Abstract
Immunosenescence contributes to a decreased capacity of the immune system to respond effectively to infections or vaccines in the elderly. The full extent of the biological changes that lead to immunosenescence are unknown, but numerous cell types involved in innate and adaptive immunity exhibit altered phenotypes and function as a result of aging. These manifestations of immunosenescence at the cellular level are mediated by dysregulation at the genetic level, and changes throughout the immune system are, in turn, propagated by numerous cellular interactions. Environmental factors, such as nutrition, also exert significant influence on the immune system with aging. While the mechanisms that govern the onset of immunosenescence are complex, systems biology approaches allow for the identification of individual contributions from each component within the system as a whole. Although there is still much to learn regarding immunosenescence, systems-level studies of vaccine responses have been highly informative and will guide the development of new vaccine candidates, novel adjuvant formulations, and immunotherapeutic drugs to improve vaccine responses among the aging population.
Keywords: immunosenescence, vaccinology, systems biology, aging, adjuvants, senolytics, immunity
Introduction
Advancements in living conditions and preventive healthcare have led to a concomitant increase in human life expectancy, with the global number of individuals aged 60 years or older expected to exceed 2 billion by 2050 [1]. This burgeoning population of elderly adults is predicted to place a massive burden on global healthcare resources, as aging is associated with a number of disease conditions (i.e., cardiovascular disease, hypertension, Alzheimer’s disease) that can result in long-term complications and significant costs [2–4]. Among these is an increased susceptibility to infectious disease, which is caused primarily by a decreased ability of the immune system to respond effectively to new pathogens or vaccines [5]. This age-associated, intrinsic failure of the immune system, termed “immunosenescence,” is an important factor in the increased morbidity and mortality associated with infectious disease in aging populations [6–8] and in the poor immune response to vaccines designed to protect against these diseases.
Immunosenescence is characterized by the inability to mount effective (protective) humoral and cellular immune responses against a pathogen or vaccine, as well as a systemic low-grade inflammatory state (termed “inflammaging”), which contributes to the dysregulation of several components of the innate and adaptive immune systems [9, 10]. Alterations in cell population numbers, as well as defects in cellular function, have all been identified as factors contributing to immunosenescence; yet, numerous studies (from both a reductionist and systems-level approach) have failed to comprehensively define the factors that lead to and govern these phenomena. The different arms of the immune system are highly interregulated, and recent advancements in immunological research have led to an appreciation for the role that the innate immune response plays in governing the development of adaptive responses [11, 12]. Furthermore, evidence has also emerged that the innate immune system has some capacity for memory-like responses that train recognition of certain pathogenic features [13]. When considering the crosstalk that occurs among these multiple components of a highly dynamic and complex biological system, it is imperative that a holistic approach be applied to fully comprehend the intricate processes that lead to immunosenescence.
Systems biology has emerged as a means to comprehensively study complex biological processes and evaluate the behavior of each system component as it relates to others [14]. With respect to the study of immune responses, we and others have applied systems-level approaches to understand how the immune system responds to vaccination and whether these data could be used to develop predictive models of vaccine efficacy [15–17]. Querec et al. pioneered the use of systems biology in a seminal study that identified molecular signatures of immunological response in individuals vaccinated against yellow fever virus [18]. Many studies since, including our own, have identified predictive associations between gene polymorphisms, gene expression, and correlates of protective immunity after vaccination [19–27].
In this review, we seek to provide a comprehensive discussion on the failure of the aging immune system, with an emphasis on the fact that perturbations at the genetic level mediate changes at the molecular and/or cellular level. Due to the interconnectivity of the immune system, these alterations in cellular activity can then influence numerous additional cell types, propagating global changes in response outcomes to infection or vaccination. By examining these relationships, the intricacies of the entire system become apparent, and models can be developed to predict outcomes based on changes within each component. We provide an overview on the applications of systems biology to the study of immunosenescence, highlighting recent advancements with respect to vaccinology as well as areas that require further study. In the final segment of this review, we discuss how the knowledge gained from studies on systems biology can be used to inform the design of new vaccine candidates that are directly engineered to stimulate robust responses in the face of immunological aging.
Immunosenescence at the cellular and molecular level
One of the primary hallmarks of immunosenescence is an age-associated shift in both the composition and function of immune cells circulating in the periphery, which is exemplified by alterations in surface marker expression, shifts in cell subtype populations, and defects in cell signaling [6, 28, 29]. In addition to dysregulation among immune cells, other cell types begin to transition toward an apoptosis-resistant, inflammatory phenotype that is incapable of replication [30, 31]. This senescence-associated secretory phenotype (SASP) is responsible for an increased production of specific cytokines, chemokines, miRNAs, and other pro-inflammatory signals that mediate immune cell migration and activation [32, 33]. The SASP feeds into the chronic, low-grade inflammatory state commonly observed among older adults, which further contributes to immune cell dysregulation. More recently, studies have found that “inflammaging” can also be governed by a number of different environmental and metabolic factors, suggesting that an individual’s diet, nutrition, and gut microbiota can all influence the magnitude and persistence of chronic inflammation. [34–36] The environmental and metabolic factors influencing “inflammaging” have been excellently reviewed elsewhere. [34–36]
Although immunosenescence is most commonly related to failures in adaptive immunity (B- and T-cells) [37–39], significant evidence has accrued that indicates aging also impacts various functions of the innate immune system [40]. Furthermore, several components of the innate immune system perform pivotal roles in priming robust adaptive responses, and defects in innate immunity have been associated with failures in long-term humoral and cellular immunity [41, 42]. In this section, we summarize age-associated alterations in function that have been observed in cells of both the innate and adaptive immune system, and we relate how these changes in cellular phenotype and the production of signaling molecules impact overall responses to infection and vaccination.
Neutrophils
Neutrophils are one of the predominant classes of phagocytes found in circulation, and they are rapidly recruited to sites of infection as a result of proinflammatory cytokine and chemokine production (i.e., IL-1, IL-8, TNF-α) by tissue-resident macrophages and endothelial cells [43]. Neutrophils migrate along these chemotactic gradients toward the site of inflammation, sampling low affinity interactions with P/E-selectins on endothelial cell surfaces [44]. Endothelial cells are also stimulated by these inflammatory cytokines to upregulate the expression of intercellular adhesion molecule (ICAM)-1 and −2 on their surface, which arrest migration near the site of infection through high affinity interactions with LFA-1 on the neutrophil surface [45]. Once neutrophils have infiltrated inflamed tissues, they mediate the clearance of pathogens through phagocytosis and the release of antimicrobial proteins from their intracellular granules; upon resolution of the infection, neutrophils undergo apoptosis.
The classical functions of neutrophils described above can be dramatically altered during the course of aging, and several studies have implicated external factors (such as nutrition and lifestyle) as additional mediators of neutrophil function. These studies have been reviewed elsewhere [46, 47], so the main focus of this discussion will relate to changes in neutrophil function directly associated with aging. Defects in aged neutrophils can be primarily related to perturbations in signal transduction, albeit in multiple pathways [48, 49]. The dysregulation of two of these signaling pathways (JAK-STAT and PI3K) and their putative effects on neutrophil function will be discussed herein.
The ability of neutrophils to migrate to and subsequently clear infections has been shown to significantly decline with age [48]. Constitutive phosphatidylinositol-3 kinase (PI3K) activity has been implicated in the dysregulation of both migration and phagocytosis, among other cellular functions [50]. PI3K signals through the phosphorylation of phosphatidylinositol 4,5-bisphosphate in response to the binding of chemokines to their cognate G-protein coupled receptors on the neutrophil surface, [51, 52] and aberrant activation of this signaling cascade has been shown to disrupt neutrophil migration along chemotactic gradients [50]. The phagocytic capacity of aged neutrophils has also been shown to be reduced due to decreased surface expression of the Fcγ receptor CD16,[53] although this is not the sole factor governing phagocytic activity, as neutrophils expressing normal levels of CD16 have also been shown to display a lower phagocytic index [54]. The ability of neutrophils to serve as primary responders to infection is further compromised by a decreased capacity to produce reactive oxygen species in response to antigenic stimuli [54]. The majority of these deficiencies in responses are attributed to alterations in the lipid membrane composition rather than changes in the levels of protein expression [49]. The concentration of phospholipid in neutrophil membranes increases with age relative to that of cholesterol and leads to an increase in membrane fluidity, which compromises the integrity of lipid rafts that are essential for the recruitment and assembly of signaling molecules such as NADPH oxidase [55, 56].
Alterations in cell signaling also play a central role in maintaining homeostasis for neutrophils. Neutrophils are naturally short-lived cells; inflammatory signals can prolong their lifespan, but after an infection has been cleared, they undergo apoptosis. Studies in vitro have shown that granulocyte-macrophage colony stimulating factor (GM-CSF) has the ability to rescue neutrophils from apoptosis in younger individuals through JAK-STAT signaling; this protective signaling capacity is abolished in neutrophils from the elderly [57]. This phenomenon has also been linked to lipid raft function, as the negative regulator SHP-1 remains associated with the GM-CSF receptor in aged neutrophils but is excluded from these domains in younger cells [58]. An increased susceptibility to apoptotic signaling with aging could lead to premature clearance of neutrophils, thereby compromising the initial responses against infections or vaccination.
While it is generally accepted that functional changes occur in neutrophils with aging, it is much less certain what happens with regard to their overall number or if shifts in subpopulations occur. Most studies have reported that for elderly individuals in good health, the number of neutrophils present in circulation does not change [59]; however, other studies have reported decreased neutrophil counts associated with aging [60]. Increased neutrophilia has been associated with a greater risk of overall morbidity and mortality, presumably due to concomitant dysregulation of neutrophil function in the chronic inflammatory environment that develops with aging [61, 62]. There is even less consensus with regard to shifts in neutrophil subpopulations and how variations among these might mediate observed alterations in neutrophil responses. Clearly, there is much to learn with regard to neutrophil biology and its associated changes with aging.
Monocytes and Macrophages
Monocytes and macrophages are also phagocytic cells that are important to the innate response against infection. Circulating monocytes are precursors to tissue-resident macrophages; upon differentiation, these macrophages serve as one of the leading lines of defense in innate immunity [63]. Macrophages are defined by their plasticity and heterogeneous functionality and, depending upon the stimulus and cytokine milieu, they can be polarized towards pro- or anti-inflammatory phenotypes. These various aspects of macrophage polarization have been reviewed in detail elsewhere [64–66]. For this discussion, we will focus only on age-related changes in monocyte/macrophage number and function that relates to the immune response against infection or vaccination.
While the majority of studies identifying age-related changes in macrophage and monocyte function have focused on rodents, there is considerable evidence suggesting that macrophage function is altered in elderly humans as well [40, 67–69]. As with neutrophils, defects in monocyte and macrophage function are predominantly mediated by the dysregulation of cellular signaling with age. Decreased cytokine production (particularly IL-6 and TNF-α) has been observed in response to various Toll-like receptor 4 (TLR4) stimuli in murine macrophages from aged mice, [41, 70] and similar results have been reported for human monocytes in response to TLR1/TLR2 stimulation [71, 72]. While changes in the level of TLR expression have been proposed as a potential mechanism for these observations, there are conflicting reports as to whether these levels increase or decrease with age [70, 73]. The mechanisms underlying defective cytokine production in monocytes and macrophages are likely far more complex and may be at least partially influenced by impaired intracellular signaling, as evidenced by the reduced activation of mitogen-activated protein kinases (MAPK) following TLR4 stimulation in macrophages from aged mice [70].
Macrophages also exhibit declines in phagocytic function with age, which has been associated with a global decline of autophagic functions (e.g., macroautophagy, microautophagy, chaperone-mediated autophagy) in numerous immune cell types with age. [74] Studies in mice have found that macrophages deficient in macroautophagy are more susceptible to bacterial infection and mount defective inflammatory responses. [75] Similarly, defective mitophagy (recycling of mitochondria) in phagocytes has been linked to dysregulation of the inflammasome, resulting in increased cytokine production that likely contributes to “inflammaging.” [76] The effects of defective autophagy in other classes of immune cells have been excellently reviewed elsewhere. [74]
In addition to dysregulation in TLR signaling and cytokine production, aged macrophages and monocytes exhibit a decreased capacity to respond to other inflammatory stimuli as well. Interferon-γ (IFN-γ) is a necessary component for macrophage activation, yet studies have shown that aged murine macrophages demonstrate decreased phosphorylation of STAT-1α in response to IFN-γ stimulation [77]. Furthermore, studies in rats have shown that the IFN-dependent production of superoxide anion is significantly inhibited in aged macrophages [78]. Monocytes and macrophages typically upregulate major histocompatibility complex (MHC) class II molecules on their surface in response to inflammatory stimuli; however, studies in human monocytes and murine macrophages have found levels of HLA and MHC class II molecules to be significantly reduced with age [79–81].
As with neutrophils, age-associated shifts in macrophage and monocyte populations are less well-defined, with several reports detailing conflicting observations. One such study has shown that “non-classical” monocytes (CD14+CD16+) are significantly increased with age but display lower expression of HLA class II molecules and chemokine receptors [82]. Interestingly, macrophages derived from these monocytes do not display any functional defects in activation or cytokine production. In contrast, a separate study identified significant expansion of CD14dimCD16bright monocytes in elderly subjects and found that these cells had an imbalanced production of cytokines in response to inflammatory stimuli [83]. The aforementioned defects in cellular signaling would be suspected to impact macrophage polarization with aging, but studies focused on this phenomenon are lacking.
NK Cells
Natural killer (NK) cells are a class of cytotoxic cells that comprise ~15% of the circulating lymphocyte population and play an important role in innate cellular immune responses [40]. NK cells are typically classified into different subsets based on surface expression of CD56 and their associated capacity for cytotoxic effector activity (CD56dim) or immunoregulatory functions (CD56bright) [84]. While traditionally recognized for their capacity to respond against intracellular pathogens during the initial hours of an immune response, there is a growing appreciation for the role that NK cells play in orchestrating adaptive responses [12, 85].
Contrary to neutrophils and monocytes, the dysregulation of NK cell function has been primarily attributed to phenotypic shifts in cellular subpopulations rather than inherent defects in receptor expression or signaling. During the course of aging, there is a progressive differentiation wherein the CD56bright population diminishes while the CD56dim population continues to expand and begins to express CD57 [86–88]. This population of CD56dimCD57+ cells exhibits high cytolytic capacity but a decreased responsiveness to cytokine signaling, which explains many of the functional differences observed with elderly NK cells [89]. Loss of the CD56bright subset leads to a decrease in regulatory cytokine and chemokine production, which compromises signaling and recruitment of other immune cells [86]. Interestingly, residual CD56bright cells compensate for the diminishing population by increasing their output of IFN-γ, but this has not been observed for other cytokines [90].
While the majority of functional changes in aged NK cells can be associated with phenotypic shifts in subpopulations, there is some evidence for dysregulated expression of protein receptors. The effects of aging on surface expression of killer immunoglobulin-like receptor (KIR) expression on NK cells have been disputed, with conflicting reports detailing age-related increases while others demonstrate no significant changes [87, 91, 92]. The expression level of CD16, an important receptor for initiating the release of cytotoxic granules, does not change with age [87, 92]; however, decreased expression of the natural cytotoxicity receptor NKp30 has been reported and has been linked to an observed decrease in cytotoxic effector function (on a per cell basis) of NK cells in elderly individuals [87, 91]. NKp30 also plays a crucial role in regulatory signaling with dendritic cells, which could further impact the development of effective adaptive immune responses to infection or vaccination.
