Summary
Background
Cabozantinib is approved for patients with metastatic renal cell carcinoma on the basis of studies done in clear-cell histology. The activity of cabozantinib in patients with non-clear-cell renal cell carcinoma is poorly characterised. We sought to analyse the antitumour activity and toxicity of cabozantinib in advanced non-clear-cell renal cell carcinoma.
Methods
We did a multicentre, international, retrospective cohort study of patients with metastatic non-clear-cell renal cell carcinoma treated with oral cabozantinib during any treatment line at 22 centres: 21 in the USA and one in Belgium. Eligibility required patients with histologically confirmed non-clear-cell renal cell carcinoma who received cabozantinib for metastatic disease during any treatment line roughly between 2015 and 2018. Mixed tumours with a clear-cell histology component were excluded. No other restrictive inclusion criteria were applied. Data were obtained from retrospective chart review by investigators at each institution. Demographic, surgical, pathological, and systemic therapy data were captured with uniform database templates to ensure consistent data collection. The main objectives were to estimate the proportion of patients who achieved an objective response, time to treatment failure, and overall survival after treatment.
Findings
Of 112 identified patients with non-clear-cell renal cell carcinoma treated at the participating centres, 66 (59%) had papillary histology, 17 (15%) had Xp11.2 translocation histology, 15 (13%) had unclassified histology, ten (9%) had chromophobe histology, and four (4%) had collecting duct histology. The proportion of patients who achieved an objective response across all histologies was 30 (27%, 95% CI 19–36) of 112 patients. At a median follow-up of 11 months (IQR 6–18), median time to treatment failure was 6·7 months (95% CI 5·5–8·6), median progression-free survival was 7·0 months (5·7–9·0), and median overall survival was 12·0 months (9·2–17·0). The most common adverse events of any grade were fatigue (58 [52%]), and diarrhoea (38 [34%]). The most common grade 3 events were skin toxicity (rash and palmar-plantar erythrodysesthesia; five [4%]) and hypertension (four [4%]). No treatment-related deaths were observed. Across 54 patients with available next-generation sequencing data, the most frequently altered somatic genes were CDKN2A (12 [22%]) and MET (11 [20%]) with responses seen irrespective of mutational status.
Interpretation
While we await results from prospective studies, this real-world study provides evidence supporting the antitumour activity and safety of cabozantinib across non-clear-cell renal cell carcinomas. Continued support of international collaborations and prospective ongoing studies targeting non-clear-cell renal cell carcinoma subtypes and specific molecular alterations are warranted to improve outcomes across these rare diseases with few evidence-based treatment options.
Funding
None.
Introduction
Renal cell carcinoma comprises a heterogeneous group of diseases with distinct histopathological and molecular features, as well as natural history.1 The predominant clear-cell subtype represents approximately 70–75% of renal cell carcinomas, whereas a continuously expanding group of varied histologies constitute the remaining 25–30%. Despite their unique pathogenesis and biological behaviours, these tumours are generally captured under the umbrella term non-clear cell. The most common type of non-clear-cell renal cell carcinoma is papillary,2 which is further stratified into histological (type 1 or 2) or molecular (mesenchymal–epithelial transition factor gene [MET] driven or MET independent) subgroups. Other rare types of renal cell carcinoma include chromophobe (≤5%), collecting duct, medullary, and translocation carcinoma (each <1%).
Given their rarity, prospective investigation has been scarce and non-clear-cell renal cell carcinoma has generally been excluded from the pivotal randomised studies of targeted therapies and immunotherapies. Most antitumour activity data are derived from retrospective studies, expanded access programmes, and prospective single-arm studies.1,3 The first prospective data were gleaned from a subset analysis of the phase 3 study of temsirolimus,4,5 which allowed non-clear-cell renal cell carcinoma and showed comparable efficacy with the clear-cell renal cell carcinoma cohort. Three subsequent randomised phase 2 trials6-8 comparing the VEGFR multityrosine kinase inhibitor sunitinib to the mTOR inhibitor everolimus included a diverse array of non-clear histologies and provided evidence that front-line sunitinib induces better progression-free survival, albeit modestly, when compared with that observed in clear-cell disease. Other VEGFR tyrosine kinase inhibitors, such as axitinib and pazopanib, have been evaluated in small prospective series and retrospective analyses showing promising activity, with the proportion of patients who achieved a response being between 27% and 38%, which is comparable to the clear-cell population.9-11 In 2018, the contemporary approach to the treatment of non-clear-cell renal cell carcinoma beyond first line was extrapolated from the clear-cell renal cell carcinoma data, and patient participation in clinical trials is encouraged.
Cabozantinib is an inhibitor of multiple tyrosine kinase receptors implicated in tumour progression, including VEGFR, MET, RET, KIT, and AXL.12 Its regulatory approval in renal cell carcinoma was based on the results of two prospective clinical trials limited to a population with clear-cell renal cell carcinoma.13,14 Only two small retrospective studies15,16 have reported on the effects of cabozantinib in non-clear-cell renal cell carcinoma, and both showed encouraging activity.
Recognising the paucity of reported activity data of cabozantinib in non-clear-cell renal cell carcinoma, we sought to investigate its antitumour activity in patients who had been treated in any line of therapy using an international collaboration to address this evidence gap, while we await the results of prospective studies.
Methods
Study design and participants
We did a multicentre, international, retrospective, cohort analysis of patients with metastatic non-clear-cell renal cell carcinoma treated with cabozantinib. Data were collected from patients treated in the USA (21 centres) and Belgium (one centre). Only de-identified data were shared with the coordinating institution (Dana-Farber Cancer Institute, Boston, MA, USA). Eligibility required patients with histologically confirmed non-clear-cell renal cell carcinoma who received cabozantinib for metastatic disease during any treatment line. Mixed tumours with clear-cell histology component were excluded. No other restrictive inclusion criteria were applied. Available baseline and on-therapy clinical and imaging data were required to assess outcomes. Each participating centre obtained regulatory approval per their institutional guidelines.
Procedures
Data were obtained from retrospective chart review by investigators at each institution roughly between 2015 and 2018. Demographic, surgical, pathological, and systemic therapy data were captured with uniform database templates to ensure consistent data collection.
For cabozantinib, information on starting dose, dose modifications and reason for discontinuation, laboratory data, and adverse events were collected. Objective response was assessed locally and categorised using general RECIST (Response Evaluation Criteria in Solid Tumors) principles. Official radiology RECIST evaluation was preferred, but if not available, the calculations were done by the site investigator. Clinical and radiological assessments were done periodically according to the standard of care of each participating centre.
Toxicity was collected retrospectively by the investigators using the Common Terminology Criteria for Adverse Events (version 4.0). Toxicities considered related to cabozantinib were recorded from the date of first dose to 30 days after the last dose. Toxicities leading to dose modifications and reasons for treatment discontinuation were collected. When available, data on somatic molecular alterations obtained via next-generation sequencing of archival tumour tissue were collected.
