Abstract
Nuclear factor of activated T cells (NFATs) is an important transcription factor for T cell activation and proliferation. Recent studies have highlighted the role of NFATs in regulating the differentiation of effector CD4 T helper (Th) subsets including Th1 and Th17 cells. Because controlling the effector T cell function is important for the treatment of autoimmune diseases, regulation of NFAT functions in T cells would be an important strategy to control the pathogenesis of autoimmune diseases. Here, we demonstrated that an NFAT inhibitory peptide, VIVIT conjugated to dNP2 (dNP2-VIVIT), a blood-brain barrier-permeable peptide, ameliorated experimental autoimmune encephalomyelitis (EAE) by inhibiting Th1 and Th17 cells, but not regulatory T (Treg) cells. dNP2-VIVIT negatively regulated spinal cord-infiltrating interleukin-17A (IL-17A) and interferon (IFN)-γ-producing CD4+ T cells without affecting the number of Foxp3+ CD4+ Treg cells, whereas dNP2-VEET or 11R-VIVIT could not significantly inhibit EAE. In comparison with cyclosporin A (CsA), dNP2-VIVIT selectively inhibited Th1 and Th17 differentiation, whereas CsA inhibited the differentiation of all T cell subsets including that of Th2 and Treg cells. Collectively, this study demonstrated the role of dNP2-VIVIT as a novel agent for the treatment of autoimmune diseases such as multiple sclerosis by regulating the functions of Th1 and Th17 cells.
Keywords: Multiple sclerosis (MS), VIVIT, T cell, Cell penetrating peptide (CPP), dNP2
Introduction
The nuclear factor of activated T cells (NFATs) proteins are important transcription factors involved in the regulation of T cell receptor (TCR) signaling and functions.1, 2, 3 To date, five NFAT family members have been identified that all share a highly conserved DNA-binding domain, NFAT1 (NFATc2 or NFATp), NFAT2 (NFATc1 or NFATc), NFAT3 (NFATc4), NFAT4 (NFATc3 or NFATx), and NFAT5 (TonEBP or OREBP).4 Whereas four of these proteins are regulated by calcium signaling, NFAT5 is calcium independent and is activated under conditions of osmotic stress. Binding of the TCR to the antigen increases intracellular calcium levels, thereby activating serine/threonine phosphatase calcineurin.5, 6, 7 Activated calcineurin binds and dephosphorylates NFATs, leading to its nuclear translocation and induction of inflammatory cytokine gene expression.6,8
Recently, the NFATs has been re-highlighted to play important roles in regulating the expression of lineage-specific transcription factors and signature cytokines of CD4 T helper subsets including T helper 1 (Th1) and Th17 cells.9, 10, 11, 12 NFAT1/NFAT4 and NFAT1/NFAT2 deficiency has been shown to impair IFN-γ production.13, 14, 15 NFAT1 has been reported to directly bind to the IFN-γ promoter,11,16 and loss of NFAT1 was found to increase the resistance to the induction of EAE and significantly decrease the levels of IFN-γ production by CNS-infiltrating CD4+ T cells.17 Likewise, in Th17 cells, NFAT1 and NFAT2 proteins directly bound to the interleukin-17 (IL-17) promoter.18,19 NFAT1-deficient T cells produced IL-17A along with IL-4 and IL-10, which are not pathogenic.17 Loss of NFAT2 in CD4+ T cells led to reduced IL-17A, IL-17F, IL-21, and RORγt expression, and NFAT1- and NFAT2-deficient (double-knockout [DKO]) mice were resistant to the induction of EAE. Moreover, NFAT1 deficiency exerted a protective effect against experimental colitis through reduced production of IL-6 and IL-17 by mucosal T lymphocytes.20 Considering the important role of NFAT signaling in T cell responses, the NFATs has been targeted for the treatment of autoimmune diseases for decades.21, 22, 23, 24 The most commonly used drugs for targeting NFATs are calcineurin inhibitors, CsA and FK506. Upon entering the cells, these inhibitors form a complex with immunophilins, cyclophilins, and FK-binding protein 12 (FKBP12), respectively.25, 26, 27 This complex directly binds to calcineurin and inhibits its phosphatase activity, thereby inhibiting NFAT dephosphorylation.28 Although calcineurin inhibitors effectively regulate T cell responses and are widely used to control the pathogenicity of autoimmune diseases and graft rejection, they have been reported to have serious drawbacks, including neurotoxicity, nephrotoxicity, and regulatory T (Treg) cell count reduction.29, 30, 31, 32
To overcome the limitations associated with calcineurin inhibitors, we developed the VIVIT peptide based on the common calcineurin-NFAT binding motif PxIxIT, which selectively regulates NFAT transcription factor.33 The VIVIT peptide effectively inhibited NFAT-dependent gene expression without affecting the calcineurin phosphatase activity.34
Here, we utilized a cell-permeable peptide, dNP2, which is a promising blood-brain-barrier-permeable peptide that can deliver cargoes into primary T cells and to the cells of the brain and spinal cord.35, 36, 37 We synthesized VIVIT conjugated with dNP2 (dNP2-VIVIT), which could strongly ameliorate EAE severity and demyelination. dNP2-VIVIT inhibited CNS invasion of CD4+ T cells and significantly reduced IL-17A and IFN-γ production. EAE inhibition by the VIVIT peptide was more significant in conjugation with dNP2, but not with 11R. In comparison with CsA, dNP2-VIVIT specifically inhibited Th1 and Th17 cell differentiation, whereas CsA inhibited all T cell subsets including the differentiation of Treg and Th2 cells. Taken together, our findings demonstrate that a blood-brain barrier-permeable peptide conjugated with VIVIT (dNP2-VIVIT) could be an effective immunomodulatory agent for treating multiple sclerosis through specific inhibition of NFATs.
