Abstract
This case report presents a 36-year old man with a disseminated sporotrichosis who presented with seizures and crusted lesions all over the body. Imaging studies revealed acute ischemic brain event with haemorrhagic transformation in right frontal lobe. Skin biopsy showed Sporothrix schenckii. He was treated with standard amphotericin B. Despite therapy, he developed consciousness loss, multiorgan-failure and eventually expired. Necropsy findings showed renal, hepatic, splenic, prostate, testicles and meningeal/cerebral involvement.
Keywords: Necropsy, Brazil, Sporotrichosis, Sporothrix brasiliensis, Sporothrix schenckii, Endemic mycoses
1. Introduction
Sporotrichosis is a granulomatous infection caused by the dimorphic fungus, Sporothrix schenckii, an endemic organism widely distributed throughout the world [1]. During the last decade there has been a significant increase in clinical cases of sporotrichosis in Brazil, particularly in the Southeast region. In Brazil an epidemic has manifested itself in humans, which correlates with transmission by infected cats [2]. Currently this mycosis should be considered an important zoonosis, especially in areas where it is endemic. Infection with the organism is caused by traumatic inoculation of the fungus from cat scratches or materials found on soil, plants and decaying vegetables [3]. This dimorphic fungus grows in a mycelial form at 25 °C, progressing to a yeast stage at 37 °C. The mycelial phase is characterized by slender, hyaline, septate and branched hyphae containing thin conidiophores whose apex forms a small vesicle with sympodially arranged denticles that produces one conidium arranged in flower-like groups. The conidia detach from the conidiophores and sometimes become bilaterally arranged side by side with the hyphae in a row. The yeast phase is pleomorphic, with spindle-shaped and/or oval cells that resemble a “cigar” [4]. Through sequencing of genes, one has been demonstrated that the species S. schenckii is a complex that consists of the following cryptic species: Sporothrix albicans, Sporothrix brasiliensis, Sporothrix globosa, Sporothrix luriei, Sporothrix mexicana and S. schenckii [5]. Most cases of sporotrichosis are localized to the skin and subcutaneous tissues. Dissemination to osteoarticular structures and viscera is uncommon and appears to occur more often among patients who have a history of alcohol abuse or immunosuppression, especially patients with AIDS. Pulmonary and disseminated forms of infection, although uncommon, can occur when S. schenckii conidia are inhaled. Meningitis occurs rarely as an isolated chronic infection in non-immunosuppressed patients, but it occurs more often during disseminated infection in immunosuppressed patients [6]. Many other diseases, including atypical mycobacterial infections, nocardiosis, and leishmaniasis, can produce lesions a clinical finding similar to those seen with sporotrichosis [7]. The diagnosis of visceral involvement with S. schenckii is often delayed when epidemiological evidence is not clear and culture of S. schenckii remains the gold standard for establishing the diagnosis of sporotrichosis [8]. Microscopic observation of the fungus on the pathological material can be performed on smears submitted to conventional stains such as Gram, Giemsa, Panótico, New methylene blue or histological sections stained by PAS, Gomori trichrome or silver Methenamine. Sporothrix sp appears as oval, round or cigar-shaped yeasts, free or in the interior of macrophages. Its cell wall is refractile and the cytoplasm can retract, giving the impression of having a capsule. In this case, care must be taken not to confuse it with Cryptococcus neoformans. In ulcerated lesions, yeasts are found in large numbers, especially in cats, while in man and other animals such as dogs and in non-ulcerated lesions a smaller number is observed, or they may not be found. Better results can be achieved by immunofluorescence with the use of antibodies labelled with fluorescein or rhodamine [9]. Treatment options for sporotrichosis include local measures (hyperthermia), SSKI, azoles (ketoconazole, itraconazole, and fluconazole), amphotericin B, and the allylamine, terbinafine [10]. Azoles have become the preferred agents for treatment of several forms of sporotrichosis, moreover voriconazole is not active in vitro against S. schenckii [11], whereas posaconazole does have activity in vitro [12]. Amphotericin B remains the treatment of choice for patients with serious or life-threatening sporotrichosis. The experience in the literature is almost entirely with amphotericin B deoxycholate, but many clinicians, including the panel members, now prefer to use lipid formulations of amphotericin B, because such formulations have fewer adverse effects [13].