Outcomes
The main objective was to evaluate the antitumour effects of cabozantinib in patients with non-clear-cell renal cell carcinoma in terms of the proportion of patients who achieved an objective response, time to treatment failure, and overall survival. Objective response was defined as the proportion of patients with complete or partial responses as the best radiological response on cabozantinib. Clinical benefit encompassed the proportion of patients who achieved an objective response plus stable disease. Time to treatment failure was calculated from treatment initiation to treatment discontinuation for any reason including progressive disease, treatment toxicity, patient preference, or death, or censored at date of last dose in patients who were still on therapy. Overall survival was calculated from initiation of treatment until death or last follow-up. Progression-free survival was also analysed and defined similarly to time to treatment failure, but only progressive disease and death were included as events; patients who were off treatment for non-progressive disease causes without further disease assessment were censored at date of last dose. Other secondary endpoints were proportion of patients who were failure-free at 6 months and 12 months, safety, and the prevalence of somatic genomic alterations by next-generation sequencing and outcomes in patients whose tumours had known genomic alterations.
Statistical analysis
As a retrospective study, no formal sample size or power calculations were done a priori. Clinical and disease characteristics were summarised as median and IQR for continuous variables, and as number and percentage for categorical variables. Responses were calculated as the percentage of patients who achieved an objective response or clinical benefit along with 95% Clopper-Pearson exact CIs. Patients with non-evaluable response by RECIST were conservatively included as non-responders. Median time to treatment failure, progression-free survival, overall survival, and 6-month and 12-month overall survival were calculated with the Kaplan-Meier method for the overall cohort and by subgroups. Subgroup analyses were descriptive in nature and no formal comparisons were done. Statistical analyses were done with SAS (version 9.4).
Role of the funding source
No specific funding source was used for this study. Discretionary funds of the investigators were used on a site-by-site basis. The corresponding author had full access to all the data and had final responsibility to submit for publication.
Results
112 patients across 22 institutions who were treated with cabozantinib for non-clear-cell renal cell carcinoma were identified. Most patients were men (85 [76%]) and had a performance status of 0–1 (82 [73%]; table 1). Median age at initiation of treatment with cabozantinib was 60 years (IQR 48–66).
Table 1:
Patient characteristics
| All patients (N=112) | |
|---|---|
| Age, years | 60 (48–66) |
| Sex | |
| Female | 27 (24%) |
| Male | 85 (76%) |
| Race | |
| White | 90 (80%) |
| African | 18 (16%) |
| Asian | 1 (<1%) |
| Unknown | 3 (3%) |
| Comorbidities* | |
| Hypertension | 60 (54%) |
| Diabetes | 18 (16%) |
| Elevated cholesterol | 24 (21%) |
| Deep vein thrombosis or pulmonary embolism | 11 (10%) |
| Atrial fibrillation | 10 (9%) |
| ECOG performance status | |
| 0 | 23 (20%) |
| 1 | 59 (53%) |
| 2–3 | 11 (10%) |
| Unknown | 19 (17%) |
| Stage at diagnosis | |
| I–III | 54 (48%) |
| IV | 58 (52%) |
| Previous nephrectomy | |
| No | 26 (23%) |
| Radical | 73 (65%) |
| Partial | 13 (12%) |
| Cytoreductive intent | 35 (41%) |
| Histology | |
| Papillary | 66 (59%) |
| Xp11·2 translocation | 17 (15%) |
| Unclassified | 15 (13%) |
| Chromophobe | 10 (9%) |
| Collecting duct | 4 (4%) |
| Sarcomatoid component | |
| Yes | 30 (27%) |
| <20% | 7 (23%) |
| 20–70% | 8 (27%) |
| >70% | 4 (13%) |
| Unknown | 11 (37%) |
| No | 51 (46%) |
| Unknown | 31 (28%) |
| Necrosis component | |
| Yes | 38 (34%) |
| No | 25 (22%) |
| Unknown | 49 (44%) |
| Fuhrman grade | |
| 1–2 | 5 (4%) |
| 3 | 35 (31%) |
| 4 | 31 (28%) |
| Unknown | 41 (37%) |
| IMDC risk group | |
| Favourable | 9 (8%) |
| Intermediate | 71 (63%) |
| Poor | 29 (26%) |
| Unknown | 3 (3%) |
| Sites of metastasest† | |
| Lymph nodes | 95 (85%) |
| Lung | 66 (59%) |
| Bone | 49 (44%) |
| Liver | 42 (38%) |
| Brain | 6 (5%) |
| Number of previous systemic therapies | |
| 0 | 22 (20%) |
| 1 | 31 (28%) |
| 2 | 32 (29%) |
| ≥3 | 27 (24%) |
| Type of previous systemic treatment | |
| Tyrosine kinase inhibitor | 39 (35%) |
| Immunotherapy | 12 (11%) |
| Tyrosine kinase inhibitor and immunotherapy | 36 (32%) |
| Other | 3 (3%) |
| None | 22 (20%) |
Data are median (IQR) or n (%). ECOG=Eastern Cooperative Oncology Group. IMDC=International Metastatic Renal Cell Carcinoma Database Consortium.
The five most frequent comorbidities are shown. More than one comorbidity per patient might be included if present.
Patients might have had more than one metastatic site.
The histopathological breakdown of the 112 patients with non-clear-cell renal cell carcinoma was papillary (66 [59%]), Xp11.2 translocation (17 [15%]), unclassified (15 [13%]), chromophobe (ten [9%]), and collecting duct (four [4%]). Of the patients with papillary tumours, 12 (18%) were type 1, 23 (35%) were type 2, and 31 (47%) were unclassified. Sarcomatoid features were noted in 30 (27%) tumours and necrosis in 38 (34%) tumours.
Breakdown by International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk group at the start of cabozantinib treatment was favourable (nine [8%] patients), intermediate (71 [63%]), and poor risk (29 [26%]). Patients most commonly had metastatic cancer in lymph nodes (95 [85%]), lung (66 [59%]), bone (49 [44%]), and liver (42 [38%]).
Cabozantinib was generally administered for the treatment of refractory disease: first line (22 [20%] patients), second line (31 [28%]), and third line or beyond (59 [53%]). In patients who had received previous systemic therapy, treatments were tyrosine kinase inhibitor (39 [35%] patients), immune checkpoint inhibitor (12 [11%]), tyrosine kinase inhibitor and immune checkpoint inhibitor (36 [32%]), and other treatments (three [3%]). Sunitinib (39 [43%] patients) and nivolumab (38 [42%]) were the most frequently used previous therapies (appendix p 1).
Median follow-up was 11 months (IQR 6–18). The proportion of patients who achieved an objective response, as determined by local investigators, was 30 (27%, 95% CI 19–36) of 112 patients, including one complete response and 29 partial responses (table 2). For 53 (47%) patients, stable disease was the best response. The proportion of patients with overall clinical benefit was 74% (95% CI 65–82). Four patients were not evaluable because of inadequate time on therapy (<8 weeks) to assess treatment response.
