Abstract
We present a case of acinar cell carcinoma of the pancreas (ACC) with metastasis to the liver in a patient who presented with complaints abdominal pain. The presentation, diagnosis, and management of a 56-year-old man with ACC are discussed here. Imaging with computerized tomography (CT) in particular is crucial in the diagnosis, which can identify the primary lesion as well as metastases. ACC should be considered in the differential as a source of abdominal, epigastric, or back pain with imaging that is suggestive of the diagnosis as prompt recognition and initiation of treatment is paramount in the overall prognosis.
Keywords: Pancreatic acinar cell carcinoma, Computed tomography, Magnetic resonance imaging, Oncologic radiology, Diagnostic radiology
Introduction
Acinar cell carcinoma (ACC) of the pancreas is a rare, malignant neoplasm that accounts for 1%-2% of all pancreatic neoplasms [1]. Acinar cells are the functional exocrine units of the pancreas and the carcinoma arises from malignant transformation of these cells. Although acinar cells are the most prevalent cell type in the pancreas, malignant transformation of these cells is very rare, estimated to account for only 1% of exocrine tumors of the pancreas [2]. Acinar cell cystadenoma is an additional example of an acinar cell neoplasm, although considered to be benign in nature.
Currently, there is no gold standard of treatment for ACC, but aggressive surgical resection with negative margins has been associated with better long-term survival [3]. In a report of 9 patients with confirmed ACC who underwent pancreatic resection, 5 of which also had adjuvant chemotherapy, the median overall survival was 31 months. Median disease-free survival was 18 months [4].
Early recognition and diagnosis are of particular importance, specifically with the use of computerized tomography (CT) and magnetic resonance imaging (MRI), confirmed with fine needle aspiration (FNA) biopsy. Nonetheless, imaging features of ACC are variable and to our knowledge there is no consensus in the literature describing specific features of this tumor. Men are affected more frequently than women with a ratio of 3.6:1, and there are no known racial associations. In a case series of 11 patients with confirmed ACC, 9 (82%) presented with epigastric and right upper quadrant pain (n = 6), jaundice (n = 1), back pain (n = 1), or painful skin nodules and arthralgias (n = 1) [3], [4], [5]. Other clinical manifestations may include elevated lipase and lytic bone lesions, but these are not specific for ACC. Based on a review of 39 patients with confirmed ACC, patients who could be treated with surgery as first-line therapy had a longer average survival time of 36 months compared with only 14 months in those who did not have surgery [6]. The intent of this report is to present the case of primary pancreatic ACC with a metastatic lesion to the liver with a positive response to chemotherapy and surgical resection.
Case report
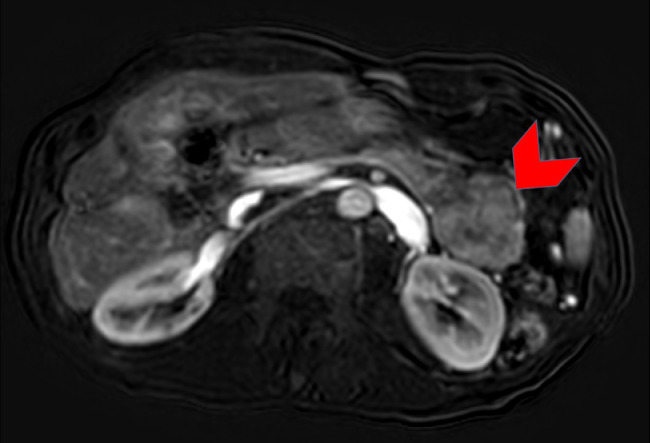
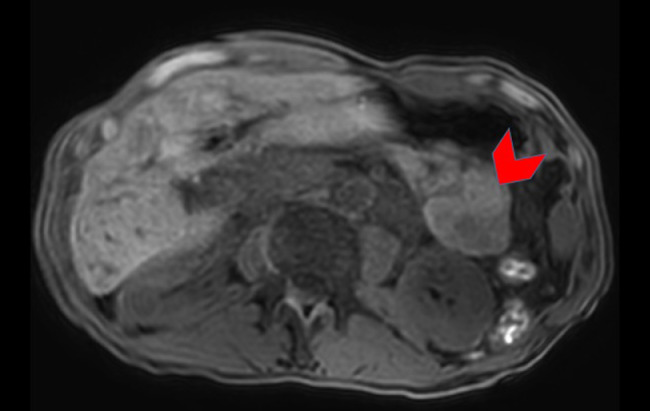
A 56-year-old man presented with progressive abdominal pain and was found to have an elevated lipase (initial lipase unavailable). A CT of the abdomen and pelvis with intravenous contrast revealed a heterogeneously enhancing 9.0 × 7.2 cm solid mass in the tail of the pancreas abutting the posterior wall of the fundus of the stomach (Fig. 1). In addition, a 4.3 × 3.8 cm mass was noted in in segment III/II of the liver (Fig. 2), presumed to be metastatic disease. An MRI performed concurrently showed that the pancreatic mass was homogenously hypointense on T2-weighted (T2W) images (Fig. 3) with marked restricted diffusion (Fig. 4) as well as heterogeneous enhancement on postcontrast T1-weighted (T1W) gradient echo (GRE) subtraction images (Fig. 5).
