1. Introduction
Titanium represents the ideal material for implant fabrication due to its excellent biocompatibility.1 However the use of titanium abutments with thin gingival biotype causes greyish hue to surrounding soft tissues. Therefore, various tooth coloured implant abutment materials have been introduced in the past. These include densely sintered alumina and zirconia.2,3 Alumina implant abutments perform well biologically as well as aesthetically, but they possess a risk of abutment fracture during clinical use at implant abutment connection site, whereas zirconia abutment has high mechanical strength due to its unique stress induced transformation toughening mechanism.4,5 Along with mechanical strength, zirconia has excellent esthetics, corrosion resistance, biocompatibility and high loading capacity, therefore it is preferred over alumina as an abutment material.6 Zirconia abutment enhance peri -implant health by reducing inflammation and less bleeding on probing as compared to titanium abutment.7
Previous studies on alveolar crestal bone loss around oral implants has shown that bone loss in first year is up to 1.5 mm and 0.2 mm in subsequent years with recession of mucosa is unavoidable in implant prosthetic treatment.8,9 A positive correlation between crestal bone loss and plaque deposition has been stated in previous clinical studies.10 Inflammatory changes due to plaque deposition on implant surfaces or abutments are similar as with the gingival and alveolar mucosa in natural teeth. Abutment material has been perceived as important factor affecting the stability of the peri-implant mucosa and crestal bone. Abrahamsson et al. stated that abutment material has important role in the reduction of alveolar crestal bone loss and soft tissue recession.11
Past studies on bacterial colonization on zirconia and titanium abutments stated that zirconia abutment had good sealing properties with significantly less bacterial counts than that of titanium abutments.12, 13, 14, 15 One in-vitro study suggested that titanium has high surface energy than zirconia. (0.0185 N/m versus 0.02662 N/m).16
Microcirculatory evaluation reveals that blood flow around zirconia abutment is almost similar to the blood flow around natural teeth. Therefore, immune function maintenance would be improved with zirconia abutments.17,18
However very few studies have compared peri-implant hard tissues with respect to titanium and zirconia abutments before the commencement of this study and the results were conflicting. Anja Zembic et al. in their study found no significant difference in peri-implant hard tissues around zirconia and titanium abutments in different sample distribution.19
To draw a more definitive conclusion, comparative clinical studies with different abutment materials, to assess their effect on peri implant crestal bone height were required. Therefore the aim of this study was to assess crestal bone level around two different abutment materials –titanium and zirconia for implant retained crowns in posterior mandibular region.
2. Materials and methods
This pilot study was design as unicentric, prospective, split mouth, single blinded and, conducted according to the Consolidated Standard of Reporting Trial (CONSORT).20 Ethical permission was obtained from institution ethics subcommittee (Ref No.: IESC/T-50/03.01.2014) before starting recruitment of the patients. World's Medical Association's Declaration of Helsinki tenets was utilized in study design. As there were no previous studies for estimation of sample size during the phase of study design, convenient sample size of 11 subjects was selected. Selected subjects were in the age range 20–45 years, having bilateral missing first mandibular molars, free of systemic diseases affecting bone or gingival health, and with positive informed consent. Individual with history of smoking, alcohol, drug or tobacco abuse, pregnancy and presence of systemic diseases or hereditary disorders affecting bone implant interface were excluded from the study.
Two titanium screw implant (3.75 mm × 11.5 mm) were placed in each subjects under local anaesthesia in standard aseptic condition. All subjects were prescribed 0.2% chlorhexidine mouth wash pre-operatively for two days till 10 days postoperatively, twice per day and received postoperative antibiotics for 5 days. The implants were left in situ for three months for osseointegration.
Punch type of second stage surgery was performed and healing abutments were placed. 2 weeks after second stage surgery implant level impression were recorded in elastomeric impression material (DENTSPLY, Germany). To fabricate metal ceramic crowns, titanium and zirconia abutment were placed on each side of the arch following computer generated randomization table (Fig. 1). The collar height of the abutments was selected such that the finish line on the abutments remains supragingivally by 0.5–1 mm. All definitive prosthesis was cemented with zinc phosphate cement (Fig. 2). Contact profilometry was used to evaluate the surface roughness (Ra-Value) of titanium abutment (190 nm range 167 nm–211nm) and zirconia abutment (186 nm range 166–214 nm). Roughness value was almost similar for both the abutments and they differ in their chemical composition only.
