Glass T, et al. Hypoxic preconditioning protects against ischemic brain injury. NeuroRx J Am Soc Exp Neurother. 2004;1(1):26–35. doi: 10.1602/neurorx.1.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vesnina ZV, Lishmanov YB, Alexandrova EA, Nesterov EA. Evaluation of Nephroprotective efficacy of hypoxic preconditioning in patients undergoing coronary artery bypass surgery. Cardiorenal Med. 2016;6(4):328–336. doi: 10.1159/000446571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li C, Xu M, Wu Y, Li Y-S, Huang W-Q, Liu K-X. Limb remote ischemic preconditioning attenuates lung injury after pulmonary resection under propofol-remifentanil anesthesia: a randomized controlled study. Anesthesiology. 2014;121(2):249–259. doi: 10.1097/ALN.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 16.Benumof JL. Intermittent hypoxia increases lobar hypoxic pulmonary vasoconstriction. Anesthesiology. 1983;58(5):399–404. doi: 10.1097/00000542-198305000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Yasuuji M, Kusunoki S, Hamada H, Kawamoto M. Intermittent reinflation is safe to maintain oxygenation without alteration of extravascular lung water during one-lung ventilation. J Clin Anesth. 2014;26(3):177–183. doi: 10.1016/j.jclinane.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Rice TW, Wheeler AP, Bernard GR, Hayden DL, Schoenfeld DA, Ware LB, et al. Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest. 2007;132(2):410–417. doi: 10.1378/chest.07-0617. [DOI] [PubMed] [Google Scholar]
- 19.Sengupta S. Post-operative pulmonary complications after thoracotomy. Indian J Anaesth. 2015;59(9):618–626. doi: 10.4103/0019-5049.165852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iyer A, Yadav S. Postoperative Care and Complications After Thoracic Surgery. Princ Pract Cardiothorac Surg [Internet]. 2013 Jun 12 [cited 2019 Feb 7]; Available from: https://www.intechopen.com/books/principles-and-practice-of-cardiothoracic-surgery/postoperative-care-and-complications-after-thoracic-surgery
- 21.Şen S, Şen S, Şentürk E, Kuman NK. Postresectional lung injury in thoracic surgery pre and intraoperative risk factors: a retrospective clinical study of a hundred forty-three cases. J Cardiothorac Surg. 2010;5:62. doi: 10.1186/1749-8090-5-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Misthos P, Katsaragakis S, Milingos N, Kakaris S, Sepsas E, Athanassiadi K, et al. Postresectional pulmonary oxidative stress in lung cancer patients. The role of one-lung ventilation. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. 2005;27(3):379–382. doi: 10.1016/j.ejcts.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 23.Misthos P, Katsaragakis S, Theodorou D, Milingos N, Skottis I. The degree of oxidative stress is associated with major adverse effects after lung resection: a prospective study. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. 2006;29(4):591–595. doi: 10.1016/j.ejcts.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 24.García-de-la-Asunción J, García-del-Olmo E, Perez-Griera J, Martí F, Galan G, Morcillo A, et al. Oxidative lung injury correlates with one-lung ventilation time during pulmonary lobectomy: a study of exhaled breath condensate and blood. Eur J Cardiothorac Surg. 2015;48(3):e37–e44. doi: 10.1093/ejcts/ezv207. [DOI] [PubMed] [Google Scholar]
- 25.Cheng Y-D, Gao Y, Zhang H, Duan C-J, Zhang C-F. Effects of OLV preconditioning and postconditioning on lung injury in thoracotomy. Asian J Surg. 2014;37(2):80–85. doi: 10.1016/j.asjsur.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Lee K-Z, Chiang S-C, Li Y-J. Mild acute intermittent hypoxia improves respiratory function in Unanesthetized rats with Midcervical contusion. Neurorehabil Neural Repair. 2017;31(4):364–375. doi: 10.1177/1545968316680494. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.


