Abstract
Background
Abnormal autocrine fibroblast growth factor 19 (FGF19) production has been observed in several types of cancers, including hepatocellular carcinoma (HCC). In this study, we investigated the potential of serum FGF19 as a novel tumor marker of HCC based on a sandwich enzyme-linked immunosorbent assay (ELISA).
Methods
The serum FGF19 levels of 304 patients with HCC was measured by ELISA. The serum levels of existing markers, including alpha-fetoprotein (AFP) and des-gamma-carboxy prothrombin (DCP) were determined by chemiluminescence enzyme immunoassay. Both diagnostic value of FGF19 and its changes after curative ablation therapy was further examined.
Results
The median FGF19 levels in controls, chronic liver disease patients, and primary HCC patients, were 78.8 pg/mL, 100.1 pg/mL, and 214.5 pg/mL, respectively. The subsequent receiver operating characteristic curves (ROC) successfully determined an optimal cut-off value of 200.0 pg/mL. The area under the ROC curve (AUC) of FGF19 for HCC detection was comparable to those of AFP and DCP. Of importance, FGF19 showed higher sensitivity for the detection of small HCC (solitary cancer with diameter < 20 mm) than those of existing markers. In addition, 43 out of 79 cases (54.4%) with normal AFP and DCP (so-called “double negative HCC”) exhibited serum FGF19 level ≥ 200 pg/mL. In 45 HCC patients treated with curative ablation therapy, serum FGF19 levels changed from 257.4 pg/mL to 112.0 pg/mL after the treatment.
Conclusion
Our findings reveal that FGF19 can be a potential novel biomarker for HCC. Although FGF19 is not necessarily a substitute for existing markers, it may help improve the prognosis in HCC patients owing to its resourceful use in various aspects of HCC management and treatment.
Keywords: FGF19, HCC, Tumor marker, AFP, RFA
Background
Hepatocellular carcinoma (HCC) is the third largest cause of cancer deaths globally, and the number of new HCC cases has been gradually increasing [1, 2]. In spite of the advances in imaging technology and therapeutic approaches, the 5-year survival rate remains at 20% [3, 4]. Tumor marker detection has been widely used for several purposes, such as comprise diagnosis, follow-up of the post-treatment clinical course, optimization of the treatment efficacy, and prediction of prognosis in a variety of cancers [5]. In HCC, three tumor markers, namely alpha-fetoprotein (AFP), AFP-L3, and des-gamma-carboxy prothrombin (DCP), have been used as serum biomarkers. Although the measurement of these markers is not necessarily needed for the establishment of a definitive diagnosis of HCC as per the guidelines proposed by American Association for the Study of Liver Diseases (AASLD), European Association for the Study of the Liver (EASL), and The Japan Society of Hepatology (JGH), these markers play a key role in monitoring for HCC onset and recurrence [6–8]. However, it is well-known that these markers often remain in the normal range, particularly in small HCC [9]. Furthermore, an unexpected elevation in these markers is sometimes observed in chronic liver disease (CLD) patients who do not have HCC. Taken together, alternative serum biomarkers with high sensitivity and specificity are required.
Fibroblast growth factors (FGFs) signal through FGF receptor (FGFR) tyrosine kinases to regulate a wide range of biological processes, including cell growth, differentiation, angiogenesis, and metabolism [10–12]. It is noteworthy that dysregulated FGF/FGFR signaling contributes to cancer development in many types of cancers [13–18]. Fibroblast growth factor 19 (FGF19), secreted from ileum, negatively regulates bile duct acid synthesis in the liver through FGFR4 activation [19, 20]. However, FGF19 production in an autocrine fashion reportedly activates FGF19/FGFR4 signaling and contributes to HCC development [21]. It has been also demonstrated that the overexpression of FGF19 and FGFR4 is associated with unfavorable prognosis in HCC patients [22]. These findings are mainly based on pathological studies, and whether serum FGF19 functions as a biomarker in HCC remains unclear.
In this study, we conducted a sandwich enzyme-linked immunosorbent assay (ELISA) to examine the serum levels of FGF19 in HCC patients. To determine the sensitivity and specificity of FGF19 as a tumor marker for HCC, the sera of controls and CLD patients were also examined. After determining of the optimal cut-off value based on the receiver operating characteristic curves (ROC), we compared the tumor detection ability of FGF19 and the existing markers. Further, we attempted to determine whether FGF19 functions as a marker of treatment efficacy in HCC treated with ablation therapy.
Methods
Patients and blood samples
Blood samples were collected from 304 patients who underwent initial treatment for HCC at the Chiba University hospital between January 2014 and December 2017. Serum samples were collected during the 1-month period before treatment initiation. To investigate the changes in the FGF19 levels, the sera of patients treated with radiofrequency ablation (RFA) were also collected. Similarly, the sera of 142 CLD patients without HCC and those of 10 non-CLD patients (controls) were also collected. After obtaining informed written consent, we analyzed the preserved blood samples and the data acquired from their medical records. This study was approved by the Research Ethics Committees of the Graduate School of Medicine, Chiba University (approval number: 3024).