Results
The Synthetic Peptide dNP2-VIVIT Ameliorates Experimental Autoimmune Encephalomyelitis
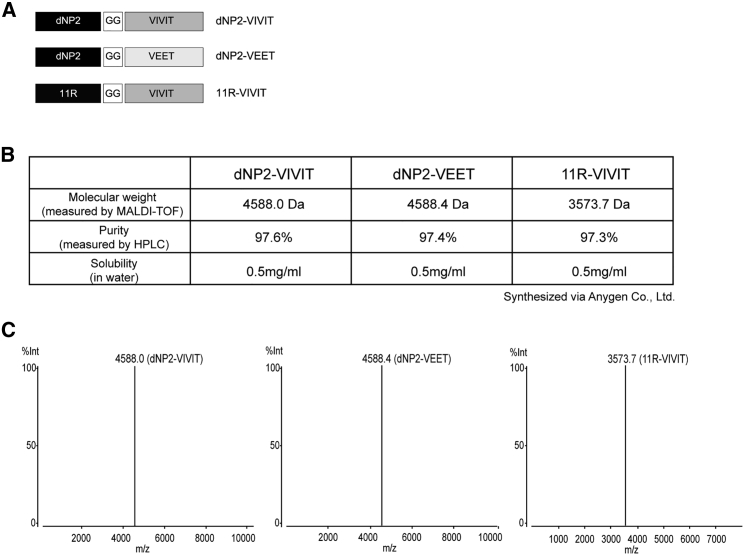
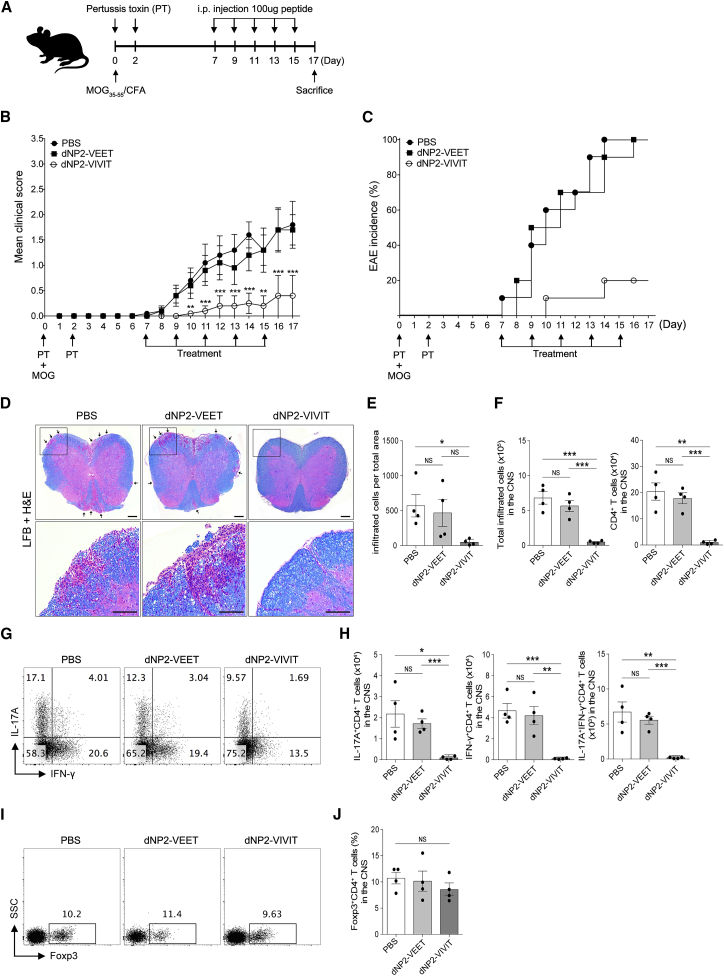
To examine the inhibitory effects of NFAT function in autoimmune encephalomyelitis, we synthesized a 40-amino-acid-long, highly pure VIVIT peptide conjugated with dNP2 (dNP2-VIVIT) (97% purity when purified by high-pressure liquid chromatography [HPLC] analysis) (Figures 1A–1C). We also constructed a negative control peptide conjugate (dNP2-VEET), which has a non-functional mutation sequence of VIVIT. In order to determine the function of NFAT-specific inhibition by dNP2-VIVIT, we used EAE, a Th1/Th17-mediated autoimmune neuroinflammation mouse model of multiple sclerosis. Eight-week-old female C57BL/6 mice were immunized with the MOG35–55 peptide on day 0 and were treated with pertussis toxin on days 0 and 2. dNP2-VIVIT or dNP2-VEET (100 μg) were intraperitoneally administered every other day starting from day 7. The mice were monitored daily for the clinical score and were sacrificed on day 17 (Figure 2A). dNP2-VIVT treatment significantly delayed EAE onset and disease progression (Figure 2B). The percentage of EAE incidence was also reduced remarkably by dNP2-VIVIT, whereas no effects were observed in dNP2-VEET-treated or PBS control mice (Figure 2C). Spinal cord tissue histology revealed reduced demyelination and cellular infiltration upon dNP2-VIVIT treatment in comparison with the control groups (Figures 2D and 2E). On examining the cellular characteristics of spinal cord cells by single-cell isolation, we found that the reduction in the total number of CNS-infiltrating cells observed upon dNP2-VIVIT treatment correlated with the reduction in the number of CD4+ T cells (Figure 2F). Importantly, dNP2-VIVIT treatment significantly decreased both the proportion and number of IL-17A- and/or IFN-γ-producing CD4+ T cells in the spinal cord (Figures 2G and 2H). However, the proportion of CD4+Foxp3+ T cells in the spinal cord was not significantly different (Figures 2I and 2J), suggesting that dNP2-VIVIT specifically inhibited encephalitogenic T cells. Taken together, these data suggest that dNP2-VIVIT could regulate pathogenic T cells and ameliorate disease progression during autoimmune neuroinflammation.
Figure 1.
Generation of NFAT Targeting Peptides
(A) Peptides (dNP2-VIVIT, dNP2-VEET, and 11R-VIVIT) were synthesized by Anygen. (B) Molecular weight was measured by AXIMA and assurance mass cytometry. (C) Peptide purity was estimated by Shimadzu HPLC LabSolution.
Figure 2.
NFAT Inhibitory Peptide dNP2-VIVIT Ameliorated Experimental Autoimmune Encephalomyelitis (EAE)
EAE was induced in 8-week-old female C57BL/6 mice by immunization with MOG in complete Freund’s adjuvant. (A) The mice were treated intraperitoneally with PBS or 100 μg dNP2-VIVIT or dNP2-VEET on day 7 after immunization, and were subsequently treated every other day. Clinical scores (B) and incidence (C) were monitored daily (n = 10 per group). Data are presented as the mean ± SEM of two independent experiments. (D) Spinal cord tissues were harvested and observed after Luxol fast blue (LFB) and H&E staining to determine demyelination and tissue inflammation levels (scale bars, 100 μM). (E) The number of spinal cord tissue-infiltrating cells was counted via ImageJ software (n = 4 per group). (F) The spinal cord cells were isolated, and IL-17A- and/or IFN-γ-expressing CD4+ T cells were analyzed by flow cytometry (G) and were counted and multiplied to determine their proportion (H). (I–J) The proportion of Foxp3-expressing CD4+ T cells was analyzed in the spinal cord (n = 4 per group). Data are presented as the mean ± SEM of one representative experiment out of two; statistical analysis by two-way ANOVA compared with PBS for (B) and two-tailed Student’s t test for (E), (H), and (J). *p < 0.05, **p < 0.01, ***p < 0.001. NS, not significant.