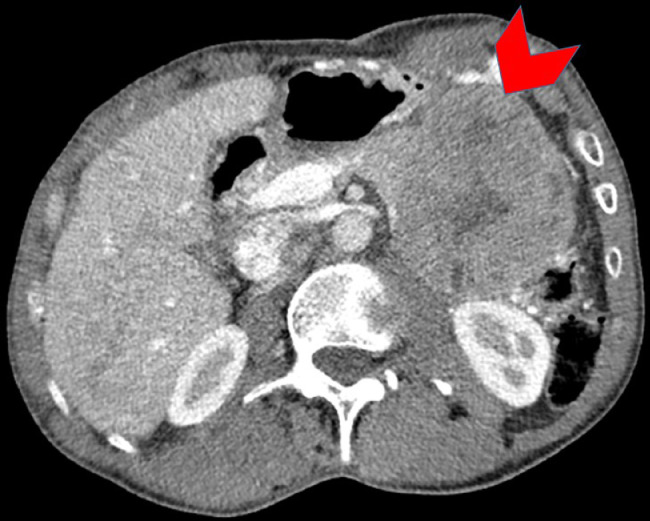
Fig. 1.
Axial contrast enhanced CT of the abdomen demonstrates a well-circumscribed heterogeneously enhancing pancreas tail mass (red arrowhead) compressing the neighboring stomach and splenic flexure of the colon. (Color version of figure is available online.)
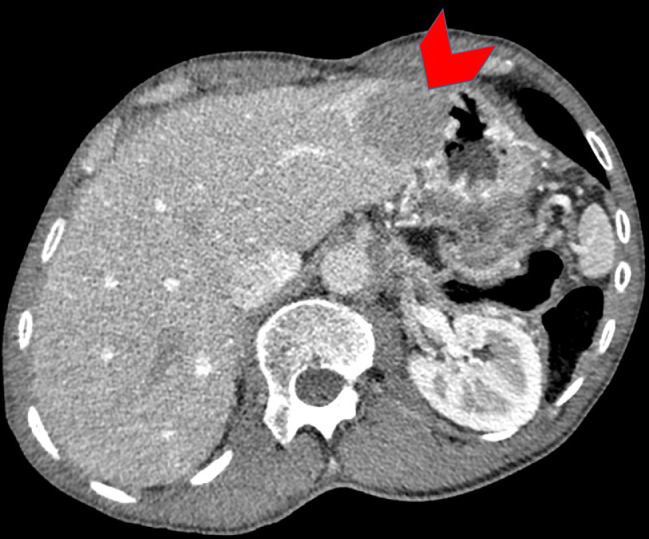
Fig. 2.
Axial contrast enhanced CT of the abdomen demonstrates a subcapsular hepatic segment II lesion (red arrowhead) highly concerning for a metastasis. (Color version of figure is available online.)
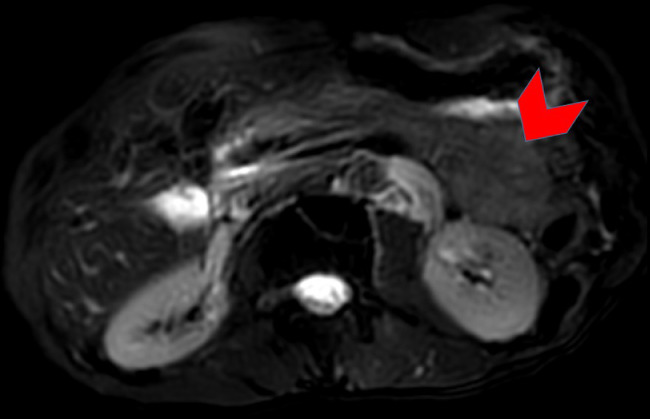
Fig. 3.