Primary HCC patients comprised 213 men (70.1%) and 91 women (29.9%), with a median age of 72 years. Liver damage was attributed to Hepatitis B virus (HBV) infection (11.2%), Hepatitis C virus (HCV) infection (51.3%), and others (37.5%) (Table 1). As per the Child–Pugh classification, 80.9% of the patients belonged to class A, 16.1% to class B, and 3.0% to class C. According to the Union for International Cancer Control (UICC) stages, the number of patients in stage I, II, III, and IV were 141 (46.4%), 72 (23.7%), 69 (22.7%), and 22 (7.2%), respectively. The CLD patients comprised 84 men (59.2%) and 58 women (40.8%), with a median age of 65 years. Liver damage was attributed to HBV infection (18.3%), HCV infection (48.6%), and others (33.1%). As per the Child–Pugh classification, 88.0% of the CLD patients belonged to class A, 9.2% to class B, and 2.8% to class C.
Table 1.
Baseline characteristics of study patients
| Characteristics | CLD patients (n = 142) | HCC patients (n = 304) | p value |
|---|---|---|---|
| Age (year)a | 65 (14) | 72 (14) | < 0.001 |
| Sex (male/female) | 84/58 | 213/91 | 0.023 |
| Etiology (HBV/HCV/Others) | 26/69/47 | 34/156/114 | 0.116 |
| Liver damage (CH/LC) | 66/76 | 52/252 | < 0.001 |
| AFP (ng/mL)a | 3.8 (3.1) | 15.7 (112.7) | < 0.001 |
| DCP (mAU/mL)a | 20 (9) | 93 (1140) | < 0.001 |
| AST (IU/L)a | 27 (23) | 47 (44) | < 0.001 |
| ALT (IU/L)a | 22 (28) | 35 (32) | < 0.001 |
| ALB (g/dL)a | 4.3 (0.5) | 3.7 (0.7) | < 0.001 |
| T-Bil (mg/dL)a | 0.9 (0.5) | 0.9 (0.6) | 0.612 |
| PLT (× 104/μL)a | 14.2 (10.2) | 11.9 (9.7) | < 0.001 |
| PT (%)a | 99 (21) | 93 (24) | 0.014 |
| Child-Pugh (A/B/C) | 125/13/4 | 246/49/9 | 0.137 |
a Data are expressed as median (interquartile range)
Abbreviations, CLD chronic liver disease, HCC hepatocellular carcinoma, HBV hepatitis B virus, HCV hepatitis C virus, CH chronic hepatitis, LC liver cirrhosis, AFP alpha-fetoprotein, DCP des-gamma-carboxy prothrombin, AST aspartate aminotransferase, ALT alanine aminotransferase, ALB albumin, T-Bil total bilirubin, PLT platelet, PT prothrombin time
Diagnosis of CLD and HCC
The diagnosis of CLDs, including chronic hepatitis and cirrhosis was based on the laboratory data, clinical manifestation, and/or histological finding [23]. HCC was diagnosed on the basis of contrast-enhanced imaging findings and/or histological analysis as per the diagnostic criteria of the AASLD [24, 25]. A solitary lesion with a diameter < 20 mm defined small HCC.
Measurement of serum FGF19, AFP, and DCP levels
Serum FGF19 levels of the primary HCC patients, CLD patients, and controls were determined using a sandwich ELISA according to the manufacturer’s instructions (R&D Systems, Inc., MN). Serum AFP and DCP levels were determined by chemiluminescence enzyme immunoassay (CLEIA) (LUMIPULSE® L2400, FUJIREBIO INC., Japan). Serum FGF19 levels of the HCC patients were measured using the sera collected during the 1-month period before the initial treatment. Moreover, the serum FGF19 levels of 45 patients treated with complete ablation were also analyzed at some point in recurrence-free period based on radiological findings.
Statistical analyses
Data are expressed as the median and interquartile range (IQR) values. Statistical differences in the quantitative valuables of the groups were determined using Wilcoxon rank sum test and Kruskal-Wallis test. Chi-square test was used for the categorical values. Correlation between each marker was determined using Spearman’s rank correlation coefficient. The area under the curve (AUC) values were determined with the ROC analysis. Recurrence-free survival (RFS) was calculated using the Kaplan-Meier method and compared using log-rank test. The level of significance was set at p < 0.05. All statistical analyses were performed using the SPSS statistical software package (SPSS version 24).
Results
Serum FGF19 levels
The result of ELISA demonstrated that the median serum FGF19 levels in controls, CLD patients, and primary HCC patients were 78.8 pg/mL, 100.1 pg/mL, and 214.5 pg/mL, respectively (Fig. 1). The FGF19 levels of HCC patients ranged from 20.0 pg/mL to 5605.8 pg/mL. HCC patients showed significantly higher serum FGF19 levels than controls (p = 0.002) and CLD patients (p < 0.001). Although CLD patients showed a tendency to have higher serum FGF19 levels than controls, the difference was not statistically significant.
Fig. 1.