Dendritic Cells
Dendritic cells are classical antigen presenting cells (APCs) and serve as the main bridge between innate and adaptive immune responses. Their primary function is to present antigens and provide activation signals to T-cells, which are essential in generating robust humoral responses as well as directly mediating cellular immunity [93]. As with other classes of immune cells, there are distinct subsets of dendritic cells specialized for various functions—for this discussion, we will briefly comment on age-related changes in the function of myeloid (mDC), plasmacytoid (pDC), and follicular (fDC) dendritic cell subsets.
The pDC subset plays an important role in the early response against viral antigens, [94] and several studies have identified age-related defects in pDC signaling and function [95–97]. Aged pDCs have a decreased capacity for the production of type I and type III IFNs, which has been linked to impaired phosphorylation of the transcription factor IRF-7 following stimulation of TLR7/TLR9 [96]. Furthermore, pDCs from aged individuals display a reduced capacity for antigen presentation, which limits the activation of CD4+ and CD8+ T-cells [96], although they retain the ability to produce some inflammatory cytokines and activation signals [97]. These age-related defects in cell signaling mirror those observed in other classes of immune cells.
Functional dysregulation in mDCs has been more widely studied, as alterations in both basic cellular functions as well as cell signaling events have been observed in elderly individuals [95, 98]. mDCs from elderly donors exhibit reduced phagocytic activity and chemotaxis in response to inflammatory stimuli, and their ability to stimulate CD4+ T-cell activation is also impaired [95]. These global defects in mDC function have been primarily attributed to a decrease in PI3K activity and have been implicated in the regulation of both DC migration and TLR signaling [99]. The overall result of decreased PI3K activity results in alternative signaling events that lead to upregulation of NF-κB, which contributes to aberrant production of pro-inflammatory cytokines (i.e., IL-6, TNF-α) even in the absence of stimulation [95]. This decline of normal cellular functions, combined with inflammatory cytokine production, severely compromises the ability of mDCs to coordinate an adaptive response in elderly individuals.
The formation of germinal centers and the proper presentation of antigens by fDCs to B-cells are critical for the development of robust, high-affinity antibodies in response to infection or vaccination [93]. Defects in germinal center reactions have been documented in elderly mice, with studies showing that the retention of antigen complexes on fDCs is lowered and the number of memory B-cells generated is subsequently reduced [100–102]. An age-related decline in the expression of the FcγRII receptor on fDCs has been implicated in the development of a defective network of DCs in germinal centers that retain fewer antigens, leading to decreases in B-cell proliferation and antibody production [101]. Collectively, these defects in dendritic cell subpopulations can have dramatic influences on the development of productive adaptive immune responses.
B- and T-Cells
While these examples have concerned defects in innate immunity, immunosenescence is largely manifested by the effects of these deficiencies on adaptive immune responses; furthermore, inherent defects in B- and T-cells can also develop with aging [39, 103, 104]. B-cells are responsible for the generation of long-lasting protective antibody responses and the development of immunological memory following infection or vaccination; consequently, dysregulation of function at numerous points throughout the B-cell lifecycle can impact their capacity to initiate a primary or secondary response [39]. The production of B-cells has been shown to wane with age, and this has been linked to a decreased frequency of progenitor cell populations [105, 106]. These progenitor cells also exhibit increased MAPK activity, which leads to turnover dysregulation of the transcription factor E47 that affects subsequent lineage progression and repertoire establishment [107, 108]. In addition to intrinsic changes in cell populations, stunted B-cell production has also been attributed to changes in the cellular microenvironment [109]. Stromal cells in the bone marrow of aged mice have been shown to produce less IL-7, which is a critical growth factor for B-cell development, and B-cell progenitors in the same mice are concurrently less responsive to IL-7 signaling [106, 110].
The reduced output of B-cells has a definitive impact on the function and homeostasis of the mature circulating B-cell population. Studies in mice have found that the total number of B-cells remains constant with age, indicating that cell turnover is slowed to account for decreased cellular output [109, 111]. This compensatory extension of the primary B-cell lifespan may contribute to some of the age-related defects in function observed among the circulating population, as cellular function wanes with age and the antigen repertoire will contract without replenishment. Spectratype analysis of CDR3 has shown that there is a significant loss of B-cell receptor (BCR) diversity among aging subjects [112], but it is not clear when the contraction of the repertoire occurs. Intrinsic differences in the progenitor repertoire have been proposed as one cause, but it is likely that changes in the repertoire are influenced by homeostatic alterations as well [113].
While the contraction of the repertoire directly affects the ability to respond to a novel immunogen, the reduced efficacy of primary responses has also been linked to intrinsic defects in class-switch recombination [114]. This is primarily due to the dysregulation of E47 and subsequent expression of activation-induced cytidine deaminase (AID), which is critical for the recombination process [115]. Studies have shown that the rate of E47 mRNA degradation is significantly increased in B-cells from elderly mice as a result of increased tristetraprolin production [116]. The decrease in BCR diversity, combined with these inherent defects in B-cell function, drastically hinders the development of protective humoral immunity in response to infectious pathogens in the elderly.
The roles of T-cell responses in adaptive immunity are also critical for the generation and maintenance of long-term immunological memory and protection. T-cells are responsible for orchestrating robust humoral immunity (CD4+) as well as directly mediating cytotoxic responses (CD8+), and defects in both arms of T-cell-mediated immunity occur with aging [117]. The overall number of circulating T-cells remains relatively constant with age, but there are dramatic age-related shifts in the composition of T-cell subpopulations that impact immune response outcomes [118–120]. Thymic involution occurs with age, directly impacting the output of naïve T-cells [119, 120]; as a result, circulating naïve T-cells undergo homeostatic proliferation into virtual memory cells, and the naïve compartment contracts [120, 121]. Memory T-cells in the periphery can be classified as two phenotypically and functionally distinct populations: central memory T-cells (TCM), which are enriched in CD4+ T-cells and primarily reside in secondary lymphoid organs; and effector memory T-cells (TEM), which are enriched in CD8+ T-cells that traffick among numerous compartments in the periphery [122].
Numerous studies implicate thymic involution as the key mediating force behind contraction of the naïve T-cell repertoire, but there is also significant evidence to suggest that persistent latent viral infections, such as cytomegalovirus (CMV), are associated with deleterious changes in the T-cell compartment with age [123, 124]. Studies have identified differences in the shrinkage of the naïve CD4+ and CD8+ T-cell pools with age that are associated with the presence or absence of CMV infection, respectively, although the mechanisms underlying this disproportionate effect of CMV infection on naïve CD4+ T-cells is unknown [123]. Chronic CMV infection has also been shown to impact the memory compartment, stimulating the oligoclonal expansion of CMV-specific memory CD8+ T-cells. [125] This inflation of CMV-specific T-cells in the periphery disproportionately limits the ability of the circulating T-cell repertoire to respond to other antigens. These associations clearly imply that other mechanisms related to CMV infection are impacting contraction of the T-cell repertoire, as thymic involution should theoretically affect both CD4+ and CD8+ populations equally.
As with dysregulation of many of the immune cells discussed to this point, alterations in protein expression levels play an important role in age-related defects of T-cells. One of the hallmarks of T-cell immunosenescence is the loss of the costimulatory receptor CD28, which is critical for complete T-cell activation [126]. Decreases in the expression levels of CD28 have been associated with weakened immune responses in the context of both infection and vaccination for elderly individuals [126–128], but this is not a result of these T-cells becoming anergic [129]. Studies have shown that the number of CD28 molecules expressed on the T-cell surface is inversely correlated with the time between cell cycle divisions in T-cells from older adults, [130] suggesting that proliferation in response to antigenic stimulation does occur but is significantly delayed in aged CD28null T-cells. This delay in proliferation may be especially relevant in the context of poor vaccine responses among older adults. While the mechanisms underlying the loss of CD28 expression with age are not fully understood, several studies have found that TNF-α can significantly modulate CD28 expression by inhibiting transcription. [131–133] These findings indicate that increased levels of TNF-α observed in older adults with chronic “inflammaging” may directly contribute to dysregulated T-cell function. A progressive accumulation of highly differentiated CD8+ TEM cells also occurs with aging; these cells characteristically exhibit a decreased capacity for proliferation and enhanced cytotoxic activity [134, 128]. This progressive accumulation of terminally differentiated memory cells also impacts the ability to respond to challenges from infection or vaccination, as these cells display hindered antigen responsiveness and limit the available repertoire against novel antigens [135, 136].
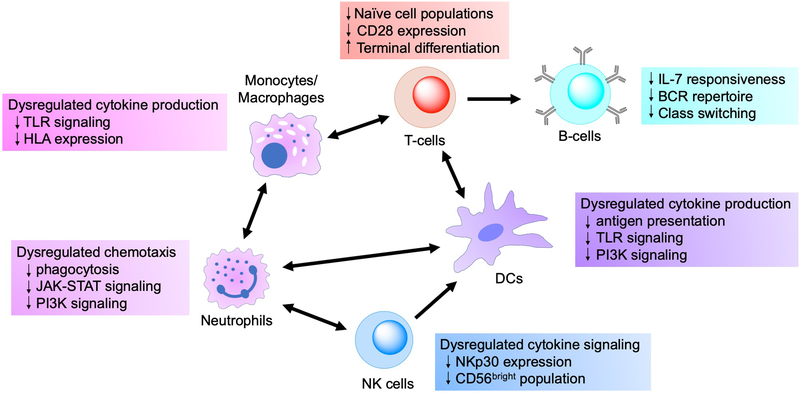
Thus, it is clear that adverse changes occur at different levels across multiple cell types involved in both innate and adaptive immunity. Moreover, these cells do not perform their functions in isolation, and alterations in the properties of one cell population can propagate through crosstalk with multiple other cell types, thereby leading to perturbations throughout the entire immune response. This interconnectivity is highlighted at numerous levels, as various cell types coordinate responses with other cell populations both within and between the innate and adaptive arms of the immune system (see Figure 1). Neutrophils secrete cytokines and chemokines that recruit macrophages and dendritic cells to sites of inflammation, and these cells in turn produce cytokines that prolong the neutrophil lifespan [40]. In addition to their interactions with neutrophils, macrophages can signal and present antigens to T-cells in certain contexts, although dendritic cells are more commonly associated with antigen presentation and T-cell activation [137]. NK cells have been found to play an important role in priming dendritic cells to accomplish this function [85]. Activated T helper-cells mediate numerous immune outcomes, most notably by providing stimulatory signals to B-cells in order to generate robust antibody responses. The preceding discussion clearly demonstrates how dysregulation among any of these features impacts the overall immune response, but the mechanisms at the genetic and gene pathway levels that mediate these alterations in cellular phenotype and function deserve further discussion.
Figure 1. Immune cell populations and the effects of immunosenescence on cellular function.
Arrows denote crosstalk/signaling between cell populations, illustrating the potential for dysfunction in a single population to affect the function of numerous other cell subsets. Innate immune cells (neutrophils, macrophages/monocytes, dendritic cells, NK cells) are all involved with signaling and recruitment of other innate cell populations during the early phases of an immune response. Macrophages and dendritic cells bridge the gap from innate to adaptive immunity via antigen presentation and stimulatory signaling to cognate T-cells, and disruption in either of these functions can drastically compromise adaptive responses. Helper T-cells are critical for subsequent development of robust B-cell antibody responses, further illustrating how dysfunction among cells at both the innate and adaptive level can affect the overall outcomes of an immune response.
Immunosenescence at the genetic level
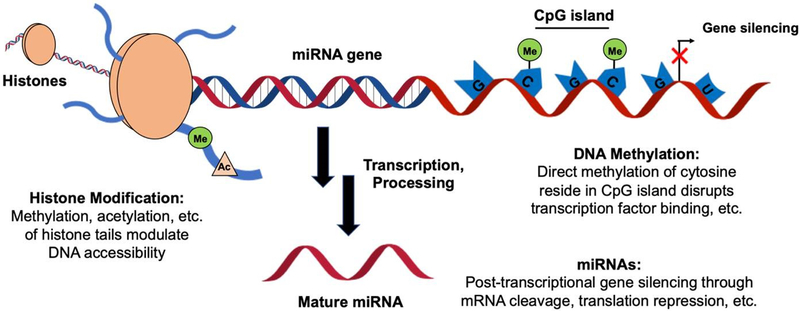
While the effects of immunosenescence are most readily observed at the cellular and molecular levels, dysregulation in biological activity during aging can often be traced to perturbations at the genetic level. While we acknowledge that external factors may also influence immunological activity with age, even these environmental elements— such as nutritional abundances/deficiencies and microbiome exposure— exert their influence on an individual’s immunome by stimulating a cellular response that ultimately manifests as a result of altered gene expression. Epigenetic mechanisms tightly regulate all biological processes by controlling gene transcription and translation, and disruptions in these mechanisms can result in an aberrant increase or decrease in protein production apparent in aging cells(see Figure 2) [138]. In this section, we will summarize changes in epigenetic regulation (DNA methylation, histone modifications, and microRNAs) that have been identified in aged cells from the innate and adaptive immune systems, and we will relate how these mediate changes at the molecular level discussed in the previous section. We will also briefly describe the use of statistical mediation analysis as an approach for evaluating how changes at the transcriptomic level govern immune response outcomes.
Figure 2. Genetic regulatory mechanisms affected by immunosenescence.
Regulation of cellular function is often mediated by changes at the genetic level. Covalent modifications on histone tails modulate the accessibility of DNA for transcription, methylation of CpG sequences in promoter regions can affect transcription factor binding, and miRNA expression can modulate gene silencing through a number of different mechanisms at the post-transcriptional level. All of these have been shown to be differentially regulated to some capacity with aging and associated with changes in immune cell function.
DNA Methylation
DNA methylation is recognized as one of the key epigenetic elements that control gene expression and is primarily limited to CpG-rich sequences clustered in the promoter region of most genes, although other methylation sites have been identified [139, 140]. Methylation of these CpG islands has the effect of inhibiting transcription factor binding or recruiting repressive protein complexes, thereby modulating gene expression [141]. Aging leads to global changes in DNA methylation throughout various cell types and tissues [142–144], and several age-related diseases are associated with changes in methylation state [145]. Alterations in DNA methylation with age have been identified in a number of different cells from the immune system, and these changes in gene regulation have been linked to significant changes in immune cell function.
Changes in methylation state appear to have definitive impacts on innate immune cell function, although studies have mostly focused on the global epigenetic signatures present in mixed populations of peripheral blood mononuclear cells (PBMCs) rather than isolated cell subsets. Monocyte differentiation is impacted, as hypomethylation of several genes in aged progenitor cells appears to decrease their phenotypic plasticity [146]. Promoter demethylation has also been linked to an increased production of TNF-α in both PBMCs and macrophages from older individuals [147], which in turn induces a low-grade inflammatory phenotype in neutrophils that contributes to “inflammaging.” Decreased methylation of the IL6 promoter in monocytes has been observed in vitro as a response to zinc deficiency, which is frequently observed among the elderly and would also likely contribute to a chronic inflammatory state in vivo [148]. NK cells display lower cytotoxic activity with aging, which is partially mediated by hypermethylation of the IFNG and IL2 promoter regions in PBMCs that limits the transcription and expression of these cytokines [149]. DNA methylation can clearly impact the function of innate immune cells and drive differentiation towards both pro- and anti-inflammatory states.
Cells from the adaptive immune system also display unique changes to their methylome with aging that can impact function, in addition to the effects exerted upon them by aging innate cell populations. Integrated epigenetic analyses have shown the development and function of aging T-cells to be dependent upon DNA methylation status [150]. The impaired functionality of CD4+CD28− T-cells has also been attributed to unique changes in DNA methylation that affect subsequent gene expression in cytokine and receptor signaling pathways [151]. The disproportionate population of regulatory T-cells that has been observed in aged mice has been linked to hypomethylation in the FOXP3 promotor, leading to expansion of this population and subsequent suppression of effector T-cell responses [152]. B-cell development and response are governed by epigenetic mechanisms in a similar manner, so it is likely that age-associated changes also affect their function [153, 154]. Indeed, our own work has identified methylation signatures in PBMC populations from elderly individuals vaccinated against seasonal influenza that correlate with humoral response outcomes [19, 155].