dNP2-VIVIT, but Not CsA, Specifically Regulates Th1 and Th17 Differentiation In Vitro
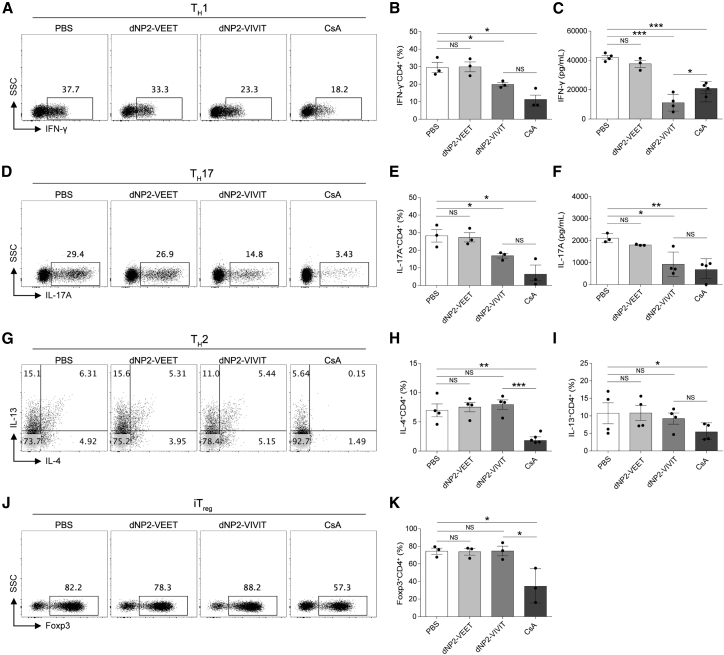
Because EAE progression is mainly caused by myelin antigen-specific Th1 and Th17 cells or because of the imbalance between effector cells and Treg cells, we further investigated dNP2-VIVIT function in CD4+ T cell differentiation in vitro. FACS-sorted naive (CD4+CD25−CD62LhighCD44low) T cells were differentiated into Th1, Th2, Th17, and iTreg cells, and their effector cytokine responses were analyzed by flow cytometry and ELISA. dNP2-VIVIT regulated Th1 (Figures 3A–3C) and Th17 differentiation (Figures 3D–3F) by significantly inhibiting effector cytokine (IFN-γ and IL-17A) production. However, dNP2-VIVIT failed to inhibit Th2 (Figures 3G–3I) or iTreg (Figures 3J and 3K) differentiation. Importantly, the calcineurin inhibitor, CsA, potently suppressed all T cell subsets (Th1, Th2, Th17, and iTreg) without showing significant toxicity (Figure S1), suggesting that the specificity of T cell differentiation by VIVIT was different from that of CsA. Collectively, these results demonstrate that NFAT inhibition by dNP2-VIVIT could specifically regulate the differentiation of Th1 and Th17 cells. This could explain the possible mechanism and advantage of dNP2-VIVIT over CsA with respect to its regulatory function in EAE pathogenesis.
Figure 3.
NFAT-Specific Inhibition by dNP2-VIVIT Suppresses Th1 and Th17 Differentiation
Naive (CD4+CD25−CD62LhighCD44low) T cells were differentiated into Th1 (A-C), Th2 (D-F), Th17 (G-I), and iTreg (J and K) skewing conditions in the presence of anti-CD3/CD28 stimulation for 5 days. Cells were incubated with 1 μM dNP2-VEET, dNP2-VIVIT, and 20 ng/mL CsA. The frequencies and concentrations of IFN-γ (A–C) and IL-17A (D–F) in the supernatants were analyzed by flow cytometry and ELISA. (G–I) The frequencies of IL-4 and IL-13 were analyzed by flow cytometry. (J and K) Foxp3-expressing cells were analyzed. Data are presented as mean ± SEM of three (n = 3) independent experiments (A–F, J, and K) or are representative of four (n = 4) independent experiments (G–I); statistical analysis by two-tailed Student’s t test. *p < 0.05, **p < 0.01, ***p < 0.001. NS, not significant.
dNP2 Is Required for Efficient VIVIT Internalization into T Cells and Alleviating EAE
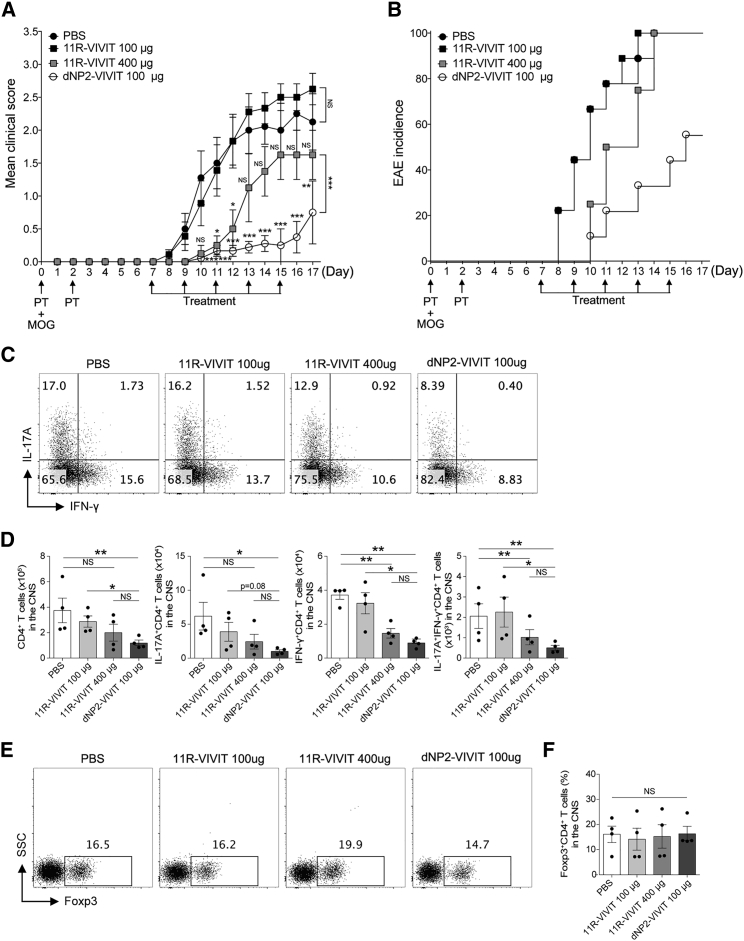
Because NFATs is critical for T cell activation, NFAT inhibition has already been attempted by using a poly arginine of 11R (11R-VIVIT) VIVIT peptide. However, no studies have been carried out to assess its ability to control T cell-mediated autoimmune disease, except for one report that demonstrated increased success rate of allogeneic islet transplantation upon treatment with 11R-VIVIT.38 Based on our previous findings, we hypothesized that the dNP2 peptide is critical for the efficient delivery of VIVIT peptide into T cells, thereby allowing inhibition of autoimmune diseases like EAE. By utilizing identical EAE designs, the in vivo efficacy of dNP2-VIVIT and 11R-VIVIT was comparatively analyzed. Upon treating mice with an equivalent amount of VIVIT peptides (100 μg), 11R-VIVIT could not control EAE onset or progression, whereas a high dose (400 μg) of this peptide showed partial effects on controlling EAE severity (Figures 4A and 4B). Remarkably, the EAE clinical scores of the mice treated with dNP2-VIVIT were significantly lower than those of that treated with 11R-VIVIT (100 μg, 400 μg) or PBS. The spinal cord-infiltrating IFN-γ- and IL-17A-producing cell numbers correlated with the clinical score of EAE disease. dNP2-VIVIT potently inhibited Th1 or Th17 cells in the CNS compared with 11R-VIVIT (100 μg), both in their proportion (Figure 4C) and the number (Figure 4D), without affecting the proportion of Treg cells (Figure 4E). Four times dose (400 μg) treatment of 11R-VIVIT showed partial reduction in the number of encephalitogenic T cells in the CNS, suggesting that dNP2 is a more effective peptide than 11R with respect to regulating T cell effector functions and EAE pathogenesis.
Figure 4.
dNP2-VIVIT Is More Efficient Than 11R-VIVIT at Alleviating Autoimmune Encephalomyelitis
EAE was induced in 8-week-old female C57BL/6 mice by immunization with MOG in complete Freund’s adjuvant. The mice were treated intraperitoneally with PBS, dNP2-VIVIT, or 11R-VIVIT on day 7 after immunization and subsequently treated every other day. Clinical scores (A) and incidence (B) were monitored daily (n = 9 for PBS, dNP2-VIVIT 100 μg, and 11R-VIVIT 100 μg; n = 4 for 11R-VIVIT 400 μg). Data are presented as the mean ± SEM of one or two independent experiments. The spinal cord cells were isolated, and IL-17A- and/or IFN-γ-expressing CD4+ T cells were analyzed by flow cytometry (C) and were counted and multiplied to determine their proportion (D). (E and F) The proportion of Foxp3-expressing CD4+ T cells was analyzed in the spinal cord (n = 4 per group). Data are presented as the mean ± SEM of one representative experiment out of two; statistical analysis by two-way ANOVA compared with PBS for (A) and two-tailed Student’s t test for (D) and (E). *p < 0.05, **p < 0.01, ***p < 0.001. NS, not significant.