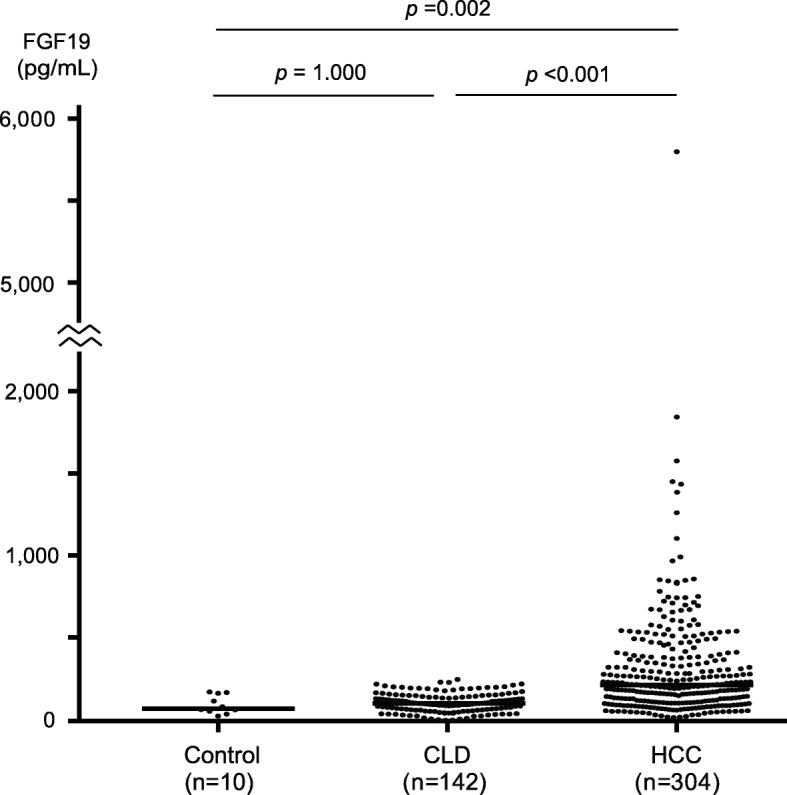
Dot plots for the serum FGF19 levels in controls, CLD patients, and HCC patients
ROC analysis
To compare the diagnostic ability of FGF19 and the existing markers, namely AFP and DCP, we subsequently conducted ROC analysis. The AUC values for FGF19, AFP, and DCP for HCC detection were 0.795, 0.827, and 0.854, respectively (Fig. 2).
Fig. 2.
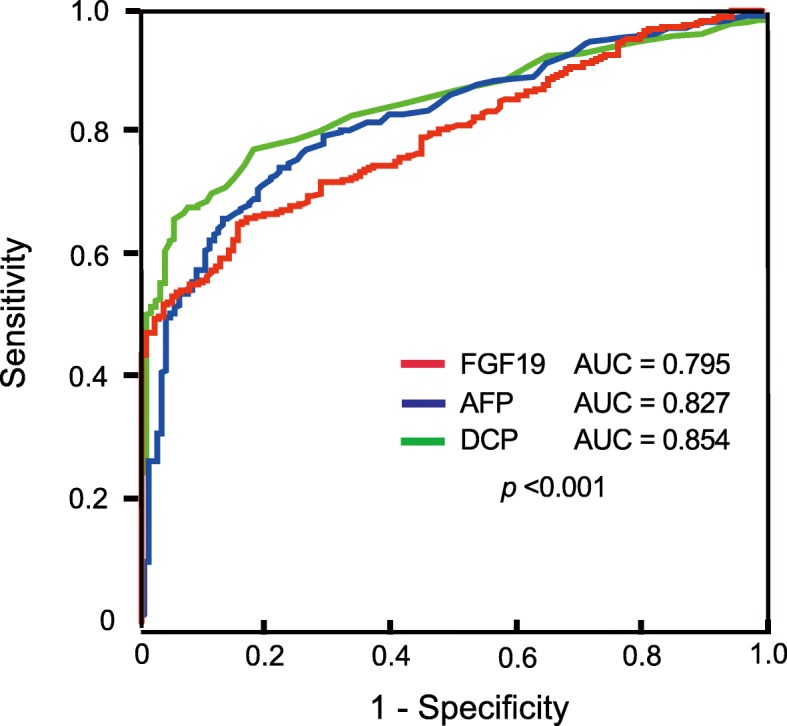
ROC curves of FGF19, AFP, and DCP for HCC diagnosis
Diagnostic value of FGF19 compared to those of AFP and DCP
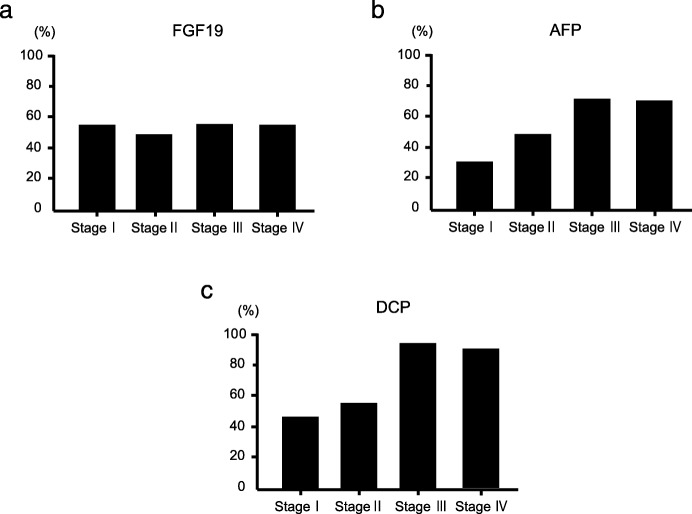
We calculated the cut-off value of FGF19 as 200 pg/mL using Youden index. According to the previous studies, those of AFP and DCP were decided as 20 ng/mL and 40 mAU/mL, respectively [26, 27]. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of FGF19 for HCC diagnosis were 53.2, 95.1, 95.9, and 48.7%, respectively (Table 2). These data appeared comparable to those of other existing markers. In particular, the sensitivity of FGF19 was significantly higher than those of other markers (55.0% for FGF19, 30.4% for AFP, and 33.3% for DCP) in patients with small HCC, defined as a solitary tumor with a diameter < 20 mm (Table 3). Thereafter, we examined the positive rate of each marker in HCC patients as per the UICC stage progression (Fig. 3). Although the sensitivities of the existing markers, including AFP, and DCP in HCC with UICC stage I were 29.1 and 44.7%, respectively, they increased with stage progression. The sensitivities of AFP and DCP in HCC with UICC stage IV were 68.2 and 90.2%, respectively. In contrast, the sensitivity of FGF19 was constant at approximately 50%, irrespective of the UICC stage (54.6% in stage I, 48.6% in stage II, 55.1% in stage III, and 54.5% in stage IV).
Table 2.
Sensitivity, specificity, PPV, NPV, and accuracy of serum FGF19 levels and the existing markers alone and in combination in all the HCC cases
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) | |
|---|---|---|---|---|---|
| Single marker | |||||
| AFP | 44.4 | 96.5 | 96.4 | 44.8 | 61.0 |
| DCP | 62.2 | 95.5 | 96.9 | 52.9 | 70.9 |
| FGF19 | 53.2 | 95.1 | 95.9 | 48.7 | 66.6 |
| Double markers | |||||
| AFP and DCP | 73.7 | 91.7 | 95.3 | 61.0 | 77.6 |
| AFP and FGF19 | 76.0 | 91.5 | 95.1 | 64.0 | 80.9 |
| DCP and FGF19 | 81.3 | 91.0 | 95.4 | 68.8 | 82.5 |
| Multiple markers | |||||