2. Case
A 36-year-old male, homeless, heavy drinker, drug abuser (marijuana, cocaine and crack), was admitted with a 6-months history of verrucous and secretive ulcers on his face, trunk, arms and legs associated with neurologic symptoms, hyporexia and weight loss. On this admission (day 0), he was admitted to our dermatological clinics and in the same day he was hospitalized. The initial evaluation was remarkable for ulcer-vegetating lesions on nose (with destruction of nasal septum), frontal and malar regions, arms, legs, shoulders and back. Signs of secondary bacterial infection were noticed. Neurologic manifestations included: imbalance sensation, seizures (reported by patient) and loss of sphincter control. There was no fever or any respiratory symptom. On clinical examination he was found with abnormal gait (marcha ebria) and incoordination of the Finger-to-nose Test (right side). Upon admission he was afebrile with normal oxygen saturation; laboratory studies revealed an elevated white blood cell count (17200/mm3), neutrophils 12900/mm3, lymphocytes 2580 mm3, anemia with a hemoglobin of 6.6 g/dl, normal platelets count (847000/μl), renal function (creatinine 0.55 mg/dL, urea 19mg/dl), hepatic function (oxaloacetic transaminase 18 U/L, pyruvic transaminase 14 U/L), C reactive protein 203.7 mg/L, RNI 1.39, negative serologies for HIV, HTLV, positive VDRL and FTA-ABS (1:16) and positive toxoplasmosis IgG. A skin biopsy was performed, and bacterial cultures initially grow Acinetobacter baumannii, with sensibility only to amikacin and polymyxin B. He was begun on empiric amoxicillin clavulanate and thiamine. On hospital day 1, he developed fever to 38.6 °C and significant apathy, a psychiatric consultation was sought for concern of the inability to communicate and to the fact he was always covered with a blanket. He was diagnosed with personality disorder, depression and addiction to multiple drugs. On hospital day 2, the patient subjectively felt worse; he developed neurological dysfunction with torpor, stiff neck, weakness and motor loss of the right side and decreased pain sensation on the left side. A brain MRI scan showed decrease of encephalic volume, disproportional to the age, area of acute/sub-acute infarction in the right brainstem, a suggestive focus of ischemic event with hemorrhagic transformation in the right frontal region. Outbreaks of signal abnormality sparse, non-specific, in supratentorial white matter. MRI also showed pan sinusitis, deposition of dense material, osteolysis and obliteration of drainage pathways of sinus. Cerebrospinal fluid analysis showed, no red blood cells and white blood cells, glucose 35.7 mg/dL; protein 292 mg/dL; DNA test for Mycobacterium tuberculosis was negative; Cryptococcus sp (Nankin ink) was negative; VDRL was negative; bacteria, mycobacteria and fungal cultures was negative. He was empirically started on phenytoin due to concern for seizures. On hospital day 3, he was begun on empiric standard amphotericin B (50 mg/day) due to concern for fungal infection and ceftriaxone due to neurosyphilis. He developed worsening hypoxemic respiratory failure with acute respiratory distress syndrome (ARDS) on day 4, requiring endotracheal intubation and full mechanical ventilator support. Culture testing was requested and Piperaciclin/tazobactam prescribed due to possible pulmonary aspiration. He was transferred to the intensive care unit. The patient progressed with hemodynamic instability, requiring vasoactive amines, oliguria and on day 7 he was non-responsive and with 3 points on Glasgow coma scale, additional CT brain scan revealed hypodensity in the right brain stem and questionable hypodense image in the left thalamus. A verbal report of the skin biopsy was released at day 10 and was consistent with Sporothrix schenckii. On day 11 he died. The official report of the fungal culture was available post mortem on day 15 of admission. Fungal identification was made by mold culture on a Sabouraud agar media. Brown pigmented filamentous colonies were seen when initially incubated at room temperature (27 °C) with the dark colored conidia arranged along the hyphae in “bouquet-like” arrangements. Growth of the yeast phase was observed when incubated at 37 °C on enriched media. An autopsy was performed 3 h after the patient's death (Day + 11). The body height was 160 cm, and the weight was 50 kg. Organs were fixed with formalin and slides of representative areas were stained with hematoxylin and eosin (HE) or periodic acid-Schiff and hematoxylin (PAS-Hematoxylin). Findings demonstrated extensive cutaneous involvement of limbs, trunk and face (Fig. 1), without lymphatic dissemination, associated with chronic granulomatous process and yeast structures compatible with Sporothrix schenckii (Fig. 2). Moreover, nasal collapse with extension of the inflammatory process to paranasal sinuses and bones that showed granulomatous myelitis. Focal involvement of the neurohypophysis, adenohypophysis pars intermedia and leptomeninges of the base, especially at the brainstem and cerebellum level, was positive for fungus research. Some fungal structures and endarteritis were also seen in a branch of basilar artery at bridge level. There were multiple small foci of encephalitis in the cerebral trunk, frontal cortex, white parietal substance and nuclei of the base on the right hemisphere. Overall, brain's meninges lesions were less expressive, with rare mononuclear exudate and fungal structures (Fig. 3, Fig. 4). Multiple lesions on necrotic-exudative and reparative phases with granulomas and fibrosis were found in the lungs (Fig. 5), cervical and mediastinal lymph nodes, kidneys, testis (Fig. 6), prostate (Fig. 7) and suprarenal glands. Other findings included global hypoxic ischemic encephalopathy, incipient liver cirrhosis, chronic pancreatitis, diffuse alveolar damage, ascites (1100 ml) and moderate to severe malnutrition.
