Abstract
Three dimensional (3D) supramolecules with giant cavities are attractive due to their wide range of applications. Herein, we used pentatopic terpyridine ligands with three types of coordination moieties to assemble two giant supramolecular hexagonal prisms with a molecular weight up to 42 608 and 43 569 Da, respectively. Within the prisms, two double-rimmed Kandinsky Circles serve as the base surfaces as well as the templates for assisting the self-sorting during the self-assembly. Additionally, hierarchical self-assembly of these supramolecular prisms into tubular-like nanostructures was fully studied by scanning tunneling microscopy (STM) and small-angle X-ray scattering (SAXS). Finally, these supramolecular prisms show good antimicrobial activities against Gram-positive pathogen methicillin-resistant Staphylococcus aureus (MRSA) and Bacillus subtilis (B. subtilis).
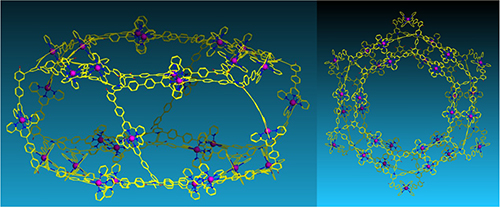
Graphical Abstract
INTRODUCTION
Self-assembly as an “order-out-of-chaos” strategy is widely utilized by nature to efficiently assemble biological structures with sophisticated functionalities.1 In the supramolecular chemistry field, coordination-driven self-assembly plays an important role in the construction of a wide variety of metallo-supramolecules in a well-controlled manner.2 Particularly, three-dimensional (3D) supramolecules3 with cavities are very attractive due to their applications in catalysis,4 stabilization of (air/water)-sensitive molecules,5 drug delivery,6 and artificial transmembrane channels.7 Among these 3D structures, supramolecular prisms have been assembled mainly through the following four strategies: (i) two-component self-assembly with multitopic subunits at the lateral/base faces hinged by ditopic motifs as the vertices or pillars;8,9 (ii) multicomponent self-assembly with lateral face motifs, base building blocks, and metal vertices;5b,c,10,11 (iii) multicomponent self-assembly with the assistance of template molecules;12 (iv) subcomponent self-assembly with linear ligands as the edges of both base and lateral face.7a,13 However, most of the ligands were designed with high symmetry to afford all the metal binding sites with the same chemical environment. It remains a challenge to introduce different coordination environments into the same ligand to further enhance the complexity of prisms.
In supramolecular chemistry, pyridinium salts have been extensively utilized as building blocks to assemble cages with good solubility in water and tunable host–guest interactions on the basis of their multiple positive charges.14 Consequently, a wide range of 3D structures, such as triangular prism,15 molecular dice,16 bowl shape cage,17 and octahedron,18 have been constructed with pyridinium moieties. In most of these cases, pyridinium groups were introduced into the backbone via pendent linkers. Without sufficient rigidity and directionality, the so-formed pyridinium ligands, however, were unable to assemble large 3D supramolecules with higher complexity through a directional bonding approach.2b Pyrylium salt–aryl primary amine condensation is an alternative synthetic approach to introduce a pyridinium group into supramolecular architectures with rigid backbones.19 Benefiting from the efficient condensation reaction as well as the modularized synthetic strategy, a series of giant 2D Kandinsky Circles (KCs) with concentric hexagon rings was obtained in our previous study, in which pyridinium groups were acting as bridges connecting different rims.20 In addition, these 2D KCs showed potent antimicrobial activity against Gram-positive pathogen methicillin-resistant Staphylococcus aureus (MRSA) through the formation of transmembrane channels.20a
Beyond 2D concentric hexagons, we herein designed and assembled hexagonal prisms based on pyrylium salts and pyridinium salts chemistry. We prepared two pentatopic 2,2′:6′,2″-terpyridine (TPY) ligands, in which the metal-TPY coordinations are settled in three different environments (Scheme 1, TPYa–c). After assembly, giant and discrete 3D supramolecular hexagonal prisms, were constructed with two KCs as the bases and the tail-anchored <TPY–Cd–TPY> linkages as the lateral edges, respectively. The obtained hexagonal prisms have a high tendency to further hierarchically assemble into 1D nanostructures by base-to-base stacking in solution. In addition, these hexagonal prisms display antimicrobial activity against Gram-positive bacteria, including MRSA and Bacillus subtilis (B. subtilis).
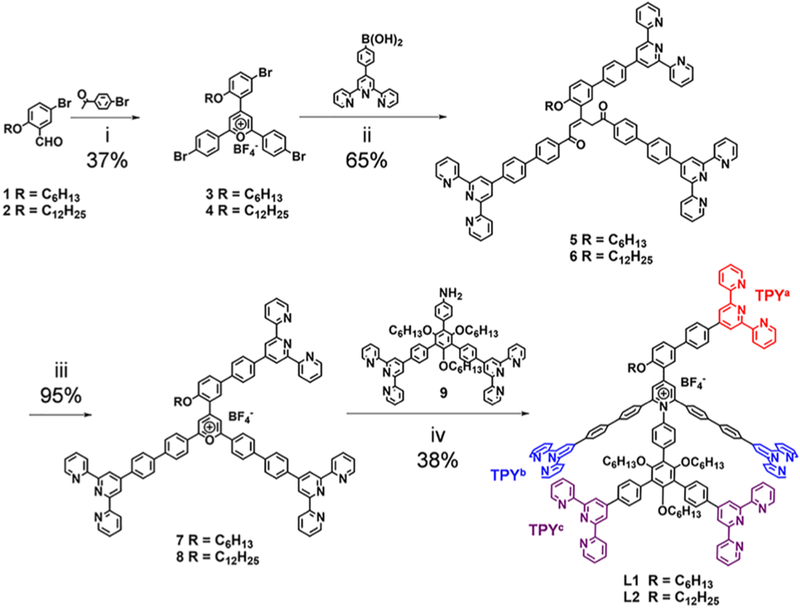
Scheme 1. Synthesis of Ligands L1 and L2a.
aConditions: (i) BF3·Et2O, 100 °C, 2 h; (ii) Pd(PPh3)4, NaHCO3, H2O, toluene, tert-butanol, 80 °C, 6 h; (iii) HBF4 (35% aqueous solution), MeOH, CHCl3, 12 h; (iv) 4 Å molecular sieve, DMSO, 120 °C, 24 h.
RESULTS AND DISCUSSION
Synthesis of the Ligands and Self-Assembly of the Complexes.
To incorporate 2D KCs into 3D hexagonal prisms, an additional TPY group is necessary to serve as the lateral edges, which act in a distinct structural role compared to the base edges. As such, the 1,3-substituted phenyl group was introduced to the ligand as a spacer, providing the anchored TPY a freely rotating axle as well as the rigid backbone with a proper intrinsic angle for adapting a vertical conformation to the hexagonal base surface during the self-assembly. Accordingly, a new pentatopic TPY ligand, L1, was designed as shown in Scheme 1 and efficiently synthesized by utilizing the pyrylium–pyridinium salts chemistry.20 During the synthesis, the key precursor diketone 5 was prepared via a 3-fold Suzuki coupling reaction based on the tribromo-pyrylium salt 3. Note that the reaction time (6 h) was one of the most crucial parameters to achieve a good yield (65%), owing to the instability of 5 under basic condition. Another ligand, L2, with a longer alkyl chain was prepared via the similar procedure.