Histone Modifications
In addition to the chemical modification of DNA, histone proteins can also be modified through numerous mechanisms to modulate chromatin structure and gene expression. Histone methylation at lysine or arginine residues can serve to regulate transcription, but other post-translational modifications (e.g., acetylation, phosphorylation, ubiquitylation,) can promote or repress gene expression as well. The overall impact on chromatin structure is not dependent upon any single modification but rather the combination of all modifications in a given region. Although studies of DNA methylation signatures in immunosenescence are well-documented, the contributions of histone modification to the senescent state are not well-defined.
Cellular function can be dramatically altered by coordinated changes in histone modification for both the innate and adaptive response. Impaired antigen presentation by macrophages has been linked to the deacetylation of histones associated with regulatory networks that ultimately repress HLA class II expression [156]. Inflammatory cytokine expression in monocytes has also been associated with histone methylation and acetylation, leading to increased production of IL-6 and TNF-α [157]; disruptions in this epigenetic program could limit response efficacy or contribute to chronic inflammation. Age-associated shifts in activated T-cell populations have also been related to increased histone modifications (H3K27Ac and H3K4me1), suggesting a role in “inflammaging” and an increased risk for autoimmune reactions [158]. Likewise, decreased histone methylation and acetylation have been linked to altered gene expression and senescence of T-cells in the thymus and spleen of aged rats [159].
MicroRNAs
Recent studies have identified greater than 1,000 small, non-coding RNA molecules that have broad functionality with respect to gene regulation [160]. These microRNAs (miRNAs) have been implicated as fundamental components of both innate and adaptive immune cell signaling [161, 162]. In innate cell populations, signaling through TLRs and other pattern recognition receptors is ultimately dependent upon miRNA function; in B- and T-lymphocytes, miRNAs have been shown to regulate broad functions from transcription factor expression to receptor signaling. Nearly all of the miRNAs involved in immune regulation are modulated during the course of aging, and numerous studies have found this dysregulation with aging to be associated with both the progression of immunosenescence as well as chronic inflammation [163].
For innate cell populations, TLR expression and function is critical for initiating responses to infection and vaccination, and perturbations in receptor signaling have been linked to changes in the expression of several miRNAs in the elderly. For example, MiR-29 is expressed in dendritic cells and macrophages, where it modulates the production of IL-6, TNF-α, and CXCL9 [164, 165]; unsurprisingly, miR-29 is highly upregulated in aged mice, indicating a potential role in “inflammaging” [166]. Alternatively, the miR-46 family plays a key role in regulating TLR signaling through negative feedback, but aberrant NF-κB activity has been shown to result in dysregulated expression of miR-46a in aged macrophages, leading to the continued production of inflammatory cytokines [167]. Studies in macrophages and DCs have also identified miR-155 as a central regulator of both the innate and adaptive immune systems, as its activity controls the expression of numerous other miRNAs [168, 169].
Expression of miR-155 is upregulated in B-cells from elderly individuals and leads to a subsequent decrease in the expression of AID; simultaneously, miR-16 is also expressed and negatively regulates expression of the transcription factor E47 [169]. This combination of miRNA regulation greatly inhibits class-switch recombination and contributes to the senescent state of elderly B-cells. MiRNA expression is also critical in the regulation of T-cell function. An age-related decrease in miR-92a expression has been shown to correlate with the contraction of the naïve CD8+ T-cell pool, possibly as a result of repeated antigen exposure which leads to terminal differentiation and exhaustion [170]. Furthermore, a decline in miR-181a levels was correlated with increased DUSP6 activity in elderly CD4+ T-cells, which impairs TCR signaling and further diminishes adaptive responses in the elderly [171].
Taken together, these studies demonstrate that perturbations at the genetic level are causative of the functional changes that manifest at the cellular level during immunosenescence. Alterations in regulation of a single gene can affect numerous pathways within a cell, ultimately impacting cellular function. Changes in the function of any one class of cells, particularly with respect to the immune system, can have far-reaching effects due to the level of cross-talk between the arms of innate and adaptive immunity. This becomes an even more complex area of study if one considers that, instead of a single gene, numerous genes across multiple cell types are being affected simultaneously as a result of aging. Mediation analysis is a comprehensive, data-driven approach that uses statistical models to evaluate how these changes at the transcriptomic level mediate the link between biological variables (e.g., vaccine type, sex, promoter methylation) and immune response outcomes. [172, 173, 26, 174] These statistical models are able to identify specific genes (or genesets) associated with a variable of interest and determine whether these genes are directly involved in mediating the downstream effects of this variable on a particular aspect of protective immunity. [175, 176] In this manner, one can begin to mechanistically examine how changes in gene expression may affect the outcome of an immune response with aging. Given the large number of interrelated components that are interacting concurrently, a systems-level approach provides for the most complete analysis to identify all of the factors contributing to immunosenescence.
Systems biology studies of immunosenescence and vaccine responses
While most vaccination strategies have been focused on children, older adults (> 60 years of age) represent a critical at-risk population for whom effective vaccination strategies are sorely lacking. The development of immunosenescence not only places these individuals at greater risk for infection but also compromises their ability to develop protective immune responses following vaccination. In order to fully understand the mechanisms that mediate immunosenescence, a fundamental understanding of the behavior of each component in the system must be established. Systems biology seeks to characterize each part in a biological system and utilize computational models to predict the behavior of the entire system. We and others have applied systems biology approaches in studying the mechanisms governing vaccine responses across different populations. We detail several of those studies herein to demonstrate this approach’s power to identify causative relationships with respect to immune responses and how findings from these studies might be applied in the development of next-generation vaccines.
Systems biology was first applied in the study of immune responses to vaccines against yellow fever by Querec et al. [18]; since this pioneering work, numerous other groups have implemented high-dimensional approaches to the study of vaccine responses and immune function. One such study identified a neutrophil-mediated IFN gene signature in the blood transcriptome that was associated with tuberculosis susceptibility and exacerbated infection [177]. Many others have also focused on tuberculosis immunity, which substantially contributed to the development of novel therapeutics and vaccines [178–180]. Pneumococcal vaccines have also been investigated using a systems-level approach, which identified early innate inflammatory gene expression signatures that correlated with peak plasmablast responses [181].
Influenza vaccination has been a major focus of systems-level studies, as it remains an important pathogen among high-risk older populations due to high morbidity and mortality rates. Pulendran and colleagues carried out a series of clinical studies over a three-year period to evaluate the innate and adaptive responses to influenza vaccination in healthy young adults [26]. From this work, they were able to identify an association between the gut microbiome and TLR5-depenedent signaling that served to prime peripheral immune cells for optimal antibody responses [25]. In an independent trial, Tan et al. employed geneset enrichment analysis to identify genes related to B-cell proliferation, and these signatures were able to accurately predict high and low responders to influenza vaccination [182]. Another group identified polymorphisms in genes related to membrane trafficking and antigen processing that were important determinants of influenza vaccine immunogenicity [183]. While much of our own work has focused on the genetic factors underlying interindividual variability of immune responses against measles, mumps, and rubella [21, 22, 27, 184–186], we have also been one of the few groups to investigate influenza vaccine responses in the elderly using systems biology [19, 155, 187–192].
Our studies of influenza vaccination in the elderly have allowed for the identification of several genesets and transcriptomic signatures that may better characterize immunosenescence. An analysis of system-wide methylation levels identified groups of CpG islands that affected the expression of several genes with established roles in humoral immunity (e.g., HLA-B and HLA-DQB2) [19]. Transcriptome-wide profiling of PBMCs following vaccination further identified several gene signatures associated with memory B-cell responses against influenza, including significant changes in pathways for lipid and cholesterol metabolism [187]. Further analysis of the transcriptome following vaccination identified several enriched genesets associated with cellular and humoral immune responses, including genes not previously associated with vaccine responses (e.g., SPON2, MATK, CST7) [191]. Extensive analysis of these elderly cohorts suggested that humoral responses to influenza vaccination were significantly associated with age-related changes in T-cell populations and function [188]. Moreover, integrated network analysis of pathways and genesets involved in chemokine signaling and T-cell activity identified several changes in gene expression that were associated with differences in DNA methylation and miRNA production and implicated in immunosenescence [155].
While it is apparent that systems-level analysis has great power in identifying causative relationships between gene regulation and global immune outcomes, there is still much we do not understand with regard to the genetic mechanisms underlying immunosenescence and vaccine responses. The studies discussed in this section serve to illustrate both of these aspects. While several studies highlighted genesets that are known to be involved in immune responses, many also identified genesets not canonically associated with immunity [187, 191]. Still others identified interrelations, such as the association of the gut microbiome with humoral influenza response [25], which would not have been readily apparent without a systems-level approach. Although it may not yet be possible to fully define all of the mechanisms contributing to immunosenescence and their subsequent effects on vaccine responses, many causative elements have been identified that could inform the development of novel vaccines to address inadequate protection in older adults.
Improving vaccination among older adults
The aging immune system limits the efficacy of current vaccines that are capable of eliciting protective responses in children and younger adults, emphasizing the need for novel vaccine formulations tailored for the growing older population [193]. The most common approach for improving immune responses in the aging population has been increasing the dose of antigen or the addition of adjuvants into vaccine formulations [194]. Recent studies have also highlighted the potential benefit of senolytic and other immunomodulatory drugs for improving vaccine responses [195]. Systems biology can serve to identify target pathways or proteins that are associated with diminished immune responses, and thus can inform the molecular design of adjuvants [196]. Once a target has been identified, molecules/formulations can be synthesized that specifically stimulate activity in these pathways (or others) in order to improve immune responses, thereby compensating for age-related deficiencies. Alternatively, senolytics selectively induce apoptosis of senescent cells and may serve to boost immune responses by the removal of inhibitory cell populations [195]. Other classes of immunomodulatory drugs have shown similar potential for remodeling cellular activity to influence immune responses. Numerous adjuvants and immunomodulatory drug formulations have already been employed in both preclinical and clinical studies in an effort to bolster immune responses in older adults (see Table 1), and we will conclude this review with a discussion on how these approaches might lead to improved vaccines for the elderly population.
Table 1.
Adjuvants currently in clinical or preclinical use for the elderly population.
| Name | Adjuvant Class | Components | Clinical Stage | References |
|---|---|---|---|---|
| MF59® | Oil-in-water emulsion | Squalene, Tween 80, Span85 | Licensed | [185–188] |
| AS03 | Oii-in-water emulsion | Squalene, polysorbate 80, α-tocopherol | Phase II (licensed during pandemic) | [189–192] |
| Montanide™ | Oil-in-water emulsion | Mineral oil, mannide monooleale surfactant | Phase I | [193, 196] |
| CAF01 | Cationic liposomes | Dimethyl dioctadecylammonium, trehalose dibehenate | Preclinical | [194, 197] |
| JVRS-100 | Cationic liposomes + DNA | DOTIM, cholesterol, plasmid DNA | Preclinical | [195] |
| Flagellin | TLR agonist | Flagellin | Preclinical | [25] |
| GLA-SE | Oil-in-water emulsion + TLR agonist | GLA, squalene | Preclinical | [200–203] |
| Imiquimod | TLR agonist | Imiquimod | Phase II/III | [204, 205] |
| CpG ODN + Flt3 ligand | TLR-agonist + DC stimulant | Plasmid DNA encoding for CpG ODN + Flt3 ligand | Preclinical | [218] |
| CpG1018 | TLR agonist | CpG ODN | Licensed | [208–213] |
| AS02V | Oil-in-water emulsion + TLR agonist + saponin | Squalene, MPL, QS-21 | Phase II | [219] |
| AS01B | Liposomes + TLR agonist + saponin | Liposomes, MPL, QS-21 | Licensed | [223–225] |
DOTIM: 1-[2-(oleoyloxy)ethyl]-2-oleyl-3-(2-hydroxyethyl)imidazolinium chloride
Flt-3: Fms-like tyrosine kinase 3
GLA: glucopyranosyl lipid A
MPL: 3-O-desacyl-4’-monophosphoryl lipid A
ODN: oligodeoxynucleotide
QS-21: Quillaja saponaria Molina, fraction 21
Adjuvants
It is apparent that failure of the immune system can occur in different capacities with aging; in order to combat immunosenescence effectively, adjuvants must be designed to stimulate robust responses that overcome these deficiencies. Most studies of novel adjuvants have focused on stimulating higher antibody responses and/or robust T-cell activation in order to achieve protection against disease. The mechanism by which these adjuvants function is critical to their efficacy, and we will briefly discuss both licensed formulations as well as those in preclinical development that have been applied against diseases disparately impacting older adults.
Influenza causes a disproportionate number of hospitalizations and deaths among the elderly population each year due to lower vaccine efficacy [197–199]. One strategy to overcome these diminished responses has been to simply increase the amount of hemagglutinin administered per dose [200]. Another mechanism has been to add adjuvants, such as the emulsion-based adjuvant MF59®, which has been shown to elicit substantially higher antibody responses in seasonal influenza vaccines that are cross-reactive with heterologous viral strains, substantially increasing its clinical efficacy [201, 202]. While the exact mechanisms of MF59® are not fully understood, it is known to induce the production of proinflammatory cytokines and chemokines involved in the innate response [203], and it has also been implicated in driving germinal center B-cell differentiation [204]. Another emulsion-based adjuvant (AS03) has been clinically evaluated for use with pandemic influenza A strains (H1N1, H7N1, and H9N2) and was found to induce significantly higher antibody responses compared to higher doses of the standard vaccine in elderly subjects, presumably due to enhanced antigen uptake and presentation in the draining lymph nodes [205–208]. Phase I clinical trials using the oil-in-water emulsion Montanide™ (ISA 51), together with influenza peptide epitopes, generated virus-specific CD8+ T-cell responses in healthy young adults [209], but this vaccine has not yet been tested in older adults. In addition to these emulsion-based adjuvants, charged liposomal formulations (CAF01, JVRS-100) have also been evaluated in preclinical animal studies and demonstrated higher immunogenicity along with the induction of broader cross-protective antibodies against heterologous influenza strains [210, 211]. These liposomal and emulsion-based adjuvant systems presumably establish an antigen depot, which slowly releases antigen and stimulates APC recruitment to the site of injection to enhance both cellular and humoral immune responses [212, 213]. While the chemotaxis and responsiveness of immune cells in older adults is compromised, enhanced antigen uptake and presentation stimulated by these adjuvant systems serve to overcome these failures of the immune system.