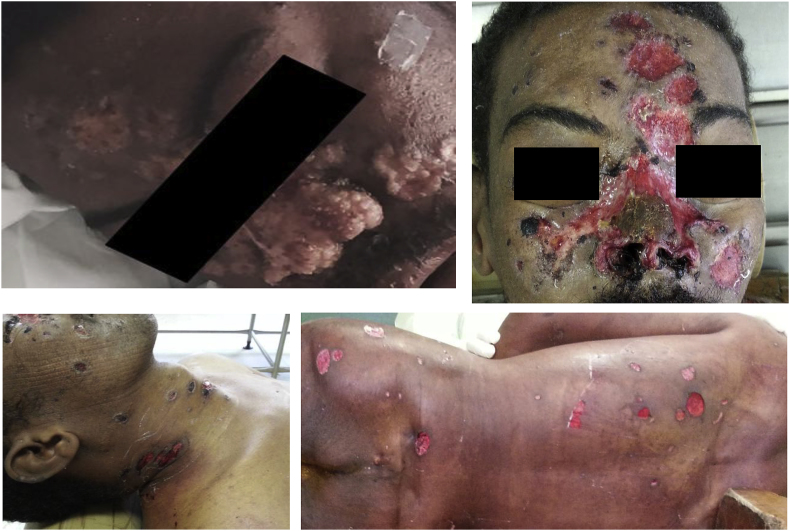
Fig. 1.
Sporotrichosis lesions at admission day (upper left) and soon prior the autopsy (face, neck and back).
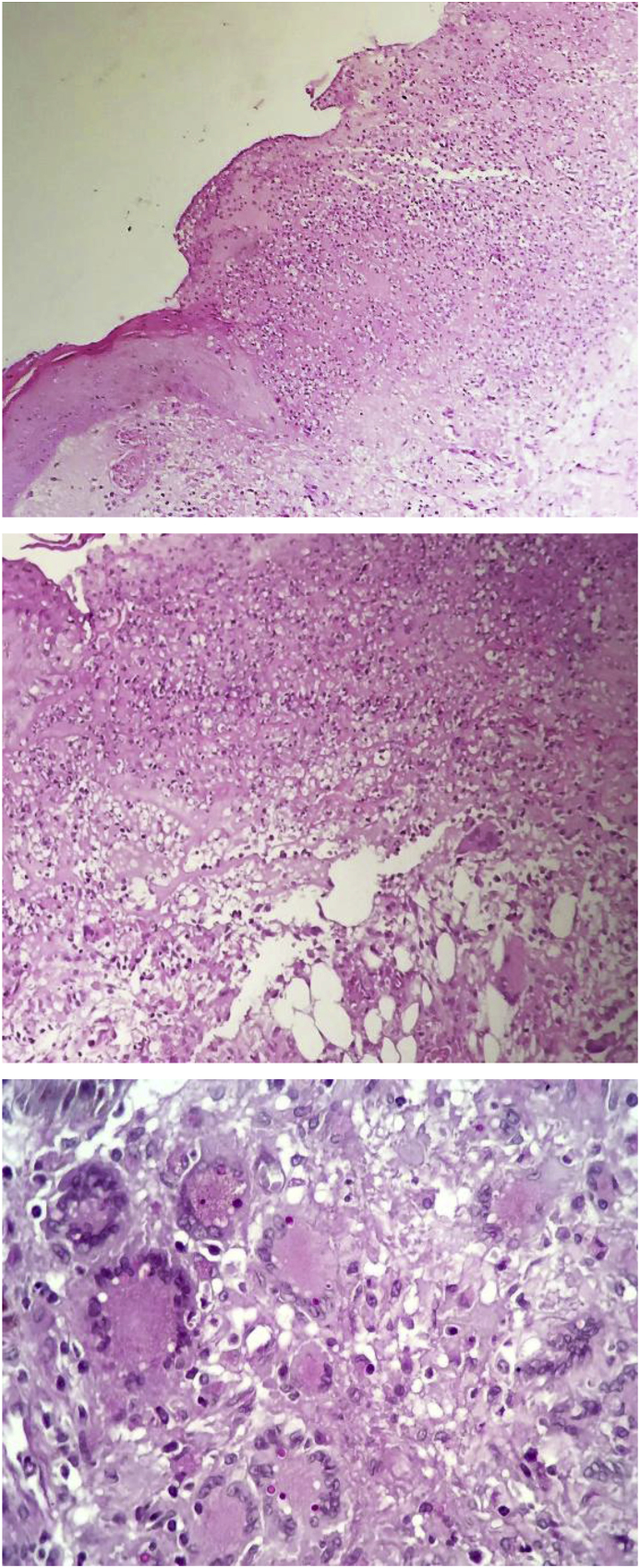
Fig. 2.
ssSkin histological section. (Upper) Cutaneous ulceration with a fibrin-leucocyte crust (PAS-Hematoxylin, 100x). (Middle) Suppurative granulomatous dermatitis with panniculitis (PAS-Hematoxylin, 200x). (Lower) Multinucleated