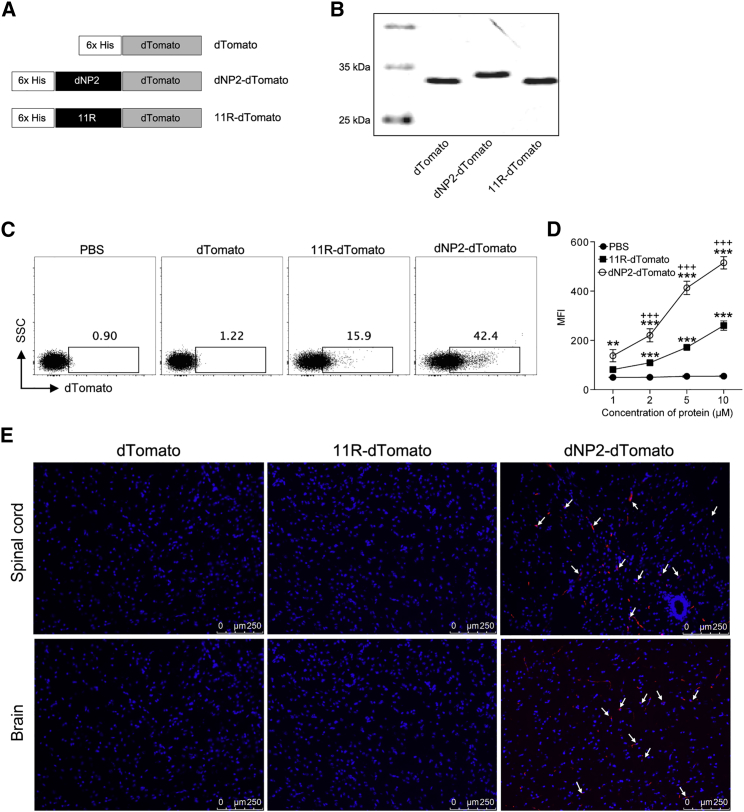
dNP2, but Not 11R, Delivers Cargo Proteins to CNS Tissues
Based on our findings that dNP2-VIVIT significantly ameliorated EAE pathogenicity, whereas 11R VIVIT did not, we hypothesized that the therapeutic effect of VIVIT peptide mediated by dNP2 would be due to the efficient cargo delivery into the CNS, by bypassing the blood-brain barrier. To visualize the intracellular protein delivery by dNP2 or 11R in vivo, we constructed plasmids expressing dTomato with or without the cell-penetrating peptides dNP2 and 11R (Figure 5A), and purified the resultant proteins as previously described (Figure 5B).37 The intracellular transduction efficiency of these purified proteins was examined by incubation with isolated naive (CD4+CD25−CD62LhighCD44low) T cells that are generally known to be hard to transfect. Flow cytometric analysis demonstrated higher transduction efficiency of dNP2-dTomato into naive CD4+ T cells relative to 11R-dTomato (Figure 5C), in a concentration-dependent manner (Figure 5D). Furthermore, we analyzed the brain and spinal cord tissue localization of dNP2-dTomato, 11R-dTomato, and dTomato proteins after 2 h of intravenous injection. Sectioned brain and spinal cord tissues were examined under a fluorescent microscope. dNP2-dTomato signal was significantly detected in the brain and spinal cord tissues, whereas 11R-dTomato and dTomato signal was barely observed (Figure 5E), suggesting that the blood-brain barrier (BBB) penetrability of dNP2 is significantly higher than that of the other controls. Therefore, we suggest that the delivery of VIVIT peptide conjugated with CNS-permeable peptide dNP2 would have an advantage with respect to inducing efficient NFAT-specific inhibition in CNS-infiltrating T cells, which might presumably allow the dNP2-VIVIT peptide to control in vivo physiology during EAE progression.
Figure 5.
Efficient CNS Cargo Protein Delivery by dNP2
(A) The dTomato, dNP2-dTomato, and 11R-dTomato constructs. (B) SDS-PAGE analysis of purified proteins. (C) Naive (CD4+CD25−CD62LhighCD44low) T cells were incubated with 10 μM dTomato, dNP2-dTomato, and 11R-dTomato for 1 h, and cells were analyzed by flow cytometry. (D) Μean fluorescence intensity (MFI) was analyzed in naive CD4+ T cells treated with various concentrations (1, 2, 5, or 10 μM) of dTomato, dNP2-dTomato, and 11R-dTomato for 1 h. (E) Eight-week-old female C57BL/6 mice were intravenously injected with 5 mg of dTomato, dNP2-dTomato, and 11R-dTomato. After 2 h, spinal cord and brain tissues were harvested and prepared as frozen slides. The nucleus was stained with Hoechst and fluorescence staining and was observed via fluorescence microscopy. Data are presented as mean ± SEM of three independent experiments (n = 3); statistical analysis by two-tailed Student’s t test. **p < 0.01, ***p < 0.001 versus PBS; +++p < 0.001 versus 11R-dTomato. NS, not significant.
Discussion
In this study, we present the successful application of the NFAT inhibitory peptide, VIVIT, for in vivo modulation of encephalitogenic T cell functions and CNS autoimmune neuroinflammation. A potent cell-penetrating peptide, dNP2, which enables efficient cargo delivery in CNS tissues allowed VIVIT to selectively regulate the differentiation of Th1 and Th17 cells and to strongly reduce EAE severity and demyelination.