Table 2:
Activity outcomes (overall cohort and subgroups)
| Overall response |
Clinical benefit |
Median time to treatment failure |
12-month overall survival |
|||
|---|---|---|---|---|---|---|
| n/N | % (95% CI) | n/N | % (95% CI) | |||
| Overall cohort | 30/112 | 27% (19–36) | 83/112 | 74% (65–82) | 6·7 (5·5– 8·6) | 51% (39–62) |
| Histology | ||||||
| Papillary | 18/66 | 27% (17–40) | 48/66 | 73% (60–83) | 6·9 (4·6–10·1) | 46% (31–60) |
| Xp11.2 translocation | 5/17 | 29% (10–56) | 14/17 | 82% (57–96) | 8·3 (4·6–NR) | 69% (36–87) |
| Unclassified | 2/15 | 13% (2–40) | 10/15 | 67% (38–88) | 6·0 (1·4–9·9) | 36% (8–67) |
| Chromophobe | 3/10 | 30% (7–65) | 7/10 | 70% (35–93) | 5·7 (1·1–7·8) | 60% (16–87) |
| Collecting duct | 2/4 | 50% (7–93) | 4/4 | 100% (40–99) | NC | NC |
| Sarcomatoid features | ||||||
| Yes | 6/30 | 20% (8–39) | 23/30 | 77% (58–90) | 5·1 (2·8–6·2) | 25% (8–47) |
| No | 13/51 | 25% (14–40) | 34/51 | 67% (52–79) | 7·4 (4·6–11·0) | 48% (31–64) |
| Treatment | ||||||
| First line | 5/22 | 23% (8–45) | 18/22 | 82% (60–95) | 7·6 (5·5–17·2) | 60% (29–81) |
| Second line or more | 25/90 | 28% (19–38) | 65/90 | 72% (62–81) | 6·2 (4·6–8·6) | 49% (36–61) |
| Type of previous systemic therapies | ||||||
| Immunotherapy | 4/12 | 33% (10–65) | 10/12 | 83% (52–98) | 5·1 (1·8–7·8) | 27% (1–66) |
| Tyrosine kinase inhibitor | 11/39 | 28% (15–45) | 24/39 | 62% (45–77) | 6·2 (3·4–10·6) | 54% (36–70) |
| Tyrosine kinase inhibitor and immunotherapy | 10/36 | 28% (14–45) | 28/36 | 78% (61–90) | 6·9 (4·6–11·0) | 48% (28–66) |
| Other | 0/3 | 0 | 3/3 | 100% (29–99) | NC | NC |
| IMDC risk group | ||||||
| Favourable | 5/9 | 56% (21–86) | 8/9 | 89% (52–99) | 11·0 (6·2–NR) | 67% (19–90) |
| Intermediate | 15/71 | 21% (12–32) | 52/71 | 73% (61–83) | 6·0 (4·6–7·8) | 51% (36–64) |
| Poor | 9/29 | 31% (15–51) | 21/29 | 72% (53–87) | 8·0 (3·7–15·9) | 46% (23–66) |
| Presence of bone metastasis* | ||||||
| Yes | 10/49 | 20% (10–34) | 38/49 | 78% (63–88) | 6·9 (4·8–9·0) | 44% (27–60) |
| No | 20/63 | 32% (21–45) | 45/63 | 71% (59–82) | 6·4 (4·6–11·0) | 57% (41–71) |
Data are n/N, % (95% CI), or months (95% CI). NR=not reached. NC=not calculated (too few patients to calculate reliably). IMDC=International Metastatic Renal Cell Carcinoma Database Consortium.
All patients with bone metastasis also had either lymph node or visceral metastases.
The patient with papillary renal cell carcinoma who achieved a complete response had previous radical nephrectomy 37 months before metastatic relapse. He had stable disease as best response to first-line pazopanib, and received it for 30 weeks before metastasectomy of skin lesions. After 3 months on cabozantinib, he had a significant partial response and by 6 months had achieved a complete response in his lung and lymph node disease. No causal molecular alterations were identified in the next-generation sequencing analysis of his metastatic lesion.
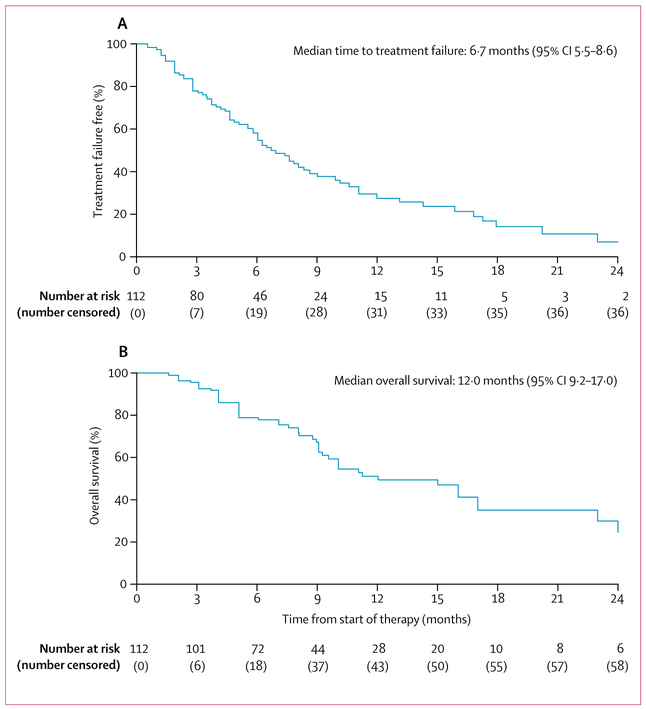
At the time of analysis, 74 patients had discontinued cabozantinib, and 38 remained on therapy. Reasons for treatment failure included progression (63 [85%] patients), toxicity (five [7%]), patient preference (two [3%]), physician preference (one [1%]), and other (three [4%]). 27 patients received subsequent systemic therapy (appendix p 1). Median time to treatment failure was 6·7 months (95% CI 5·5–8·6; figure 1). The proportion of patients who were treatment failure free at 6 months was 55% (44–64) and at 12 months was 27% (18–38). Median progression-free survival was 7·0 months (95% CI 5·7–9·0). Median overall survival was 12·0 months (9·2–17·0) and 50 patients had died (figure 1).Overall survival at 6 months was 79% (70–86) and at 12 months was 51% (39–62).