Axial fat suppressed T2-weighted turbo spin-echo image through the abdomen shows a well-circumscribed homogeneously low signal intensity mass within the tail of the pancreas (red arrowhead). No associated high signal intensity components evident. (Color version of figure is available online.)
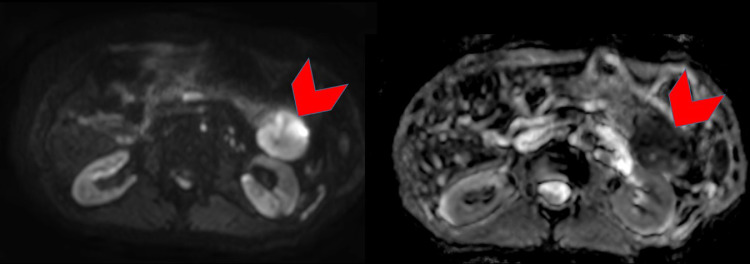
Fig. 4.
Axial diffusion-weighted image (left) with b value of 800 s/mm2 and apparent diffusion coefficient (ADC) map (right). The lesion (red arrowheads) shows restriction diffusion seen as high signal intensity on the diffusion weighted image and low signal intensity on the corresponding ADC map. (Color version of figure is available online.)
Fig. 5.
Axial 3D gradient-echo fat-suppressed T1-weighted image without contrast of the shows near homogeneous intermediate signal intensity of the pancreatic tail mass (red arrowhead). (Color version of figure is available online.)
Interventional radiology performed a biopsy of the liver mass under ultrasonographic guidance. The core biopsy specimen, strained with H&E and immunohistochemistry, demonstrated metastatic tumor cells arranged in groups of acinar type structures. The tumor cells were positive for Cytokeratin 7 (CK), Pan CK, trypsin, and chymotrypsin and were negative for CK 20, chromogranin, synaptophysin, and classification determinant 56 (CD). The tumor cells were also negative for mucicarmine and showed diastase resistance cytoplasmic granules. These findings are consistent with metastatic ACC of pancreatic origin. The tumor marker, carbohydrate antigen (CA) 19-9, was elevated at 82 U/mL (normal range: 0-37 U/mL). After the diagnosis of ACC was made, he was referred to oncology for treatment.
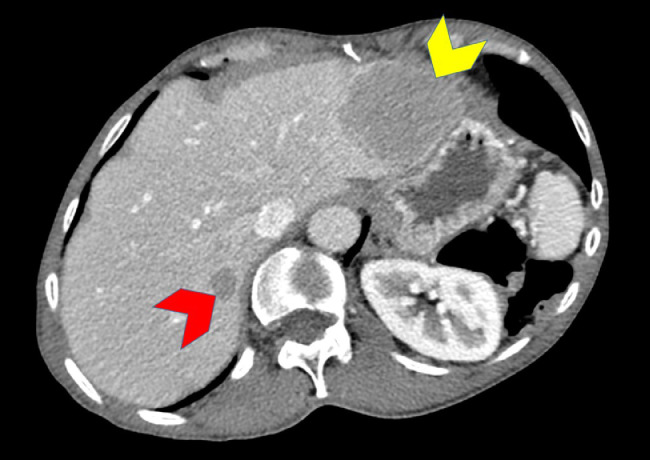
Chemotherapy with fluorouracil continuous infusion/levcovrin/oxaliplatin (FOLFOX) was initiated with 26 rounds completed over 14 months with variable days between treatments. A repeat CT of the abdomen and pelvis with intravenous contrast 17 months following the initial discovery showed a stable 4.9 × 3.8 cm pancreatic mass. However, a new 1.2 × 1 cm lesion most compatible with new metastatic disease was now present within segment VII of the liver and the known lesion segment III/II lesion increased in size to 5.8 × 4.4 cm (Fig. 6).
Fig. 6.
Axial subtraction portal venous phase contrast-enhanced 3D gradient-echo fat-suppressed T1-weighted image demonstrates diffuse enhancement of the pancreatic tail mass with some central heterogeneity (red arrowhead). (Color version of figure is available online.)
The following day, a distal pancreatectomy, liver lobectomy, and splenectomy were performed. Negative margins were obtained in both the pancreatectomy specimen as well as that of the resected portion of the liver. He remains under the care of the oncology and surgical services.