| AFP, DCP, and FGF19 | 87.5 | 87.2 | 94.0 | 76.3 | 85.7 |
Table 3.
Sensitivity, specificity, PPV, NPV, and accuracy of serum FGF19 levels and the existing markers alone and in combination in small HCC cases
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) | |
|---|---|---|---|---|---|
| Single marker | |||||
| AFP | 30.4 | 96.5 | 80.7 | 74.1 | 74.9 |
| DCP | 33.3 | 95.5 | 79.3 | 73.4 | 71.1 |
| FGF19 | 55.0 | 95.1 | 84.4 | 79.9 | 82.0 |
| Double markers | |||||
| AFP and DCP | 53.6 | 91.7 | 77.1 | 79.2 | 75.4 |
| AFP and FGF19 | 68.1 | 91.5 | 79.7 | 85.5 | 83.9 |
| DCP and FGF19 | 66.7 | 91.0 | 79.3 | 84.0 | 79.1 |
| Multiple markers | |||||
| AFP, DCP, and FGF19 | 75.4 | 87.2 | 75.3 | 87.2 | 80.1 |
Fig. 3.
Sensitivities of FGF19, AFP, and DCP for HCC detection as per the UICC stages. Although FGF19 (a) remained constant at approximately 50% independent of UICC stages, AFP (b), and DCP (c) showed high sensitivity in the advanced stages but not in the early stages
Supplementary effect of FGF19 on HCC detection when used with the existing markers
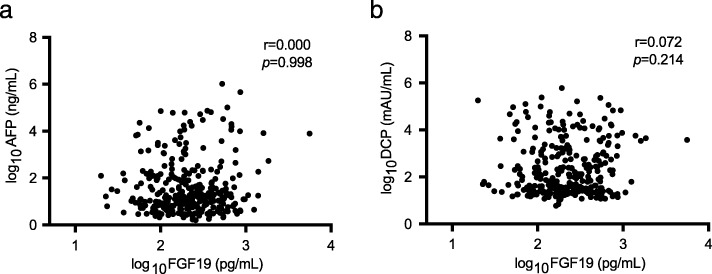
We estimated the relationship between FGF19 and the existing markers. Spearman’s rank correlation coefficient analysis demonstrated no significant correlation of the serum FGF19 level with AFP or DCP (Fig. 4). Therefore, we examined an additional effect of FGF19 measurement on the existing markers of HCC detection. As expected, the additional FGF19 measurement resulted in increased sensitivity and NPV and a mild decrease in the specificity and PPV. In all the HCC cases analyzed, the addition of FGF19 measurement to AFP or DCP increased the sensitivity from 44.4 to 76.0% and 62.2 to 81.3%, respectively. Although the sensitivity of the combined use of AFP and DCP was 73.7%, the added measurement of FGF19 increased the sensitivity to 87.5% (Table 2). A similar marked trend was observed in patients with small HCC (Table 3). In the analyses of small HCC, the addition of FGF19 to AFP or DCP increased the sensitivity from 30.4 to 68.1% and 33.3 to 66.7%, respectively. It is noteworthy that the sensitivity of the combined use of AFP and DCP was only 53.6%, while that with AFP, DCP, and FGF19 was up to 75.4%. Thus, not only FGF19 measurement, but also the combined analyses of FGF19 and the existing markers could contribute to HCC diagnosis, especially in patients with small HCC.