giant cells containing oval structures, Periodic acid-Schiff positive, consistent with fungal cells (PAS-Hematoxylin, 400x).
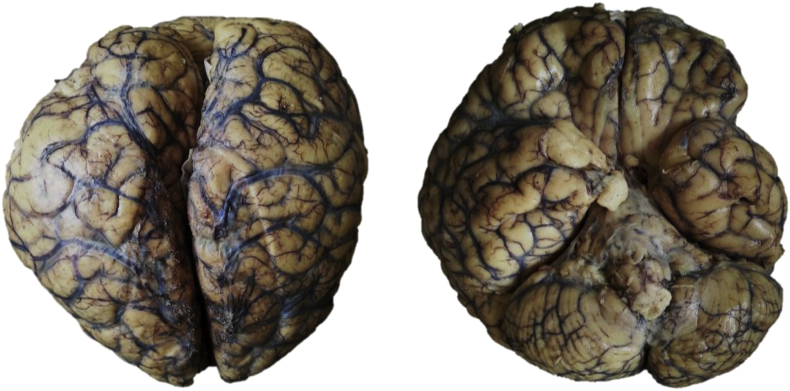
Fig. 3.
Brain after formalin fixation. Upper view: Diffuse vascular congestion and opacified leptomeninges. Botton view: Leptomeningeal fibrin deposition around brainstem and diffuse vascular congestion.
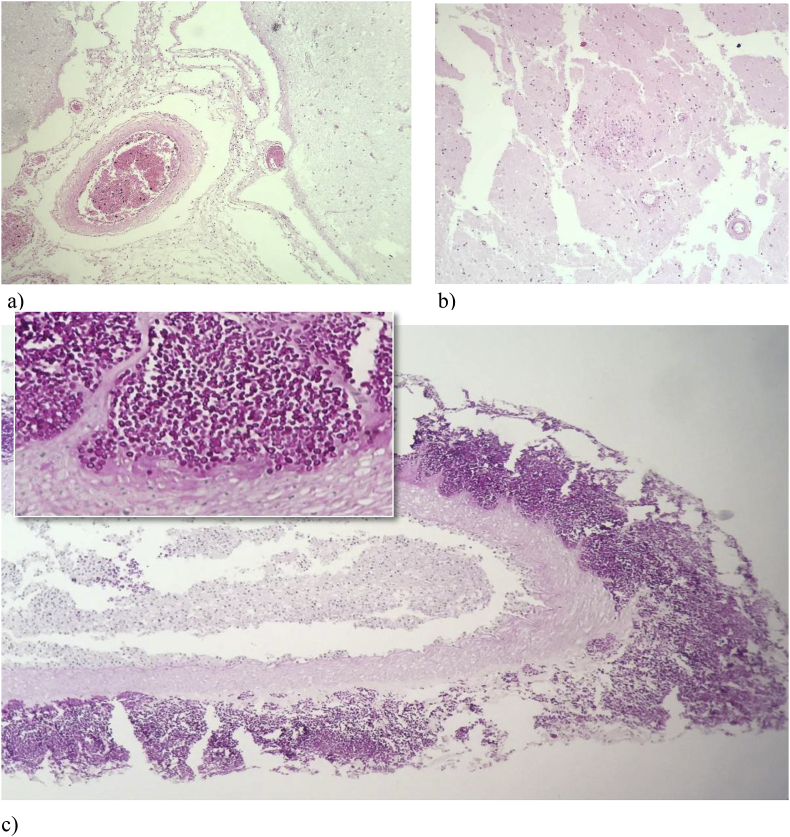
Fig. 4.