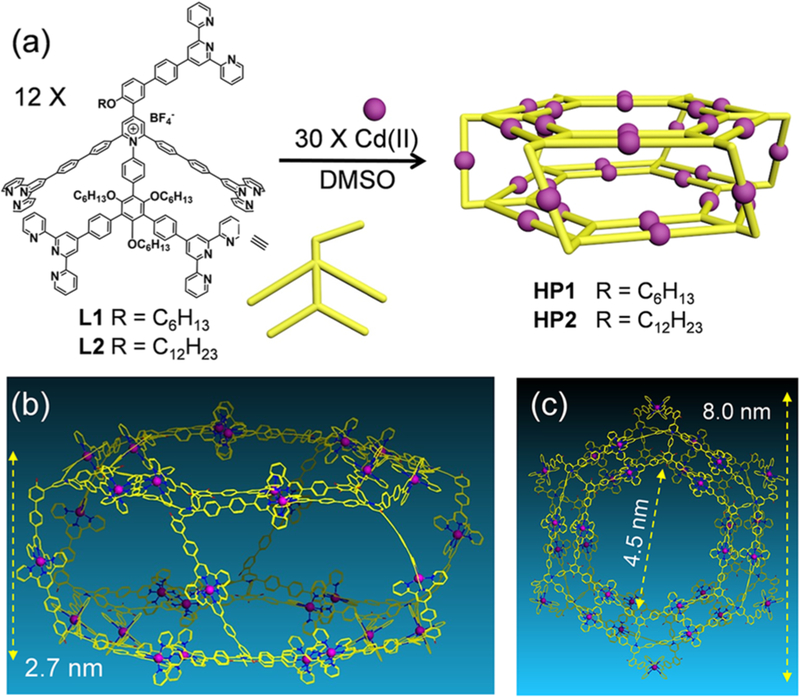
With the ligands in hand, another crucial requirement of forming the desired prism is a precise narcissistic self-sorting of the three distinct types of TPY groups to form <TPYa–Cd(II)–TPYa>, <TPYb–Cd(II)–TPYb>, and <TPYc–Cd(II)–TPYc> coordination sites during the self-assembly. Without fully excluding the possibilities of undesired coordination, polymeric complex instead of discrete structure could be formed during the assembly. To address the above concerns, self-assembly of the pentatopic ligands with Cd(II) ion was performed because of its higher reversibility.20a The self-assembly was performed in DMSO (10 mg/mL of L1 or L2) at 80 °C for 12 h with the stoichiometric ratio of ligand/metal = 2/5 (Figure 1a).
Figure 1.
(a) Self-assembly of hexagonal prisms HP1 and HP2; (b) side view and (c) top view of the representative energy-minimized structures from molecular modeling of HP1/2; alkyl chains were omitted for clarity.
Characterization of Supramolecules.
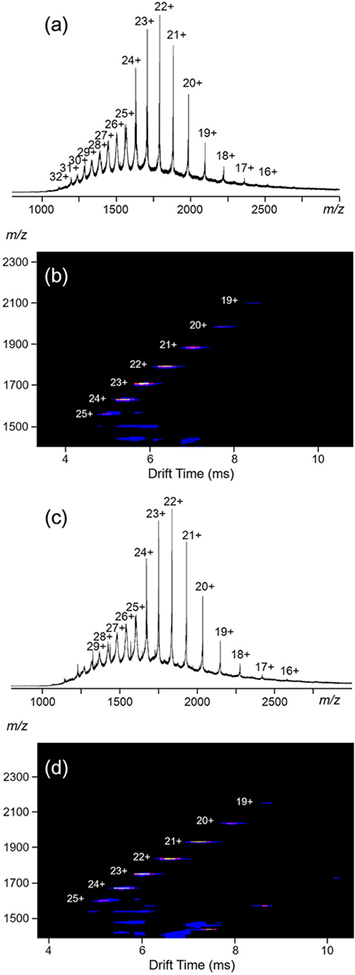
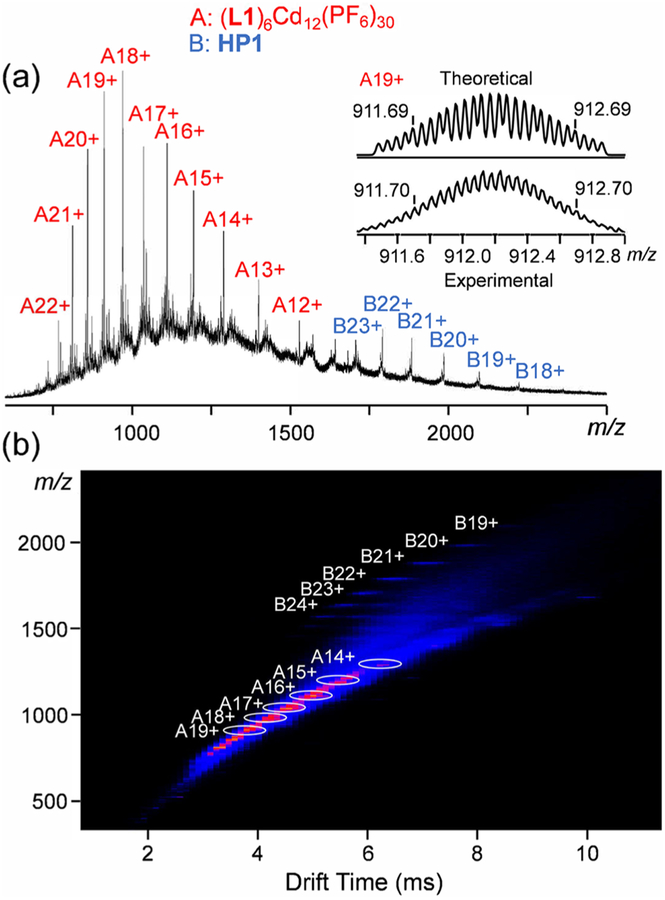
Electrospray ionization-mass spectrometry (ESI-MS) was first utilized to characterize the obtained complexes (the anions were exchanged to PF6−), as shown in Figure 2. Figure 2a (HP1) shows one dominant set of peaks with continuous charge states ranging from 16+ to 32+, due to successive loss of the counterions, PF6−. A very similar spectrum of HP2 is observed (Figure 2c) with slight shifts of the peaks toward higher m/z region. After deconvolution, the average molar masses of HP1 and HP2 are 42 608 and 43 569 Da, respectively, matching well with their expected chemical compositions [(C164H139N16O4)12Cd30(PF6)72] (HP1) and [(C170H151N16O4)12Cd30(PF6)72] (HP2), ruling out the possibilities of forming other undesired complexes. These two supramolecules are among the largest metallo-supramolecules ever reported.21 The traveling wave ion mobility-mass spectrum (TWIM-MS)20a,22 of HP1 (Figure 2b) displays a series of bands with narrowly distributed drifting time at each charge state, indicating no other isomers or conformers exist. HP2 also displays similar TWIM-MS spectrum shown in Figure 2d.
Figure 2.
(a, c) ESI-MS and (b, d) TWIM-MS plots (m/z vs drift time) of HP1 and HP2, respectively.
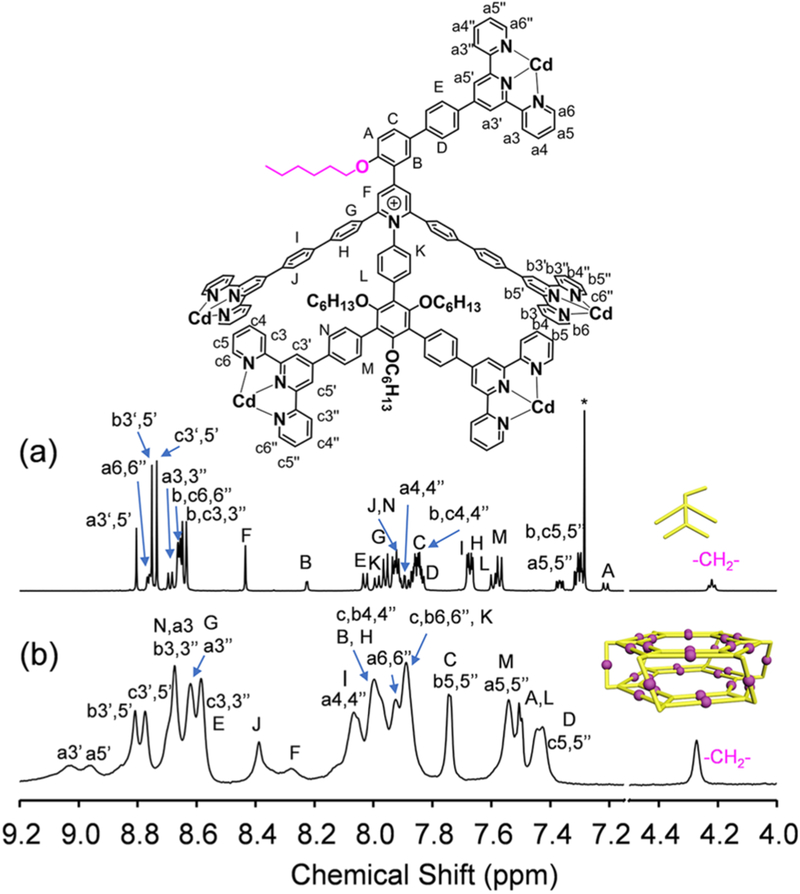
NMR spectroscopy was further employed to identify the structural information on the complexes by comparing with their corresponding ligands. Figure 3 displays the 1H NMR spectra of HP1 and L1. L1 exhibits three different sets of TPY protons with an integration ratio of 1/2/2 (Figure 3a), corresponding to the TPY groups in the tail (TPYa), the outer rim (TPYb), and the inner rim (TPYc) of the ligand, respectively. After coordination, characteristic upfield shifts of all TPY-H6,6″ signals are observed with Δδ= 0.8–1.0 ppm (Figure 3b), which is attributed to the electron shielding effect of the <TPY–Cd(II)–TPY> coordination environments.23 Also, much broader spectrum is displayed after coordination, due to the lower tumbling motion of the protons in the large complex.21 Notably, four sets of distinct TPY-H3′,5′ signals were clearly observed in the spectrum of HP1 instead of three sets in the case of L1. The additional set of signals is caused by the splitting of TPY-Ha3′,5′ after the formation of the <TPY–Cd(II)–TPY> lateral edges. One possible reason for the splitting is probably ascribed to the steric hindrance of the lateral faces, which blocks the free rotation of the TPY group and further slows down the tumbling motion of TPY-Ha3′,5′. As a result, much broader TPY-Ha3′,5′ signals are observed, compared with TPY-Hb,c3′,5′ signals. 1H NMR spectrum of HP2 shows exactly the same set of signals in the aromatic region (Figure S41).