Over the last decade, NFAT transcription factor has been targeted for the treatment of autoimmune diseases because of its critical role in calcium signaling initiated by TCR engagement. Considering the important role of T cells in autoimmune disease, modulation of calcium signaling-mediated T cell regulation was targeted by developing calcineurin inhibitors such as cyclosporine A (CsA) and tacrolimus (FK506). CsA and tacrolimus are used in autoimmune diseases including rheumatoid arthritis, membranous nephropathy, and systemic lupus erythematosus.21,23,39,40 Previous studies revealed that CsA treatment suppressed the effector function of Th17 cells in patients with rheumatoid arthritis and Sjögren’s syndrome.41,42 Moreover, a recent study reported that tacrolimus can be effective for maintaining remission in patients with rheumatoid arthritis.43 Although these chemical inhibitors have been used in clinical applications against human autoimmune diseases, they have serious disadvantages such as neurotoxicity and nephrotoxicity.29,30 Calcineurin inhibitors directly suppress the calcineurin phosphatase activity, and thus they broadly affect all of the signaling pathways associated with calcineurin.29 Calcineurin has been reported to be highly expressed in brain tissues and is known to modulate the Ca2+ influx by binding to the inositol triphosphate (IP3) and ryanodine receptors.44,45 Calcineurin also affects the activity of gamma aminobutyric acid (GABA) and N-methyl-d-aspartate (NMDA) receptors, thereby regulating neurotransmitter recycling and exocytosis.46, 47, 48 Treatment with calcineurin inhibitors upregulated the expression of transforming growth factor β (TGF-β) and endothelin, which induced nephrotoxic effects such as endothelial dysfunction, impaired glomerular filtration, systemic hypertension, and tubular fibrosis.49, 50, 51, 52 Moreover, recent studies have reported that calcineurin inhibitors negatively affected Treg cell proliferation and function, which have a pivotal role in immune tolerance.31,32
To overcome the limitations associated with the currently used calcineurin inhibitors, we developed the VIVIT peptide. The VIVIT peptide selectively regulates NFATs through interaction with the conserved calcineurin NFAT binding motif PxlxlT without affecting calcineurin phosphatase activity.33,34 For efficient in vivo therapeutic application, previous studies utilized various cell-permeable forms of the VIVIT peptide. One study reported that the cell-penetrating peptide (CPP) 11 arginine-conjugated VIVIT successfully increased transplant survival in islet transplanted mice.38 Another cell-penetrating peptide known as Sim-2-conjugated VIVIT inhibited IL-2 production in an ovalbumin (OVA)-induced asthma model.53 However, none of the VIVIT conjugates studied so far have reached clinical trials, implying that the existing CPP-based VIVIT peptides may have lower delivery efficiency in hard-to-transfect cells like human primary cells or T cells. Moreover, considering the important role of NFATs in autoimmune diseases, NFAT-specific inhibition by VIVIT should be further investigated in suitable autoimmune animal models. We designed a drug peptide dNP2-VIVIT, which could effectively penetrate primary T cells and inhibit NFAT-dependent gene expression without affecting the calcineurin phosphatase activity. dNP2-VIVIT successfully inhibited the pathogenic function of CNS-infiltrating encephalitogenic T cells and displayed potent therapeutic effects with respect to autoimmune neuroinflammation. Importantly, whereas the CsA treatment strongly inhibited all effector T cell differentiation (Th1, Th2, Th17, and iTreg) in vitro, dNP2-VIVIT selectively inhibited the differentiation of Th1 and Th17, but not of Th2 or Treg cells. A recent study revealed that although high-dose CsA (125 mg/kg) markedly decreased EAE severity, withdrawal of CsA induced EAE relapse.54 Because Treg cells play a key role in immune system balance, CsA-mediated non-specific inhibition of both Treg cells and effector T cells may explain the rebound effect induced by a high dose of CsA. In our study, we confirmed that dNP2-VIVIT ameliorated EAE severity and incidence without affecting Treg cell functions in vivo, revealing that dNP2-VIVIT can serve as a novel agent to regulate pathogenic Th1 or Th17 cell functions to control autoimmune diseases.
Multiple sclerosis (MS) is a human autoimmune disease characterized by demyelination and axonal loss in the CNS.55 Studies on EAE, a model of MS, have demonstrated that myelin-specific Th1 and Th17 cells that cross the BBB are the major mediators of autoimmune encephalomyelitis.56 Moreover, the importance of TCR-independent innate-like Th17 cells in the pathogenicity of EAE have been recently reported.57 Because the NFATs has been recently reported to actively regulate lineage-specific transcription factors and signature cytokines of CD4 T helper subsets including Th1 and Th17 cells,9, 10, 11 NFATs can be a potent therapeutic target for MS. However, effective therapeutic agents that are to be used for the treatment of CNS-related diseases should be able to successfully traverse the BBB and blood-spinal cord barrier (BSCB). Various strategies have been developed to bypass the BBB/BSCB; however, the challenge of delivering an effective therapeutic drug to the CNS is formidable.58 In the current study, we conjugated the VIVIT peptide with dNP2, which can efficiently penetrate the BBB and deliver cargoes to the CNS.37 We found that compared with 11R, dNP2 showed higher delivery efficiency with respect to entering the primary naive T cells and CNS tissues. Moreover, dNP2-VIVIT efficiently inhibited EAE progression, whereas 11R-VIVIT did not, presumably because of the difference in their delivery efficiency.
Taken together, our study collectively suggests that this is the first study to successfully regulate autoimmune neuroinflammation by targeting NFATs. Thus, we hope that dNP2-VIVIT can be further developed as a therapeutic agent against MS and other human autoimmune diseases.
Materials and Methods
Mice
C57BL/6J mice were purchased from Orient Bio. Mice used in this study were between 6 and 9 weeks of age. All mice were maintained in the specific pathogen-free facility at Hanyang University, and all animal protocols used in this study were approved by the Animal Experimentation Ethics Committee of Hanyang University. All experiments were performed according to the guidelines of the Institutional Animal Care and Use Committees of Hanyang University.
Peptide Synthesis
dNP2-VIVIT, dNP2-VEET, and 11R-VIVIT were synthesized by Anygen. The peptides were synthesized with 97% purity; purity was confirmed by HPLC analysis.
Cell Isolation and Differentiation
Naive (CD4+CD25−CD62LhighCD44low) T cells from the spleens and lymph nodes of 6- to 9-week-old mice were isolated using a Naive T Cell Isolation Kit II (Miltenyi Biotec). Purified naive CD4+ T cells were stimulated with plate-bound anti-CD3/CD28 (2 μg/mL; BD Biosciences). The cells were incubated in the presence of the following cytokines for 3 or 5 days: anti-IL-4 neutralizing antibody (5 μg/mL; BD Biosciences), IL-2 (50 U/mL; PeproTech), and IL-12 (2 ng/mL; PeproTech) for Th1; anti-IFN-γ neutralizing antibody (5 μg/mL; BD Biosciences), IL-4 (30 ng/mL; BD Biosciences), and IL-2 (50 U/mL; PeproTech) for Th2 cells; anti-IL-4/IFN-γ neutralizing antibody (5 μg/mL; BD Biosciences), TGF-β (1 ng/mL; R&D Systems), IL-6 (30 ng/mL; BD Biosciences), IL-1β (20 ng/mL; R&D Systems), and IL-23 (20 ng/mL; R&D Systems) for Th17; IL-2 (100 U/mL; PeproTech) and TGF-β (5 ng/mL; R&D Systems) for iTreg cells. Th2 cells were harvested at day 5 and were further reactivated by plate-bound anti-CD3/CD28 (2 μg/mL) for 24 h. Cells were incubated with dNP2-VEET (1 μM), dNP2-VIVIT (1 μM), and CsA (20 ng/mL; Calbiochem).
Flow Cytometry
Cell staining was performed using the following monoclonal antibodies: anti-CD4 (RM4-5; eBioscience), anti-CD25 (PC61.5; eBioscience), anti-CD44 (IM7; BioLegend), and anti-CD62L (MEL-14; BioLegend). For intracellular staining, cells were first re-stimulated with a cell stimulation cocktail (00-4975-03; eBioscience) for 4 h at 37°C, after which staining of cell surface markers was performed. After staining of the surface markers, the cells were fixed and permeabilized. Intracellular staining was performed using the following monoclonal antibodies: anti-IL-17A (eBio17B7; eBioscience), anti-IFN-γ (XMG1.2; eBioscience), anti-IL-4 (11B11; BioLegend), anti-IL-13 (eBio13A; eBioscience), and anti-Foxp3 (FJK-16s; eBioscience). Stained cells were detected by flow cytometry (FACSCanto II; BD Biosciences), and data were analyzed using FlowJo software version 10.0.7 (Tree Star).