Fig. 1.
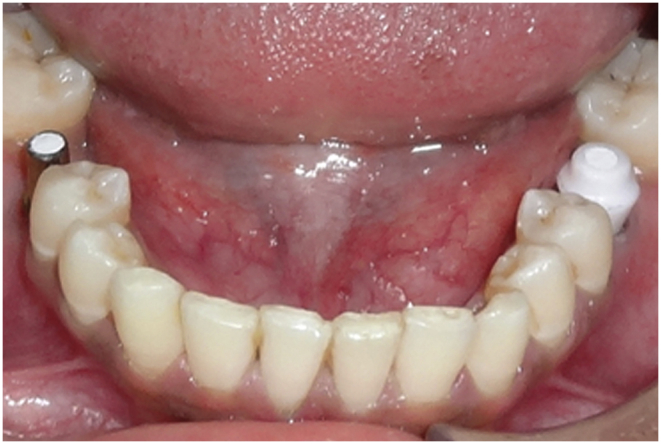
Titanium and zirconia abutment placed.
Fig. 2.
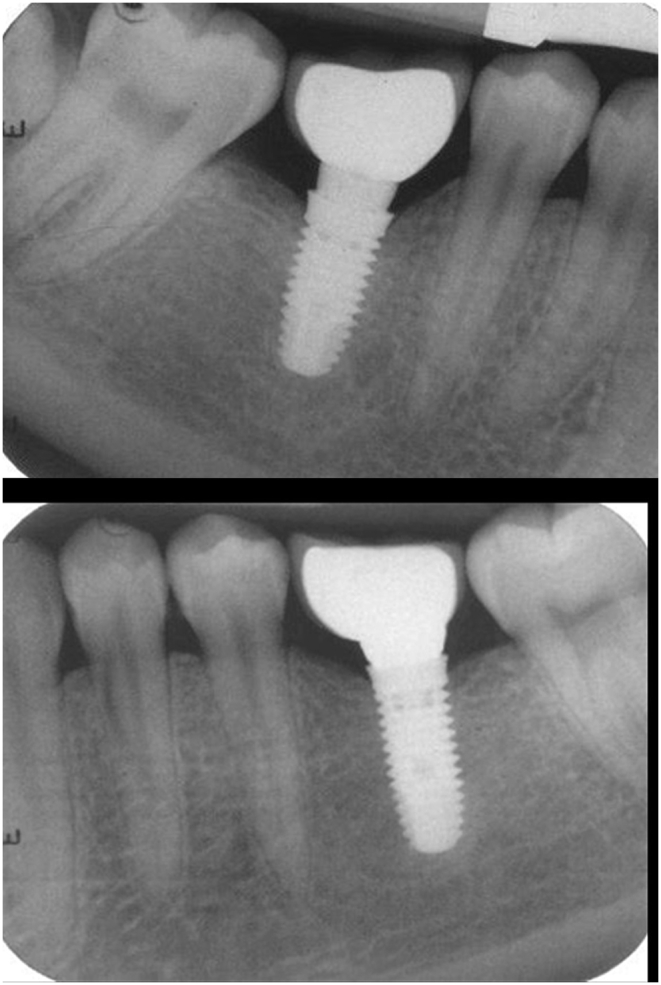
IOPA x ray after cementation of final prosthesis.
Crestal bone height (CBH) was measured using intra oral preoperative x-ray (IOPA) of the implant site taken with long cone parallel beam technique using re-positional film holders (XCP-ORA positioning system, RINN, Dentsply, Germany) to control projection geometry. Long cone paralleling technique was used with intraoral radiography instrument (RINN instrument) to position and stabilize the film in the mouth and the x-ray cone. Radiograph exposure parameters were kept 65-9-kV, 7.5–10 mA, and 0.22–0.5s. Automatic processor were utilize for films developing. IOPA's were taken immediately after abutment placement and crown cementation (Fig. 2) and at subsequent intervals of 3 and 12 months. Radiographs were scanned, digitized in jpg format, and stored in a personal computer. Blinding of examiner was done by randomly numbering the radiographic pictures. Measurement scale was calibrated by the available dental implant length. Distance between shoulder of implant and first bone to implant contact (BIC) was calculated with pixel/mm ratio. Two set of measurement were obtained by two different examiners independently in darkened room to have accurate and unbiased measurements.21 These measurements were tabulated according to the time interval and group allocated. Mean of both set of measurements were used for final analysis.
