Abstract
Lymphagiomatosis are rare benign malformations of the lymphatic system. They are more commonly seen during childhood and are frequently asymptomatic and incidentally found in the adult patient. We report a case of a 31-year-old male who presented initially with melena. Computer tomography scan revealed multiple confluent, fluid-density lesions encasing the retroperitoneum and mesentery. A laparotomy and incisional biopsy of the mesenteric lesion was performed. Histologic examination demonstrated fibrofatty tissue with prominent, thick-walled endothelial-lined vessels. The histologic and computer tomography findings were consistent with a diagnosis of retroperitoneal and mesenteric lymphangiomatosis. The patient was subsequently discharged home well.
Keywords: Abdomen, Computer tomography scan, Lymphangiomatosis, Melena, Surgery
Introduction
Lymphangiomatosis are rare, benign malformations of the lymphatic vessels. It is usually diagnosed during childhood and are usually incidentally diagnosed in asymtomatic adults. These lesions are preferentially located in the neck and axilla, and abdominal lymphagiomatosis are uncommonly encountered. We report a case of a 31-year-old male presenting with melena and incidentally diagnosed with retroperitoneal and mesenteric lymphangiomatosis.
Case presentation
A 31-year-old male presented to our institution with a one day history of melena for investigation. The patient had a background of recurrent gastrointestinal (GI) bleeding as evidenced by 2 separate episodes of melena that occurred a month and a week prior to this presentation. Oesophagogastroduodenoscopy performed during those episodes revealed a Forrest III D1 ulcer and a Forrest IA D1 ulcer respectively. These were successfully treated endoscopically with hemoclips and adrenaline injection. Physical examination did not reveal any abnormalities apart from conjunctival pallor. No abdominal masses were noted. Significant laboratory investigations included anemia (Hemoglobin 6.5 g/dL) and hypoproteinemia (Albumin 20 g/L). Coagulation profile was within normal limits.
An urgent oesophagogastroduodenoscopy was performed which did not reveal any active bleeding. Subsequently, patient developed further episodes of melena requiring a repeat attempt at endoscopic hemostasis. This was unsuccessful and a computer tomography (CT) angiogram was performed for the patient with a view for interventional radiological management of the bleeding D1 ulcer. However, this did not demonstrate any active bleeding. The patient's bleeding spontaneously resolved thereafter and he was maintained on proton pump inhibitors.
Incidentally, the CT angiogram revealed multiple confluent, fluid-density lesions encasing the mesenteric and retroperitoneal vessels (Figs. 1 and 2). Given the radiological findings, our initial concern was to evaluate for chronic infection or malignancy. Infective markers including tests for tuberculosis and human immunodeficiency virus were negative. A pan-CT scan of the patient demonstrated lobulated, fluid density cystic lesions around the mesenteric root encasing the mesenteric vessels (see Fig. 2). No other lesions in the rest of the body were identified on the pan-CT. Bone marrow aspirate did not reveal any lymphoproliferative disorder.
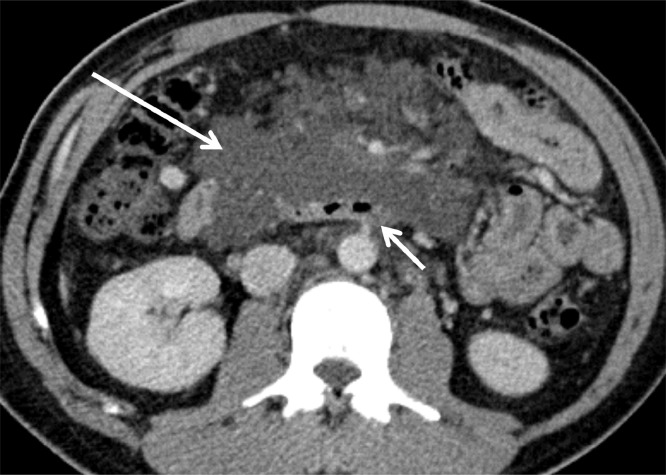
Fig. 1.