Figure 1:
Kaplan-Meier estimates of time to treatment failure (A) and overall survival (B)
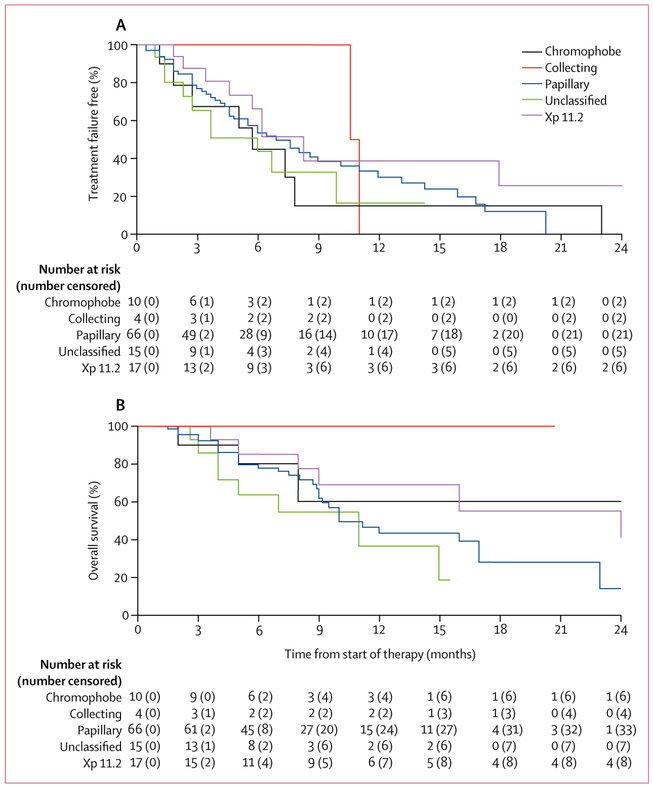
The proportion of patients who achieved an objective response varied among the different histologies: 27% (95% CI 17–40; 18 of 66) with papillary, 29% (10–56; five of 17) with Xp11.2 translocation, 30% (7–65; three of ten) with chromophobe, 50% (7–93; two of four) with collecting duct, and 13% (2–40; two of 15) unclassified (table 2; figure 2). Similar trends were observed in time to treatment failure and overall survival. In the 30 patients whose tumours had sarcomatoid features, the proportion of patients who achieved an objective response was 20% (95% CI 8–39; six of 30), median time to treatment failure was 5·1 months (95% CI 2·8–6·2), and 12-month overall survival was 25% (95% CI 8–47). Among the 51 patients whose tumours did not have sarcomatoid features, the proportion of patients who achieved an objective response was 25% (95% CI 14–40; 13 of 51), median time to treatment failure was 7·4 months (95% CI 4·6–11·0), and 12-month overall survival was 48% (95% CI 31–64). According to IMDC risk, patients with favourable-risk disease (n=9) had the longest time to treatment failure of 11·0 months (95% CI 6·2–not reached), whereas time to treatment failure for patients with intermediate-risk disease (n=71) was 6·0 months (4·6–7·8), and for those with poor-risk disease (n=29) was 8·0 months (3·7–15·9).
Figure 2:
Kaplan-Meier estimates of time to treatment failure (A) and overall survival (B) by histology subgroup
No patient had bone-only disease, but for the 49 patients whose metastatic sites included bone, the proportion of patients who achieved an objective response was 20% (95% CI 10–34; clinical benefit in 78% [95% CI 63–88]) and 12-month overall survival was 44% (95% CI 27–60), whereas in the 63 patients with non-bone metastases (eg, lung, node, liver) the proportion of patients who achieved an objective response was 32% (95% CI 21–45; clinical benefit in 71% [95% CI 59–82]) and 12-month overall survival was 57% (95% CI 41–71).
When stratified by line of systemic therapy, treatment naive versus previously treated, the proportion of patients who achieved an objective response was 23% (95% CI 8–45) versus 28% (19–38), and 12-month overall survival was 60% (95% CI 29–81) versus 49% (36–61), respectively. When analysed according to type of previous therapies, the proportion of patients who achieved an objective response was 28% (95% CI 15–45) in those who had tyrosine kinase inhibitor therapy only, 33% (10–65) in those who had immunotherapy only, and 28% (14–45) in those who had both therapies; and 12-month overall survival was 54% (95% CI 36–70) versus 27% (1–66) versus 48% (28–66), respectively.
Cabozantinib was initiated at the standard dose of 60 mg per day in 93 (83%) patients. In 19 (17%) patients, treatment was started at a reduced dose of 40 mg per day; two patients were increased to 60 mg per day later in their treatment course. The most common treatment-related adverse events of any grade noted in more than 5% of patients were fatigue (58 [52%]), diarrhoea (38 [34%]), skin toxicity (rash and palmar-plantar erythrodysesthesia; 35 [31%]), nausea (33 [29%]), hypertension (31 [28%]), transaminitis (24 [21%]), mucositis (22 [20%]), hypothyroidism (17 [15%]), vomiting (11 [10%]) and thrombocytopenia (seven [6%]; table 3). 19 (17%) patients had grade 3 events, the most frequent were skin toxicity (five [4%]) and hypertension (four [4%]). Only five (7%) patients discontinued because of treatment toxicity; however, nearly half (51 [46%]) required dose reductions because of treatment-related adverse events (appendix p 2), and cabozantinib was interrupted temporarily (then resumed) or permanently at some point in 44 (39%) patients. 69 deaths occurred (63 because of progressive disease and six within 1 month of discontinuing cabozantinib without progressive disease recorded). No deaths related to cabozantinib toxicity were observed.
Table 3:
Overall incidence of adverse events considered related to cabozantinib in the total population
| Grade 1–2 | Grade 3 | Unknown grade |
|
|---|---|---|---|
| Fatigue | 42 (38%) | 2 (2%) | 14 (13%) |
| Diarrhoea | 26 (23%) | 3 (3%) | 9 (8%) |
| Skin toxicity* | 18 (16%) | 5 (4%) | 12 (11%) |
| Nausea | 24 (21%) | 0 | 9 (8%) |
| Hypertension | 22(20%) | 4 (4%) | 5 (4%) |
| Transaminitis | 19 (17%) | 1 (1%) | 4 (4%) |
| Mucositis | 14 (13%) | 1 (1%) | 7 (6%) |
| Hypothyroidism | 12 (11%) | 0 | 5 (4%) |
| Vomiting | 6 (5%) | 0 | 5 (4%) |
| Thrombocytopenia | 6 (5%) | 1 (1%) | 0 |
| Dyspnoea | 3 (3%) | 0 | 2 (2%) |
| Proteinuria | 0 | 1 (1%) | 2 (2%) |
| Neutropenia | 3 (3%) | 0 | 0 |
| Other | 5 (4%) | 3 (3%) | 10 (9%) |
Data are n (%). N=112. Includes adverse events after the date of first dose and including 30 days after the date of last dose of cabozantinib. Patients with multiple events in the same category are counted only once in that category.
Patients with events in more than one category are counted in each of those categories. No grade 4 or 5 adverse events were reported.
Skin toxicity included rash and palmar-plantar erythrodysesthesia.
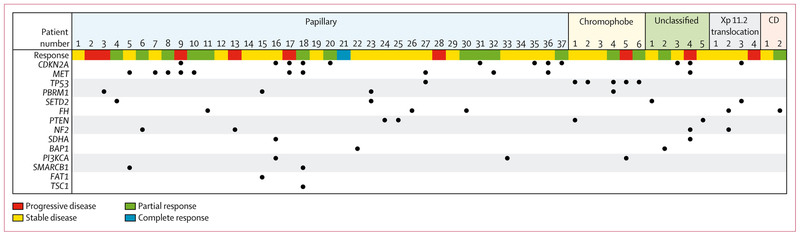
Next-generation sequencing data were available for 54 (48%) of 112 patients: 20 from primary tumour, 31 from metastases, and three from unknown site. It was most frequently available in papillary tumours (n=37) with some information in chromophobe (n=6), unclassified (n=5), Xp11.2 translocation (n=4), and collecting duct (n=2) tumours (figure 3). The most common somatic genetic alterations across all tumours were CDKN2A (12 [22%]) followed by MET (11 [20%]), TP53 (six [11%]), FH (five [9%]), the chromatin-modifying genes PBRM1, SETD2, PTEN, and NF2 (four [7%] each). Of the 37 papillary tumours, ten (27%) had alterations in MET and nine (24%) in CDKN2A. TP53 alterations were present in five (83%) of six chromophobe tumours. Four patients, including the youngest patient (age 14 years), developed a papillary renal cell carcinoma in the context of hereditary leiomyomatosis and renal cell carcinoma syndrome. Three of these patients had next-generation sequencing data showing an FH alteration in their tumour; only two had concurrent germline testing that confirmed FH germline mutations.