Fig. 4.
Correlation of the serum FGF19 levels with AFP and DCP. Spearman’s rank correlation coefficient analyses showed no correlation of serum FGF19 levels with AFP (a) or DCP (b)
FGF19 as a marker for response to treatment and recurrence prediction
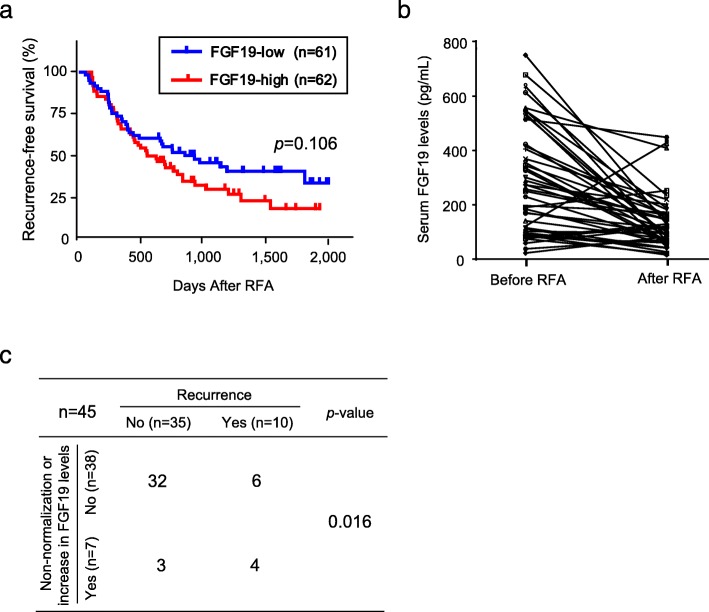
Among the 304 HCC patients, 123 were treated with RFA, those had achieved complete response based on post-RFA imaging findings. Subsequently, they were subjected to the prognostic analyses. Although there were no statistical differences, Kaplan-Meier analyses showed a trend that RFS in patients with high serum FGF19 levels (≥200 pg/mL) is shorter than those in patients with low serum FGF19 levels (p = 0.106, Fig. 5a). We then focused on the changes in FGF19 levels. For 45 of the 123 patients, paired serum samples were prepared for the further analyses (Fig. 5b). Among them, 25 patients with high serum FGF19 levels (≥200 pg/mL) showed a decrease in the FGF19 levels diminished after the ablation therapy. Although 5 patients maintained elevated FGF19 levels after the ablation, 2 patients developed recurrence within a year of RFA. Among the 20 HCC patients with low FGF19 levels, 2 showed an increase in the serum FGF19 levels (≥200 pg/mL) after the treatment. Subsequently, 1 patient experienced recurrence within a year of RFA. The recurrence rates in patients with non-normalization and an unexpected increase in the FGF19 levels after the ablation therapy were significantly higher than those in patients without them (p = 0.016, Fig. 5c).
Fig. 5.
Utilities of FGF19 as a biomarker for recurrence prediction. (a) Cumulative RFS rate based on serum FGF19 levels before ablation therapy. (b) Changes in the serum FGF19 levels after the ablation therapy against HCC. (c) Relationship between non-normalization and an unexpected increase in the FGF19 levels after the ablation therapy and early recurrence
Discussion
Recent advances in research that has used next-generation sequencers has enabled the detection of genomic aberrations [28]. Thus, substantial chromosomal and genetic abnormalities of the driver genes have been reported in a variety of cancers, including HCC [29]. Among them, focal amplification of the FGF19 gene, located on chromosome locus 11q13, has been detected in 20% of all clinical HCC samples [30]. In contrast, immunohistochemical analyses have demonstrated that FGF19 overexpression is observed in approximately 50% of all HCC cases [31]. These findings indicates that FGF19 overexpression in HCC tissues may not be accompanied by its copy number gain. Considering that FGF19 is a serum secretory protein produced by HCC cells in an autocrine loop fashion, we investigated the efficacy of serum FGF19 as a tumor marker.
First, the serum FGF19 levels of HCC patients were measured using a sandwich ELISA. As expected, the serum FGF19 levels in HCC patients were significantly higher than those in controls (p = 0.002) and in CLD patients (p < 0.001). Given that FGFs are associated with pulmonary fibrosis and renal fibrosis [32, 33], we investigated the influence of the FGF19 serum levels on liver fibrosis. Consequently, Spearman’s rank correlation coefficient analyses showed no relationship of FGF19 with hyaluronic acid or FIB-4 index (data not shown). These results indicate the possibility that FGF19 functions as a marker of HCC rather than that of severe fibrosis.