a) Leptomeningitis: mild inflammatory infiltrate in the leptomeninges (HE, 200x). b) Granulomatous encephalitis (HE, 200x). c) Numerous oval structures, Periodic acid-Schiff positive, around and in a leptomeningeal vessel wall (HE, 200x and 400x).
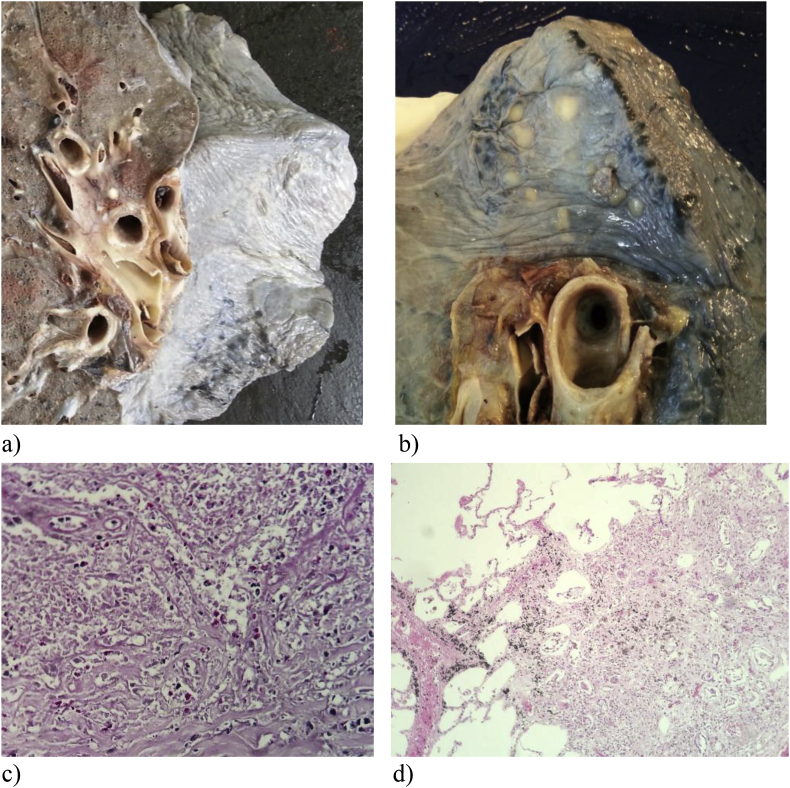
Fig. 5.
Lung (upper lobe) after formalin fixation. a) Whitish nodules on the surface of the lung. b) Cut surface showing whitish nodules around the lung hilum. c) Granulomatous interstitial pneumonitis (HE, 200x). d) Oval structures, Periodic acid-Schiff positive, near and inside pulmonary vessel wall (HE, 400x).
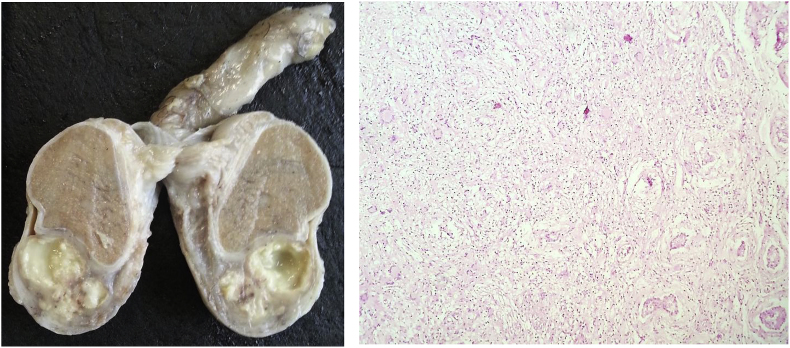
Fig. 6.