The data thus generated were subjected to statistical analysis using Statistical Software for Social Sciences, Version16 (IBM, Chicago, IL.). A repeated measure ANOVA was used for intra group analysis of data for crestal bone loss followed by bonferroni correction. The paired t-test was used for intergroup analysis of crestal bone loss. Power of the study was more than 80% and P values of less than 0.05 were considered as statistically significant.
3. Results
Study shows 100% survival rate for the implants irrespective of the abutment used. The mean crestal bone height around implants with titanium and zirconia abutments was evaluated and compared at three different time intervals i.e. baseline (immediately after abutment placement), 3-months and 12-months after abutment placement (Table 1). There was statistically significant reduction in CBH around implants with both titanium and zirconia abutments at various time intervals. (P < 0.05) (Table 1). Comparison of change in CBH for both the abutments showed that the mean difference from baseline to 12-months was significantly lower for zirconia abutment (0.487 ± 0.159) as compared to titanium abutment (0.621 ± 0.207), while rest of the mean differences at different time intervals for both the abutments were statistically insignificant.(P > 0.05) (Table 2).
Table 1.
Intragroup comparison of change in crestal bone levels (in millimeters) for titanium and zirconia abutment at different time intervals.
| Surface | Time Interval | Titanium |
Zirconia |
||
|---|---|---|---|---|---|
| Mean difference | P - value | Mean difference | P-value | ||
| Proximal surface | 0–3 Months | 0.320 | 0.003 | 0.202 | 0.003 |
| 3–12 Months | 0.346 | 0.003 | 0.285 | 0.003 | |
| 0–12 Months | 0.621 | 0.003 | 0.487 | 0.003 | |
Table 2.
Comparison of change in crestal bone levels (in millimetres)for both titanium and zirconia abutments at different time intervals.
| Time interval | Mean Difference± SD (mm) |
P value | ||
|---|---|---|---|---|
| Titanium | Zirconia | |||
| Proximal surfaces | 0–3 Months | 0.32 ± 0.889 | 0.202 ± 0.121 | 0.1 |
| 3–12 Months | 0.346 ± 0.189 | 0.285 ± 0.115 | 0.3 | |
| 0–12 Months | 0.621 ± 0.207 | 0.487 ± 0.159 | 0.02 | |
4. Discussion
The present study showed 100% survival of implants with both zirconia and titanium abutment, similar finding were reported in literature.22 Results showed that there was significant reduction in crestal bone level on proximal aspects of implants with both titanium and zirconia abutments over a period of 12 months. However the crestal bone loss was less for zirconia as compared to titanium abutment at 12 months.
These clinical finding were in accordance with previous study which reported increase bone loss and inflammation around implants with titanium abutment than with zirconia abutments in different sample distribution.14,23 A recent systematic review and similar studies also shows increase inflammation around titanium abutments, that further leads to crestal bone loss.7,24
The less reduction in crestal bone level with zirconia abutment may be due to difference in surface energy of both the materials. Since zirconia has less surface energy, it showed decreased plaque accumulation compared with titanium.16
In a study on biological response of soft tissue to zirconia and titanium abutments, it founds significantly greater blood flow in free gingival around zirconia abutment as compare to titanium abutment. Thus, zirconia abutments promote microcirculatory dynamics in peri-implant mucosa that is close to that of natural teeth.17 Increase in blood circulation in peri-implant soft and hard tissue leads to improved immune response and that will further results in decreased bone destruction. Peri-implant crevicular fluid (PICF) around titanium abutments shows increase levels of leptin than that of zirconia abutment.18 This can also be responsible for more bone loss at titanium abutments than that of zirconia abutments.
Thus, from the above discussion and result of present study it can be stated that zirconia can be used as an abutment material for single tooth implant restorations in posterior regions. However the result obtained in the present study cannot be generalised on long term performance of zirconia abutments because of limited sample size and short observational period. Progressive aging has lead to reduction in zirconia physical properties. Some vitro studies suggested that during simulated aging process there is decrease in fracture toughness (50%) of zirconia in humid environment and it also effect gingival cell attachment and proliferation properties.19,25, 26, 27
In the present study, zirconia abutments used were of metallic internal implant-abutment connection. Presently various new designs of zirconia abutments are available with different implant abutment connections. It may influence differences in their clinical performance. More studies comparing zirconia and titanium abutment required with long follow up period required for definitive conclusion regarding the choice of materials.