Figure 3: Next-generation sequencing results by histology and radiological response.
Response as defined by Response Evaluation Criteria in Solid Tumors, version 1.1. CD=collecting duct.
Of the 12 patients whose tumours had CDKN2A aberrations, four papillary carcinomas achieved a partial response. Among the FH-altered renal cell carcinomas (n=5), one (20%) patient with collecting duct and two (40%) patients with papillary renal cell carcinoma had a partial response. Of the ten MET-altered papillary renal cell carcinomas, four (40%) partial responses were observed.
Discussion
Using data from a multicentre collaboration, we characterised the activity and toxicity of cabozantinib in non-clear-cell renal cell carcinoma in the largest, retrospective cohort study reported, to our knowledge. Cabozantinib showed encouraging antitumour activity, with a proportion of patients who achieved an objective response of 27% (30 of 112 patients) across all subtypes of non-clear-cell renal cell carcinoma, both treatment naive and previously treated.
Cabozantinib is an established efficacious agent in clear-cell renal cell carcinoma based on the results of two large randomised trials. METEOR13 was a phase 3 trial of cabozantinib compared with everolimus in patients who had received previous antiangiogenic therapy. Cabozantinib showed a significant improvement in progression-free survival (7·4 months [95% CI 5·6–9·1] vs 3·9 months [3·7–5·1]), overall survival (21·4 months [18–7-not estimable] vs 16·5 months [14·7–18·8]), and proportion of patients who achieved an objective response (17% [95% CI 13–22] vs 3% [2–3]). In CABOSUN,14 a phase 2 study comparing cabozantinib with sunitinib as first-line therapy in patients with intermediate-risk and poor-risk disease by IMDC, cabozantinib improved median progression-free survival (8·2 months [95% CI 6·2–8·8] vs 5·6 months [3·4–8·1]) and the proportion of patients who achieved an objective response (33% [95% CI 23–44] vs 12% [5·4–21·0]). Neither randomised study permitted non-clear-cell disease. Only two small, retrospective series exploring the efficacy of cabozantinib in non-clear-cell renal cell carcinoma have been reported. One single-institution study15 of 30 patients showed encouraging efficacy with a median progression-free survival of 8·6 months and a median overall survival of 25·4 months across both treatment-naive (n=3) and previously treated disease (n=27). A second multi-institution Italian study16 of 17 patients, who had relapsed after previous systemic treatments, reported a median progression-free survival of 7·8 months with cabozantinib.
Our study cohort consisted of heavily pretreated patients, with 24% having had three or more previous systemic therapies and 89% having poor-risk or intermediate-risk disease. Nevertheless, robust clinical activity, comparable to that seen in the pivotal trials of cabozantinib in clear-cell disease, was observed across all non-clear-cell subtypes and lines of treatment, which might reflect the broad biological activity of cabozantinib in antagonising multiple oncogenic pathways. These results suggest that the antitumour activity of cabozantinib is not restricted to clear-cell histology.
Our data also permitted exploratory evaluation of subsets of disease of clinical importance, such as bone metastases and sarcomatoid histology. Bone metastases are associated with poor prognosis in renal cell carcinoma.17 Recent data18 have revealed that cabozantinib can be especially active in this subgroup, probably because of the role of MET in modulating the activity of osteoblasts and osteoclasts. Although fewer patients with bone metastases achieved objective responses and overall survival in our study compared with those without, 78% achieved clinical benefit in terms of disease stability for a median of 6·9 months, suggesting clinically meaningful disease control in this poor-risk subgroup. As expected, patients with sarcomatoid features and poor-risk and intermediate-risk disease were associated with poorer outcomes than those without sarcomatoid features and favourable risk disease in our study, necessitating continued clinical investigation into more effective drugs and enhanced understanding of biology in these subgroups.
Genomic analyses have revealed the rich and diverse spectrum of genetic and epigenetic changes across the different types of kidney cancers.19-23 In our cohort, CDKN2A was the most frequent genomic alteration detected. Loss of CDKN2A has been associated with a worse prognosis in renal cell carcinoma independent of the histology.19 However, in our study, the subgroup of patients with CDKN2A tumour alterations had similar outcomes compared with the overall population. MET alterations are frequently found in papillary renal cell carcinomas, and MET was the most frequently altered gene in our papillary cohort.20-22 Responses to drugs targeting MET such as foretinib, savolitinib, and crizotinib have been observed in the MET-altered papillary renal cell carcinomas, providing a strong rationale for biomarker-based studies. 24-26 The responses observed in the MET-altered non-clear-cell renal cell carcinomas were similar to previous prospective trial data in clear-cell renal cell carcinoma, provide insight into efficacy in a non-clinical trial population of non-clear-cell renal cell carcinomas, and support MET targeting with cabozantinib in MET-positive papillary carcinomas.
Ultimately, prospective investigation is paramount, and cabozantinib is being studied in trials such as PAPMET () and SAVOIR (). PAPMET is a randomised phase 2 trial comparing the efficacy of tyrosine kinase inhibitors that target VEGFR, MET, or both in an unselected population of papillary tumours. SAVOIR is a phase 3 study of MET-driven papillary cancers and will evaluate a selective MET-inhibitor savolitinib compared with sunitinib.
Further efforts are underway to assess the efficacy of cabozantinib in other rare histologies, such as collecting duct carcinoma. One case report revealed a partial metabolic response by PET after 3 months of first-line therapy with cabozantinib in a patient with multiple bone metastases.27 In our cohort of four patients with collecting duct tumours, we observed encouraging disease control (50% objective response, 100% clinical benefit); however, this finding is caveated by the small sample size. BONSAI (), a single-arm phase 2 trial evaluating cabozantinib in untreated collecting duct renal cell carcinoma, will provide more definitive data.
Enhanced understanding of the drivers of biological pathways of these rare tumours are needed to improve clinical outcomes. Further prospective studies to clarify the role of molecular tumour profiling as a tool to further classify non-clear-cell renal cell carcinomas, guide selection of optimal therapy, and inform the development of targeted therapies is warranted. From our results and across the literature, promising candidates include CDK4/6 inhibitors in tumours with CDKN2A loss28,29 or EZH2 inhibitors in tumours with SWI/SNF chromatin remodelling pathway mutations.30 Defects in DNA-repair pathways have been linked hereditary leiomyomatosis and renal cell carcinoma syndrome, suggesting potential susceptibility to PARP inhibitors.31
Therapies targeting the programmed cell death protein 1 (PD-1) axis are broadly used in renal cell carcinoma. Two retrospective studies32,33 showed antitumour activity in the non-clear-cell renal cell carcinoma subgroup, which generally was excluded from the initial trials. Prospective studies are evaluating the PD-1-targeted therapies in non-clear-cell renal cell carcinoma (, , ), including one study evaluating the combination of nivolumab and ipilimumab plus cabozantinib, where at least one patient with sarcomatoid renal cell carcinoma has achieved a partial response ().