Ideal biomarkers should be highly sensitive and specific to enable early detection of HCC. Our results demonstrated that the sensitivity of FGF19 was highest among that of the existing markers, followed by that of DCP in all HCC patients, and was highest in patients with small liver cancer. Existing markers, such as AFP and DCP, often remain in the normal range in HCC patients, not only in the early stage, but also in the advanced stage [34, 35]. Although the sensitivities of AFP and DCP were 29.1 and 44.7% in HCC with UICC stage I, respectively, there was an increasing trend with stage progression. In contrast, the sensitivity of FGF19 was constant at approximately 50%, irrespective of the UICC stage. It is noteworthy that in patients with stage I, the sensitivity of FGF19 was significantly higher than those of AFP and DCP.
The specificity of FGF19 was comparable to those of the other markers in both all the patients with HCC and patients with small HCC. However, the FGF19 levels in CLD patients were mildly but not significantly elevated as compared to those in controls. Although bile acid is produced from cholesterol in the liver, hepatic bile acid synthetic levels and its secretion are tightly regulated by enterohepatic circulation [36]. However, cholestasis and liver dysfunction increase the concentrated bile acid in both the blood and bile and induce hepatocyte injury [37]. Similar to HCC cells, normal hepatocytes produce FGF19 in an autocrine fashion to protect hepatocytes from the cytotoxicity of bile acid in mice model [38]. Considering that FGF19 inhibits bile acid synthesis via the downregulation of cholesterol 7 alpha-hydroxylase (Cyp7a) [39], a mild increase in the serum FGF19 levels of CLD patients may be responsible for the negative feedback of elevated serum bile acid levels.
Because there was no significant correlation of the serum FGF19 levels and the existing markers such as AFP or DCP, we then examined whether FGF19 measurement in addition to that of these markers could improve the HCC detection rate. As expected, the addition of FGF19 measurement increased the sensitivity of HCC detection. Of importance, 79 of the 304 cases (26.0%) showed negativity for AFP and DCP, 43 cases (14.1%) could be detected using FGF19 measurement. Moreover, from the 32 cases (out of 69 cases, 46.4%) negative for AFP and DCP in patients with small HCC, 15 cases (21.7%) showed abnormal elevation in the serum FGF19 levels. Whereas AFP and DCP are markers that are comparatively highly specific for HCC, increased serum FGF19 levels have been reported in several types of cancers [40]. This is the point which requires attention.
Unlike the existing markers, FGF19 is a functional protein that is responsible for essential intracellular signal of HCC. FGF19 plays an important role in the proliferation of both, tumor cells and endothelial cells; therefore, it is believed to be promising therapeutic target molecules [41]. In fact, anti-FGF19 antibody treatment is reported to reduce the growth of colon tumor xenografts and prevent HCC development in FGF19 transgenic mice [42]. Furthermore, molecular-targeted drugs for advanced HCC, including sorafenib, regorafenib, and lenvatinib, are categorized as multikinase inhibitors; FGFR4, a receptor for FGF19, is one of the most important therapeutic targets [43, 44]. The activation in FGF19/FGFR4 signaling contributes to sorafenib resistance; therefore, abnormal FGF19 production may be associated with the treatment effect of these drugs [45]. Further analyses are necessary to clarify this issue.
Conclusion
FGF19 functions as a tumor marker for HCC detection, especially for small HCC. The combined use of FGF19 with AFP and DCP increases the diagnostic accuracy of HCC. Further, FGF19 could be a marker for monitoring the therapeutic effect and making a prognostic prediction.
Acknowledgements
Not applicable.
Abbreviations
- AFP
Alpha-fetoprotein
- AFP-L3
Lens culinaris agglutinin-reactive fraction of alpha-fetoprotein
- ALT
Alanine aminotransferase
- AUC
Area under the curve
- CLD
Chronic liver disease
- CLEIA
Chemiluminescence enzyme immunoassay
- DCP
Des-gamma-carboxy prothrombin
- FGF19
Fibroblast growth factor 19
- FGFR4
Fibroblast growth factor receptor 4
- HBV
Hepatitis B virus
- HCC
Hepatocellular carcinoma
- HCV
Hepatitis C virus
- IQR
Interquartile range
- NPV
Negative predictive value
- OS
Overall survival
- PPV
Positive predictive value
- RFA
Radiofrequency ablation
- RFS
Recurrence-free survival
- ROC
Receiver operating curve
- UICC
Union for international cancer control
Authors’ contributions
T.M., T.C., H.K., and K.K. conducted study concept and design. T.M., T.S., Y.K., K.K., S.K., M.K., S.O., E.S., Y.O., S.N., T.K., H.M., and N.K. collected samples. T.M., H.K., J.A., K.K., R.N., and R.M. analyzed and interpreted data obtained. T.C. performed statistical analyses and manuscript preparation. T.K., H.M., and N.K. edited and reviewed manuscript. All authors have read and approved the final version of the manuscript.