Figure 3.
1H NMR spectra (600 MHz, 300 K) of (a) L1 in CDCl3 (the asterisk (*) represents the solvent residue of CHCl3) and (b) HP1 in d6-DMSO.
A 2D diffusion-ordered NMR spectroscopy (2D DOSY) analysis of HP1 in d6-DMSO reveals a single band at logD ≈ – 10.5 (Figure S47); HP2 also displays one dominant signal band with the same logD value (Figure S48). After conducting calculations by using the modified Stocks–Einstein equation based on the column model (detailed calculating procedure is summarized in the Supporting Information),24 the experimental diameter of the base is found to be 7.0 nm, and the height of the prism is 2.5 nm. Such sizes are comparable to the predicted results (8.0 nm in base diameter, and 2.7 nm in height) from the molecular modeling of these hexagonal prisms (Figure 1b,c). The other NMR spectroscopic results are summarized in Figures S1–28 and 35–46, including 2D COSY, 2D NOESY, and 13C NMR spectra (see Supporting Information).
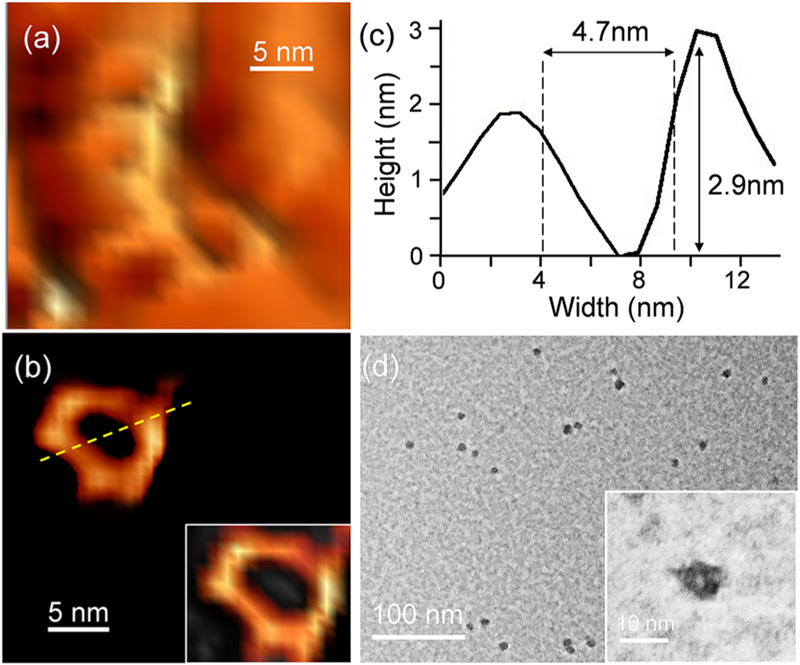
To obtain more shape and size information, atomic force microscopy (AFM) and transmission electron microscopy (TEM) were applied to image individual hexagonal prisms. AFM images were first collected on a fresh mica surface by drop casting a diluted DMF solution (3.5 × 10−6 mol/L) of HP1 for a short time (1 min) and then washing and drying the surface, to avoid aggregation. Particles with ring-like structures were observed under AFM with uniform diameters (Figure 4a,b, Figure S49) by utilizing an ultrasharp cantilever tip (1–2 nm) to reduce the broadening effect. Detailed analysis of the size and shape of one selected particle (Figure 4c) reveals a hexagonal ring with an outer rim diameter at ca. 9 nm and an inner rim diameter at 4.7 nm, comparable to the modeling size (Figure 1b,c). The average height of these particles is around 2.5 nm, corresponding to the height of one prism molecule. In addition, individual dots with uniform diameters were also observed under TEM, as shown in Figure 4d. The zoomed-in TEM image (Figure 4d inset) shows a ring-like structure, which can also be assigned to the base of HP1 with a comparable size (ca. 8 nm in diameter).
Figure 4.
AFM images of (a) individual HP1 on mica surface, (b) a selected single HP1 molecule and its 3D image (right bottom inset), (c) cross-section of the molecule shown in image b, (d) TEM images of individual HP1, zoomed-in image in inset.
Investigation of the Hierarchical Self-Assembly Behavior.
In our previous report, we found that KCs were able to hierarchically self-assemble into tubular-like nanostructures through the face-to-face packing.20a With the similar KCs structure acting as the bases, we speculated HP1 could similarly pack into nanostructures through base-to-base packing, which might provide us additional structural information on the prism. Gels were obtained by slow diffusion of ethyl acetate (the poor solvent) into the DMSO solution of HP1, then investigated by TEM (Figures S50). Worm-like nanostructures with uniform diameters at ca. 8 nm were observed, and most of them further interacted with each other to form bigger aggregation without well-defined pattern.
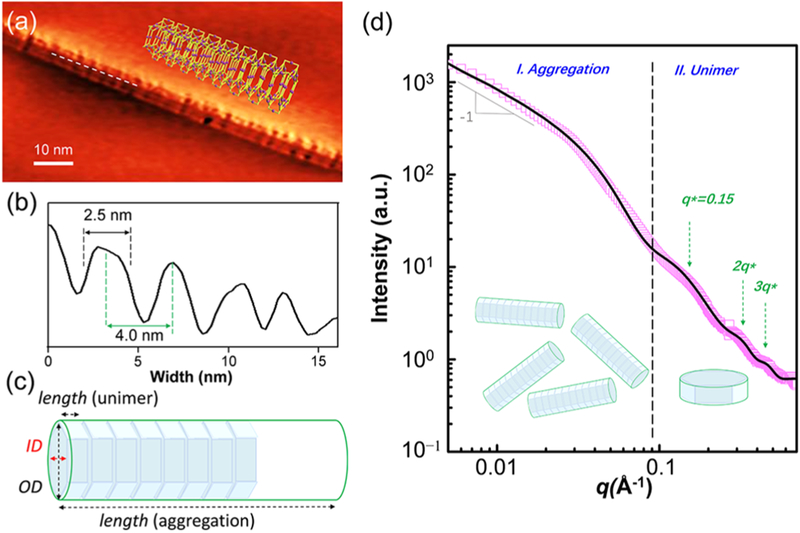
To obtain more detailed information on the nanostructures, scanning tunneling microscopy (STM) was utilized to image the samples on a highly oriented pyrolytic graphite (HOPG) surface. Acetonitrile instead of ethyl acetate was used as the aggregation solvent, due to its better solubility of the supramolecules, in order to get well-dispersed nanostructures on the surface (the detailed sample preparation procedure was summarized in the Supporting Information). A tubular-like nanostructure was observed as shown in Figure 5a with a uniform diameter around 8 nm, corresponding to the diameter of the KC base surface. Breakages on the nanowire were observed owing to the spacing between prisms. The periodic distribution of the bright areas and the gaps observed on the nanowires suggested the base-to-base packing of the prisms in the 1D nanostructure. The measured thickness of each repeating substructure in the nanostructure (marked distance in Figure 5b) was around 2.5 nm, which was well consistent with the predicted height of each supramolecular prism (Figure 1b). (More STM images of the nanostructures were included in Figure S51.)