Cabozantinib’s toxicity profile has been well documented from the clear-cell renal cell carcinoma experience. No treatment-related deaths, or new or unexpected toxicities were observed in our cohort of patients with non-clear-cell renal cell carcinoma. In the phase 3 METEOR trial13 evaluating cabozantinib versus everolimus in patients who had progressed after VEGF-targeted therapy, cabozantinib doses were reduced in 60% of the patients (to 40 mg or 20 mg from 60 mg). In the randomised phase 2 trial evaluating cabozantinib versus sunitinib in the first-line setting, dose reductions occurred in 46% of patients treated with cabozantinib.14 In our real-world study that spanned patients who were treatment naive and who had refractory disease, the proportion of dose reductions was 46%, which is in line with these prospective randomised trials in the clear-cell population. In our study, only 7% discontinued treatment because of toxicity suggesting the adverse events were generally manageable, despite the poorer risk clinical features of our cohort. We observed fewer all grade adverse events and grade 3 or 4 adverse events compared with published trials. However, our more favourable toxicity results might be attributed to the retrospective nature of the analyses (eg, missing data) and to greater experience with these agents (after years of use in clear-cell disease) with subsequent improved toxicity management and appropriate dosing modifications by treating physicians, who are specialised in renal cell carcinoma.34
Limitations of our study include the retrospective nature of the analyses with resultant potential selection bias. The heterogeneous population including multiple histological subtypes with varied natural histories, grouping of types 1 and 2 papillary carcinoma as a single entity, and absence of some aggressive subtypes such as medullary carcinoma might have introduced additional bias. However, non-clear-cell renal cell carcinomas are rare tumours and the cohort included in this study is well representative of the population seen in clinical practice. Our study also lacked central pathological and radiographic review, which might have affected eligibility and tumour response assessment. These flaws might have been tempered by inclusion of patients from centres with strong genitourinary oncology expertise. We used the metric time to treatment failure rather than progression-free survival as one of our main endpoints because it reflects real-world clinical practice; often the determination to discontinue treatment is based on more than just tumour progression, including the physician’s judgment of clinical benefit, toxicity, and patient tolerability. Finally, although this is the largest series yet reported, our ability to assess the clinical effect of tumour genomic alterations on the efficacy of cabozantinib was limited by the small numbers and variability in genomic platforms employed.
In our multi-institutional, retrospective experience, we found cabozantinib to be active in the control of metastatic non-clear-cell renal cell carcinoma. In the absence of available prospective data, our study provides additional evidence for the safety and potential activity of cabozantinib in patients across the metastatic non-clear-cell renal cell carcinoma spectrum. Given the varied histological and molecular subtypes, collaboration and participation in ongoing prospective clinical trials assessing the antitumour efficacy of cabozantinib and other novel agents in non-clear-cell renal cell carcinoma are imperative to improve clinical outcomes in these rare diseases.
Supplementary Material
Research in context.
Evidence before the study
We searched PubMed, Google Scholar, and abstracts from the American Society of Clinical Oncology and European Society of Medical Oncology annual meetings with the terms “non-clear cell”, “renal cell carcinoma”, and “cabozantinib” for papers published between Jan 1, 2000, and Nov 1, 2018. Trials of cabozantinib in renal cell carcinoma did not include patients with non-clear-cell histologies. Only two small retrospective studies encompassing fewer than 50 patients with non-clear-cell tumours have been reported that showed encouraging preliminary activity. Prospective clinical trials evaluating the efficacy of cabozantinib in these rare types of renal cell carcinoma are underway.
Added value of this study
Ongoing prospective studies will take years to produce results; in the meantime, comprehensive retrospective series can provide important data to guide clinical management.
This international, multicentre, retrospective study summarises data on the clinical activity and safety of cabozantinib in 112 patients with non-clear-cell renal cell carcinoma, and, to our knowledge, is the largest reported effort to date.
Implications of all the available evidence
Our study suggests that the antitumour activity of cabozantinib is not limited to the clear-cell renal cell carcinoma subgroup and that some patients with non-clear-cell histology can achieve significant clinical benefit. Encouraging antitumoural activity was observed across all non-clear-cell subtypes. In the absence of prospective data, this study provides support for the use of cabozantinib in real-world clinical practice in patients with non-clear-cell renal cell carcinoma, for whom effective treatment options are few and a standard second-line therapy is absent. We highlight the crucial need to support prospective clinical trials and international collaborations to improve outcomes in these rare and heterogeneous group of diseases amassed under the umbrella term non-clear-cell renal cell carcinoma.
Acknowledgments
No specific funding source was used for this study. Discretionary funds of the investigators were used on a site-by-site basis.