Discussion
Introduction
ACC is a rare subtype of pancreatic cancer accounting for approximately 1%-2% of all pancreatic neoplasms. Although ACC can originate from any part of the pancreas, most cases including those reported by Hsu et al, occur in the pancreatic head with a higher incidence [7]. In most studies, approximately 50% of cases present with metastatic disease at the time of diagnosis with the most common site being the liver. Surgical resection is the best treatment for localized ACC [8].
Presentation
The average age at diagnosis is approximately 59 years with a range 20-88 years. Men are more commonly affected than women with a ratio of 2:1. Although most ACCs arise sporadically, rare cases diagnosed in the context of Lynch syndrome or familial adenomatous polyposis has been described [9]. Patients with ACC who present at a younger age are more likely to be candidates for resection with the potential for curative outcomes [10]. Studies following patients with surgically resected ACC have shown significantly better survival of greater than 40% at 5 years when compared to those who underwent chemotherapy alone [11]. For pancreatic adenocarcinoma, in which patients are able to undergo surgical resection, 5-year survival is estimated to be 27%; in patients with locally advanced or metastatic disease the median survival is typically less than 12 months [12].
Imaging
A differential diagnosis including ACC should be considered in patients when CT imaging reveals a bulky, ovoid, heterogeneous predominantly solid mass with or without clear margins in the pancreas or peripancreatic fat. Other differential diagnostic possibilities for such imaging findings would include ductal adenocarcinoma, metastatic disease, endocrine tumors, solid pseudopapillary tumors, and mesenchymal tumors. The MRI features of the ACC in this case were consistent with a solid composition with some hemorrhage (Fig. 3, Fig. 4, Fig. 5) Though nonspecific, these findings were concordant with those often reported in the literature. Our case however did not reveal any evidence of a tumoral cystic component which has been reported [3,13]. In an analysis of 6 histologically-confirmed ACC cases, the tumors were distributed in the head (n = 4), body (n = 1), and tail (n = 1) of the pancreas, this lesion was located in the pancreatic tail. Four of those cases (67%) were uniformly or partially well-defined with thin, enhancing capsules, similar to this case. Central cystic components were found in 5 tumors (83%). Two of the tumors (33%) exhibited intratumor hemorrhage and 1 tumor (17%) had amorphous tumoral calcification [5]. There was no evidence of intratumoral hemorrhage in our case. (Fig. 7). In a study of 865 patients with ACC performed by Schmidt et al, the median tumor size was 6.9 cm (vs 4.6 cm in ductal cell carcinoma [DCC] with 32.1% having nodal metastasis (vs 48% in DCC) and 47% having high-grade tumors (vs. 37.3% in DCC) [14]
Fig. 7.
Axial contrast-enhanced CT of the abdomen after chemotherapy shows interval increase in size of previously detected hepatic segment II metastasis (yellow arrowhead), and a new hepatic segment VIII (red arrowhead) metastasis. (Color version of figure is available online.)
Diagnosis
Diagnosis of ACC is confirmed with histologic and immunohistochemical analysis. Studies show that antibodies directed against trypsin and BCL10 are superior in terms of sensitivity. Acinar enzymes, especially trypsin and chymotrypsin, are demonstrable by immunohistochemistry in essentially all cases of ACC [6,7]. ACCs may also express CK7 and CK19, traditionally known as markers of ductal adenocarcinomas [7].
In this case, an FNA specimen was obtained from the liver and stained positive for trypsin and CK7. These findings, along with CT imaging, confirmed the diagnosis of metastatic ACC. CA19-9 has been used as a tumor marker to measure the recurrence of some types of pancreatic cancer. Although in this particular patient, the CA 19-9 was elevated, this is not usually the case in ACC [2].
Management
The current standard of treatment is aggressive surgical resection with negative margins. There is limited data in regard to adjunct chemotherapy. However, there is evidence in the literature supporting the addition of chemotherapy in the treatment of ACC with varying responses.
While there are no standard chemotherapy regimens currently being used, a case series of seven patients with confirmed ACC showed that substantial antitumor activity was observed for treatment regimens containing 5-FU and oxaliplatin with partial responses or prolonged disease stabilizations of greater than 12 months observed in 6 out of the 7 patients (86%). Activity was also observed for single-agent 5-FU and its oral prodrugs [15].
Conclusion
In conclusion, ACC remains a rare subtype of pancreatic cancer with limited data and no consensus on a gold standard of treatment. Although surgical resection with negative margins remains the mainstay of treatment for those with resectable disease, there appears to be some benefit to adjunct chemotherapy, in particular, with 5-FU. Early diagnosis with the aid of CT imaging is imperative to the long-term survivability of ACC. Additionally, FNA biopsy followed by histological and immunohistochemical analysis has been recognized as satisfactory methods for confirmatory diagnosis.