Figure 5.
(a) STM images of tubular-like nanostructure on a HOPG surface; (b) cross-section of the line marked on the nanostructure shown in panel a. (c) The hollow cylinder model of the aggregated tubular-like nanostructure is hierarchically self-assembled by a hexagonal prism unimer. (d) 1-D SAXS of HP1 in acetonitrile solution (2.5 × 10−4 mol/L): (□) experimental data. The solid black line represents the best fit result.
One dimensional small angle X-ray scattering (1D-SAXS) was further used to study HP1 and its aggregates in acetonitrile solution (2.5 × 10−4 mol/L), and the results are summarized in Figure 5d. As expected, a q−1 decay was observed stemming from the low-q region beyond the probing range (Figure 5d, region I), suggesting the existence of long cylindrical objects with the average length over 1000 Å, consistent with the nanostructures observed in the STM images. Apparently, the HP1 aggregates in acetonitrile with the base-to-base configuration prior to the incubation process on the HOPG surface for STM imaging. Apart from the tubular-like structure, scattering signals corresponding to individual HP1 unimers were also observed at a higher q region (Figure 5d, region II) with three quasi-Bragg peaks located between 0.15 and 0.5 Å−1 corresponding to the high order harmonics (q*, 2q*, and 3q*), likely originating from the well-defined repeat spacing, d of the hexagonal prisms in the cylindrical aggregate. As a result, the best fitting model contains the contributions from discoidal shells, hollow cylinders, and three Gaussian peaks (Figure 5c). As shown in Figure 5d, the best fit agrees well with the experimental data, and the fitting parameters are listed in Table S1. The best fits for the prism height, outer rim, and inner rim diameters are (2.5 ± 0.2), (6.8 ± 1.1), and (4.0 ± 0.5) nm, respectively, which are consistent with the molecular modeling results (Figure 1b,c). Moreover, the cylindrical aggregates share the same inner rim and outer rim diameter with those of the unimer, except for an ultralong length. The uniform d derived from q* as is also consistent with the STM result (Figure 5b).
Study of the Self-Assembly Mechanism.
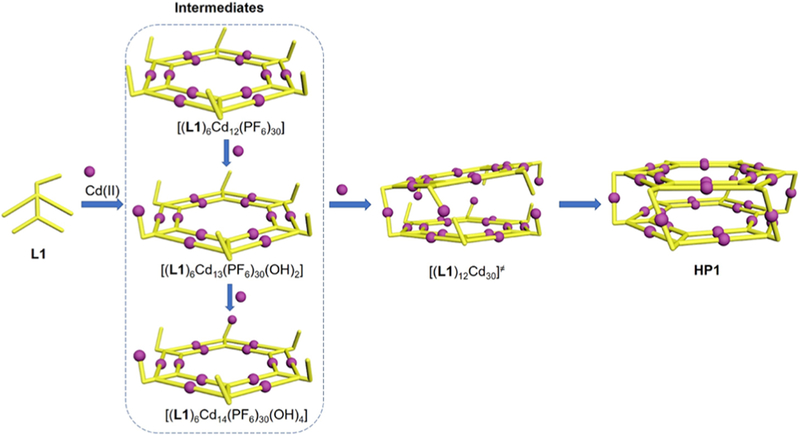
After characterization of the desired supramolecular hexagonal prisms, we further studied the self-assembly mechanism of these prism structures. Briefly, the self-assembly of L1 with Cd(II) (molar ratio: 2/5) was performed under a milder condition (i.e., 2 mg/mL of L1 in DMSO, 50 °C for 3 h) in order to determine the intermediate state of the assembling procedure. The ESI-MS spectrum (the anions were transferred to PF6−) shows two dominant sets of sharp peaks (Figure 6a), located at the lower m/z (700–1500 Da) and higher m/z (1500–2300 Da) regions, respectively. After deconvolution, the averaged molar mass of the signals at the lower m/z region was 20 094 Da, which exactly fits the chemical composition of [(L1)6Cd12(PF6)30]. In addition, the isotope pattern of each charge state (12+ to 22+) agrees well with the corresponding simulated one (Figure 6 inset and Figure S52). Such a chemical composition is assigned to a double-rimmed KC with six tail-anchored metal-free TPY groups (Scheme 2). It can be viewed as an intermediate prior to the formation of our desired prism structure (HP1). The peaks at a higher m/z region are assigned to HP1 with 18+ to 23+ charge. The coexistence of KC intermediates with HP1 was further confirmed by the TWIM-MS spectrum (Figure 6b) with two sets of signals. The signal bands at the lower m/z region with relative shorter drifting time are attributed to the intermediates, while the well split signal bands at higher m/z region correspond to the hexagonal prism structure with longer drift time. Between [(L1)6Cd12 (PF6)30] and HP1, other intermediates such as [(L1)6Cd13(PF6)30(OH)2] and [(L1)6Cd14(PF6)30(OH)4] (Scheme 2) were also detected by isotope analysis of mass spectra (Figures S53–S54). Every two of these KC intermediates together with a proper amount of Cd(II) finally formed HP1 by forming the lateral edges as the scaffold of the 3D structure. Such a stepwise assembly mechanism leads to precise self-sorting of the three types of TPY groups into three distinct coordination environments (Scheme 2). As a result, the KC rings play dual roles during the formation of the supramolecular hexagonal prisms by acting as the structural base surface and the template to guide the formation of the lateral edges.
Figure 6.
(a) ESI-MS and (b) TWIM-MS (m/z vs drift time) of the self-assembly of L1 with Cd(II) under a milder condition. Both intermediates (A series of signals) and HP1 (B series of signals) were observed; (right inset of a) theoretical and experimental isotope patterns of A19+.
Scheme 2.
Proposed Self-Assembly Mechanism of L1 Coordinating with Cd(II) to form HP1
Antimicrobial Activity.
It is expected that these 3D prisms might show antimicrobial activity against Gram-positive bacteria for the following reasons: (i) the high density of positive charges on these supramolecules provides high affinity with the negatively charged cell envelope of the Gram-positive bacteria;25 (ii) pyridinium containing molecules are widely applied as antimicrobial agents;26 (iii) the prisms may act as transmembrane channels to disrupt the bacterial membrane.20a As a result, the antibacterial activities of HP1/2 were evaluated with two Gram-positive bacteria, MRSA and B. subtilis, (Table 1)
Table 1.
Antimicrobial Activity and Selectivity of the Ligands and Supramolecules
| HP1 | HP2 | L1 | L2 | Cd(NO3)2 | |
|---|---|---|---|---|---|
| MRSA (IC50, μg/mL) | 1 | 1 | 3 | 3 | >25 |
| B. subtilis (IC50, μg/mL) | 3 | 2 | None | None | 3 |
| hemolysis (HC50, μg/mL) | >250 | >250 | >250 | >250 | >250 |
| selectivity HC50/IC50 (MASA) | >250 | >250 | >80 | >80 | >10 |
| selectivity HC50/IC50 (B. subtilis) | >80 | >120 | N/A | N/A | >80 |
Both HP1/2 showed antimicrobial activity on MRSA with IC50s as 1 μg/mL (ca. 22 nmol/L), which was lower than the IC50s of the corresponding ligands (3 μg/mL, 1200 nmol/L) and the control group, Cd(NO3)2·4H2O, (IC50 > 25 μg/mL, 8.1 × 105 nmol/L). Compared with the previous reported 2D KCs,20a HP1/2 displayed lower antibacterial activity, probably due to their low solubilities and high aggregation tendency proven by TEM/STM/SAXS studies. These features caused the precipitation or aggregation of HP1/2 in contact with the growth medium before their effective interaction with MRSA cells. As a result, supramolecular prisms with better water-solubility are expected to possess enhanced antimicrobial activity.