Footnotes
Declaration of interests
NMC reports personal fees from Bayer and Pfizer WX reports personal fees from Bayer. MAB reports research founding from BMS, Nektar, Sanofi, Incyte, AstraZeneca, Genentech, Bayer, Tricon, Pfizer, and Seattle Genetics; and personal fees for advisory boards from Exelixis and Nektar. DMG reports personal fees for advisory boards from Exelixis. WS reports grant funding from AstraZeneca, Bayer, Bristol-Myers Squibb, Esai, Genentech, Pfizer, AbbVie, Astellas, Boehringer Ingelheim, Calithera, Clovis, Exilixis, Janssen, Merck, Seattle Genetics, Tesaro, and XP4 Pharmaceuticals; and personal fees from AstraZeneca, Bayer, Bristol-Myers Squibb, Caremark/CVS, Esai, Genentech, Pfizer, and Sotio. YZ reports personal fees for advisory board from Amgen, Roche Diagnostics, Novartis, Johnson & Johnson, Eisai, Exelixis, Pfizer, and Castle Bioscience. VN reports personal fees from Exelixis.BB reports personal fees from Ipsen, Merck, Amgen, Pfizer; and grant funding from BMS. RRM reports grant funding from Bayer and Pfizer; and personal fees for advisory board from Exelixis, BMS, Novartis, Tempus, and Janssen. RP reports grant funding from Ferring; and personal fees from AstraZeneca, BMS, Dendreon, Exelixis, Genentech/Roche, Merck, Sanofi-Genzyme, Jounce, EMD Serono, and Argos Therapeutics; and non-financial support from BMS, Dendreon, and Genentech/Roche. ETL reports grant funding from Exelixis. TLR reports grant funding from National Institute of Health. NV reports personal fees from Boehringer lngelheim. MRH reports personal fees from Argos, AstraZeneca, Exelixis, Genentech, and Pfizer; and grant funding from Bristol-Myers Squibb, Exelixis, Genentech, Merck, and Pfizer. AM reports personal fees for advisory board from Genetech/Roche; and institutional support for clinical trials from Acerta Pharma, Genentech/Roche, Seattle Genetics, Mirati Therapeutics, Bristol-Myers Squibb, and Roche. ERP reports grant funding from AstraZeneca, Bristol-Myers Squibb, Merck, Peloton, and Pfizer; and consulting funding from BMS, Exelexis, Genentech, Merck, Clovis, and Pfizer. UV reports grant funding and personal fees from Exelixis. SG reports grant funding from Pfizer, Merck, Agensys, BMS, Novartis, Bayer, and Eisai; and personal fees from AstraZeneca, Bayer, BMS, Novartis, Pfizer, Genentech, Exelixis, Janssen, Corvus, and Sanofi/Genzyme. NH reports personal fees from Merck, Novartis, Armo Biosciences, Pfizer, and BMS; and clinical research funding from Merck, SFJ Pharmaceuticals, and BMS. NA reports personal fees from consultancy from Astellas, AstraZeneca, Argos, BMS, Bayer, Clovis, Eisai, Exelixis, EMD Serono, Ely Lilly, Genentech, Merck, Medivation, Novartis, Nektar, and Pfizer; and grant research funding from Active Biotech, AstraZeneca, Bavarian Nordic, BMS, Calithera, Celldex, Eisai, Exelixis, Genetech, GlaxoSmithKline, Immunomedics, Janssen, Medivation, Merck, NewLink Genetics, Novartis, Pfizer, Prometheus, Rexahn, Sanofi, Takeda, and Tracon. SKP reports personal fees for consultancy and research from Pfizer, Novartis, AVEO, Myriad Genetics, Genentech, Exelixis, BMS, Astellas, and Medivation. DYCH reports grant funding and personal fees from BMS, Pfizer, Novartis, Ipsen, Exilexis, and Eisai. DB reports personal fees from BMS, Pfizer, and AstraZeneca. TKC reports grant funding, personal fees, and non-financial support from Pfizer and Exelixis; grant funding and personal fees from AstraZeneca, Bayer, BMS, Cerulean, Esai, Foundation Medicine, Exelixis, Genentech, Roche, GlaxoSmithKline, Merck, Novartis, Peloton, Pfizer, Prometheus Labs, Corvus, and Ipsen; grant funding from Tracon, Calithera, and Takeda outside the submitted work; and personal fees from Alligent, Up-to-Date, National Comprehensive Cancer Network, Analysis Group, Michael J Hennessy Associates (Healthcare Communications Company and several brands such as OnClive and PER), L-path, Kidney Cancer Journal, Clinical Care Options, Platform Q, Navinata Healthcare, Harborside Press, American Society of Medical Oncology, New England Journal of Medicine, and Lancet Oncology. LCH reports personal fees from Exelixis Bayer, Genentech, Dendreon, Pfizer, Medivation/Astellas, Kew Group, Corvus, Merck, Novartis, Michael J Hennessy Associates (Healthcare Communications Company and several brands such as OncLive and PER), and Jounce; grants from Bayer, Sotio, Bristol-Myers Squib, Merck, Takeda, Dendreon/Valient, Janssen, Medivation/Astellas, Genentech, Pfizer, and non-financial support from Bayer and Sanofi. All other authors declare no competing interests.
Contributor Information
Nieves Martínez Chanzá, Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA, USA.
Wanling Xie, Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA, USA.
Mehmet Asim Bilen, Winship Cancer Institute, Emory University, Atlanta, GA, USA.
Hannah Dzimitrowicz, Duke Cancer Center, Durham, NC, USA.
Jarred Burkart, Department of Medicine, Division of Medical Oncology, The Ohio State University Comprehensive Cancer Center, Columbus, OH, USA.
Daniel M Geynisman, Fox Chase Cancer Center, Philadelphia, PA, USA.
Archana Balakrishnan, Barbara Ann Karmanos Cancer Institute, Detroit, MI, USA.
I Alex Bowman, University of Texas Southwestern Medical Center, Dallas, TX, USA.
Rohit Jain, Roswell Park Cancer Institute, Buffalo, NY, USA.
Walter Stadler, University of Chicago Comprehensive Cancer Center, Chicago, IL, USA.
Yousef Zakharia, Holden Comprehensive Cancer Center, University of Iowa, Iowa City, IA, USA.
Vivek Narayan, Abramson Cancer Center, Philadelphia, PA, USA.
Benoit Beuselinck, Leuven Cancer Institute, Universitair Ziekenhuis, Leuven, Leuven, Belgium.
Rana R McKay, Moores Cancer Center, University of California San Diego, La Jolla, CA, USA.
Abhishek Tripathi, Stephenson Cancer Center, Oklahoma City, OK, USA.
Russell Pachynski, Washington University School of Medicine, St Louis, MO, USA.
Andrew W Hahn, Huntsman Cancer Institute, Salt Lake City, UT, USA.
JoAnn Hsu, City of Hope Comprehensive Cancer Center, Duarte, CA, USA.
Sumit A Shah, Stanford Cancer Institute, Palo Alto, CA, USA.
Elaine T Lam, University of Colorado Cancer Center, Aurora, CO, USA.
Tracy L Rose, University of North Carolina, Lineberger Cancer Comprehensive Center, Chapel Hill, NC, USA.
Anthony E Mega, Lifespan Cancer Institute, Alpert Medical School, Brown University, Providence, RI, USA.
Nicholas Vogelzang, Comprehensive Cancer Centers of Nevada, Las Vegas, NV, USA.
Michael R Harrison, Duke Cancer Center, Durham, NC, USA.
Amir Mortazavi, Department of Medicine, Division of Medical Oncology, The Ohio State University Comprehensive Cancer Center, Columbus, OH, USA.
Elizabeth R Plimack, Fox Chase Cancer Center, Philadelphia, PA, USA.
Ulka Vaishampayan, Barbara Ann Karmanos Cancer Institute, Detroit, MI, USA.
Hans Hammers, University of Texas Southwestern Medical Center, Dallas, TX, USA.
Saby George, Roswell Park Cancer Institute, Buffalo, NY, USA.
Naomi Haas, Abramson Cancer Center, Philadelphia, PA, USA.
Neeraj Agarwal, Huntsman Cancer Institute, Salt Lake City, UT, USA.
Sumanta K Pal, City of Hope Comprehensive Cancer Center, Duarte, CA, USA.
Sandy Srinivas, Stanford Cancer Institute, Palo Alto, CA, USA.
Benedito A Carneiro, Lifespan Cancer Institute, Alpert Medical School, Brown University, Providence, RI, USA.
Daniel Y C Heng, Tom Baker Cancer Centre, University of Calgary, Calgary, AB, Canada.
Dominick Bosse, The Ottawa Hospital Cancer Center, University of Ottawa, Ottawa, ON, Canada.
Toni K Choueiri, Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA, USA.
Lauren C Harshman, Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA, USA.