5. Conclusions
According to the result of this study titanium implant abutment junction shows time dependent change in CBH irrespective of the abutment material. Zirconia abutment on titanium implants lead to lesser reduction in CBH as compare to titanium abutment in one year study.
Source of funding
This research did not receive any specific grant from funding agencies in the public, commercial or not for profit sector.
Declaration of competing interest
The authors declare that they have no conflicts of interest.
Acknowledgment
The authors are grateful for the help from staff of Centre for Dental Education and Research, All India Institute of Medical Sciences, New Delhi, India for helping during study completion.
References
- 1.Scheller H., Urgell J.P., Kultje C. A 5-year multicenter study on implant-supported single crown restorations. Int J Oral Maxillofac Implant. 1998;13:212–218. [PubMed] [Google Scholar]
- 2.Sailer I., Zembic A., Jung R.E., Hämmerle C.H.F., Mattiola A. Single-tooth implant reconstructions: esthetic factors influencing the decision between titanium and zirconia abutments in anterior regions. Eur J Esthetic Dent. 2007;2 296–10. [PubMed] [Google Scholar]
- 3.Schmalz G., Garhammer P. Biological interactions of dental cast alloys with oral tissues. Dent Mater. 2002;18 doi: 10.1016/s0109-5641(01)00063-x. 396–06. [DOI] [PubMed] [Google Scholar]
- 4.Belser U.C., Schmid B., Higginbottom F., Buser D. Outcome analysis of implant restorations located in the anterior maxilla: a review of the recent literature. Int J Oral Maxillofac Implant. 2004;19:30–42. [PubMed] [Google Scholar]
- 5.Scott H.G. Phase relationships in the zirconia-yttria system. J Mater Sci. 1975;10:1527–1535. [Google Scholar]
- 6.Manicone P.F., Rossi Iommetti P., Raffaelli L. Biological considerations on the use of zirconia for dental devices. Int J Immunopathol Pharmacol. 2007;20:9–12. doi: 10.1177/039463200702001s03. [DOI] [PubMed] [Google Scholar]
- 7.Sanz-Martín I., Sanz-Sánchez I., Carrillo de Albornoz A., Figuero E., Sanz M. Effects of modified abutment characteristics on peri-implant soft tissue health: a systematic review and meta-analysis. Clin Oral Implant Res. 2017;29(1):118–129. doi: 10.1111/clr.13097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albrektsson T., Zarb G., Worthington P., Eriksson A.R. The long-term efficacy of currently used dental implants: a review and proposed criteria of success. Int J Oral Maxillofac Implant. 1986;1:11–25. [PubMed] [Google Scholar]
- 9.De Bruyn H., Vandeweghe S., Ruyffelaert C., Cosyn J., Sennerby L. Radiographic evaluation of modern oral implants with emphasis on crestal bone level and relevance to peri-implant health. Periodontol 2000. 2013;62(1):256–270. doi: 10.1111/prd.12004. [DOI] [PubMed] [Google Scholar]
- 10.Oh T.-J., Yoon J., Misch C.E., Wang H.-L. The causes of early implant bone loss: myth or science? J Periodontol. 2002;73:322–333. doi: 10.1902/jop.2002.73.3.322. [DOI] [PubMed] [Google Scholar]
- 11.Abrahamsson I., Berglundh T., Glantz P.O., Lindhe J. The mucosal attachment at different abutments. An experimental study in dogs. J Clin Periodontol. 1998;25:721–727. doi: 10.1111/j.1600-051x.1998.tb02513.x. [DOI] [PubMed] [Google Scholar]
- 12.Rimondini L., Cerroni L., Carrassi A., Torricelli P. Bacterial colonization of zirconia ceramic surfaces: an in vitro and in vivo study. Int J Oral Maxillofac Implant. 2002;17:793–798. [PubMed] [Google Scholar]
- 13.Scarano A., Piattelli M., Caputi S., Favero G.A., Piattelli A. Bacterial adhesion on commercially pure titanium and zirconium oxide disks: an in vivo human study. J Periodontol. 2004;75:292–296. doi: 10.1902/jop.2004.75.2.292. [DOI] [PubMed] [Google Scholar]
- 14.do Nascimento C., Pita M., Santos E. Microbiome of titanium and zirconia dental implants abutments. Dent Mater. 2016;32(1) doi: 10.1016/j.dental.2015.10.014. 93-1. [DOI] [PubMed] [Google Scholar]