Experimental Autoimmune Encephalomyelitis
C57BL/6J mice were immunized with 100 μg of MOG35–55 peptide in complete Freund’s adjuvant (Hooke Laboratories). At 0 and 24 h after immunization, the mice were intraperitoneally treated with 100 ng of pertussis toxin (PTX) (Hooke Laboratories). dNP2-VIVIT (100 μg), dNP2-VEET (100 μg), and 11R-VIVIT (100 μg or 400 μg) were diluted with PBS and intraperitoneally administered every other day starting from day 7. Animals were scored daily for signs of clinical disease as follows: partially limp tail, 0.5; completely limp tail, 1; limp tail and waddling gait, 1.5; paralysis of one hindlimb, 2; paralysis of one hindlimb and partial paralysis of the other hindlimb, 2.5; paralysis of both hindlimbs, 3; ascending paralysis, 3.5; paralysis of trunk, 4; moribund, 4.5; and death, 5. On either day 16 or 17, the mice were euthanized and the lymphocytes in the spinal cord were isolated by Percoll (GE Healthcare) density-gradient centrifugation. Absolute cell numbers of the isolated lymphocytes from the spinal cord were counted using hematology analyzer (Beckman Coulter) and further multiplied to determine the proportion of cytokine-producing T cells. For histological analysis, paraffin blocks of spinal cord tissues were de-paraffinized and immersed for combination staining (Luxol fast blue and H&E). The infiltrated cells in the white matter region of spinal cord tissues were counted using ImageJ software 1.48v.
Purification of Recombinant Proteins
CPP-conjugated fluorescent proteins were purified by using bacterial systems as previously described.37 In brief, DNAs were cloned into pRSET-B vector, and Escherichia coli BL21 (DE3) Star and CodonPlus cells were transformed with the constructed plasmids. Transformed colonies were inoculated in Luria-Bertani media broth (LB broth) containing ampicillin, and protein expression in bacteria was induced by adding isopropyl-β-D-thiogalactopyranoside (IPTG; Sigma-Aldrich). The sample was purified by AKTA pure (GE Healthcare) fast protein liquid chromatography (FPLC) through Nickel-6× His tag affinity chromatography. Purified protein was desalted by using PD-10 columns (Amersham). Purified proteins were stored at −80°C, and their concentrations were measured right before the experiments.
In Vitro Delivery Efficiency of dNP2
Naive (CD4+CD25−CD62LhighCD44low) T cells from the spleens and lymph nodes of 6- to 9-week-old mice were isolated using a Naive T Cell Isolation Kit II (Miltenyi Biotec). Purified naive CD4+ T cells were cultured in 96-well plates at 2.5 × 105 per well. Cells were incubated with dTomato, 11R-dTomato, and dNP2-dTomato proteins for 1 h. Following incubation, the cells were harvested and washed with PBS. Intracellular fluorescence was detected by flow cytometry (FACSCanto II; BD Biosciences), and data were analyzed using FlowJo software version 10.0.7 (Tree Star).
Imaging of Brain and Spinal Cord
C57BL/6J mice were intravenously injected with 5 mg of dTomato, dNP2-dTomato, and 11R-dTomato. Two hours after the injection, the mice were euthanized and the tissues were harvested. All harvested tissues were washed with PBS and fixed with 4% paraformaldehyde. The tissues were then frozen using O.C.T. compound (WAKO Chemical). The frozen blocks of tissues were cut into 7-μm-thick slices using cryostat (Thermo Scientific) and examined via fluorescence microscopy (Leica Microsystems).
Statistics
Data were analyzed using a two-tailed Student’s t test or two-way ANOVA using GraphPad Prism, version 6.0 (GraphPad Software). p values <0.05 were considered statistically significant.
Author Contributions
J.-M.C. conceived, designed, and supervised the study. L.-K.K. performed most of the experiments. H.-G.L., L.-K.K., and J.-M.C. analyzed and discussed the data. H.-G.L. wrote the draft manuscript. J.-M.C. wrote and revised the manuscript.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
This research was supported by the Bio & Medical Technology Development Program (NRF-2017M3A9C8027972) and by the Basic Science Research Program (NRF-2019R1A2C3006155) of the National Research Foundation (NRF) funded by the Korean Government.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omtm.2019.10.006.
Supplemental Information
References
- 1.Shaw J.P., Utz P.J., Durand D.B., Toole J.J., Emmel E.A., Crabtree G.R. Identification of a putative regulator of early T cell activation genes. Science. 1988;241:202–205. doi: 10.1126/science.3260404. [DOI] [PubMed] [Google Scholar]
- 2.Chen L., Rao A., Harrison S.C. Signal integration by transcription-factor assemblies: interactions of NF-AT1 and AP-1 on the IL-2 promoter. Cold Spring Harb. Symp. Quant. Biol. 1999;64:527–531. doi: 10.1101/sqb.1999.64.527. [DOI] [PubMed] [Google Scholar]
- 3.Henderson D.J., Naya I., Bundick R.V., Smith G.M., Schmidt J.A. Comparison of the effects of FK-506, cyclosporin A and rapamycin on IL-2 production. Immunology. 1991;73:316–321. [PMC free article] [PubMed] [Google Scholar]
- 4.Macian F. NFAT proteins: key regulators of T-cell development and function. Nat. Rev. Immunol. 2005;5:472–484. doi: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]
- 5.Hogan P.G., Lewis R.S., Rao A. Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annu. Rev. Immunol. 2010;28:491–533. doi: 10.1146/annurev.immunol.021908.132550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feske S. Calcium signalling in lymphocyte activation and disease. Nat. Rev. Immunol. 2007;7:690–702. doi: 10.1038/nri2152. [DOI] [PubMed] [Google Scholar]
- 7.Trama J., Lu Q., Hawley R.G., Ho S.N. The NFAT-related protein NFATL1 (TonEBP/NFAT5) is induced upon T cell activation in a calcineurin-dependent manner. J. Immunol. 2000;165:4884–4894. doi: 10.4049/jimmunol.165.9.4884. [DOI] [PubMed] [Google Scholar]
- 8.Hogan P.G., Chen L., Nardone J., Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17:2205–2232. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- 9.Kiani A., Viola J.P., Lichtman A.H., Rao A. Down-regulation of IL-4 gene transcription and control of Th2 cell differentiation by a mechanism involving NFAT1. Immunity. 1997;7:849–860. doi: 10.1016/s1074-7613(00)80403-3. [DOI] [PubMed] [Google Scholar]
- 10.Rengarajan J., Tang B., Glimcher L.H. NFATc2 and NFATc3 regulate T(H)2 differentiation and modulate TCR-responsiveness of naïve T(H)cells. Nat. Immunol. 2002;3:48–54. doi: 10.1038/ni744. [DOI] [PubMed] [Google Scholar]