Funding
This work is partially supported by grants from the Japan Society for the Promotion of Science (JSPS, #16 K09340) and the Program for Basic and Clinical Research on Hepatitis from Japan Agency for Medical Research and Development (AMED, #JP18fk0210014). The funding bodies were not involved in the study design, data acquisition, interpretation of the results, or writing of the manuscript.
Availability of data and materials
Data is available upon request.
Ethics approval and consent to participate
Informed written consent was obtained from all subjects. This study was approved by the Research Ethics Committees of the Graduate School of Medicine, Chiba University (approval number: 3024).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Takahiro Maeda and Hiroaki Kanzaki contributed equally to this work.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, et al. Global Cancer statistics. CA Cancer J Clin [Internet] 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Kawano Y, Sasaki A, Kai S, et al. Short- and long-term outcomes after hepatic resection for hepatocellular carcinoma with concomitant esophageal varices in patients with cirrhosis. Ann Surg Oncol. 2008;15:1670–1676. doi: 10.1245/s10434-008-9880-7. [DOI] [PubMed] [Google Scholar]
- 4.Stefaniuk P, Cianciara J, Wiercinska-Drapalo A. Present and future possibilities for early diagnosis of hepatocellular carcinoma. World J Gastroenterol. 2010;16:418–424. doi: 10.3748/wjg.v16.i4.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schrohl AS, Holten-Andersen M, Sweep F, et al. Tumor Markers. Mol Cell Proteomics. 2003;2:378–387. doi: 10.1074/mcp.R300006-MCP200. [DOI] [PubMed] [Google Scholar]
- 6.Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 7.Galle PR, Forner A, Llovet JM, et al. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 8.Kokudo N, Hasegawa K, Akahane M, et al. Evidence-based clinical practice guidelines for hepatocellular carcinoma: the Japan Society of Hepatology 2013 update (3rd JSH-HCC guidelines) Hepatol Res. 2015;45:123–127. doi: 10.1111/hepr.12512. [DOI] [PubMed] [Google Scholar]
- 9.Yuen MF, Lai CL. Serological markers of liver cancer. Best Pract Res Clin Gastroenterol. 2005;19:91–99. doi: 10.1016/j.bpg.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Itoh N, Ornitz DM. Evolution of the Fgf and Fgfr gene families. Trends Genet. 2004;20:563–569. doi: 10.1016/j.tig.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–149. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Schlessinger J. Common and distinct elements in cellular signaling via EGF and FGF receptor. Science. 2004;306:1506–1507. doi: 10.1126/science.1105396. [DOI] [PubMed] [Google Scholar]
- 13.Richelda R, Ronchetti D, Baldini L, et al. A novel chromosomal translocation t (4;14)(p16.3;q32) in multiple myeloma involves the fibroblast growth-factor receptor 3 gene. Blood. 1997;90:4062–4070. doi: 10.1182/blood.V90.10.4062. [DOI] [PubMed] [Google Scholar]
- 14.Zaharieva BM, Simon R, Diener PA, et al. High-throughput tissue microarray analysis of 11qI3 gene amplification (CCND1, FGF3, FGF4, EMS1) in urinary bladder cancer. J Pathol. 2003;201:603–608. doi: 10.1002/path.1481. [DOI] [PubMed] [Google Scholar]
- 15.Xiao S, Nalabolu SR, Aster JC, et al. FGFR1 is fused with a novel zinc-finger gene, ZNF198, in the t (8;13) leukaemia/lymphoma syndrome. Nat Genet. 1998;18:84–87. doi: 10.1038/ng0198-84. [DOI] [PubMed] [Google Scholar]
- 16.Shimokawa T, Furukawa Y, Sakai M, et al. Involvement of the FGF18 gene in colorectal carcinogenesis, as a novel downstream target of the β-catenin/T-cell factor complex. Cancer Res. 2003;63:6116–6120. [PubMed] [Google Scholar]
- 17.Ruohola JK, Viitanen TP, Valve EM, et al. Enhanced invasion and tumor growth of fibroblast growth factor 8b-overexpressing MCF-7 human breast cancer cells. Cancer Res. 2001;61:4229–4237. [PubMed] [Google Scholar]
- 18.Gowardhan B, Douglas DA, Mathers ME, et al. Evaluation of the fibroblast growth factor system as a potential target for therapy in human prostate cancer. Br J Cancer. 2005;92:320–327. doi: 10.1038/sj.bjc.6602274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Potthoff MJ, Kliewer SA, Mangelsdorf DJ. Endocrine fibroblast growth factors 15/19 and 21 : from feast to famine. Genes Dev. 2012;26:312–324. doi: 10.1101/gad.184788.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kir S, Kliewer SA, Mangelsdorf DJ. Roles of FGF19 in liver metabolism roles of FGF19 in liver metabolism. Cold Spring Harb Symp Quant Biol. 2011;76:139–144. doi: 10.1101/sqb.2011.76.010710. [DOI] [PubMed] [Google Scholar]
- 21.Miura S, Mitsuhashi N, Shimizu H, et al. Fibroblast growth factor 19 expression correlates with tumor progression and poorer prognosis of hepatocellular carcinoma. BMC Cancer. 2012;12:56. doi: 10.1186/1471-2407-12-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hyeon J, Ahn S, Lee JJ, et al. Expression of fibroblast growth factor 19 is associated with recurrence and poor prognosis of hepatocellular carcinoma. Dig Dis Sci. 2013;58:1916–1922. doi: 10.1007/s10620-013-2609-x. [DOI] [PubMed] [Google Scholar]