Testis after formalin fixation. Testicular abscesses. Granulomatous orchitis (HE, 200x).
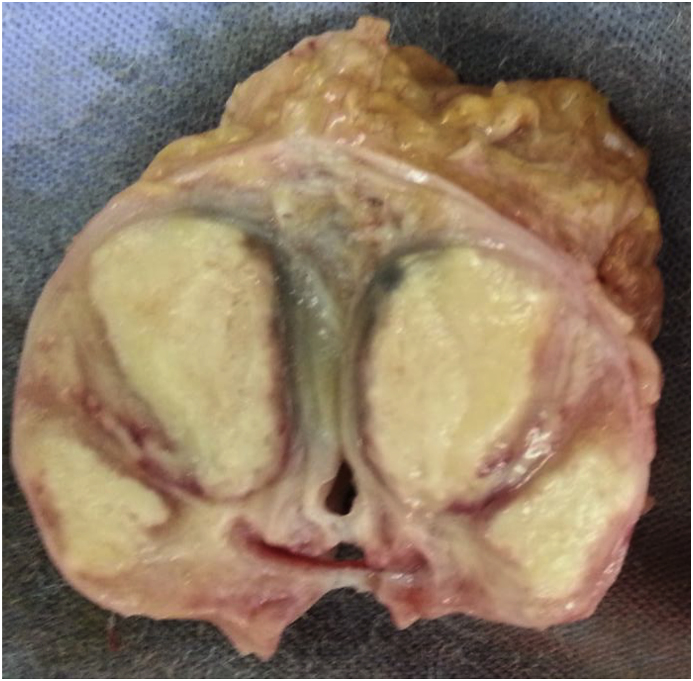
Fig. 7.
Prostate after formalin fixation with abscesses.
3. Discussion
The occurrence of sporotrichosis is predominantly associated in workers who deal with contaminated plants and soil. However, since 1998, in Brazil, an epidemic zoonotic transmission by felines has been reported and continues to spread over country nowadays [14]. In our city, Belo Horizonte, in the Southeast of Brazil, this case occurred in 2015 and seems to be in the beginning of the epidemic. This patient was a homeless person who had contact with contaminated cats. The disseminated characteristics of this case were not associated with an immunocompromising underling disease but probably with a malnutrition and alcoholism and a delaying in the medical assessment. Various clinical presentations were described, however a disseminated fatal disease as presented here is not common in humans but described in felines [15]. Sporotrichosis is typically chronic lymphocutaneous disorder that may have several polymorphisms. Correct diagnosis requires high level of clinical suspicion and a close history is essential. Our case showed atypical verrucous lesions with nasal and face evolvement mostly seen in cats [15]. Humans show more frequently hands and arms lesions. The nervous system evolvement is rarely reported and a fatal evolution is not an expected outcome for this disease. Paixão et al. reported a disseminated illness with brain evolvement presenting exudative hydrocephalus in a patient with advanced AIDS however the patient presented a good evolution e survived [16]. Therefore, it is of paramount importance to clinically suspect the mycosis even in unusual locations and disseminated serious diseases as presented here. Whereas the disease is endemic in Brazil and its miscellaneous clinical presentations, it should always be listed among the possible diagnoses. The purpose of this report is to show that patients with disseminated severe disease and or with some immunosuppressive factor can present atypical forms and that if not rapidly and effectively treated they can have a fatal outcome.
Declaration of competing interest
There are none.
Acknowledgements
Assistance provided by Autopsy laboratory of Faculdade de Medicina of UFMG was greatly appreciated.