- 11.Lee D.U., Avni O., Chen L., Rao A. A distal enhancer in the interferon-gamma (IFN-gamma) locus revealed by genome sequence comparison. J. Biol. Chem. 2004;279:4802–4810. doi: 10.1074/jbc.M307904200. [DOI] [PubMed] [Google Scholar]
- 12.Lee J.U., Kim L.K., Choi J.M. Revisiting the Concept of Targeting NFAT to Control T Cell Immunity and Autoimmune Diseases. Front. Immunol. 2018;9:2747. doi: 10.3389/fimmu.2018.02747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erb K.J., Twardzik T., Palmetshofer A., Wohlleben G., Tatsch U., Serfling E. Mice deficient in nuclear factor of activated T-cell transcription factor c2 mount increased Th2 responses after infection with Nippostrongylus brasiliensis and decreased Th1 responses after mycobacterial infection. Infect. Immun. 2003;71:6641–6647. doi: 10.1128/IAI.71.11.6641-6647.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fonseca B.P., Olsen P.C., Coelho L.P., Ferreira T.P., Souza H.S., Martins M.A., Viola J.P. NFAT1 transcription factor regulates pulmonary allergic inflammation and airway responsiveness. Am. J. Respir. Cell Mol. Biol. 2009;40:66–75. doi: 10.1165/rcmb.2007-0102OC. [DOI] [PubMed] [Google Scholar]
- 15.Kiani A., García-Cózar F.J., Habermann I., Laforsch S., Aebischer T., Ehninger G., Rao A. Regulation of interferon-gamma gene expression by nuclear factor of activated T cells. Blood. 2001;98:1480–1488. doi: 10.1182/blood.v98.5.1480. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal S., Avni O., Rao A. Cell-type-restricted binding of the transcription factor NFAT to a distal IL-4 enhancer in vivo. Immunity. 2000;12:643–652. doi: 10.1016/s1074-7613(00)80215-0. [DOI] [PubMed] [Google Scholar]
- 17.Dietz L., Frommer F., Vogel A.L., Vaeth M., Serfling E., Waisman A., Buttmann M., Berberich-Siebelt F. NFAT1 deficit and NFAT2 deficit attenuate EAE via different mechanisms. Eur. J. Immunol. 2015;45:1377–1389. doi: 10.1002/eji.201444638. [DOI] [PubMed] [Google Scholar]
- 18.Gomez-Rodriguez J., Sahu N., Handon R., Davidson T.S., Anderson S.M., Kirby M.R., August A., Schwartzberg P.L. Differential expression of interleukin-17A and -17F is coupled to T cell receptor signaling via inducible T cell kinase. Immunity. 2009;31:587–597. doi: 10.1016/j.immuni.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hermann-Kleiter N., Meisel M., Fresser F., Thuille N., Müller M., Roth L., Katopodis A., Baier G. Nuclear orphan receptor NR2F6 directly antagonizes NFAT and RORγt binding to the Il17a promoter. J. Autoimmun. 2012;39:428–440. doi: 10.1016/j.jaut.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weigmann B., Lehr H.A., Yancopoulos G., Valenzuela D., Murphy A., Stevens S., Schmidt J., Galle P.R., Rose-John S., Neurath M.F. The transcription factor NFATc2 controls IL-6-dependent T cell activation in experimental colitis. J. Exp. Med. 2008;205:2099–2110. doi: 10.1084/jem.20072484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mok C.C. Calcineurin inhibitors in systemic lupus erythematosus. Best Pract. Res. Clin. Rheumatol. 2017;31:429–438. doi: 10.1016/j.berh.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 22.Azzi J.R., Sayegh M.H., Mallat S.G. Calcineurin inhibitors: 40 years later, can’t live without …. J. Immunol. 2013;191:5785–5791. doi: 10.4049/jimmunol.1390055. [DOI] [PubMed] [Google Scholar]
- 23.Wells G., Haguenauer D., Shea B., Suarez-Almazor M.E., Welch V.A., Tugwell P. Cyclosporine for rheumatoid arthritis. Cochrane Database Syst. Rev. 2000;2000:CD001083. doi: 10.1002/14651858.CD001083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanifin J.M., Ling M.R., Langley R., Breneman D., Rafal E. Tacrolimus ointment for the treatment of atopic dermatitis in adult patients: part I, efficacy. J. Am. Acad. Dermatol. 2001;44(Suppl 1):S28–S38. doi: 10.1067/mjd.2001.109810. [DOI] [PubMed] [Google Scholar]
- 25.Fischer G., Wittmann-Liebold B., Lang K., Kiefhaber T., Schmid F.X. Cyclophilin and peptidyl-prolyl cis-trans isomerase are probably identical proteins. Nature. 1989;337:476–478. doi: 10.1038/337476a0. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi N., Hayano T., Suzuki M. Peptidyl-prolyl cis-trans isomerase is the cyclosporin A-binding protein cyclophilin. Nature. 1989;337:473–475. doi: 10.1038/337473a0. [DOI] [PubMed] [Google Scholar]
- 27.Harding M.W., Galat A., Uehling D.E., Schreiber S.L. A receptor for the immunosuppressant FK506 is a cis-trans peptidyl-prolyl isomerase. Nature. 1989;341:758–760. doi: 10.1038/341758a0. [DOI] [PubMed] [Google Scholar]
- 28.Bram R.J., Hung D.T., Martin P.K., Schreiber S.L., Crabtree G.R. Identification of the immunophilins capable of mediating inhibition of signal transduction by cyclosporin A and FK506: roles of calcineurin binding and cellular location. Mol. Cell. Biol. 1993;13:4760–4769. doi: 10.1128/mcb.13.8.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bechstein W.O. Neurotoxicity of calcineurin inhibitors: impact and clinical management. Transpl. Int. 2000;13:313–326. doi: 10.1007/s001470050708. [DOI] [PubMed] [Google Scholar]
- 30.Abramowicz D., Wissing K.M., Broeders N. Nephrotoxicity of calcineurin inhibitors: new therapeutic approaches. Transplant. Proc. 2000;32(Suppl 1A):3S–5S. doi: 10.1016/s0041-1345(00)00807-1. [DOI] [PubMed] [Google Scholar]
- 31.Miroux C., Morales O., Ghazal K., Othman S.B., de Launoit Y., Pancré V., Conti F., Delhem N. In vitro effects of cyclosporine A and tacrolimus on regulatory T-cell proliferation and function. Transplantation. 2012;94:123–131. doi: 10.1097/TP.0b013e3182590d8f. [DOI] [PubMed] [Google Scholar]
- 32.Miroux C., Moralès O., Carpentier A., Dharancy S., Conti F., Boleslowski E., Podevin P., Auriault C., Pancré V., Delhem N. Inhibitory effects of cyclosporine on human regulatory T cells in vitro. Transplant. Proc. 2009;41:3371–3374. doi: 10.1016/j.transproceed.2009.08.043. [DOI] [PubMed] [Google Scholar]
- 33.Aramburu J., Yaffe M.B., López-Rodríguez C., Cantley L.C., Hogan P.G., Rao A. Affinity-driven peptide selection of an NFAT inhibitor more selective than cyclosporin A. Science. 1999;285:2129–2133. doi: 10.1126/science.285.5436.2129. [DOI] [PubMed] [Google Scholar]
- 34.Aramburu J., Garcia-Cózar F., Raghavan A., Okamura H., Rao A., Hogan P.G. Selective inhibition of NFAT activation by a peptide spanning the calcineurin targeting site of NFAT. Mol. Cell. 1998;1:627–637. doi: 10.1016/s1097-2765(00)80063-5. [DOI] [PubMed] [Google Scholar]