In addition, to expand the antimicrobial spectrum of this type of supramolecule, B. subtilis was used to evaluate the new agents. HP1/2 showed obvious antimicrobial activity against B. subtilis, while L1/2 did not exhibit potent activity (Table 1). Note that the weight-based IC50 value of Cd(II) control was comparable to the values of HP1/2. However, considering the much lower weight percentage of Cd(II) in HP1 (8.0%) and HP2 (7.8%) than in Cd(NO3)2·4H2O (37%), as well as the strong chelation of Cd(II) with the pentatopic TPY ligands in HP1/2, the antibacterial potency of the supramolecules should be mainly derived from the ensembles rather than individual component.
The red-blood-cell hemolysis studies of HP1/2 show negligible hemolytic toxicity (HC50 higher than 250 μg/mL), leading to their good antimicrobial selectivity toward MRSA and B. subtilis. We speculated that such a good selectivity should be based on the stronger electrostatic interaction of cationic HP1/2 with the negative charged surfaces of the Gram-positive bacteria,25 than with the zwitterionic surfaces of the mammalian cells.27
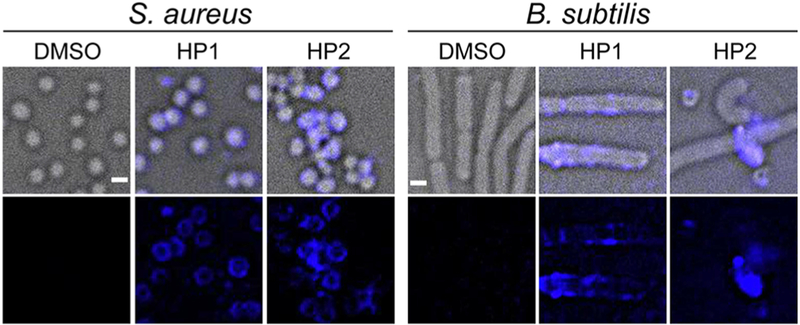
3D deconvolution fluorescence microscopy, a combination of optical and computational techniques to maximize the observed resolution and signal from a biological specimen,28 was further employed to monitor the location of HP1/2 during the growth inhibition in S. aureus and B. subtilis. Benefiting from the strong fluorescence (FL) nature of these two supramolecules (the FL spectra of HP1/2 are recorded in Figures S55–S56), no external dye is needed to visualize the cell membrane. As shown in Figure 7, strong fluorescence was observed on the surface of both S. aureus and B. subtilis after being treated with HP1/2. In addition, in both cases, significant fluorescence signal was absent outside of the cells. These results indicate the high selectivity and binding affinity of the antimicrobial materials for the Gram-positive bacteria, and then inhibit the growth of MASA and B. subtilis.
Figure 7.
3D deconvolution fluorescence microscopy images of bacteria cells with and without treatment of HP1/HP2 (4 μmol/L, DMSO as the control agent). Scale bar: 1 μm.
CONCLUSIONS
We were able to construct giant metallo-supramolecular hexagonal prisms with pentatopic terpyridine ligands, which displayed three different coordination environments during the self-assembly. Such a design fully takes advantage of the rigid scaffold of the pyridinium-aryl group and its facile synthesis. In addition, the detailed study of the self-assembly process reveals that the double-rimmed KCs are the key intermediates required to form a hexagonal prism architecture. The assembled supramolecular hexagonal prisms had a strong tendency to form a tubular-like nanostructure in solution as evidenced by STM imaging and SAXS study. Furthermore, the pyridinium salt containing supramolecular prisms showed potent antimicrobial activities against two Gram-positive bacteria, possibly due to their high binding affinity on the membrane of these bacteria. Overall, through deep understanding of the design and self-assembly process, this study may shed more light on designing more sophisticated 3D supramolecular architectures with desired functions.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the support from NIH (R01GM128037 to X.L.; 5R01AL110098–05 to B.X; and R35GM133617 to P.E.), and partial support through University of South Florida Nexus Initiative (UNI) Award. The SAXS data were collected with the help of Dr. Lin Yang, at the 16ID-LiX Beamline, National Synchrotron Light Source II, Brookhaven National Laboratory (BNL), NY, USA, through a beamtime proposal (BAG-302208). The LiX beamline is part of the Life Science Biomedical Technology Research resource, jointly supported by the National Institutes of Health, National Institute of General Medical Sciences, under Grant P41 GM111244, and by the Department of Energy Office of Biological and Environmental Research under Grant KP1605010, with additional support from NIH Grant S10 OD012331. NSLS-II is a U.S. Department of Energy (DOE) Office of Science User Facility, operated for the DOE Office of Science by Brookhaven National Laboratory under Contract No. DESC0012704.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.9b08484.
Synthetic details, molecular modeling, ligand and complex characterization, including 1H NMR, 13C NMR, 2D COSY, 2D NOESY, 2D DOSY, ESI-MS, UV–vis, emission spectra and additional STM images (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Whitesides GM; Grzybowski B Self-Assembly at All Scales. Science 2002, 295 (5564), 2418–2421. [DOI] [PubMed] [Google Scholar]
- (2).(a) Leininger S; Olenyuk B; Stang PJ Self-Assembly of Discrete Cyclic Nanostructures Mediated by Transition Metals. Chem. Rev 2000, 100 (3), 853–908. [DOI] [PubMed] [Google Scholar]; (b) Chakrabarty R; Mukherjee PS; Stang PJ Supramolecular Coordination: Self-Assembly of Finite Two- and Three-Dimensional Ensembles. Chem. Rev 2011, 111 (11), 6810–6918. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Cook TR; Stang PJ Recent Developments in the Preparation and Chemistry of Metallacycles and Metallacages via Coordination. Chem. Rev 2015, 115 (15), 7001–7045. [DOI] [PubMed] [Google Scholar]
- (3).(a) Zarra S; Wood DM; Roberts DA; Nitschke JR Molecular Containers in Complex Chemical Systems. Chem. Soc. Rev 2015, 44 (2), 419–432. [DOI] [PubMed] [Google Scholar]; (b) Dalgarno SJ; Power NP; Atwood JL Metallo-Supramolecular Capsules. Coord. Chem. Rev 2008, 252 (8), 825–841. [Google Scholar]
- (4).(a) Yoshizawa M; Tamura M; Fujita M Diels-Alder in Aqueous Molecular Hosts: Unusual Regioselectivity and Efficient Catalysis. Science 2006, 312 (5771), 251–254. [DOI] [PubMed] [Google Scholar]; (b) Park JS; Karnas E; Ohkubo K; Chen P; Kadish KM; Fukuzumi S; Bielawski CW; Hudnall TW; Lynch VM; Sessler JL Ion-Mediated Electron Transfer in a Supramolecular Donor-Acceptor Ensemble. Science 2010, 329 (5997), 1324–1327. [DOI] [PubMed] [Google Scholar]; (c) Kaphan DM; Levin MD; Bergman RG; Raymond KN; Toste FD A Supramolecular Microenvironment Strategy for Transition Metal Catalysis. Science 2015, 350 (6265), 1235–1238. [DOI] [PubMed] [Google Scholar]; (d) Cullen W; Misuraca MC; Hunter CA; Williams NH; Ward MD Highly Efficient Catalysis of the Kemp Elimination in the Cavity of a Cubic Coordination Cage. Nat. Chem 2016, 8, 231–236. [DOI] [PubMed] [Google Scholar]; (e) Wang Q-Q; Gonell S; Leenders SHAM; Dürr M; Ivanovic-Burmazovic I; Reek JNH Self-Assembled Nanospheres with Multiple Endohedral Binding Sites Pre-Organize Catalysts and Substrates for Highly Efficient Reactions. Nat. Chem 2016, 8, 225–230. [DOI] [PubMed] [Google Scholar]; (f) Marcos V; Stephens AJ; Jaramillo-Garcia J; Nussbaumer AL; Woltering SL; Valero A; Lemonnier J-F; Vitorica-Yrezabal IJ; Leigh DA Allosteric Initiation and Regulation of Catalysis with a Molecular Knot. Science 2016, 352 (6293), 1555–1559. [DOI] [PubMed] [Google Scholar]