TLR agonists are a promising class of adjuvants that can be implemented effectively from the knowledge gained in systems biology studies [214, 215]. TLR agonists directly stimulate pathways critical for early immune responses, and signaling through these pathways has been shown to decline significantly with aging in certain cell populations. Stimulating TLR pathways may serve not only to boost waning immune responses, but also may be used to directly initiate signaling that influences specific immune response outcomes. The identification of TLR5-mediated priming of the immune system for effective influenza vaccine responses stands as a primary example [25]; if signaling through a particular pathway is a known requirement for robust immune responses, a specific TLR agonist (i.e., flagellin) can be formulated with a vaccine. Glucopyranosyl lipid A in a squalene-based emulsion (GLA-SE) has been investigated as a TLR4 agonist in combination with influenza vaccines and was shown to enhance cytokine production and T-cell responses in older adults [216, 217]. While the effects of GLA-SE on antibody titer are unclear, there is consensus that the adjuvant induces a broader antibody repertoire [218, 219]. Imiquimod is another synthetic TLR7/8 agonist that has been employed in preclinical studies to enhance the immunogenicity of influenza vaccines [220]. A clinical trial found that topical administration of imiquimod prior to intradermal injection greatly increased the immunogenicity of the influenza vaccine, but it had no effect on the vaccine when it was delivered intramuscularly [221]. Unmethylated CpG-rich oligonucleotides have also been administered with vaccine formulations to stimulate innate immune responses through TLR9 activation [222, 223]. The newly developed adjuvant 1018 is a short, 22-base oligonucleotide sequence that contains immunostimulatory CpG motifs and has been shown to induce rapid antibody responses in older adults, presumably by overcoming immunosenescence through robust activation of TLR9 signaling [224–226]. The recently approved hepatitis B vaccine (HEPLISAV-B®) is a mixture of recombinant surface antigen from hepatitis B virus and CpG 1018, and this formulation has shown tremendous immunogenicity in both healthy and hyporesponsive individuals [227, 228, 225, 226]. Clinical studies have shown that individuals administered a 4-week, two-dose regimen of HEPLISAV-B® developed higher protective antibody titers compared to those on a 24-week, three-dose regimen for the approved alum-containing Engerix-B® vaccine [229, 227, 225, 226].
Among older adults, infection with Streptococcus pneumoniae is another common cause of upper respiratory disease that can lead to several debilitating conditions, including bacteremia and meningitis [230]. A carbohydrate vaccine that is protective against 23 different pneumococcal serotypes has been licensed for use in elderly adults [231], and a 13-valent conjugate vaccine that elicits more robust T cell-dependent responses has also been introduced [232]. A randomized clinical trial demonstrated that elderly patients receiving the conjugate vaccine suffered significantly fewer episodes (75%) of pneumococcal disease compared to subjects receiving a placebo [233]. Due to the low immunogenicity of carbohydrate vaccines, antigenic peptides from pneumococcal surface protein (PspA) have been explored as candidates for intranasal vaccination in combination with nucleic acid-based adjuvants. Dual delivery of CpG oligodeoxynucleotides and plasmid DNA encoding for the dendritic cell ligand Flt3 stimulated increased recruitment and activation of DCs in aged mice, which conferred protection against S. pneumoniae infection [234]. Trials have also explored other pneumococcal proteins (pneumococcal histidine triad D and pneumolysin) as potential vaccine candidates in combination with an emulsion (AS02V) of monophosphoryl lipid A and the saponin QS-21; these formulations exhibited acceptable safety profiles and gave rise to enhanced immune responses in a cohort of elderly adults [235].
Herpes zoster (shingles) is another disease that disproportionately affects elderly populations due to the reactivation of latent varicella zoster virus (VZV) in the dorsal root ganglia, which can lead to long-term complications such as post-herpetic neuralgia (PHN) [236]. Virus-specific T-cell responses typically prevent reactivation of the virus early in the life, but the onset of immunosenescence limits the functional capacity of T-cells [237]. A live-attenuated zoster vaccine was developed and licensed for use in older adults to protect against the incidence of PHN, but vaccine efficacy was markedly limited in subjects over 60 years of age. [238] Furthermore, long-term studies have shown that immune responses induced by this vaccine wane over time [239]. More recently, a vaccine containing recombinant VZV glycoprotein E (gE) and the liposomal adjuvant AS01B (Shingrix™) was licensed, whichdemonstrated markedly superior seroconversion rates in older adults compared to the live attenuated vaccine. [240] The efficacy of Shingrix™ has been shown to be significantly higher than that of the live attenuated vaccine, especially among adults older than 70 years of age. [241, 242] The protective responses elicited by the recombinant vaccine in older adultsalso exhibit significant durability, with longitudinal studies showing persistence for at least 9 years following vaccination [243, 242]. Recent evidence has indicated that gE-specific CD4+ and CD8+ memory T-cell responses are stronger in subjects receiving the adjuvanted vaccine, suggesting that these cells mediate these durable immune responses [244]. The resounding clinical success of Shingrix™ highlights the important role of adjuvants in vaccine formulations designed for use in older adults.
While several adjuvant systems have been licensed for clinical use in order to improve vaccine responses among the elderly, the knowledge gaps that remain in our understanding of immunosenescence limit our ability to tailor adjuvant formulations with precision. Furthermore, the mechanism of action for many of the adjuvant systems currently employed in the clinic or undergoing preclinical studies are not well understood. In order to develop the most effective vaccine formulations for use in the elderly, comprehensive studies directly comparing adjuvants with the same vaccine are warranted. While a few systems-level studies have undertaken comparative evaluations of adjuvant formulations [245, 246, 224], this area of research remains largely unexplored. High-dimensional studies focused on the mechanisms of action for adjuvants currently in clinical and preclinical use will allow us to better understand how these vaccines function to stimulate robust immune responses in older individuals, and may also provide a general mechanistic understanding for how certain classes of adjuvants engage the immune system. Similarly, systems-level studies of immunosenescence— both in animal models of aging and human subjects— will allow for elucidation of the biological mechanisms underlying declines in immune cell function with age, and with specific pathways or proteins identified, novel adjuvants can be designed at the molecular level to engage specific receptors in signaling pathways that compensate for failures in other cellular functions and overcome immunosenescence.
Senolytics and Immunomodulatory Drugs
In addition to adjuvants, several other pharmacological strategies that involve modulation of the immune system or elimination of senescent cell populations are currently being explored to improve immune responses in older adults. Senolytic drugs selectively induce apoptosis in senescent cells without harming healthy cell populations and have shown promise for treating chronic age-related conditions in preclinical studies. The tyrosine kinase inhibitor dasatinib, along with the flavonoid quercetin, have both demonstrated the capacity to eliminate senescent adipocyte progenitors and endothelial cells, respectively [247]. Administration of the drugs to aged mice reduced the senescent cell burden and mitigated the onset of age-related pathologies, demonstrating their potential for clinical application; however, these and many other senolytics remain untested in the field of vaccinology. A limited number of studies have reported on the beneficial effects of small molecule administration in combination with influenza vaccination. Treatment of an elderly cohort with the mTOR inhibitor RAD001 led to enhanced vaccine responses, possibly due to changes in cellular metabolism and increased expression of an IFN-dependent geneset [248]. Administration of the histamine receptor agonist nizatidine enhanced both humoral and cellular vaccine responses in a mouse model of influenza [249], providing further evidence for the use of small molecule immunomodulators in vaccine development.
There are numerous other classes of immunomodulatory drugs that have been evaluated for the treatment of cancers and autoimmune disorders, yet have the potential for manipulation of immune cell populations and signaling events in order to bolster vaccine responses. Low-dose administration of cyclophosphamide was shown to deplete regulatory T-cells and led to an expansion of tumor-specific effector T-cells in a clinical trial for colorectal cancer [250]; it is possible that the same treatment may improve vaccine responses in older adults. Similar to the application of mTOR inhibitors, inhibitors of vascular endothelial growth factor (VEGF) signaling, have also been evaluated for their effect on immunological responses. Inhibition of VEGF was associated with an increase in DCs and effector T-cells, which were shown to mediate vaccine efficacy in a murine melanoma model [251], and it is possible this effect may extend beyond tumor-infiltrating cells. Small molecule modulators of cytokine function have also been explored in the context of inflammatory autoimmune disease, providing even more examples of molecular targets that could be harnessed for improving vaccine responses in older adults [252].
Conclusion
Defects in the immune system develop with age and contribute to the reduced immunogenicity and efficacy of vaccines in the elderly population. Changes in gene regulation or gene expression mediate phenotypic changes in numerous cell types, leading to altered cellular function and dysregulation of cell signaling. As the innate and adaptive immune systems are comprised of an intricately connected network of different cell types, changes in even a single cell subset can result in a domino effect throughout the entire biological system; however, immunosenescence typically develops in parallel across numerous cell types, leading to even more complex outcomes. The basic biological changes that accompany an aging immune system are still not fully understood, and further studies are necessary in order to develop more effective vaccine formulations. In particular, systems biology allows for the study of high-dimensional datasets in toto in order to fully understand multicomponent processes and their interactions, such as those governing immunosenescence and vaccine responses in older adults. Such studies of these underlying genetic and epigenetic mechanisms are needed and have the potential to inform the rational design of novel candidate vaccines, adjuvants, or immunomodulatory therapeutuics that can stimulate robust immune responses in the elderly. Systems-level studies focused on vaccine formulations that have been largely effective in older adults (e.g. Shingrix™, HEPLISAV-B®, FLUAD™) are lacking and will be instrumental for informing novel vaccine design, as any immune signatures that are identified will inform upon the mechanism by which these vaccines stimulate robust immunity and may prove to be more broadly informative of pathways or cell subsets that should be targeted in the development of vaccines for the elderly.
Table 2.
Senolytic and immunomodulatory agents currently in clinical or preclinical use.
| Name | Drug Class | Clinical Stage | References |
|---|---|---|---|
| Dasatinib | Tyrosine kinase inhibitor | Preclinical | [229] |
| Quercetin | Flavonoid | Preclinical | [229] |
| RAD001 | mTOR inhibitor | Phase II | [230] |
| Nizatidine | Histamine receptor agonist | Preclinical | [231] |
| Cyclophosphamide | Immunosuppressant | Phase I/II | [232] |
| sVEGFR1/R2 | VEGF inhibitor | Preclinical | [233] |
mTOR: mammalian target of rapamycin
VEGF: vascular endothelial growth factor
ACKNOWLEDGEMENTS
We thank Caroline L. Vitse for her editorial assistance.
FUNDING
Research reported in this publication was supported by the National Institute of Allergy And Infectious Diseases of the National Institutes of Health under award numbers U01AI089859 and R01AI132348. This research has been reviewed by the Mayo Clinic Conflict of Interest Review Board and was conducted in compliance with Mayo Clinic Conflict of Interest policies.
List of abbreviations
- SASP
Senescence-associated secretory phenotype
- MAPK
Mitogen-activated protein kinases
- ICAM
Intercellular adhesion molecule
- PI3K
Phosphatidylinositol-3 kinase
- GM-CSF
Granulocyte-macrophage colony stimulating factor
- IFN-γ
Interferon-γ
- MHC
Histocompatibility complex
- NK
Natural killer
- KIR
Killer immunoglobulin-like receptor
- APCs
Antigen presenting cells
- mDC
Myeloid dendritic cell subset
- pDC
Plasmacytoid dendritic cell subset
- fDC
Follicular dendritic cell subset
- BCR
B-cell receptor
- AID
Activation-induced cytidine deaminase
- TCM
Central memory T-cells
- TEM
Effector memory T-cells
- CMV
Cytomegalovirus
- PBMCs
Peripheral blood mononuclear cells
- miRNAs
MicroRNAs
- GLA-SE
Glucopyranosyl lipid A in a squalene-based emulsion
- PspA
Pneumococcal surface protein
- VZV
Varicella zoster virus
- PHN
Post-herpetic neuralgia
- gE
Glycoprotein E
- VEGF
Vascular endothelial growth factor
Footnotes
COMPETING INTERESTS
Dr. Poland is the chair of a Safety Evaluation Committee for novel investigational vaccine trials being conducted by Merck Research Laboratories. Dr. Poland offers consultative advice on vaccine development to Merck & Co. Inc., Avianax, Adjuvance, Valneva, Medicago, Sanofi Pasteur, GlaxoSmithKline, and Emergent Biosolutions. Drs. Poland and Ovsyannikova hold patents related to vaccinia and measles peptide vaccines. Dr. Kennedy holds a patent related to vaccinia peptide vaccines. Dr. Kennedy has received research funding from Merck Research Laboratories to study waning immune responses to mumps vaccine. These activities have been reviewed by the Mayo Clinic Conflict of Interest Review Board and are conducted in compliance with Mayo Clinic Conflict of Interest policies. All other authors declare no competing interests.