References
- 1.Werner A.H., Werner B.E. Sporotrichosis in man and animal. Int. J. Dermatol. 1994;33:692–700. doi: 10.1111/j.1365-4362.1994.tb01512.x. [DOI] [PubMed] [Google Scholar]
- 2.Da Rosa A.C.M., Scroferneker M.L., Vettorato R. Epidemiology of sporotrichosis: a study of 304 cases in Brazil. J. Am. Acad. Dermatol. 2005;52:451–459. doi: 10.1016/j.jaad.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 3.Barros M.B.L., Schubach A.O., do Valle A.C.F., Gutierrez Galhardo M.C., Conceição-Silva F., Schubach T.M. Cat-transmitted sporotrichosis epidemic in Rio de Janeiro, Brazil: description of a series of cases. Clin. Infect. Dis. 2004;38:529–535. doi: 10.1086/381200. [DOI] [PubMed] [Google Scholar]
- 4.Lopes-Bezerra L., Schubach A., Costa R. Sporothrix schenckii and sporotrichosis. An. Acad. Bras. Cienc. 2006;78:293–308. doi: 10.1590/s0001-37652006000200009. [DOI] [PubMed] [Google Scholar]
- 5.Marimon R., Cano J., Gené J., Sutton D.A., Kawasaki M., Guarro J. Sporothrix brasiliensis, S. globosa, and S. mexicana, three new Sporothrix species of clinical interest. J. Clin. Microbiol. 2007;45:3198–3206. doi: 10.1128/JCM.00808-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scott E.N., Kaufman L., Brown A.C., Muchmore H.G. Serologic studies in the diagnosis and management of meningitis due to Sporothrix schenckii. N. Engl. J. Med. 1987;317:935–940. doi: 10.1056/NEJM198710083171505. [DOI] [PubMed] [Google Scholar]
- 7.RAJr Smego, Castiglia M., Asperilla M.O. Lymphocutaneous syndrome: a review of non-sporothrix causes. Medicine (Baltim.) 1999;78:38–63. doi: 10.1097/00005792-199901000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Kwon-Chung K.J., Bennett J.E. Sporotrichosis. In: Kwon-Chung K.J., Bennett J.E., editors. Medical Mycology. Lea & Febiger; Philadelphia: 1992. pp. 707–729. [Google Scholar]
- 9.Barros M.B.L., Almeida Paes R., Schubach A.O. Sporothrix schenckii and Sporotrichosis. Clin. Microbiol. Rev. 2011;24:633–654. doi: 10.1128/CMR.00007-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kauffman C.A. Old and new therapies for sporotrichosis. Clin. Infect. Dis. 1995;21:981–985. doi: 10.1093/clinids/21.4.981. [DOI] [PubMed] [Google Scholar]
- 11.McGinnis M.R., Nordoff N., Li R.K., Pasarell L., Warnock D.W. Sporothrix schenckii sensitivity to voriconazole, itraconazole and amphotericin B. Med. Mycol. 2001;39:369–371. doi: 10.1080/mmy.39.4.369.371. [DOI] [PubMed] [Google Scholar]
- 12.Espinel-Ingroff A. Comparison of in vitro activities of the new triazole SCH56592 and the echinocandins MK-0991 (L-743,872) and LY303366 against opportunistic filamentous and dimorphic fungi and yeasts. J. Clin. Microbial. 1998;36:2950–2956. doi: 10.1128/jcm.36.10.2950-2956.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Groll A.H., Giri N., Petraitis V. Comparative efficacy and distribution of lipid formulations of amphotericin B in experimental Candida albicans infection of the central nervous system. J. Infect. Dis. 2000;182:274–282. doi: 10.1086/315643. [DOI] [PubMed] [Google Scholar]
- 14.Silva M.B., Costa M.M., Torres C.C., Galhardo M.C., Valle A.C., Magalhães Mde A. Esporotricose urbana: epidemia negligenciada no Rio de Janeiro, Brasil/Urban sporotrichosis: a neglected epidemic in Rio de Janeiro, Brazil. Cad. Saúde Pública. 2012;28:1867–1880. doi: 10.1590/s0102-311x2012001000006. [DOI] [PubMed] [Google Scholar]
- 15.Sanchotene K.O., Madrid I.M., Klafke G.B., Bergamashi M., Della Terra P.P., Rodrigues A.M. Sporothrix brasiliensis outbreaks and the rapid emergence of feline sporotrichosis. Mycoses. 2015;58:652–658. doi: 10.1111/myc.12414. [DOI] [PubMed] [Google Scholar]
- 16.Paixão A.G., Galhardo M.C.G., Almeida-Paes R., Nunes E.P., Gonçalves L.C., Chequer G.L. AIDS Res. Ther. 2015;12:16. doi: 10.1186/s12981-015-0051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]