References
- 1.Bechade D, Desjardin M, Salmon E, Desolneux G, Becouarn Y, Evrard S. Pancreatic Acinar Cell Carcinoma. Case Rep Gastroenterol. 2016;10(1):174–180. doi: 10.1159/000445867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Solcia E., Capella C., Kloppel G. Tumors of the exocrine pancreas. In: Rosai J., Sorbin L., editors. Atlas of tumor pathology. Armed Forces Institute of Pathology; Washington, DC: 1997. pp. 31–144. [Google Scholar]
- 3.Chaudhary P. Acinar Cell Carcinoma of the Pancreas: A Literature Review and Update. Indian J Surg. 2015;77(3):226–231. doi: 10.1007/s12262-014-1049-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butturini G, Pisano M, D’Onofrio M, Auriemma A, Bassi C. Aggressive approach to acinar cell carcinoma of the pancreas: a single institution experience and a literature review. Langenbecks Arch Surg. 2011;396(3):363–369. doi: 10.1007/s00423-010-0706-2. [DOI] [PubMed] [Google Scholar]
- 5.Tatli S., Mortele K. CT and MRI features of pure acinar cell carcinoma of the pancreas in adults. AJR. 2005;184:511–519. doi: 10.2214/ajr.184.2.01840511. [DOI] [PubMed] [Google Scholar]
- 6.Holden K.D., Klimstra D.S. clinical characteristics and outcomes from an institutional series of acinar cell carcinoma of the pancreas and related tumors. J Clin Oncol. 2002;20(24):4673–4678. doi: 10.1200/JCO.2002.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Hsu M, Pan K, Chu S, Hung C, Wu R, Tseng J. CT and MRI features of acinar cell carcinoma of the pancreas with pathological correlations. Clin Radiol. 2010;65(3):223–229. doi: 10.1016/j.crad.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Chaudhary P., Ranjan G., Chaudhary A., Tiwari A.K., Arora M.P. Acinar cell carcinoma: a rare pancreatic malignancy. Clin Pract. 2013;3(2):e18. doi: 10.4081/cp.2013.e18. Published May 20, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.La Rosa S., Sessa F., Capella C. Acinar cell carcinoma of the pancreas: overview of clinicopathologic features and insights into the molecular pathology. Front Med (Lausanne) 2015 doi: 10.3389/fmed.2015.00041. 2:41. Published June 15, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wisnoski N, Townsend C, Nealon W, Freeman J, Riall T. 672 patients with acinar cell carcinoma of the pancreas: a population-based comparison to pancreatic adenocarcinoma. Surgery. 2008;144(2):141–148. doi: 10.1016/j.surg.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Kitagami H, Kondo S, Hirano S, Kawakami H, Egawa S, Tanaka M. Acinar cell carcinoma of the pancreas: clinical analysis of 115 patients from Pancreatic Cancer Registry of Japan Pancreas Society. Pancreas. 2007;35(1):42–46. doi: 10.1097/mpa.0b013e31804bfbd3. [DOI] [PubMed] [Google Scholar]
- 12.Cancer Research UK.2017. Survival pancreatic cancer. Cancer research UK. Available from: http://www.cancerresearchuk.org/about-cancer/pancreatic-cancer/survival.
- 13.Schmidt C.M., Matos J.M., Bentrem D.J. Acinar cell carcinoma of the pancreas in the United States: prognostic factors and comparison to ductal cell carcinoma. J Gastrointestinal Surg. 2008;12:2078. doi: 10.1007/s11605-008-0705-6. [DOI] [PubMed] [Google Scholar]
- 14.Tian L, Lv XF, Dong J, Zhou J, Zhang Y, Xi SY. Clinical features and CT/MRI findings of pancreatic acinar cell carcinoma. Int J Clin Exp Med. 2015;8(9):14846–14854. Published September 15, 2015. [PMC free article] [PubMed] [Google Scholar]
- 15.Kruger S, Haas M, Burger P, Ormanns S, Modest D, Westphalen C. Acinar cell carcinoma of the pancreas: a rare disease with different diagnostic and therapeutic implications than ductal adenocarcinoma. J Cancer Res Clin Oncol. 2016;142(12):2585–2591. doi: 10.1007/s00432-016-2264-7. [DOI] [PubMed] [Google Scholar]