References
- 1.Boraschi D, Italiani P. Immunosenescence and vaccine failure in the elderly: strategies for improving response. Immunology Letters. 2014;162(1 Pt B):346–53. doi: 10.1016/j.imlet.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Jaul E, Barron J. Age-Related Diseases and Clinical and Public Health Implications for the 85 Years Old and Over Population. Front Public Health. 2017;5:335. doi: 10.3389/fpubh.2017.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buford TW. Hypertension and aging. Ageing Res Rev. 2016;26:96–111. doi: 10.1016/j.arr.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niccoli T, Partridge L. Ageing as a risk factor for disease. Current biology: CB. 2012;22(17):R741–52. doi: 10.1016/j.cub.2012.07.024. [DOI] [PubMed] [Google Scholar]
- 5.Pera A, Campos C, Lopez N, Hassouneh F, Alonso C, Tarazona R et al. Immunosenescence: Implications for response to infection and vaccination in older people. Maturitas. 2015. doi: 10.1016/j.maturitas.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Gruver AL, Hudson LL, Sempowski GD. Immunosenescence of ageing. J Pathol. 2007;211(2):144–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pawelec G. Immunosenescence and vaccination. Immun Ageing. 2005;2:16. doi: 10.1186/1742-4933-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma Y, Fang M. Immunosenescence and age-related viral diseases. Sci China Life Sci. 2013;56(5):399–405. doi: 10.1007/s11427-013-4478-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xia S, Zhang X, Zheng S, Khanabdali R, Kalionis B, Wu J et al. An Update on Inflamm-Aging: Mechanisms, Prevention, and Treatment. J Immunol Res. 2016;2016:8426874. doi: 10.1155/2016/8426874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franceschi C, Bonafe M, Valensin S, Olivieri F, De LM, Ottaviani E et al. Inflamm-aging. An evolutionary perspective on immunosenescence. AnnNYAcadSci. 2000;908:244–54. [DOI] [PubMed] [Google Scholar]
- 11.Schenten D, Medzhitov R. The control of adaptive immune responses by the innate immune system. Advances in Immunology. 2011;109:87–124. doi: 10.1016/B978-0-12-387664-5.00003-0. [DOI] [PubMed] [Google Scholar]
- 12.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327(5963):291–5. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franceschi C, Salvioli S, Garagnani P, de Eguileor M, Monti D, Capri M. Immunobiography and the Heterogeneity of Immune Responses in the Elderly: A Focus on Inflammaging and Trained Immunity. Front Immunol. 2017;8:982. doi: 10.3389/fimmu.2017.00982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chuang HY, Hofree M, Ideker T. A decade of systems biology. Annu Rev Cell Dev Biol. 2010;26:721–44. doi: 10.1146/annurev-cellbio-100109-104122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pulendran B, Li S, Nakaya HI. Systems vaccinology. Immunity. 2010;33(4):516–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oberg AL, Kennedy RB, Li P, Ovsyannikova IG, Poland GA. Systems biology approaches to new vaccine development. Current Opinion in Immunology. 2011;23(3):436–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haralambieva IH, Poland GA. Vaccinomics, predictive vaccinology and the future of vaccine development. Future Microbiol. 2010;5:1757–60. [DOI] [PubMed] [Google Scholar]
- 18.Querec TD, Akondy RS, Lee EK, Cao W, Nakaya HI, Teuwen D et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat Immunol. 2009;10(1):116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zimmermann MT, Oberg AL, Grill DE, Ovsyannikova IG, Haralambieva IH, Kennedy RB et al. System-Wide Associations between DNA-Methylation, Gene Expression, and Humoral Immune Response to Influenza Vaccination. PLos ONE. 2016;11(3):e0152034. doi: 10.1371/journal.pone.0152034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voigt EA, Haralambieva IH, Larrabee BL, Kennedy RB, Ovsyannikova IG, Schaid DJ et al. Polymorphisms in the Wilms Tumor Gene Are Associated With Interindividual Variations in Rubella Virus-Specific Cellular Immunity After Measles-Mumps-Rubella II Vaccination. The Journal of Infectious Diseases. 2018;217(4):560–6. doi: 10.1093/infdis/jix538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haralambieva IH, Ovsyannikova IG, Kennedy RB, Larrabee BR, Zimmermann MT, Grill DE et al. Genome-Wide Associations of CD46 and IFI44L Genetic Variants with Neutralizing Antibody Response to Measles Vaccine. Human Genetics. 2017;136(4):421–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kennedy RB, Ovsyannikova IG, Haralambieva IH, Lambert ND, Pankratz VS, Poland GA. Genome-wide SNP associations with rubella-specific cytokine responses in measles-mumps-rubella vaccine recipients. Immunogenetics. 2014;66(7–8):493–9. doi: 10.1007/s00251-014-0776-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ovsyannikova IG, Pankratz VS, Salk HM, Kennedy RB, Poland GA. HLA alleles associated with the adaptive immune response to smallpox vaccine: a replication study. Human genetics. 2014;133(9):1083–92. doi: 10.1007/s00439-014-1449-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Furman D, Davis MM. New approaches to understanding the immune response to vaccination and infection. Vaccine. 2015;33(40):5271–81. doi: 10.1016/j.vaccine.2015.06.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oh JZ, Ravindran R, Chassaing B, Carvalho FA, Maddur MS, Bower M et al. TLR5-mediated sensing of gut microbiota is necessary for antibody responses to seasonal influenza vaccination. Immunity. 2014;41(3):478–92. doi: 10.1016/j.immuni.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakaya HI, Wrammert J, Lee EK, Racioppi L, Marie-Kunze S, Haining WN et al. Systems biology of seasonal influenza vaccination in humans. NatImmunol. 2011;12(8):786–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haralambieva IH, Kennedy RB, Simon WL, Goergen KM, Grill DE, Ovsyannikova IG et al. Differential miRNA expression in B cells is associated with inter-individual differences in humoral immune response to measles vaccination. PLos ONE. 2018;13(1):e0191812. doi: 10.1371/journal.pone.0191812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Del Giudice G, Goronzy JJ, Grubeck-Loebenstein B, Lambert PH, Mrkvan T, Stoddard JJ et al. Fighting against a protean enemy: immunosenescence, vaccines, and healthy aging. NPJ Aging Mech Dis. 2018;4:1. doi: 10.1038/s41514-017-0020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ongradi J, Kovesdi V. Factors that may impact on immunosenescence: an appraisal. ImmunAgeing. 2010;7:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. The Journal of clinical investigation. 2013;123(3):966–72. doi: 10.1172/JCI64098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LeBrasseur NK, Tchkonia T, Kirkland JL. Cellular Senescence and the Biology of Aging, Disease, and Frailty. Nestle Nutr Inst Workshop Ser. 2015;83:11–8. doi: 10.1159/000382054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu M, Palmer AK, Ding H, Weivoda MM, Pirtskhalava T, White TA et al. Targeting senescent cells enhances adipogenesis and metabolic function in old age. Elife. 2015;4:e12997. doi: 10.7554/eLife.12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coppe JP, Kauser K, Campisi J, Beausejour CM. Secretion of vascular endothelial growth factor by primary human fibroblasts at senescence. The Journal of Biological Chemistry. 2006;281(40):29568–74. doi: 10.1074/jbc.M603307200. [DOI] [PubMed] [Google Scholar]
- 34.Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. 2018;14(10):576–90. doi: 10.1038/s41574-018-0059-4. [DOI] [PubMed] [Google Scholar]
- 35.Calder PC, Bosco N, Bourdet-Sicard R, Capuron L, Delzenne N, Dore J et al. Health relevance of the modification of low grade inflammation in ageing (inflammageing) and the role of nutrition. Ageing Res Rev. 2017;40:95–119. doi: 10.1016/j.arr.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 36.Franceschi C, Garagnani P, Morsiani C, Conte M, Santoro A, Grignolio A et al. The Continuum of Aging and Age-Related Diseases: Common Mechanisms but Different Rates. Front Med (Lausanne). 2018;5:61. doi: 10.3389/fmed.2018.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu W, Larbi A. Markers of T Cell Senescence in Humans. Int J Mol Sci. 2017;18(8). doi: 10.3390/ijms18081742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hakim FT, Gress RE. Immunosenescence: deficits in adaptive immunity in the elderly. Tissue Antigens. 2007;70(3):179–89. doi: 10.1111/j.1399-0039.2007.00891.x. [DOI] [PubMed] [Google Scholar]
- 39.Cancro MP, Hao Y, Scholz JL, Riley RL, Frasca D, Dunn-Walters DK et al. B cells and aging: molecules and mechanisms. Trends Immunol. 2009;30(7):313–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Solana R, Tarazona R, Gayoso I, Lesur O, Dupuis G, Fulop T. Innate immunosenescence: effect of aging on cells and receptors of the innate immune system in humans. Seminars in Immunology. 2012;24(5):331–41. doi: 10.1016/j.smim.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 41.Shaw AC, Panda A, Joshi SR, Qian F, Allore HG, Montgomery RR. Dysregulation of human Toll-like receptor function in aging. Ageing ResRev. 2011;10(3):346–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Panda A, Arjona A, Sapey E, Bai F, Fikrig E, Montgomery RR et al. Human innate immunosenescence: causes and consequences for immunity in old age. Trends Immunol. 2009;30(7):325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumar V, Sharma A. Neutrophils: Cinderella of innate immune system. Int Immunopharmacol. 2010;10(11):1325–34. doi: 10.1016/j.intimp.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 44.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nature reviews Immunology. 2007;7(9):678–89. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 45.Lefort CT, Ley K. Neutrophil arrest by LFA-1 activation. Front Immunol. 2012;3:157. doi: 10.3389/fimmu.2012.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pae M, Meydani SN, Wu D. The role of nutrition in enhancing immunity in aging. Aging Dis. 2012;3(1):91–129. [PMC free article] [PubMed] [Google Scholar]
- 47.Kovacs EJ, Palmer JL, Fortin CF, Fulop T Jr., Goldstein DR, Linton PJ. Aging and innate immunity in the mouse: impact of intrinsic and extrinsic factors. Trends in immunology. 2009;30(7):319–24. doi: 10.1016/j.it.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fortin CF, McDonald PP, Lesur O, Fulop T Jr. Aging and neutrophils: there is still much to do. Rejuvenation Res. 2008;11(5):873–82. doi: 10.1089/rej.2008.0750. [DOI] [PubMed] [Google Scholar]
- 49.Fulop T, Larbi A, Douziech N, Fortin C, Guerard KP, Lesur O et al. Signal transduction and functional changes in neutrophils with aging. Aging Cell. 2004;3(4):217–26. doi: 10.1111/j.1474-9728.2004.00110.x. [DOI] [PubMed] [Google Scholar]
- 50.Sapey E, Greenwood H, Walton G, Mann E, Love A, Aaronson N et al. Phosphoinositide 3-kinase inhibition restores neutrophil accuracy in the elderly: toward targeted treatments for immunosenescence. Blood. 2014;123(2):239–48. doi: 10.1182/blood-2013-08-519520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoo SK, Deng Q, Cavnar PJ, Wu YI, Hahn KM, Huttenlocher A. Differential regulation of protrusion and polarity by PI3K during neutrophil motility in live zebrafish. Dev Cell. 2010;18(2):226–36. doi: 10.1016/j.devcel.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohta Y, Hartwig JH, Stossel TP. FilGAP, a Rho- and ROCK-regulated GAP for Rac binds filamin A to control actin remodelling. Nat Cell Biol. 2006;8(8):803–14. doi: 10.1038/ncb1437. [DOI] [PubMed] [Google Scholar]
- 53.Simell B, Vuorela A, Ekstrom N, Palmu A, Reunanen A, Meri S et al. Aging reduces the functionality of anti-pneumococcal antibodies and the killing of Streptococcus pneumoniae by neutrophil phagocytosis. Vaccine. 2011;29(10):1929–34. doi: 10.1016/j.vaccine.2010.12.121. [DOI] [PubMed] [Google Scholar]
- 54.Sauce D, Dong Y, Campillo-Gimenez L, Casulli S, Bayard C, Autran B et al. Reduced Oxidative Burst by Primed Neutrophils in the Elderly Individuals Is Associated With Increased Levels of the CD16bright/CD62Ldim Immunosuppressive Subset. J Gerontol A Biol Sci Med Sci. 2017;72(2):163–72. doi: 10.1093/gerona/glw062. [DOI] [PubMed] [Google Scholar]
- 55.Alvarez E, Ruiz-Gutierrez V, Sobrino F, Santa-Maria C. Age-related changes in membrane lipid composition, fluidity and respiratory burst in rat peritoneal neutrophils. Clinical and Experimental Immunology. 2001;124(1):95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guichard C, Pedruzzi E, Dewas C, Fay M, Pouzet C, Bens M et al. Interleukin-8-induced priming of neutrophil oxidative burst requires sequential recruitment of NADPH oxidase components into lipid rafts. The Journal of Biological Chemistry. 2005;280(44):37021–32. doi: 10.1074/jbc.M506594200. [DOI] [PubMed] [Google Scholar]
- 57.Fortin CF, Larbi A, Dupuis G, Lesur O, Fulop T Jr. GM-CSF activates the Jak/STAT pathway to rescue polymorphonuclear neutrophils from spontaneous apoptosis in young but not elderly individuals. Biogerontology. 2007;8(2):173–87. doi: 10.1007/s10522-006-9067-1. [DOI] [PubMed] [Google Scholar]
- 58.Fortin CF, Larbi A, Lesur O, Douziech N, Fulop T Jr. Impairment of SHP-1 down-regulation in the lipid rafts of human neutrophils under GM-CSF stimulation contributes to their age-related, altered functions. Journal of Leukocyte Biology. 2006;79(5):1061–72. doi: 10.1189/jlb.0805481. [DOI] [PubMed] [Google Scholar]
- 59.Wenisch C, Patruta S, Daxbock F, Krause R, Horl W. Effect of age on human neutrophil function. Journal of Leukocyte Biology. 2000;67(1):40–5. [DOI] [PubMed] [Google Scholar]
- 60.De Martinis M, Modesti M, Ginaldi L. Phenotypic and functional changes of circulating monocytes and polymorphonuclear leucocytes from elderly persons. Immunology and cell biology. 2004;82(4):415–20. doi: 10.1111/j.0818-9641.2004.01242.x. [DOI] [PubMed] [Google Scholar]
- 61.Ferrando-Martinez S, Romero-Sanchez MC, Solana R, Delgado J, de la Rosa R, Munoz-Fernandez MA et al. Thymic function failure and C-reactive protein levels are independent predictors of all-cause mortality in healthy elderly humans. Age (Dordr). 2013;35(1):251–9. doi: 10.1007/s11357-011-9341-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Drew W, Wilson DV, Sapey E. Inflammation and neutrophil immunosenescence in health and disease: Targeted treatments to improve clinical outcomes in the elderly. Experimental Gerontology. 2018;105:70–7. doi: 10.1016/j.exger.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 63.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327(5966):656–61. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Murray PJ. Macrophage Polarization. Annu Rev Physiol. 2017;79:541–66. doi: 10.1146/annurev-physiol-022516-034339. [DOI] [PubMed] [Google Scholar]
- 65.Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233(9):6425–40. doi: 10.1002/jcp.26429. [DOI] [PubMed] [Google Scholar]
- 66.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41(1):14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hajishengallis G. Too old to fight? Aging and its toll on innate immunity. Mol Oral Microbiol. 2010;25(1):25–37. doi: 10.1111/j.2041-1014.2009.00562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Agarwal S, Busse PJ. Innate and adaptive immunosenescence. Annals of allergy, asthma & immunology: official publication of the American College of Allergy, Asthma, & Immunology. 2010;104(3):183–90; quiz 90–2, 210. doi: 10.1016/j.anai.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 69.Plackett TP, Boehmer ED, Faunce DE, Kovacs EJ. Aging and innate immune cells. Journal of Leukocyte Biology. 2004;76(2):291–9. doi: 10.1189/jlb.1103592. [DOI] [PubMed] [Google Scholar]