- (5).(a) Mal P; Breiner B; Rissanen K; Nitschke JR White Phosphorus Is Air-Stable Within a Self-Assembled Tetrahedral Capsule. Science 2009, 324 (5935), 1697–1699. [DOI] [PubMed] [Google Scholar]; (b) Sawada T; Yoshizawa M; Sato S; Fujita M Minimal Nucleotide Duplex Formation in Water through Enclathration in Self-Assembled Hosts. Nat. Chem 2009, 1, 53. [DOI] [PubMed] [Google Scholar]; (c) Sawada T; Fujita M A Single Watson-Crick G·C Base Pair in Water: Aqueous Hydrogen Bonds in Hydrophobic Cavities.J. Am. Chem. Soc 2010, 132 (20), 7194–7201. [DOI] [PubMed] [Google Scholar]
- (6).(a) Samanta SK; Moncelet D; Briken V; Isaacs L Metal-Organic Polyhedron Capped with Cucurbit[8]uril Delivers Doxorubicin to Cancer Cells. J. Am. Chem. Soc 2016, 138 (43), 14488–14496. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Samanta SK; Quigley J; Vinciguerra B; Briken V; Isaacs L Cucurbit [7] uril Enables Multi-Stimuli-Responsive Release from the Self-Assembled Hydrophobic Phase of a Metal Organic Polyhedron. J. Am. Chem. Soc 2017, 139 (26), 9066–9074. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Zheng Y-R; Suntharalingam K; Johnstone TC; Lippard SJ Encapsulation of Pt(IV) Prodrugs within a Pt(II) Cage for Drug Delivery. Chem. Sci 2015, 6 (2), 1189–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).(a) Haynes CJE; Zhu J; Chimerel C; Hernández-Ainsa S; Riddell IA; Ronson TK; Keyser UF; Nitschke JR Blockable Zn10L15 Ion Channels through Subcomponent Self-Assembly. Angew. Chem., Int. Ed 2017, 56 (48), 15388–15392. [DOI] [PubMed] [Google Scholar]; (b) Kawano R; Horike N; Hijikata Y; Kondo M; Carné-Sánchez A; Larpent P; Ikemura S; Osaki T; Kamiya K; Kitagawa S; Takeuchi S; Furukawa S Metal-Organic Cuboctahedra for Synthetic Ion Channels with Multiple Conductance States. Chem 2017, 2 (3), 393–403. [Google Scholar]
- (8).(a) Fujita N; Biradha K; Fujita M; Sakamoto S; Yamaguchi K A Porphyrin Prism: Structural Switching Triggered by Guest Inclusion. Angew. Chem., Int. Ed 2001, 40 (9), 1718–1721. [PubMed] [Google Scholar]; (b) Suzuki K; Kawano M; Fujita M Solvato-Controlled Assembly of Pd3L6 and Pd4L8 Coordination “Boxes. Angew. Chem.,Int. Ed 2007, 46 (16), 2819–2822. [DOI] [PubMed] [Google Scholar]; (c) Bar AK; Chakrabarty R; Mostafa G; Mukherjee PS Self-Assembly of a Nanoscopic Pt12Fe12 Hetero-metallic Open Molecular Box Containing Six Porphyrin Walls. Angew. Chem., Int. Ed 2008, 47 (44), 8455–8459. [DOI] [PubMed] [Google Scholar]; (d) Caskey DC; Yamamoto T; Addicott C; Shoemaker RK; Vacek J; Hawkridge AM; Muddiman DC; Kottas GS; Michl J; Stang PJ Coordination-Driven Face-Directed Self-Assembly of Trigonal Prisms. Face-Based Conformational Chirality. J. Am. Chem. Soc 2008, 130 (24), 7620–7628. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Vacek J; Caskey DC; Horinek D; Shoemaker RK; Stang PJ; Michl J Pyridine Ligand Rotation in Self-Assembled Trigonal Prisms. Evidence for Intracage Solvent Vapor Bubbles. J. Am. Chem. Soc 2008, 130 (24), 7629–7638. [DOI] [PubMed] [Google Scholar]; (f) Bar AK; Mohapatra S; Zangrando E; Mukherjee PS A Series of Trifacial Pd6 Molecular Barrels with Porphyrin Walls. Chem. - Eur. J 2012, 18 (31), 9571–9579. [DOI] [PubMed] [Google Scholar]; (g) Yang J; Bhadbhade M; Donald WA; Iranmanesh H; Moore EG; Yan H; Beves JE Self-Assembled Supramolecular Cages Containing Ruthenium (II) Polypyridyl Complexes. Chem. Commun 2015, 51 (21), 4465–4468. [DOI] [PubMed] [Google Scholar]
- (9).(a) Kuehl CJ; Yamamoto T; Seidel SR; Stang PJ Self-Assembly of Molecular Prisms via an Organometallic “Clip. Org. Lett 2002, 4 (6), 913–915. [DOI] [PubMed] [Google Scholar]; (b) Kryschenko YK; Seidel SR; Muddiman DC; Nepomuceno AI; Stang PJ Coordination-Driven Self-Assembly of Supramolecular Cages: Heteroatom-Containing and Complementary Trigonal Prisms. J. Am. Chem. Soc 2003, 125 (32), 9647–9652. [DOI] [PubMed] [Google Scholar]; (c) Ghosh S; Mukherjee PS Self-Assembly of a Nanoscopic Prism via a New Organometallic Pt3 Acceptor and Its Fluorescent Detection of Nitroaromatics. Organometallics 2008, 27 (3), 316–319. [Google Scholar]; (d) Oldacre AN; Friedman AE; Cook TR A Self-Assembled Cofacial Cobalt Porphyrin Prism for Oxygen Reduction Catalysis. J. Am. Chem. Soc 2017, 139 (4), 1424–1427. [DOI] [PubMed] [Google Scholar]; (e) Sun S-S; Lees AJ One-Step Self-Assembly Organometallic Molecular Cages from 11 Components. Chem. Commun 2001, No. 1, 103–104. [Google Scholar]; (f) Ballester P; Oliva AI; Costa A; Deyà PM; Frontera A; Gomila RM; Hunter CA DABCO-Induced Self-Assembly of a Trisporphyrin Double-Decker Cage: Thermodynamic Characterization and Guest Recognition. J. Am. Chem. Soc 2006, 128 (16), 5560–5569. [DOI] [PubMed] [Google Scholar]; (g) Dinolfo PH; Coropceanu V; Brédas J-L; Hupp JT A New Class of Mixed-Valence Systems with Orbitally Degenerate Organic Redox Centers. Examples Based on Hexa-Rhenium Molecular Prisms. J. Am. Chem. Soc 2006, 128 (39), 12592–12593. [DOI] [PubMed] [Google Scholar]; (h) Govindaswamy P; Linder D; Lacour J; Süss-Fink G; Therrien B Self-Assembled Hexanuclear Arene Ruthenium Metallo-Prisms with Unexpected Double Helical Chirality. Chem. Commun 2006, No. 45, 4691–4693. [DOI] [PubMed] [Google Scholar]; (i) Oliva AI; Ventura B; Würthner F; Camara-Campos A; Hunter CA; Ballester P; Flamigni L Self-Assembly of Double-Decker Cages Induced by Coordination of Perylene Bisimide with a Trimeric Zn Porphyrin: Study of the Electron Transfer Dynamics between the two Photoactive Components. Dalton Trans 2009, No. 20, 4023–4037. [DOI] [PubMed] [Google Scholar]; (j) Mirtschin S; Slabon-Turski A; Scopelliti R; Velders AH; Severin K A Coordination Cage with an Adaptable Cavity Size. J. Am. Chem. Soc 2010, 132 (40), 14004–14005. [DOI] [PubMed] [Google Scholar]