- 23.Heidelbaugh JJ, Bruderly M. Cirrhosis and chronic liver failure: part I. Diagnosis and evaluation Am Fam Physician. 2006;74:756–762. [PubMed] [Google Scholar]
- 24.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 25.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toyoda H, Kumada T, Kiriyama S, et al. Prognostic significance of simultaneous measurement of three tumor markers in patients with hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2006;4:111–117. doi: 10.1016/S1542-3565(05)00855-4. [DOI] [PubMed] [Google Scholar]
- 27.Zhu K, Dai Z, Zhou J. Biomarkers for hepatocellular carcinoma: progression in early diagnosis, prognosis, and personalized therapy. Biomark Res. 2013;1:10. doi: 10.1186/2050-7771-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodwin S, McPherson JD, McCombie WR. Coming of age: ten years of next-generation sequencing technologies. Nat Rev Genet. 2016;17:333–351. doi: 10.1038/nrg.2016.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vogelstein B, Papadopoulos N, Velculescu VE, et al. Cancer genome landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sawey ET, Chanrion M, Cai C, et al. Identification of a therapeutic strategy targeting amplified FGF19 in liver cancer by Oncogenomic screening. Cancer Cell. 2011;19:347–358. doi: 10.1016/j.ccr.2011.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Capurro M, Wanless IR, Sherman M, et al. Glypican-3: a novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology. 2003;125:89–97. doi: 10.1016/S0016-5085(03)00689-9. [DOI] [PubMed] [Google Scholar]
- 32.Chaudhary NI, Roth GJ, Hilberg F, et al. Inhibition of PDGF, VEGF and FGF signalling attenuates fibrosis. Eur Respir J. 2007;29:976–985. doi: 10.1183/09031936.00152106. [DOI] [PubMed] [Google Scholar]
- 33.Strutz F, Zeisberg M, Hemmerlein B, et al. Basic fibroblast growth factor expression is increased in human renal fibrogenesis and may mediate autocrine fibroblast proliferation. Kidney Int. 2000;57:1521–1538. doi: 10.1046/j.1523-1755.2000.00997.x. [DOI] [PubMed] [Google Scholar]
- 34.Bertino G, Ardiri A, Malaguarnera M, et al. Hepatocellualar carcinoma serum markers. Semin Oncol. 2012;39:410–433. doi: 10.1053/j.seminoncol.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 35.Toyoda H, Kumada T, Tada T, et al. Tumor markers for hepatocellular carcinoma: simple and significant predictors of outcome in patients with HCC. Liver Cancer. 2015;4:126–136. doi: 10.1159/000367735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiang JYL, Ferrell JM. Bile acid metabolism in liver pathobiology. Gene Expr. 2018;18:71–87. doi: 10.3727/105221618X15156018385515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ashby K, Navarro Almario EE, Tong W, et al. Review article: therapeutic bile acids and the risks for hepatotoxicity. Aliment Pharmacol Ther. 2018;47:1623–1638. doi: 10.1111/apt.14678. [DOI] [PubMed] [Google Scholar]
- 38.John Y. L. Chiang. Bile acids: regulation of synthesis. J Lipid Res. 2009;50:1955–1966. doi: 10.1194/jlr.M800184-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song KH, Li T, Owsley E, et al. Bile acids activate fibroblast growth factor 19 signaling in human hepatocytes to inhibit cholesterol 7alpha-hydroxylase gene expression. Hepatology. 2009;49:297–305. doi: 10.1002/hep.22627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin BC, Desnoyers LR. FGF19 and Cancer. Adv Exp Med Biol. 2012;728:183–194. doi: 10.1007/978-1-4614-0887-1_12. [DOI] [PubMed] [Google Scholar]
- 41.Repana D, Ross P. Targeting FGF19/FGFR4 pathway: a novel therapeutic strategy for hepatocellular carcinoma. Diseases. 2015;3:294–305. doi: 10.3390/diseases3040294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng N, Wei W, Wang Z. Emerging roles of FGF signaling in hepatocellular carcinoma. Transl Cancer Res. 2016;5:1–6. doi: 10.21037/tcr.2016.05.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chae YK, Ranganath K, Hammerman PS, et al. Inhibition of the fibroblast growth factor receptor (FGFR) pathway: the current landscape and barriers to clinical application. Oncotarget. 2017;8:16052–16074. doi: 10.18632/oncotarget.14109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tohyama O, Matsui J, Kodama K, et al. Antitumor activity of lenvatinib (e7080): an angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J Thyroid Res. 2014;2014:638747. doi: 10.1155/2014/638747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao L, Wang X, Tang Y, et al. FGF19/FGFR4 signaling contributes to the resistance of hepatocellular carcinoma to sorafenib. J Exp Clin Cancer Res. 2017;36:8. doi: 10.1186/s13046-016-0478-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available upon request.