- 70.Boehmer ED, Goral J, Faunce DE, Kovacs EJ. Age-dependent decrease in Toll-like receptor 4-mediated proinflammatory cytokine production and mitogen-activated protein kinase expression. Journal of Leukocyte Biology. 2004;75(2):342–9. doi: 10.1189/jlb.0803389. [DOI] [PubMed] [Google Scholar]
- 71.Nyugen J, Agrawal S, Gollapudi S, Gupta S. Impaired functions of peripheral blood monocyte subpopulations in aged humans. Journal of Clinical Immunology. 2010;30(6):806–13. doi: 10.1007/s10875-010-9448-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van DD, Mohanty S, Thomas V, Ginter S, Montgomery RR, Fikrig E et al. Age-associated defect in human TLR-1/2 function. J Immunol. 2007;178(2):970–5. [DOI] [PubMed] [Google Scholar]
- 73.Renshaw M, Rockwell J, Engleman C, Gewirtz A, Katz J, Sambhara S. Cutting edge: impaired Toll-like receptor expression and function in aging. J Immunol. 2002;169(9):4697–701. [DOI] [PubMed] [Google Scholar]
- 74.Cuervo AM, Macian F. Autophagy and the immune function in aging. Current Opinion in Immunology. 2014;29:97–104. doi: 10.1016/j.coi.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Castillo EF, Dekonenko A, Arko-Mensah J, Mandell MA, Dupont N, Jiang S et al. Autophagy protects against active tuberculosis by suppressing bacterial burden and inflammation. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(46):E3168–76. doi: 10.1073/pnas.1210500109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nature immunology. 2011;12(3):222–30. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yoon P, Keylock KT, Hartman ME, Freund GG, Woods JA. Macrophage hypo-responsiveness to interferon-gamma in aged mice is associated with impaired signaling through Jak-STAT. Mechanisms of Ageing and Development. 2004;125(2):137–43. doi: 10.1016/j.mad.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 78.Davila DR, Edwards CK 3rd, Arkins S, Simon J, Kelley KW. Interferon-gamma-induced priming for secretion of superoxide anion and tumor necrosis factor-alpha declines in macrophages from aged rats. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 1990;4(11):2906–11. [DOI] [PubMed] [Google Scholar]
- 79.Herrero C, Marques L, Lloberas J, Celada A. IFN-gamma-dependent transcription of MHC class II IA is impaired in macrophages from aged mice. The Journal of clinical investigation. 2001;107(4):485–93. doi: 10.1172/JCI11696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Herrero C, Sebastian C, Marques L, Comalada M, Xaus J, Valledor AF et al. Immunosenescence of macrophages: reduced MHC class II gene expression. Experimental Gerontology. 2002;37(2–3):389–94. [DOI] [PubMed] [Google Scholar]
- 81.Villanueva JL, Solana R, Alonso MC, Pena J. Changes in the expression of HLA-class II antigens on peripheral blood monocytes from aged humans. Disease Markers. 1990;8(2):85–91. [PubMed] [Google Scholar]
- 82.Seidler S, Zimmermann HW, Bartneck M, Trautwein C, Tacke F. Age-dependent alterations of monocyte subsets and monocyte-related chemokine pathways in healthy adults. BMC immunology. 2010;11:30. doi: 10.1186/1471-2172-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sadeghi HM, Schnelle JF, Thoma JK, Nishanian P, Fahey JL. Phenotypic and functional characteristics of circulating monocytes of elderly persons. Experimental Gerontology. 1999;34(8):959–70. [DOI] [PubMed] [Google Scholar]
- 84.Wendt K, Wilk E, Buyny S, Buer J, Schmidt RE, Jacobs R. Gene and protein characteristics reflect functional diversity of CD56dim and CD56bright NK cells. Journal of Leukocyte Biology. 2006;80(6):1529–41. doi: 10.1189/jlb.0306191. [DOI] [PubMed] [Google Scholar]
- 85.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331(6013):44–9. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chidrawar SM, Khan N, Chan YL, Nayak L, Moss PA. Ageing is associated with a decline in peripheral blood CD56bright NK cells. Immun Ageing. 2006;3:10. doi: 10.1186/1742-4933-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Le Garff-Tavernier M, Beziat V, Decocq J, Siguret V, Gandjbakhch F, Pautas E et al. Human NK cells display major phenotypic and functional changes over the life span. Aging Cell. 2010;9(4):527–35. doi: 10.1111/j.1474-9726.2010.00584.x. [DOI] [PubMed] [Google Scholar]
- 88.Bjorkstrom NK, Riese P, Heuts F, Andersson S, Fauriat C, Ivarsson MA et al. Expression patterns of NKG2A, KIR, and CD57 define a process of CD56dim NK-cell differentiation uncoupled from NK-cell education. Blood. 2010;116(19):3853–64. doi: 10.1182/blood-2010-04-281675. [DOI] [PubMed] [Google Scholar]
- 89.Lopez-Verges S, Milush JM, Pandey S, York VA, Arakawa-Hoyt J, Pircher H et al. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK-cell subset. Blood. 2010;116(19):3865–74. doi: 10.1182/blood-2010-04-282301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hayhoe RP, Henson SM, Akbar AN, Palmer DB. Variation of human natural killer cell phenotypes with age: identification of a unique KLRG1-negative subset. Human Immunology. 2010;71(7):676–81. doi: 10.1016/j.humimm.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 91.Almeida-Oliveira A, Smith-Carvalho M, Porto LC, Cardoso-Oliveira J, Ribeiro AS, Falcao RR et al. Age-related changes in natural killer cell receptors from childhood through old age. Human Immunology. 2011;72(4):319–29. [DOI] [PubMed] [Google Scholar]
- 92.Lutz CT, Moore MB, Bradley S, Shelton BJ, Lutgendorf SK. Reciprocal age related change in natural killer cell receptors for MHC class I. Mechanisms of Ageing and Development. 2005;126(6–7):722–31. doi: 10.1016/j.mad.2005.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mildner A, Jung S. Development and function of dendritic cell subsets. Immunity. 2014;40(5):642–56. doi: 10.1016/j.immuni.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 94.Lande R, Gilliet M. Plasmacytoid dendritic cells: key players in the initiation and regulation of immune responses. Annals of the New York Academy of Sciences. 2010;1183:89–103. doi: 10.1111/j.1749-6632.2009.05152.x. [DOI] [PubMed] [Google Scholar]
- 95.Agrawal A, Gupta S. Impact of aging on dendritic cell functions in humans. Ageing Res Rev. 2011;10(3):336–45. doi: 10.1016/j.arr.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sridharan A, Esposo M, Kaushal K, Tay J, Osann K, Agrawal S et al. Age-associated impaired plasmacytoid dendritic cell functions lead to decreased CD4 and CD8 T cell immunity. Age (Dordr). 2011;33(3):363–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Agrawal A, Agrawal S, Tay J, Gupta S. Biology of dendritic cells in aging. Journal of Clinical Immunology. 2008;28(1):14–20. doi: 10.1007/s10875-007-9127-6. [DOI] [PubMed] [Google Scholar]
- 98.Della Bella S, Bierti L, Presicce P, Arienti R, Valenti M, Saresella M et al. Peripheral blood dendritic cells and monocytes are differently regulated in the elderly. Clinical immunology. 2007;122(2):220–8. doi: 10.1016/j.clim.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 99.Agrawal A, Agrawal S, Cao JN, Su H, Osann K, Gupta S. Altered innate immune functioning of dendritic cells in elderly humans: a role of phosphoinositide 3-kinase-signaling pathway. Journal of Immunology. 2007;178(11):6912–22. [DOI] [PubMed] [Google Scholar]
- 100.Aydar Y, Balogh P, Tew JG, Szakal AK. Age-related depression of FDC accessory functions and CD21 ligand-mediated repair of co-stimulation. European Journal of Immunology. 2002;32(10):2817–26. doi:. [DOI] [PubMed] [Google Scholar]
- 101.Aydar Y, Balogh P, Tew JG, Szakal AK. Altered regulation of Fc gamma RII on aged follicular dendritic cells correlates with immunoreceptor tyrosine-based inhibition motif signaling in B cells and reduced germinal center formation. Journal of Immunology. 2003;171(11):5975–87. [DOI] [PubMed] [Google Scholar]
- 102.Aydar Y, Balogh P, Tew JG, Szakal AK. Follicular dendritic cells in aging, a “bottle-neck” in the humoral immune response. Ageing Res Rev. 2004;3(1):15–29. doi: 10.1016/j.arr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 103.Dorrington MG, Bowdish DM. Immunosenescence and novel vaccination strategies for the elderly. Front Immunol. 2013;4:171. doi: 10.3389/fimmu.2013.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim C, Fang F, Weyand CM, Goronzy JJ. The life cycle of a T cell after vaccination - where does immune ageing strike? Clinical and Experimental Immunology. 2017;187(1):71–81. doi: 10.1111/cei.12829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sherwood EM, Xu W, Riley RL. B cell precursors in senescent mice exhibit decreased recruitment into proliferative compartments and altered expression of Bcl-2 family members. Mechanisms of Ageing and Development. 2003;124(2):147–53. [DOI] [PubMed] [Google Scholar]
- 106.Stephan RP, Sanders VM, Witte PL. Stage-specific alterations in murine B lymphopoiesis with age. International Immunology. 1996;8(4):509–18. [DOI] [PubMed] [Google Scholar]
- 107.King AM, Van der Put E, Blomberg BB, Riley RL. Accelerated Notch-dependent degradation of E47 proteins in aged B cell precursors is associated with increased ERK MAPK activation. Journal of Immunology. 2007;178(6):3521–9. [DOI] [PubMed] [Google Scholar]
- 108.Van der Put E, Frasca D, King AM, Blomberg BB, Riley RL. Decreased E47 in senescent B cell precursors is stage specific and regulated posttranslationally by protein turnover. Journal of Immunology. 2004;173(2):818–27. [DOI] [PubMed] [Google Scholar]
- 109.Labrie JE 3rd, Sah AP, Allman DM, Cancro MP, Gerstein RM. Bone marrow microenvironmental changes underlie reduced RAG-mediated recombination and B cell generation in aged mice. The Journal of experimental medicine. 2004;200(4):411–23. doi: 10.1084/jem.20040845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stephan RP, Lill-Elghanian DA, Witte PL. Development of B cells in aged mice: decline in the ability of pro-B cells to respond to IL-7 but not to other growth factors. Journal of Immunology. 1997;158(4):1598–609. [PubMed] [Google Scholar]
- 111.Kline GH, Hayden TA, Klinman NR. B cell maintenance in aged mice reflects both increased B cell longevity and decreased B cell generation. Journal of Immunology. 1999;162(6):3342–9. [PubMed] [Google Scholar]
- 112.Gibson KL, Wu YC, Barnett Y, Duggan O, Vaughan R, Kondeatis E et al. B-cell diversity decreases in old age and is correlated with poor health status. Aging Cell. 2009;8(1):18–25. doi: 10.1111/j.1474-9726.2008.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Guerrettaz LM, Johnson SA, Cambier JC. Acquired hematopoietic stem cell defects determine B-cell repertoire changes associated with aging. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(33):11898–902. doi: 10.1073/pnas.0805498105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Frasca D, Landin AM, Riley RL, Blomberg BB. Mechanisms for decreased function of B cells in aged mice and humans. Journal of Immunology. 2008;180(5):2741–6. [DOI] [PubMed] [Google Scholar]
- 115.Frasca D, Van der Put E, Riley RL, Blomberg BB. Reduced Ig class switch in aged mice correlates with decreased E47 and activation-induced cytidine deaminase. Journal of Immunology. 2004;172(4):2155–62. [DOI] [PubMed] [Google Scholar]
- 116.Frasca D, Landin AM, Alvarez JP, Blackshear PJ, Riley RL, Blomberg BB. Tristetraprolin, a negative regulator of mRNA stability, is increased in old B cells and is involved in the degradation of E47 mRNA. Journal of Immunology. 2007;179(2):918–27. [DOI] [PubMed] [Google Scholar]
- 117.Tu W, Rao S. Mechanisms Underlying T Cell Immunosenescence: Aging and Cytomegalovirus Infection. Front Microbiol. 2016;7:2111. doi: 10.3389/fmicb.2016.02111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Naylor K, Li G, Vallejo AN, Lee WW, Koetz K, Bryl E et al. The influence of age on T cell generation and TCR diversity. Journal of Immunology. 2005;174(11):7446–52. [DOI] [PubMed] [Google Scholar]
- 119.Britanova OV, Putintseva EV, Shugay M, Merzlyak EM, Turchaninova MA, Staroverov DB et al. Age-related decrease in TCR repertoire diversity measured with deep and normalized sequence profiling. Journal of Immunology. 2014;192(6):2689–98. doi: 10.4049/jimmunol.1302064. [DOI] [PubMed] [Google Scholar]
- 120.Nikolich-Zugich J. Ageing and life-long maintenance of T-cell subsets in the face of latent persistent infections. Nature reviews Immunology. 2008;8(7):512–22. doi: 10.1038/nri2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Goronzy JJ, Fang F, Cavanagh MM, Qi Q, Weyand CM. Naive T cell maintenance and function in human aging. Journal of Immunology. 2015;194(9):4073–80. doi: 10.4049/jimmunol.1500046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mueller SN, Gebhardt T, Carbone FR, Heath WR. Memory T cell subsets, migration patterns, and tissue residence. Annual Review of Immunology. 2013;31:137–61. doi: 10.1146/annurev-immunol-032712-095954. [DOI] [PubMed] [Google Scholar]
- 123.Wertheimer AM, Bennett MS, Park B, Uhrlaub JL, Martinez C, Pulko V et al. Aging and cytomegalovirus infection differentially and jointly affect distinct circulating T cell subsets in humans. Journal of Immunology. 2014;192(5):2143–55. doi: 10.4049/jimmunol.1301721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pawelec G, Derhovanessian E. Role of CMV in immune senescence. Virus Research. 2011;157(2):175–9. doi: 10.1016/j.virusres.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 125.Kim J, Kim AR, Shin EC. Cytomegalovirus Infection and Memory T Cell Inflation. Immune Netw. 2015;15(4):186–90. doi: 10.4110/in.2015.15.4.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Moro-Garcia MA, Alonso-Arias R, Lopez-Larrea C. When Aging Reaches CD4+ T-Cells: Phenotypic and Functional Changes. Front Immunol. 2013;4:107. doi: 10.3389/fimmu.2013.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Saurwein-Teissl M, Lung TL, Marx F, Gschosser C, Asch E, Blasko I et al. Lack of antibody production following immunization in old age: association with CD8(+)CD28(−) T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J Immunol. 2002;168(11):5893–9. [DOI] [PubMed] [Google Scholar]
- 128.Goronzy JJ, Fulbright JW, Crowson CS, Poland GA, O’Fallon WM, Weyand CM. Value of immunological markers in predicting responsiveness to influenza vaccination in elderly individuals. J Virol. 2001;75(24):12182–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Herndler-Brandstetter D, Ishigame H, Flavell RA. How to define biomarkers of human T cell aging and immunocompetence? Front Immunol. 2013;4:136. doi: 10.3389/fimmu.2013.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Witkowski JM, Bryl E. Paradoxical age-related cell cycle quickening of human CD4(+) lymphocytes: a role for cyclin D1 and calpain. Experimental Gerontology. 2004;39(4):577–85. doi: 10.1016/j.exger.2003.10.028. [DOI] [PubMed] [Google Scholar]
- 131.Goronzy JJ, Li G, Yu M, Weyand CM. Signaling pathways in aged T cells - a reflection of T cell differentiation, cell senescence and host environment. Seminars in Immunology. 2012;24(5):365–72. doi: 10.1016/j.smim.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bryl E, Vallejo AN, Matteson EL, Witkowski JM, Weyand CM, Goronzy JJ. Modulation of CD28 expression with anti-tumor necrosis factor alpha therapy in rheumatoid arthritis. Arthritis and Rheumatism. 2005;52(10):2996–3003. [DOI] [PubMed] [Google Scholar]
- 133.Lewis DE, Merched-Sauvage M, Goronzy JJ, Weyand CM, Vallejo AN. Tumor necrosis factor-alpha and CD80 modulate CD28 expression through a similar mechanism of T-cell receptor-independent inhibition of transcription. J BiolChem. 2004;279(28):29130–8. [DOI] [PubMed] [Google Scholar]