- 35.Lim S., Lee J.A., Koo J.H., Kang T.G., Ha S.J., Choi J.M. Cell Type Preference of a Novel Human Derived Cell-Permeable Peptide dNP2 and TAT in Murine Splenic Immune Cells. PLoS ONE. 2016;11:e0155689. doi: 10.1371/journal.pone.0155689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lim S., Kirkiles-Smith N.C., Pober J.S., Bothwell A.L.M., Choi J.M. Regulation of human T cell responses by dNP2-ctCTLA-4 inhibits human skin and microvessel graft rejection. Biomaterials. 2018;183:128–138. doi: 10.1016/j.biomaterials.2018.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lim S., Kim W.J., Kim Y.H., Lee S., Koo J.H., Lee J.A., Yoon H., Kim D.H., Park H.J., Kim H.M. dNP2 is a blood-brain barrier-permeable peptide enabling ctCTLA-4 protein delivery to ameliorate experimental autoimmune encephalomyelitis. Nat. Commun. 2015;6:8244. doi: 10.1038/ncomms9244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noguchi H., Matsushita M., Okitsu T., Moriwaki A., Tomizawa K., Kang S., Li S.T., Kobayashi N., Matsumoto S., Tanaka K. A new cell-permeable peptide allows successful allogeneic islet transplantation in mice. Nat. Med. 2004;10:305–309. doi: 10.1038/nm994. [DOI] [PubMed] [Google Scholar]
- 39.Yamaguchi M., Ando M., Yamamoto R., Akiyama S., Kato S., Katsuno T., Kosugi T., Sato W., Tsuboi N., Yasuda Y. Patient age and the prognosis of idiopathic membranous nephropathy. PLoS ONE. 2014;9:e110376. doi: 10.1371/journal.pone.0110376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alfaadhel T., Cattran D. Management of Membranous Nephropathy in Western Countries. Kidney Dis. (Basel) 2015;1:126–137. doi: 10.1159/000437287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang C., Zhang J., Yang B., Wu C. Cyclosporin A inhibits the production of IL-17 by memory Th17 cells from healthy individuals and patients with rheumatoid arthritis. Cytokine. 2008;42:345–352. doi: 10.1016/j.cyto.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 42.Wang K., Shi L., Yu Z., Deng Z., He A., Li S., Liu L. Cyclosporine A Suppresses the Activation of the Th17 Cells in Patients with Primary Sjögren’s Syndrome. Iran. J. Allergy Asthma Immunol. 2015;14:198–207. [PubMed] [Google Scholar]
- 43.Naniwa T., Iwagaitsu S., Kajiura M. Efficacy of add-on tacrolimus on methotrexate to maintain clinical remission after rediscontinuation of a tumor necrosis factor inhibitor in rheumatoid arthritis patients who relapsed shortly after discontinuation of the same tumor necrosis factor inhibitor due to clinical remission. Mod. Rheumatol. 2017;27:29–34. doi: 10.3109/14397595.2016.1174394. [DOI] [PubMed] [Google Scholar]
- 44.Snyder S.H., Lai M.M., Burnett P.E. Immunophilins in the nervous system. Neuron. 1998;21:283–294. doi: 10.1016/s0896-6273(00)80538-3. [DOI] [PubMed] [Google Scholar]
- 45.Cameron A.M., Steiner J.P., Roskams A.J., Ali S.M., Ronnett G.V., Snyder S.H. Calcineurin associated with the inositol 1,4,5-trisphosphate receptor-FKBP12 complex modulates Ca2+ flux. Cell. 1995;83:463–472. doi: 10.1016/0092-8674(95)90124-8. [DOI] [PubMed] [Google Scholar]
- 46.Chen S.R., Hu Y.M., Chen H., Pan H.L. Calcineurin inhibitor induces pain hypersensitivity by potentiating pre- and postsynaptic NMDA receptor activity in spinal cords. J. Physiol. 2014;592:215–227. doi: 10.1113/jphysiol.2013.263814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martina M., Mozrzymas J.W., Boddeke H.W., Cherubini E. The calcineurin inhibitor cyclosporin A-cyclophilin A complex reduces desensitization of GABAA-mediated responses in acutely dissociated rat hippocampal neurons. Neurosci. Lett. 1996;215:95–98. [PubMed] [Google Scholar]
- 48.Kumashiro S., Lu Y.F., Tomizawa K., Matsushita M., Wei F.Y., Matsui H. Regulation of synaptic vesicle recycling by calcineurin in different vesicle pools. Neurosci. Res. 2005;51:435–443. doi: 10.1016/j.neures.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 49.Khanna A.K., Cairns V.R., Becker C.G., Hosenpud J.D. Transforming growth factor (TGF)-beta mimics and anti-TGF-beta antibody abrogates the in vivo effects of cyclosporine: demonstration of a direct role of TGF-beta in immunosuppression and nephrotoxicity of cyclosporine. Transplantation. 1999;67:882–889. doi: 10.1097/00007890-199903270-00016. [DOI] [PubMed] [Google Scholar]
- 50.Khanna A., Cairns V., Hosenpud J.D. Tacrolimus induces increased expression of transforming growth factor-beta1 in mammalian lymphoid as well as nonlymphoid cells. Transplantation. 1999;67:614–619. doi: 10.1097/00007890-199902270-00021. [DOI] [PubMed] [Google Scholar]
- 51.Kakita T., Hasegawa K., Iwai-Kanai E., Adachi S., Morimoto T., Wada H., Kawamura T., Yanazume T., Sasayama S. Calcineurin pathway is required for endothelin-1-mediated protection against oxidant stress-induced apoptosis in cardiac myocytes. Circ. Res. 2001;88:1239–1246. doi: 10.1161/hh1201.091794. [DOI] [PubMed] [Google Scholar]
- 52.Slowinski T., Subkowski T., Diehr P., Bachert D., Fritsche L., Neumayer H.H., Hocher B. Interaction of the endothelin system and calcineurin inhibitors after kidney transplantation. Clin. Sci. (Lond.) 2002;103(Suppl 48):396S–398S. doi: 10.1042/CS103S396S. [DOI] [PubMed] [Google Scholar]
- 53.Choi J.M., Sohn J.H., Park T.Y., Park J.W., Lee S.K. Cell permeable NFAT inhibitory peptide Sim-2-VIVIT inhibits T-cell activation and alleviates allergic airway inflammation and hyper-responsiveness. Immunol. Lett. 2012;143:170–176. doi: 10.1016/j.imlet.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 54.Saitoh K., Kon S., Nakatsuru T., Inui K., Ihara T., Matsumoto N., Kitai Y., Muromoto R., Matsuda T. Anti-IL-17A blocking antibody reduces cyclosporin A-induced relapse in experimental autoimmune encephalomyelitis mice. Biochem. Biophys. Rep. 2016;8:139–145. doi: 10.1016/j.bbrep.2016.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trapp B.D., Ransohoff R., Rudick R. Axonal pathology in multiple sclerosis: relationship to neurologic disability. Curr. Opin. Neurol. 1999;12:295–302. doi: 10.1097/00019052-199906000-00008. [DOI] [PubMed] [Google Scholar]
- 56.Goverman J. Autoimmune T cell responses in the central nervous system. Nat. Rev. Immunol. 2009;9:393–407. doi: 10.1038/nri2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee H.G., Lee J.U., Kim D.H., Lim S., Kang I., Choi J.M. Pathogenic function of bystander-activated memory-like CD4+ T cells in autoimmune encephalomyelitis. Nat. Commun. 2019;10:709. doi: 10.1038/s41467-019-08482-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen Y., Liu L. Modern methods for delivery of drugs across the blood-brain barrier. Adv. Drug Deliv. Rev. 2012;64:640–665. doi: 10.1016/j.addr.2011.11.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.