References
- 1.Giles RH, Choueiri TK, Heng DY, et al. Recommendations for the management of rare kidney cancers. Eur Urol 2017; 72: 974–83. [DOI] [PubMed] [Google Scholar]
- 2.Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO classification of tumours of the urinary system and male genital organs—part A: renal, penile, and testicular tumours. Eur Urol 2016; 70: 93–105. [DOI] [PubMed] [Google Scholar]
- 3.Fernández-Pello S, Hofmann F, Tahbaz R, et al. A systematic review and meta-analysis comparing the effectiveness and adverse effects of different systemic treatments for non-clear cell renal cell carcinoma. Eur Urol 2017; 71: 426–36. [DOI] [PubMed] [Google Scholar]
- 4.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 2007; 356: 2271–81. [DOI] [PubMed] [Google Scholar]
- 5.Dutcher JP, de Souza P, McDermott C, et al. Effect of temsirolimus versus interferon-alpha on outcome of patients with advanced renal cell carcinoma of different tumor histologies. Med Oncol 2009; 26: 202–09. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong AJ, Halabi S, Eisen T, et al. Everolimus versus sunitinib for patients with metastatic non-clear cell renal cell carcinoma (ASPEN): a multicentre, open-label, randomised phase 2 trial. Lancet Oncol 2016; 17: 378–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tannir NM, Jonasch E, Albiges L, et al. Everolimus versus sunitinib prospective evaluation in metastatic non-clear cell renal cell carcinoma (ESPN): a randomized multicenter phase 2 trial. Eur Urol 2016; 69: 866–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Motzer RJ, Barrios CH, Kim TM, et al. Phase II randomized trial comparing sequential first-line everolimus and second-line sunitinib versus first-line sunitinib and second-line everolimus in patients with metastatic renal cell carcinoma. J Clin Oncol 2014; 32: 2765–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park I, Lee SH, Lee JL. A multicenter phase II trial of axitinib in patients with recurrent or metastatic non-clear-cell renal cell carcinoma who had failed prior treatment with temsirolimus. Clin Genitourin Cancer 2018; 16: e997–1002. [DOI] [PubMed] [Google Scholar]
- 10.Jung KS, Lee SJ, Park SH, et al. Pazopanib for the treatment of non-clear cell renal cell carcinoma: a single-arm, open-label, multicenter, phase II study. Cancer Res Treat 2018; 50: 488–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buti S, Bersanelli M, Maines F, et al. First-line pazopanib in non-clear-cell renal carcinoma: the Italian retrospective multicenter PANORAMA study. Clin Genitourin Cancer 2017; 15: e609–14. [DOI] [PubMed] [Google Scholar]
- 12.Yakes FM, Chen J, Tan J, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther 2011; 10: 2298–308. [DOI] [PubMed] [Google Scholar]
- 13.Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol 2016; 17: 917–27 [DOI] [PubMed] [Google Scholar]
- 14.Choueiri TK, Halabi S, Sanford BL, et al. Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: The Alliance A031203 CABOSUN Trial. J Clin Oncol 2017; 35: 591–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campbell MT, Bilen MA, Shah AY, et al. Cabozantinib for the treatment of patients with metastatic non-clear cell renal cell carcinoma: a retrospective analysis. Eur J Cancer 2018; 104: 188–94. [DOI] [PubMed] [Google Scholar]
- 16.Prisciandaro M, Ratta R, Massari F, et al. Safety and efficacy of cabozantinib for metastatic nonclear renal cell carcinoma: real-world data from an Italian managed access program. Am J Clin Oncol 2019; 42: 42–5. [DOI] [PubMed] [Google Scholar]
- 17.McKay RR, Lin X, Perkins JJ, Heng DY, Simantov R, Choueiri TK. Prognostic significance of bone metastases and bisphosphonate therapy in patients with renal cell carcinoma. Eur Urol 2014; 66: 502–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Escudier B, Powles T, Motzer RJ, et al. Cabozantinib, a new standard of care for patients with advanced renal cell carcinoma and bone metastases? Subgroup analysis of the METEOR trial. J Clin Oncol 2018; 36: 765–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ricketts CJ, De Cubas AA, Fan H, et al. The cancer genome atlas comprehensive molecular characterization of renal cell carcinoma. Cell Rep 2018; 23: 3698. [DOI] [PubMed] [Google Scholar]
- 20.The Cancer Genome Atlas Research Network, Linehan WM, Spellman PT, et al. Comprehensive molecular characterization of papillary renal-cell carcinoma. N Engl J Med 2016; 374: 135–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Durinck S, Stawiski EW, Pavia-Jimenez A, et al. Spectrum of diverse genomic alterations define non-clear cell renal carcinoma subtypes. Nat Genet 2015; 47: 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis CF, Ricketts CJ, Wang M, et al. The somatic genomic landscape of chromophobe renal cell carcinoma. Cancer Cell 2014; 26: 319–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malouf GG, Monzon FA, Couturier J, et al. Genomic heterogeneity of translocation renal cell carcinoma. Clin Cancer Res 2013; 19: 4673–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choueiri TK, Vaishampayan U, Rosenberg JE, et al. Phase II and biomarker study of the dual MET/VEGFR2 inhibitor foretinib in patients with papillary renal cell carcinoma. J Clin Oncol 2013; 31: 181–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schoffski P, Wozniak A, Escudier B, et al. Crizotinib achieves long-lasting disease control in advanced papillary renal-cell carcinoma type 1 patients with MET mutations or amplification. EORTC 90101 CREATE trial. Eur J Cancer 2017; 87: 147–63. [DOI] [PubMed] [Google Scholar]
- 26.Choueiri TK, Plimack E, Arkenau HT, et al. Biomarker-based phase II trial of savolitinib in patients with advanced papillary renal cell cancer. J Clin Oncol 2017; 35: 2993–3001. [DOI] [PubMed] [Google Scholar]
- 27.Mennitto A, Verzoni E, Peverelli G, Alessi A, Procopio G. Management of metastatic collecting duct carcinoma: an encouraging result in a patient treated with cabozantinib. Clin Genitourin Cancer 2018; 16: e521–23. [DOI] [PubMed] [Google Scholar]
- 28.Hamilton E and Infante JR. Targeting CDK4/6 in patients with cancer. Cancer Treat Rev 2016; 45: 129–38. [DOI] [PubMed] [Google Scholar]
- 29.Pal SK, Ali SM, Ross J, Choueiri TK, Chung JH. Exceptional response to palbociclib in metastatic collecting duct carcinoma bearing a cDkN2A homozygous deletion. JCO Precis Oncol 2017; 1: 1–5. [DOI] [PubMed] [Google Scholar]
- 30.Kim KH, Roberts CW Targeting EZH2 in cancer. Nat Med 2016; 22: 128–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sulkowski PL, Sundaram RK, Oeck S, et al. Krebs-cycle-deficient hereditary cancer syndromes are defined by defects in homologous-recombination DNA repair. Nat Genet 2018; 50: 1086–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKay R, Bossé D, Xie W, et al. The clinical activity of PD-1/PD-l1 inhibitors in metastatic non-clear cell renal cell carcinoma. Cancer Immunol Res 2018; 6: 758–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koshkin VS, Barata PC, Zhang T, et al. Clinical activity of nivolumab in patients with non-clear cell renal cell carcinoma. J Immunother Cancer 2018; 6: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gerendash BS, Creel PA. Practical management of adverse events associated with cabozantinib treatment in patients with renal-cell carcinoma. OncoTargets Ther 2017; 10: 5053–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.