- 134.Koch S, Larbi A, Derhovanessian E, Ozcelik D, Naumova E, Pawelec G. Multiparameter flow cytometric analysis of CD4 and CD8 T cell subsets in young and old people. Immun Ageing. 2008;5:6. doi: 10.1186/1742-4933-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ouyang Q, Wagner WM, Zheng W, Wikby A, Remarque EJ, Pawelec G. Dysfunctional CMV-specific CD8(+) T cells accumulate in the elderly. Experimental Gerontology. 2004;39(4):607–13. doi: 10.1016/j.exger.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 136.Saule P, Trauet J, Dutriez V, Lekeux V, Dessaint JP, Labalette M. Accumulation of memory T cells from childhood to old age: central and effector memory cells in CD4(+) versus effector memory and terminally differentiated memory cells in CD8(+) compartment. Mechanisms of Ageing and Development. 2006;127(3):274–81. [DOI] [PubMed] [Google Scholar]
- 137.Geissmann F, Gordon S, Hume DA, Mowat AM, Randolph GJ. Unravelling mononuclear phagocyte heterogeneity. Nature reviews Immunology. 2010;10(6):453–60. doi: 10.1038/nri2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Putiri EL, Robertson KD. Epigenetic mechanisms and genome stability. Clin Epigenetics. 2011;2(2):299–314. doi: 10.1007/s13148-010-0017-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet. 2012;13(7):484–92. doi: 10.1038/nrg3230. [DOI] [PubMed] [Google Scholar]
- 140.Lee SM, Choi WY, Lee J, Kim YJ. The regulatory mechanisms of intragenic DNA methylation. Epigenomics. 2015;7(4):527–31. doi: 10.2217/epi.15.38. [DOI] [PubMed] [Google Scholar]
- 141.Jones PA, Takai D. The role of DNA methylation in mammalian epigenetics. Science. 2001;293(5532):1068–70. doi: 10.1126/science.1063852. [DOI] [PubMed] [Google Scholar]
- 142.Farre P, Jones MJ, Meaney MJ, Emberly E, Turecki G, Kobor MS. Concordant and discordant DNA methylation signatures of aging in human blood and brain. Epigenetics Chromatin. 2015;8:19. doi: 10.1186/s13072-015-0011-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14(10):R115. doi: 10.1186/gb-2013-14-10-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Weidner CI, Wagner W. The epigenetic tracks of aging. Biol Chem. 2014;395(11):1307–14. doi: 10.1515/hsz-2014-0180. [DOI] [PubMed] [Google Scholar]
- 145.Johnson AA, Akman K, Calimport SR, Wuttke D, Stolzing A, de Magalhaes JP. The role of DNA methylation in aging, rejuvenation, and age-related disease. Rejuvenation Res. 2012;15(5):483–94. doi: 10.1089/rej.2012.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bocker MT, Hellwig I, Breiling A, Eckstein V, Ho AD, Lyko F. Genome-wide promoter DNA methylation dynamics of human hematopoietic progenitor cells during differentiation and aging. Blood. 2011;117(19):e182–9. doi: 10.1182/blood-2011-01-331926. [DOI] [PubMed] [Google Scholar]
- 147.Gowers IR, Walters K, Kiss-Toth E, Read RC, Duff GW, Wilson AG. Age-related loss of CpG methylation in the tumour necrosis factor promoter. Cytokine. 2011;56(3):792–7. doi: 10.1016/j.cyto.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 148.Wong CP, Rinaldi NA, Ho E. Zinc deficiency enhanced inflammatory response by increasing immune cell activation and inducing IL6 promoter demethylation. Mol Nutr Food Res. 2015;59(5):991–9. doi: 10.1002/mnfr.201400761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kutza J, Murasko DM. Effects of aging on natural killer cell activity and activation by interleukin-2 and IFN-alpha. Cellular Immunology. 1994;155(1):195–204. doi: 10.1006/cimm.1994.1112. [DOI] [PubMed] [Google Scholar]
- 150.Zhao M, Qin J, Yin H, Tan Y, Liao W, Liu Q et al. Distinct epigenomes in CD4(+) T cells of newborns, middle-ages and centenarians. Sci Rep. 2016;6:38411. doi: 10.1038/srep38411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Suarez-Alvarez B, Rodriguez RM, Schlangen K, Raneros AB, Marquez-Kisinousky L, Fernandez AF et al. Phenotypic characteristics of aged CD4(+) CD28(null) T lymphocytes are determined by changes in the whole-genome DNA methylation pattern. Aging Cell. 2017;16(2):293–303. doi: 10.1111/acel.12552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Garg SK, Delaney C, Toubai T, Ghosh A, Reddy P, Banerjee R et al. Aging is associated with increased regulatory T-cell function. Aging Cell. 2014;13(3):441–8. doi: 10.1111/acel.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Parra M. Epigenetic events during B lymphocyte development. Epigenetics. 2009;4(7):462–8. [DOI] [PubMed] [Google Scholar]
- 154.Bao Y, Cao X. Epigenetic Control of B Cell Development and B-Cell-Related Immune Disorders. Clin Rev Allergy Immunol. 2016;50(3):301–11. doi: 10.1007/s12016-015-8494-7. [DOI] [PubMed] [Google Scholar]
- 155.Kennedy RB, Ovsyannikova IG, Haralambieva IH, Oberg AL, Zimmermann MT, Grill DE et al. Immunosenescence-related transcriptomic and immunologic changes in older individuals following influenza vaccination. Front Immunol. 2016;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chang YC, Chen TC, Lee CT, Yang CY, Wang HW, Wang CC et al. Epigenetic control of MHC class II expression in tumor-associated macrophages by decoy receptor 3. Blood. 2008;111(10):5054–63. doi: 10.1182/blood-2007-12-130609. [DOI] [PubMed] [Google Scholar]
- 157.Kleinnijenhuis J, Quintin J, Preijers F, Joosten LA, Ifrim DC, Saeed S et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(43):17537–42. doi: 10.1073/pnas.1202870109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Dozmorov MG, Coit P, Maksimowicz-McKinnon K, Sawalha AH. Age-associated DNA methylation changes in naive CD4(+) T cells suggest an evolving autoimmune epigenotype in aging T cells. Epigenomics. 2017;9(4):429–45. doi: 10.2217/epi-2016-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sidler C, Woycicki R, Ilnytskyy Y, Metz G, Kovalchuk I, Kovalchuk O. Immunosenescence is associated with altered gene expression and epigenetic regulation in primary and secondary immune organs. Front Genet. 2013;4:211. doi: 10.3389/fgene.2013.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Jung HJ, Suh Y. MicroRNA in Aging: From Discovery to Biology. Curr Genomics. 2012;13(7):548–57. doi: 10.2174/138920212803251436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jasiulionis MG. Abnormal Epigenetic Regulation of Immune System during Aging. Front Immunol. 2018;9:197. doi: 10.3389/fimmu.2018.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Roy S, Sen CK. MiRNA in innate immune responses: novel players in wound inflammation. Physiological genomics. 2011;43(10):557–65. doi: 10.1152/physiolgenomics.00160.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dai R, Ahmed SA. MicroRNA, a new paradigm for understanding immunoregulation, inflammation, and autoimmune diseases. Transl Res. 2011;157(4):163–79. doi: 10.1016/j.trsl.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Brain O, Owens BM, Pichulik T, Allan P, Khatamzas E, Leslie A et al. The intracellular sensor NOD2 induces microRNA-29 expression in human dendritic cells to limit IL-23 release. Immunity. 2013;39(3):521–36. doi: 10.1016/j.immuni.2013.08.035. [DOI] [PubMed] [Google Scholar]
- 165.Cobos Jimenez V, Bradley EJ, Willemsen AM, van Kampen AH, Baas F, Kootstra NA. Next-generation sequencing of microRNAs uncovers expression signatures in polarized macrophages. Physiological genomics. 2014;46(3):91–103. doi: 10.1152/physiolgenomics.00140.2013. [DOI] [PubMed] [Google Scholar]
- 166.Ugalde AP, Ramsay AJ, de la Rosa J, Varela I, Marino G, Cadinanos J et al. Aging and chronic DNA damage response activate a regulatory pathway involving miR-29 and p53. The EMBO journal. 2011;30(11):2219–32. doi: 10.1038/emboj.2011.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Jiang M, Xiang Y, Wang D, Gao J, Liu D, Liu Y et al. Dysregulated expression of miR-146a contributes to age-related dysfunction of macrophages. Aging Cell. 2012;11(1):29–40. doi: 10.1111/j.1474-9726.2011.00757.x. [DOI] [PubMed] [Google Scholar]
- 168.Dueck A, Eichner A, Sixt M, Meister G. A miR-155-dependent microRNA hierarchy in dendritic cell maturation and macrophage activation. FEBS Letters. 2014;588(4):632–40. doi: 10.1016/j.febslet.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 169.Frasca D, Diaz A, Romero M, Ferracci F, Blomberg BB. MicroRNAs miR-155 and miR-16 Decrease AID and E47 in B Cells from Elderly Individuals. Journal of Immunology. 2015;195(5):2134–40. doi: 10.4049/jimmunol.1500520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ohyashiki M, Ohyashiki JH, Hirota A, Kobayashi C, Ohyashiki K. Age-related decrease of miRNA-92a levels in human CD8+ T-cells correlates with a reduction of naive T lymphocytes. Immun Ageing. 2011;8(1):11. doi: 10.1186/1742-4933-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Li G, Yu M, Lee WW, Tsang M, Krishnan E, Weyand CM et al. Decline in miR-181a expression with age impairs T cell receptor sensitivity by increasing DUSP6 activity. Nature Medicine. 2012;18(10):1518–24. doi: 10.1038/nm.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Voigt EA, Ovsyannikova IG, Kennedy RB, Grill DE, Goergen KM, Schaid DJ et al. Sex Differences in Older Adults’ Immune Responses to Seasonal Influenza Vaccination. Front Immunol. 2019;10:180. doi: 10.3389/fimmu.2019.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lambert ND, Ovsyannikova IG, Pankratz VS, Jacobson RM, Poland GA. Understanding the immune response to seasonal influenza vaccination in older adults: a systems biology approach. Expert Rev Vaccines. 2012;11(8):985–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Li S, Rouphael N, Duraisingham S, Romero-Steiner S, Presnell S, Davis C et al. Molecular signatures of antibody responses derived from a systems biology study of five human vaccines. Nature immunology. 2014;15(2):195–204. doi: 10.1038/ni.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.VanderWeele TJ. Explanation in causal inference: developments in mediation and interaction. International Journal of Epidemiology. 2016;45(6):1904–8. doi: 10.1093/ije/dyw277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.VanderWeele TJ. Marginal structural models for the estimation of direct and indirect effects. Epidemiology. 2009;20(1):18–26. doi: 10.1097/EDE.0b013e31818f69ce. [DOI] [PubMed] [Google Scholar]
- 177.O’Garra A. Systems Approach to Understand the Immune Response in Tuberculosis: An Iterative Process between Mouse Models and Human Disease. Cold Spring Harb Symp Quant Biol. 2013. doi: 10.1101/sqb.2013.78.020172. [DOI] [PubMed] [Google Scholar]
- 178.Wang CC, Zhu B, Fan X, Gicquel B, Zhang Y. Systems approach to tuberculosis vaccine development. Respirology. 2013;18(3):412–20. doi: 10.1111/resp.12052. [DOI] [PubMed] [Google Scholar]
- 179.Young D, Stark J, Kirschner D. Systems biology of persistent infection: tuberculosis as a case study. Nat Rev Microbiol. 2008;6(7):520–8. doi: 10.1038/nrmicro1919. [DOI] [PubMed] [Google Scholar]
- 180.Day J, Schlesinger LS, Friedman A. Tuberculosis research: going forward with a powerful “translational systems biology” approach. Tuberculosis (Edinb). 2010;90(1):7–8. doi: 10.1016/j.tube.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Obermoser G, Presnell S, Domico K, Xu H, Wang Y, Anguiano E et al. Systems scale interactive exploration reveals quantitative and qualitative differences in response to influenza and pneumococcal vaccines. Immunity. 2013;38(4):831–44. doi: 10.1016/j.immuni.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tan Y, Tamayo P, Nakaya H, Pulendran B, Mesirov JP, Haining WN. Gene signatures related to B-cell proliferation predict influenza vaccine-induced antibody response. European Journal of Immunology. 2014;44(1):285–95. doi: 10.1002/eji.201343657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Franco LM, Bucasas KL, Wells JM, Nino D, Wang X, Zapata GE et al. Integrative genomic analysis of the human immune response to influenza vaccination. Elife. 2013;2:e00299. doi: 10.7554/eLife.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Haralambieva IH, Gibson MJ, Kennedy RB, Ovsyannikova IG, Warner ND, Grill DE et al. Characterization of rubella-specific humoral immunity following two doses of MMR vaccine using proteome microarray technology. PLos ONE. 2017;12(11):e0188149. doi: 10.1371/journal.pone.0188149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Haralambieva IH, Lambert ND, Ovsyannikova IG, Kennedy RB, Larrabee BR, Pankratz VS et al. Associations between single nucleotide polymorphisms in cellular viral receptors and attachment factor-related genes and humoral immunity to rubella vaccination. PloS one. 2014;9(6):e99997. doi: 10.1371/journal.pone.0099997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lambert ND, Haralambieva IH, Kennedy RB, Ovsyannikova IG, Pankrantz VS, Poland GA. Polymorphisms in HLA-DPB1 are associated with differences in rubella-specific humoral immunity after vaccination. Journal of Infectious Diseases. 2015;211(6):898–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Haralambieva IH, Ovsyannikova IG, Kennedy RB, Zimmermann MT, Grill DE, Oberg AL et al. Transcriptional signatures of influenza A/H1N1-specific IgG memory-like B cell response in older individuals. Vaccine. 2016;34(34):3993–4002. doi: 10.1016/j.vaccine.2016.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Haralambieva IH, Painter SD, Kennedy RB, Ovsyannikova IG, Lambert ND, Goergen KM et al. The Impact of Immunosenescence on Humoral Immune Response Variation after Influenza A/H1N1 Vaccination in Older Subjects. PLos ONE. 2015;10(3):e0122282. doi: 10.1371/journal.pone.0122282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kennedy RB, Simon WL, Gibson MJ, Goergen KM, Grill DE, Oberg AL et al. The composition of immune cells serves as a predictor of adaptive immunity in a cohort of 50- to 74-year-old adults. Immunology. 2016;148(3):266–75. doi: 10.1111/imm.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Poland GA, Ovsyannikova IG, Kennedy RB, Lambert ND, Kirkland JL. A systems biology approach to the effect of aging, immunosenescence and vaccine response. Current Opinion in Immunology. 2014;29C:62–8. doi: 10.1016/j.coi.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Voigt EA, Grill DE, Zimmermann MT, Simon WL, Ovsyannikova IG, Kennedy RB et al. Transcriptomic signatures of cellular and humoral immune responses in older adults after seasonal influenza vaccination identified by data-driven clustering. Sci Rep. 2018;8(1):739. doi: 10.1038/s41598-017-17735-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zimmermann MT, Kennedy RB, Grill DE, Oberg AL, Goergen KM, Ovsyannikova IG et al. Integration of Immune Cell Populations, mRNA-Seq, and CpG Methylation to Better Predict Humoral Immunity to Influenza Vaccination: Dependence of mRNA-Seq/CpG Methylation on Immune Cell Populations. Front Immunol. 2017;8:445. doi: 10.3389/fimmu.2017.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Weinberger B. Vaccines for the elderly: current use and future challenges. Immun Ageing. 2018;15:3. doi: 10.1186/s12979-017-0107-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Weinberger B. Adjuvant strategies to improve vaccination of the elderly population. Curr Opin Pharmacol. 2018;41:34–41. doi: 10.1016/j.coph.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 195.Kirkland JL, Tchkonia T. Cellular Senescence: A Translational Perspective. EBioMedicine. 2017;21:21–8. doi: 10.1016/j.ebiom.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.O’Hagan DT, Friedland LR, Hanon E, Didierlaurent AM. Towards an evidence based approach for the development of adjuvanted vaccines. Current Opinion in Immunology. 2017;47:93–102. doi: 10.1016/j.coi.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 197.Molinari NA, Ortega-Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, Weintraub E et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25(27):5086–96. [DOI] [PubMed] [Google Scholar]