- (10).(a) Wang M; Zheng Y-R; Ghosh K; Stang PJ Metallosupramolecular Tetragonal Prisms via Multicomponent Coordination-Driven Template-Free Self-Assembly. J. Am. Chem. Soc 2010, 132 (18), 6282–6283. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zheng Y-R; Zhao Z; Wang M; Ghosh K; Pollock JB; Cook TR; Stang PJ A Facile Approach toward Multicomponent Supramolecular Structures: Selective Self-Assembly via Charge Separation. J. Am. Chem. Soc 2010, 132 (47), 16873–16882. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Shi Y; Sánchez-Molina I; Cao C; Cook TR; Stang PJ Synthesis and Photophysical Studies of Self-Assembled Multicomponent Supramolecular Coordination Prisms Bearing Porphyrin Faces. Proc. Natl. Acad. Sci. U. S. A 2014, 111 (26), 9390–9395. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Yan X; Cook TR; Wang P; Huang F; Stang PJ Highly Emissive Platinum (II) Metallacages. Nat. Chem 2015, 7, 342. [DOI] [PubMed] [Google Scholar]; (e) Ye Y; Cook TR; Wang S-P; Wu J; Li S; Stang PJ Self-Assembly of Chiral Metallacycles and Metallacages from a Directionally Adaptable BINOL-Derived Donor. J. Am. Chem. Soc 2015, 137 (37), 11896–11899. [DOI] [PubMed] [Google Scholar]; (f) Yan X; Wang M; Cook TR; Zhang M; Saha ML; Zhou Z; Li X; Huang F; Stang PJ Light-Emitting Superstructures with Anion Effect: Coordination-Driven Self-Assembly of Pure Tetraphenylethylene Metallacycles and Metallacages. J. Am. Chem. Soc 2016, 138 (13), 4580–4588. [DOI] [PubMed] [Google Scholar]; (g) Yu G; Cook TR; Li Y; Yan X; Wu D; Shao L; Shen J; Tang G; Huang F; Chen X; Stang PJ Tetraphenylethene-Based Highly Emissive Metallacage as a Component of Theranostic Supramolecular Nanoparticles. Proc. Natl. Acad. Sci. U. S. A 2016, 113 (48), 13720–13725. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Zhang M; Saha ML; Wang M; Zhou Z; Song B; Lu C; Yan X; Li X; Huang F; Yin S; Stang PJ Multicomponent Platinum(II) Cages with Tunable Emission and Amino Acid Sensing. J. Am. Chem. Soc 2017, 139 (14), 5067–5074. [DOI] [PubMed] [Google Scholar]; (i) Lu C; Zhang M; Tang D; Yan X; Zhang Z; Zhou Z; Song B; Wang H; Li X; Yin S; Sepehrpour H; Stang PJ Fluorescent Metallacage-Core Supramolecular Polymer Gel Formed by Orthogonal Metal Coordination and Host-Guest Interactions. J. Am. Chem. Soc 2018, 140 (24), 7674–7680. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Bar AK; Mostafa G; Mukherjee PS A Pd6 Molecular Cage via Multicomponent Self-Assembly Incorporating Both Neutral and Anionic Linkers. Inorg. Chem 2010, 49 (17), 7647–7649. [DOI] [PubMed] [Google Scholar]
- (11).(a) Schmittel M; He B; Mal P Supramolecular Multi-component Self-Assembly of Shape-Adaptive Nanoprisms: Wrapping up C60 with Three Porphyrin Units. Org. Lett 2008, 10 (12), 2513–2516. [DOI] [PubMed] [Google Scholar]; (b) Gaikwad S; Lai Saha M; Samanta D; Schmittel M Five-Component Trigonal Nanoprism with Six Dynamic Corners. Chem. Commun 2017, 53 (57), 8034–8037. [DOI] [PubMed] [Google Scholar]; (c) Liu D; Chen M; Li Y; Shen Y; Huang J; Yang X; Jiang Z; Li X; Newkome GR; Wang P Vertical Assembly of Giant Double- and Triple-Decker Spoked Wheel Supramolecular Structures. Angew. Chem., Int. Ed 2018, 57 (43), 14116–14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).(a) Kumazawa K; Biradha K; Kusukawa T; Okano T; Fujita M Multicomponent Assembly of a Pyrazine-Pillared Coordination Cage That Selectively Binds Planar Guests by Intercalation. Angew. Chem., Int. Ed 2003, 42 (33), 3909–3913. [DOI] [PubMed] [Google Scholar]; (b) Yoshizawa M; Ono K; Kumazawa K; Kato T; Fujita M Metal-Metal d-d Interaction through the Discrete Stacking of Mononuclear M(II) Complexes (M = Pt, Pd, and Cu) within an Organic-Pillared Coordination Cage. J. Am. Chem. Soc 2005, 127 (31), 10800–10801. [DOI] [PubMed] [Google Scholar]; (c) Ono K; Yoshizawa M; Kato T; Watanabe K; Fujita M Porphine Dimeric Assemblies in Organic-Pillared Coordination Cages. Angew. Chem., Int. Ed 2007, 46 (11), 1803–1806. [DOI] [PubMed] [Google Scholar]; (d) Ono K; Yoshizawa M; Akita M; Kato T; Tsunobuchi Y; Ohkoshi S.-i.; Fujita M Spin Crossover by Encapsulation. J. Am. Chem. Soc 2009, 131 (8), 2782–2783. [DOI] [PubMed] [Google Scholar]; (e) Yamauchi Y; Yoshizawa M; Akita M; Fujita M Discrete Stack of an Odd Number of Polarized Aromatic Compounds Revealing the Importance of Net vs. Local Dipoles. Proc. Natl. Acad. Sci. U. S. A 2009, 106 (26), 10435–10437. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Osuga T; Murase T; Ono K; Yamauchi Y; Fujita M [m × n] Metal Ion Arrays Templated by Coordination Cages. J. Am. Chem. Soc 2010, 132 (44), 15553–15555. [DOI] [PubMed] [Google Scholar]; (g) Yamauchi Y; Yoshizawa M; Akita M; Fujita M Engineering Double to Quintuple Stacks of a Polarized Aromatic in Confined Cavities. J. Am. Chem. Soc 2010, 132 (3), 960–966. [DOI] [PubMed] [Google Scholar]; (h) Yamauchi Y; Hanaoka Y; Yoshizawa M; Akita M; Ichikawa T; Yoshio M; Kato T; Fujita M m × n Stacks of Discrete Aromatic Stacks in Solution. J. Am. Chem. Soc 2010, 132 (28), 9555–9557. [DOI] [PubMed] [Google Scholar]; (i) Sato S; Morohara O; Fujita D; Yamaguchi Y; Kato K; Fujita M Parallel-Stacked Aromatic Hosts for Orienting Small Molecules in a Magnetic Field: Induced Residual Dipolar Coupling by Encapsulation. J. Am. Chem. Soc 2010, 132 (11), 3670–3671. [DOI] [PubMed] [Google Scholar]; (j) Carpenter JP; McTernan CT; Ronson TK; Nitschke J R Anion Pairs Template a Trigonal Prism with Disilver Vertices. J. Am. Chem. Soc 2019, 141 (29), 11409–11413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).(a) Riddell IA; Smulders MMJ; Clegg JK; Hristova YR; Breiner B; Thoburn JD; Nitschke JR Anion-induced reconstitution of a self-assembling system to express a chloride-binding Co10L15 pentagonal prism. Nat. Chem 2012, 4, 751–756. [DOI] [PubMed] [Google Scholar]; (b) Kieffer M; Pilgrim BS; Ronson TK; Roberts DA; Aleksanyan M; Nitschke JR Perfluorinated Ligands Induce Meridional Metal Stereochemistry to Generate M8L12, M10L15, and M12L18 Prisms. J. Am. Chem. Soc 2016, 138 (21), 6813–6821. [DOI] [PubMed] [Google Scholar]; (c) Riddell IA; Ronson TK; Clegg JK; Wood CS; Bilbeisi RA; Nitschke JR Cation- and Anion-Exchanges Induce Multiple Distinct Rearrangements within Metallosupramolecular Architectures. J. Am. Chem. Soc 2014, 136 (26), 9491–9498. [DOI] [PubMed] [Google Scholar]; (d) Zarra S; Clegg JK; Nitschke JR Selective Assembly and Disassembly of a Water-Soluble Fe10L15 Prism. Angew. Chem., Int. Ed 2013, 52 (18), 4837–4840. [DOI] [PubMed] [Google Scholar]
- (14).Dale EJ; Vermeulen NA; Juricek M; Barnes JC; Young RM; Wasielewski MR; Stoddart JF Supramolecular Explorations: Exhibiting the Extent of Extended Cationic Cyclophanes. Acc. Chem. Res 2016, 49 (2), 262–273. [DOI] [PubMed] [Google Scholar]