- 15.Şen N., Şermet I., Gürler N. Sealing capability and marginal fit of titanium versus zirconia abutments with different connection designs. J Adv Prosthodont. 2019;11(2):105. doi: 10.4047/jap.2019.11.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salihoglu U., Boynuegri D., Engin D., Duman A.N., Gokalp P., Balos K. Bacterial adhesion and colonization differences between zirconium oxide and titanium alloys: an in vivo human study. Int J Oral Maxillofac Implant. 2011;26:101–107. [PubMed] [Google Scholar]
- 17.Kajiwara N., Masaki C., Mukaibo T., Kondo Y., Nakamoto T., Hosokawa R. Soft tissue biological response to zirconia and metal implant abutments compared with natural tooth: microcirculation monitoring as a novel bioindicator. Implant Dent. 2015;24:37–41. doi: 10.1097/ID.0000000000000167. [DOI] [PubMed] [Google Scholar]
- 18.Barwacz Christopher A., Brogden Kim A., Stanford Clark M. Comparison of pro-inflammatory cytokines and bone metabolism mediators around titanium and zirconia dental implant abutments following a minimum of 6 months of clinical function. Clin Oral Implant Res. 2015;26:35–41. doi: 10.1111/clr.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zembic A., Sailer I., Jung R.E., Siegenthaler D., Holderegger C., Hämmerle C.H. Randomized controlled clinical trial of customized zirconia and titanium implant abutments for canine and posterior single-tooth implant reconstructions: preliminary results at 1 year of function. Clin Oral Implant Res. 2009;20:219–225. doi: 10.1111/j.1600-0501.2008.01636.x. [DOI] [PubMed] [Google Scholar]
- 20.Schulz K.F., Altman D.G., Moher D., CONSORT Group CONSORT 2010 Statement: updated guidelines for reporting parallel group randomized trials. Trials. 2010;11:32. doi: 10.1097/AOG.0b013e3181d9d421. [DOI] [PubMed] [Google Scholar]
- 21.Van Eekeren P., Tahmaseb A., Wismeijer D. Crestal bone changes in macrogeometrically similar implants with the implant-abutment connection at the crestal bone level or 2.5 mm above: a prospective randomized clinical trial. Clin Oral Implant Res. 2015;27(12):1479–1484. doi: 10.1111/clr.12581. [DOI] [PubMed] [Google Scholar]
- 22.Pjetursson B., Zarauz C., Strasding M., Sailer I., Zwahlen M., Zembic A. A systematic review of the influence of the implant-abutment connection on the clinical outcomes of ceramic and metal implant abutments supporting fixed implant reconstructions. Clin Oral Implant Res. 2018;29:160–183. doi: 10.1111/clr.13362. [DOI] [PubMed] [Google Scholar]
- 23.Hu M., Chen J., Pei X., Han J., Wang J. Network meta-analysis of survival rate and complications in implant-supported single crowns with different abutment materials. J Dent. 2019;88:103115. doi: 10.1016/j.jdent.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Nothdurft F. All-ceramic zirconium dioxide implant abutments for single-tooth replacement in the posterior region: a 5-year outcome report. Int J Prosthodont (IJP) 2019;32(2):177–181. doi: 10.11607/ijp.6115. [DOI] [PubMed] [Google Scholar]
- 25.Studart A.R., Filser F., Kocher P., Gauckler L.J. Fatigue of zirconia under cyclic loading in water and its implications for the design of dental bridges. Dent Mater. 2007;1:106–114. doi: 10.1016/j.dental.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 26.Zembic A., Sailer I., Jung R., Hämmerle C. Randomized-controlled clinical trial of customized zirconia and titanium implant abutments for single-tooth implants in canine and posterior regions: 3-year results. Clin Oral Implant Res. 2009;20(8):802–808. doi: 10.1111/j.1600-0501.2009.01717.x. [DOI] [PubMed] [Google Scholar]
- 27.Pandoleon P., Bakopoulou A., Papadopoulou L., Koidis P. Evaluation of the biological behaviour of various dental implant abutment materials on attachment and viability of human gingival fibroblasts. Dent Mater. 2019;35(7):1053–1063. doi: 10.1016/j.dental.2019.04.010. [DOI] [PubMed] [Google Scholar]