- 198.Bonmarin I, Belchior E, Levy-Bruhl D. Impact of influenza vaccination on mortality in the French elderly population during the 2000–2009 period. Vaccine. 2015;33(9):1099–101. doi: 10.1016/j.vaccine.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 199.Molbak K, Espenhain L, Nielsen J, Tersago K, Bossuyt N, Denissov G et al. Excess mortality among the elderly in European countries, December 2014 to February 2015. Euro Surveill. 2015;20(11). [DOI] [PubMed] [Google Scholar]
- 200.DiazGranados CA, Dunning AJ, Kimmel M, Kirby D, Treanor J, Collins A et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. The New England Journal of Medicine. 2014;371(7):635–45. doi: 10.1056/NEJMoa1315727. [DOI] [PubMed] [Google Scholar]
- 201.Del Giudice G, Hilbert AK, Bugarini R, Minutello A, Popova O, Toneatto D et al. An MF59-adjuvanted inactivated influenza vaccine containing A/Panama/1999 (H3N2) induced broader serological protection against heterovariant influenza virus strain A/Fujian/2002 than a subunit and a split influenza vaccine. Vaccine. 2006;24(16):3063–5. [DOI] [PubMed] [Google Scholar]
- 202.Ansaldi F, Bacilieri S, Durando P, Sticchi L, Valle L, Montomoli E et al. Cross-protection by MF59-adjuvanted influenza vaccine: neutralizing and haemagglutination-inhibiting antibody activity against A(H3N2) drifted influenza viruses. Vaccine. 2008;26(12):1525–9. doi: 10.1016/j.vaccine.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 203.O’Hagan DT, Ott GS, De Gregorio E, Seubert A. The mechanism of action of MF59 - an innately attractive adjuvant formulation. Vaccine. 2012;30(29):4341–8. doi: 10.1016/j.vaccine.2011.09.061. [DOI] [PubMed] [Google Scholar]
- 204.Cioncada R, Maddaluno M, Vo HTM, Woodruff M, Tavarini S, Sammicheli C et al. Vaccine adjuvant MF59 promotes the intranodal differentiation of antigen-loaded and activated monocyte-derived dendritic cells. PLos ONE. 2017;12(10):e0185843. doi: 10.1371/journal.pone.0185843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Nicholson KG, Abrams KR, Batham S, Clark TW, Hoschler K, Lim WS et al. Immunogenicity and safety of a two-dose schedule of whole-virion and AS03A-adjuvanted 2009 influenza A (H1N1) vaccines: a randomised, multicentre, age-stratified, head-to-head trial. The Lancet Infectious diseases. 2011;11(2):91–101. doi: 10.1016/S1473-3099(10)70296-6. [DOI] [PubMed] [Google Scholar]
- 206.Madan A, Ferguson M, Rheault P, Seiden D, Toma A, Friel D et al. Immunogenicity and safety of an AS03-adjuvanted H7N1 vaccine in adults 65years of age and older: A phase II, observer-blind, randomized, controlled trial. Vaccine. 2017;35(15):1865–72. doi: 10.1016/j.vaccine.2017.02.057. [DOI] [PubMed] [Google Scholar]
- 207.Madan A, Collins H, Sheldon E, Frenette L, Chu L, Friel D et al. Evaluation of a primary course of H9N2 vaccine with or without AS03 adjuvant in adults: A phase I/II randomized trial. Vaccine. 2017;35(35 Pt B):4621–8. doi: 10.1016/j.vaccine.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 208.Garcon N, Vaughn DW, Didierlaurent AM. Development and evaluation of AS03, an Adjuvant System containing alpha-tocopherol and squalene in an oil-in-water emulsion. Expert Review of Vaccines. 2012;11(3):349–66. doi: 10.1586/erv.11.192. [DOI] [PubMed] [Google Scholar]
- 209.Pleguezuelos O, Robinson S, Stoloff GA, Caparros-Wanderley W. Synthetic Influenza vaccine (FLU-v) stimulates cell mediated immunity in a double-blind, randomised, placebo-controlled Phase I trial. Vaccine. 2012;30(31):4655–60. doi: 10.1016/j.vaccine.2012.04.089. [DOI] [PubMed] [Google Scholar]
- 210.Christensen D, Christensen JP, Korsholm KS, Isling LK, Erneholm K, Thomsen AR et al. Seasonal Influenza Split Vaccines Confer Partial Cross-Protection against Heterologous Influenza Virus in Ferrets When Combined with the CAF01 Adjuvant. Front Immunol. 2017;8:1928. doi: 10.3389/fimmu.2017.01928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Liu F, Sun X, Fairman J, Lewis DB, Katz JM, Levine M et al. A cationic liposome-DNA complexes adjuvant (JVRS-100) enhances the immunogenicity and cross-protective efficacy of pre-pandemic influenza A (H5N1) vaccine in ferrets. Virology. 2016;492:197–203. doi: 10.1016/j.virol.2016.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.van Doorn E, Liu H, Huckriede A, Hak E. Safety and tolerability evaluation of the use of Montanide ISA51 as vaccine adjuvant: A systematic review. Hum Vaccin Immunother. 2016;12(1):159–69. doi: 10.1080/21645515.2015.1071455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.van Dissel JT, Joosten SA, Hoff ST, Soonawala D, Prins C, Hokey DA et al. A novel liposomal adjuvant system, CAF01, promotes long-lived Mycobacterium tuberculosis-specific T-cell responses in human. Vaccine. 2014;32(52):7098–107. doi: 10.1016/j.vaccine.2014.10.036. [DOI] [PubMed] [Google Scholar]
- 214.Gnjatic S, Sawhney NB, Bhardwaj N. Toll-like receptor agonists: are they good adjuvants? Cancer J. 2010;16(4):382–91. doi: 10.1097/PPO.0b013e3181eaca65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Mifsud EJ, Tan AC, Jackson DC. TLR Agonists as Modulators of the Innate Immune Response and Their Potential as Agents Against Infectious Disease. Front Immunol. 2014;5:79. doi: 10.3389/fimmu.2014.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Weinberger B, Joos C, Reed SG, Coler R, Grubeck-Loebenstein B. The stimulatory effect of the TLR4-mediated adjuvant glucopyranosyl lipid A is well preserved in old age. Biogerontology. 2016;17(1):177–87. doi: 10.1007/s10522-015-9576-x. [DOI] [PubMed] [Google Scholar]
- 217.Behzad H, Huckriede AL, Haynes L, Gentleman B, Coyle K, Wilschut JC et al. GLA-SE, a synthetic toll-like receptor 4 agonist, enhances T-cell responses to influenza vaccine in older adults. The Journal of Infectious Diseases. 2012;205(3):466–73. doi: 10.1093/infdis/jir769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Coler RN, Baldwin SL, Shaverdian N, Bertholet S, Reed SJ, Raman VS et al. A synthetic adjuvant to enhance and expand immune responses to influenza vaccines. PLos ONE. 2010;5(10):e13677. doi: 10.1371/journal.pone.0013677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Desbien AL, Dubois Cauwelaert N, Reed SJ, Bailor HR, Liang H, Carter D et al. IL-18 and Subcapsular Lymph Node Macrophages are Essential for Enhanced B Cell Responses with TLR4 Agonist Adjuvants. Journal of Immunology. 2016;197(11):4351–9. doi: 10.4049/jimmunol.1600993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Zhang AJ, Li C, To KK, Zhu HS, Lee AC, Li CG et al. Toll-like receptor 7 agonist imiquimod in combination with influenza vaccine expedites and augments humoral immune responses against influenza A(H1N1)pdm09 virus infection in BALB/c mice. Clinical and vaccine immunology: CVI. 2014;21(4):570–9. doi: 10.1128/CVI.00816-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Hung IF, Zhang AJ, To KK, Chan JF, Li P, Wong TL et al. Topical imiquimod before intradermal trivalent influenza vaccine for protection against heterologous non-vaccine and antigenically drifted viruses: a single-centre, double-blind, randomised, controlled phase 2b/3 trial. The Lancet Infectious diseases. 2016;16(2):209–18. doi: 10.1016/S1473-3099(15)00354-0. [DOI] [PubMed] [Google Scholar]
- 222.Bode C, Zhao G, Steinhagen F, Kinjo T, Klinman DM. CpG DNA as a vaccine adjuvant. Expert Review of Vaccines. 2011;10(4):499–511. doi: 10.1586/erv.10.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Maisonneuve C, Bertholet S, Philpott DJ, De Gregorio E. Unleashing the potential of NOD- and Toll-like agonists as vaccine adjuvants. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(34):12294–9. doi: 10.1073/pnas.1400478111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Campbell JD. Development of the CpG Adjuvant 1018: A Case Study. Methods Mol Biol. 2017;1494:15–27. doi: 10.1007/978-1-4939-6445-1_2. [DOI] [PubMed] [Google Scholar]
- 225.Janssen JM, Jackson S, Heyward WL, Janssen RS. Immunogenicity of an investigational hepatitis B vaccine with a toll-like receptor 9 agonist adjuvant (HBsAg-1018) compared with a licensed hepatitis B vaccine in subpopulations of healthy adults 18–70 years of age. Vaccine. 2015;33(31):3614–8. doi: 10.1016/j.vaccine.2015.05.070. [DOI] [PubMed] [Google Scholar]
- 226.Heyward WL, Kyle M, Blumenau J, Davis M, Reisinger K, Kabongo ML et al. Immunogenicity and safety of an investigational hepatitis B vaccine with a Toll-like receptor 9 agonist adjuvant (HBsAg-1018) compared to a licensed hepatitis B vaccine in healthy adults 40–70 years of age. Vaccine. 2013;31(46):5300–5. doi: 10.1016/j.vaccine.2013.05.068. [DOI] [PubMed] [Google Scholar]
- 227.Jackson S, Lentino J, Kopp J, Murray L, Ellison W, Rhee M et al. Immunogenicity of a two-dose investigational hepatitis B vaccine, HBsAg-1018, using a toll-like receptor 9 agonist adjuvant compared with a licensed hepatitis B vaccine in adults. Vaccine. 2018;36(5):668–74. doi: 10.1016/j.vaccine.2017.12.038. [DOI] [PubMed] [Google Scholar]
- 228.Barry M, Cooper C. Review of hepatitis B surface antigen-1018 ISS adjuvant-containing vaccine safety and efficacy. Expert Opin Biol Ther. 2007;7(11):1731–7. doi: 10.1517/14712598.7.11.1731. [DOI] [PubMed] [Google Scholar]
- 229.Halperin SA, Ward B, Cooper C, Predy G, Diaz-Mitoma F, Dionne M et al. Comparison of safety and immunogenicity of two doses of investigational hepatitis B virus surface antigen co-administered with an immunostimulatory phosphorothioate oligodeoxyribonucleotide and three doses of a licensed hepatitis B vaccine in healthy adults 18–55 years of age. Vaccine. 2012;30(15):2556–63. doi: 10.1016/j.vaccine.2012.01.087. [DOI] [PubMed] [Google Scholar]
- 230.Henriques-Normark B, Tuomanen EI. The pneumococcus: epidemiology, microbiology, and pathogenesis. Cold Spring Harb Perspect Med. 2013;3(7). doi: 10.1101/cshperspect.a010215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Falkenhorst G, Remschmidt C, Harder T, Hummers-Pradier E, Wichmann O, Bogdan C. Effectiveness of the 23-Valent Pneumococcal Polysaccharide Vaccine (PPV23) against Pneumococcal Disease in the Elderly: Systematic Review and Meta-Analysis. PLos ONE. 2017;12(1):e0169368. doi: 10.1371/journal.pone.0169368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Hak E, Grobbee DE, Sanders EA, Verheij TJ, Bolkenbaas M, Huijts SM et al. Rationale and design of CAPITA: a RCT of 13-valent conjugated pneumococcal vaccine efficacy among older adults. Neth J Med. 2008;66(9):378–83. [PubMed] [Google Scholar]
- 233.Bonten MJ, Huijts SM, Bolkenbaas M, Webber C, Patterson S, Gault S et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. The New England Journal of Medicine. 2015;372(12):1114–25. doi: 10.1056/NEJMoa1408544. [DOI] [PubMed] [Google Scholar]
- 234.Fukuyama Y, King JD, Kataoka K, Kobayashi R, Gilbert RS, Hollingshead SK et al. A combination of Flt3 ligand cDNA and CpG oligodeoxynucleotide as nasal adjuvant elicits protective secretory-IgA immunity to Streptococcus pneumoniae in aged mice. Journal of Immunology. 2011;186(4):2454–61. doi: 10.4049/jimmunol.1002837. [DOI] [PubMed] [Google Scholar]
- 235.Pauksens K, Nilsson AC, Caubet M, Pascal TG, Van Belle P, Poolman JT et al. Randomized controlled study of the safety and immunogenicity of pneumococcal vaccine formulations containing PhtD and detoxified pneumolysin with alum or adjuvant system AS02V in elderly adults. Clinical and vaccine immunology: CVI. 2014;21(5):651–60. doi: 10.1128/CVI.00807-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Mallick-Searle T, Snodgrass B, Brant JM. Postherpetic neuralgia: epidemiology, pathophysiology, and pain management pharmacology. J Multidiscip Healthc. 2016;9:447–54. doi: 10.2147/JMDH.S106340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Oxman MN. Herpes zoster pathogenesis and cell-mediated immunity and immunosenescence. J Am Osteopath Assoc. 2009;109(6 Suppl 2):S13–7. [PubMed] [Google Scholar]
- 238.Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. NEnglJ Med. 2005;352(22):2271–84. [DOI] [PubMed] [Google Scholar]
- 239.Tseng HF, Harpaz R, Luo Y, Hales CM, Sy LS, Tartof SY et al. Declining Effectiveness of Herpes Zoster Vaccine in Adults Aged >/=60 Years. The Journal of Infectious Diseases. 2016;213(12):1872–5. doi: 10.1093/infdis/jiw047. [DOI] [PubMed] [Google Scholar]
- 240.James SF, Chahine EB, Sucher AJ, Hanna C. Shingrix: The New Adjuvanted Recombinant Herpes Zoster Vaccine. The Annals of Pharmacotherapy. 2018;52(7):673–80. doi: 10.1177/1060028018758431. [DOI] [PubMed] [Google Scholar]
- 241.Cunningham AL, Lal H, Kovac M, Chlibek R, Hwang SJ, Diez-Domingo J et al. Efficacy of the Herpes Zoster Subunit Vaccine in Adults 70 Years of Age or Older. The New England Journal of Medicine. 2016;375(11):1019–32. doi: 10.1056/NEJMoa1603800. [DOI] [PubMed] [Google Scholar]
- 242.Lal H, Cunningham AL, Godeaux O, Chlibek R, Diez-Domingo J, Hwang SJ et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. The New England Journal of Medicine. 2015;372(22):2087–96. doi: 10.1056/NEJMoa1501184. [DOI] [PubMed] [Google Scholar]
- 243.Schwarz TF, Volpe S, Catteau G, Chlibek R, David MP, Richardus JH et al. Persistence of immune response to an adjuvanted varicella-zoster virus subunit vaccine for up to year nine in older adults. Hum Vaccin Immunother. 2018;14(6):1370–7. doi: 10.1080/21645515.2018.1442162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Levin MJ, Kroehl ME, Johnson MJ, Hammes A, Reinhold D, Lang N et al. Th1 memory differentiates recombinant from live herpes zoster vaccines. The Journal of clinical investigation. 2018. doi: 10.1172/JCI121484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Knudsen NP, Olsen A, Buonsanti C, Follmann F, Zhang Y, Coler RN et al. Different human vaccine adjuvants promote distinct antigen-independent immunological signatures tailored to different pathogens. Sci Rep. 2016;6:19570. doi: 10.1038/srep19570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Olafsdottir TA, Lindqvist M, Nookaew I, Andersen P, Maertzdorf J, Persson J et al. Comparative Systems Analyses Reveal Molecular Signatures of Clinically tested Vaccine Adjuvants. Sci Rep. 2016;6:39097. doi: 10.1038/srep39097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14(4):644–58. doi: 10.1111/acel.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Mannick JB, Morris M, Hockey HP, Roma G, Beibel M, Kulmatycki K et al. TORC1 inhibition enhances immune function and reduces infections in the elderly. Sci Transl Med. 2018;10(449). doi: 10.1126/scitranslmed.aaq1564. [DOI] [PubMed] [Google Scholar]
- 249.Wang S, Wu B, Xue J, Wang M, Chen R, Wang B. Nizatidine, a small molecular compound, enhances killed H5N1 vaccine cell-mediated responses and protects mice from lethal viral challenge. Hum Vaccin Immunother. 2014;10(2):461–8. doi: 10.4161/hv.27165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Scurr M, Pembroke T, Bloom A, Roberts D, Thomson A, Smart K et al. Low-Dose Cyclophosphamide Induces Antitumor T-Cell Responses, which Associate with Survival in Metastatic Colorectal Cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2017;23(22):6771–80. doi: 10.1158/1078-0432.CCR-17-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Li B, Lalani AS, Harding TC, Luan B, Koprivnikar K, Huan Tu G et al. Vascular endothelial growth factor blockade reduces intratumoral regulatory T cells and enhances the efficacy of a GM-CSF-secreting cancer immunotherapy. Clinical cancer research: an official journal of the American Association for Cancer Research. 2006;12(22):6808–16. doi: 10.1158/1078-0432.CCR-06-1558. [DOI] [PubMed] [Google Scholar]
- 252.Sundberg TB, Xavier RJ, Schreiber SL, Shamji AF. Small-molecule control of cytokine function: new opportunities for treating immune disorders. Curr Opin Chem Biol. 2014;23:23–30. doi: 10.1016/j.cbpa.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]