- (15).(a) Hafezi N; Holcroft JM; Hartlieb KJ; Dale EJ; Vermeulen NA; Stern CL; Sarjeant AA; Stoddart JF Modulating the Binding of Polycyclic Aromatic Hydrocarbons Inside a Hexacationic Cage by Anion-π Interactions. Angew. Chem., Int. Ed 2014, 54 (2), 456–461. [DOI] [PubMed] [Google Scholar]; (b) Dale EJ; Vermeulen NA; Thomas AA; Barnes JC; Juríček M; Blackburn AK; Strutt NL; Sarjeant AA; Stern CL; Denmark SE; Stoddart JF ExCage. J. Am. Chem. Soc 2014, 136 (30), 10669–10682. [DOI] [PubMed] [Google Scholar]
- (16).Roy B; Zangrando E; Mukherjee PS Self-Assembly of a Redox Active Water Soluble Pd6L8 ‘Molecular Dice’. Chem. Commun 2016, 52 (24), 4489–4492. [DOI] [PubMed] [Google Scholar]
- (17).(a) Yazaki K; Kishi N; Akita M; Yoshizawa M A Bowl-Shaped Organic Host Using Bispyridine Ligands: Selective Encapsulation of Carbonyl Guests in Water. Chem. Commun 2013, 49 (16), 1630–1632. [DOI] [PubMed] [Google Scholar]; (b) Kurihara K; Yazaki K; Akita M; Yoshizawa M A Switchable Open/Closed Polyaromatic Macrocycle that Shows Reversible Binding of Long Hydrophilic Molecules. Angew. Chem., Int. Ed 2017, 56 (38), 11360–11364. [DOI] [PubMed] [Google Scholar]
- (18).Cai L-X; Li S-C; Yan D-N; Zhou L-P; Guo F; Sun Q-F Water-Soluble Redox-Active Cage Hosting Polyoxometalates for Selective Desulfurization Catalysis. J. Am. Chem. Soc 2018, 140 (14), 4869–4876. [DOI] [PubMed] [Google Scholar]
- (19).(a) Katritzky AR; Manzo RH Kinetics and Mechanism of the Reactions of Primary Amines with Pyrylium Cations. J. Chem. Soc., Perkin Trans. 2 1981, 2 (3), 571–575. [Google Scholar]; (b) Katritzky AR; Manzo RH; Lloyd JM; Patel RC Mechanism of the Pyrylium/Pyridinium Ring Interconversion. Mild Preparative Conditions for Conversion of Amines into Pyridinium Ions. Angew. Chem., Int. Ed. Engl 1980, 19 (4), 306–306. [Google Scholar]
- (20).(a) Wang H; Qian X; Wang K; Su M; Haoyang W-W; Jiang X; Brzozowski R; Wang M; Gao X; Li Y; Xu B; Eswara P; Hao X-Q; Gong W; Hou J-L; Cai J; Li X Supramolecular Kandinsky Circles with High Antibacterial Activity. Nat. Commun 2018, 9 (1), 1815. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wang M; Wang K; Wang C; Huang M; Hao X-Q; Shen M-Z; Shi G-Q; Zhang Z; Song B; Cisneros A; Song M-P; Xu B; Li X Self-Assembly of Concentric Hexagons and Hierarchical Self-Assembly of Supramolecular Metal-Organic Nanoribbons at the Solid/Liquid Interface. J. Am. Chem. Soc 2016, 138 (29), 9258–9268. [DOI] [PubMed] [Google Scholar]; (c) Wang H; Li Y; Yu H; Song B; Lu S; Hao X-Q; Zhang Y; Wang M; Hla S-W; Li X Combining Synthesis and Self-Assembly in One Pot To Construct Complex 2D Metallo-Supramolecules Using Terpyridine and Pyrylium Salts. J. Am. Chem. Soc 2019, 141 (33), 13187–13195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).(a) Sun Q-F; Iwasa J; Ogawa D; Ishido Y; Sato S; Ozeki T; Sei Y; Yamaguchi K; Fujita M Self-Assembled M24L48 Polyhedra and Their Sharp Structural Switch upon Subtle Ligand Variation. Science 2010, 328 (5982), 1144–1147. [DOI] [PubMed] [Google Scholar]; (b) Fujita D; Ueda Y; Sato S; Mizuno N; Kumasaka T; Fujita M Self-Assembly of Tetravalent Goldberg Polyhedra from 144 Small Components. Nature 2016, 540, 563. [DOI] [PubMed] [Google Scholar]; (c) Fujita D; Ueda Y; Sato S; Yokoyama H; Mizuno N; Kumasaka T; Fujita M Self-Assembly of M30L60 Icosidodecahedron. Chem 2016, 1 (1), 91–101. [Google Scholar]
- (22).(a) Brocker ER; Anderson SE; Northrop BH; Stang PJ; Bowers MT Structures of Metallosupramolecular Coordination Assemblies Can. Be Obtained by Ion Mobility Spectrometry-Mass Spectrometry. J. Am. Chem. Soc 2010, 132 (38), 13486–13494. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Li X; Chan Y-T; Newkome GR; Wesdemiotis C Gradient Tandem Mass Spectrometry Interfaced with Ion Mobility Separation for the Characterization of Supramolecular Architectures. Anal. Chem 2011, 83 (4), 1284–1290. [DOI] [PubMed] [Google Scholar]; (c) Thalassinos K; Grabenauer M; Slade SE; Hilton GR; Bowers MT; Scrivens JH Characterization of Phosphorylated Peptides Using Traveling Wave-Based and Drift Cell Ion Mobility Mass Spectrometry. Anal. Chem 2009, 81 (1), 248–254. [DOI] [PubMed] [Google Scholar]
- (23).(a) Wang J-L; Li X; Lu X; Hsieh IF; Cao Y; Moorefield CN; Wesdemiotis C; Cheng SZD; Newkome GR Stoichiometric Self-Assembly of Shape-Persistent 2D Complexes: A Facile Route to a Symmetric Supramacromolecular Spoked Wheel. J. Am. Chem. Soc 2011, 133 (30), 11450–11453. [DOI] [PubMed] [Google Scholar]; (b) Lu X; Li X; Cao Y; Schultz A; Wang J-L; Moorefield CN; Wesdemiotis C; Cheng SZD; Newkome GR Self-Assembly of a Supramolecular, Three-Dimensional, Spoked, Bicycle-like Wheel. Angew. Chem., Int. Ed 2013, 52 (30), 7728–7731. [DOI] [PubMed] [Google Scholar]
- (24).(a) Giuseppone N; Schmitt J-L; Allouche L; Lehn J-M DOSY NMR Experiments as a Tool for the Analysis of Constitutional and Motional Dynamic Processes: Implementation for the Driven Evolution of Dynamic Combinatorial Libraries of Helical Strands. Angew. Chem., Int. Ed 2008, 47 (12), 2235–2239. [DOI] [PubMed] [Google Scholar]; (b) Schulze BM; Watkins DL; Zhang J; Ghiviriga I; Castellano RK Estimating the Shape and size of Supramolecular Assemblies by Variable Temperature Diffusion Ordered Spectroscopy. Org. Biomol. Chem 2014, 12 (40), 7932–7936. [DOI] [PubMed] [Google Scholar]
- (25).Weidenmaier C; Peschel A Teichoic Acids and Related Cell-Wall Glycopolymers in Gram-Positive Physiology and Host Interactions. Nat. Rev. Microbiol 2008, 6, 276. [DOI] [PubMed] [Google Scholar]
- (26).(a) Sambhy V; Peterson BR; Sen A Antibacterial and Hemolytic Activities of Pyridinium Polymers as a Function of the Spatial Relationship between the Positive Charge and the Pendant Alkyl Tail. Angew. Chem., Int. Ed 2008, 47 (7), 1250–1254. [DOI] [PubMed] [Google Scholar]; (b) Docherty KM; Kulpa CF Jr. Toxicity and Antimicrobial Activity of Imidazolium and Pyridinium Ionic Liquids. Green Chem 2005, 7 (4), 185–189. [Google Scholar]
- (27).Nederberg F; Zhang Y; Tan JPK; Xu K; Wang H; Yang C; Gao S; Guo XD; Fukushima K; Li L; Hedrick JL; Yang Y-Y Biodegradable Nanostructures with Selective Lysis of Microbial Membranes. Nat. Chem 2011, 3, 409. [DOI] [PubMed] [Google Scholar]
- (28).Sarder P; Nehorai A Deconvolution Methods for 3-D Fluorescence Microscopy Images. IEEE Signal Process. Mag 2006, 23 (3), 32–45. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.