Contrast-enhanced axial sections of the abdomen reveal lobulated, fluid-density cystic lesions in the root of the mesentery (long arrow). These cause mild mass effect with displacement of the bowel loops. Note the lack of obstruction on the adjacent third part of duodenum (short arrow) despite the size of the lesion
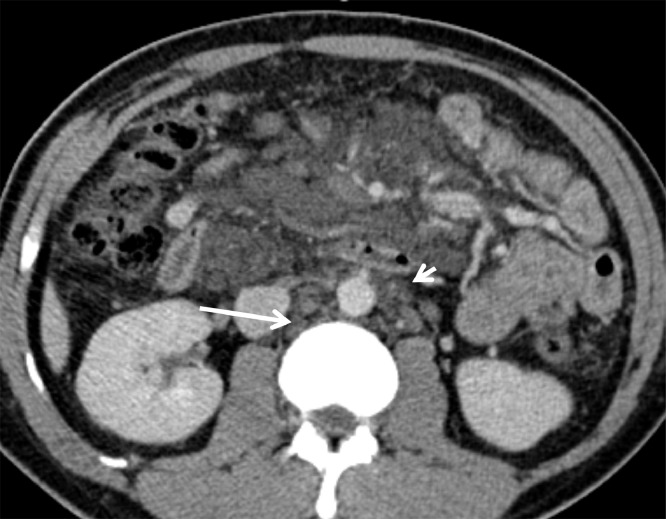
Fig. 2.
Low attenuation retroperitoneal cystic lesions are similarly noted in the retrocaval (arrow) and para-aortic (arrowhead) regions
The patient subsequently underwent elective exploratory laparotomy to obtain tissue samples for histologic analysis. Intraoperatively, diffusely swollen mesenteric tissue with multiple enlarged lymphatic channels were noted. Serous lymphatic fluid was also seen leaking out under pressure after incisional biopsy of the lesion. In view of the extent of mesenteric involvement and presence of dense adhesions, the operation was concluded after the biopsy. Histopathological examination showed fibrofatty tissue with prominent, thick-walled endothelial-lined vessels consistent with a diagnosis of lymphangiomatosis.
Postoperatively, the patient recovered well apart from having postoperative ileus which resolved with bowel rest and drip and suck regimen. There were no more episodes of melena. He was discharged uneventfully on the eighth postoperative day. The patient was reviewed 6 weeks after discharge and found to be asymptomatic with good appetite and bowel habits.
Discussion
Definition
Lymphangiomatosis are rare, benign proliferative malformations of the lymphatic system. They consist of endothelium-lined spaces surrounded by a connective tissue stroma of varying thickness containing lymphoid tissue, round cells and smooth muscle [1]. Up to 90% of cases are diagnosed within the first 2 years of life [2] and it is rarely diagnosed in adults [1], [3]. There is no gender predilection and no familial tendency [2]. Although the exact etiology of the disease remains unclear, its occurrence mainly in the paediatric age group has led researchers to postulate that the disease is due to a congenital abnormality of the lymphatic system [3]. Other secondary causes of lymphangiomatosis include abdominal trauma, surgery, radiation, lymphatic obstruction or inflammatory processes [4].
Lymphangiomatosis have been reported in almost all body organs but the brain, which does not contain lymphatic tissue [5]. The vast majority (95%) of lesions are located in the neck (known as “cystic hygromas”) and axilla, with the remaining 5% scattered throughout the body [6]. Abdominal lymphangiomatosis account for less than 1% of all cases and typically affect the retroperitoneum. Intraperitoneal sites include the mesentery and omentum, with the small bowel mesentery accounting for 70% of intraperitoneal disease [7]. Of note, our patient had retroperitoneal and mesenteric disease consistent with data reported in the literature.
Symptoms/presentation
There are no pathognomonic symptoms or signs of abdominal lymphangiomatosis and routine laboratory tests are nonspecific. Abdominal pain, distention or a palpable abdominal mass are possible findings but patients can present in a variety of ways. As these lesions are essentially pockets of fluid, patients are often asymptomatic and incidentally diagnosed only after abdominal imaging. Alternatively, the patient may present acutely with pain, GI bleeding, intestinal obstruction or bowel perforation [8], [9]. Uncommon but potentially life-threatening complications include traumatic cyst rupture, cyst torsion, intestinal volvulus or severe GI bleeding [10].
There are a handful of case reports in the literature [1], [11], [12] describing adult patients having abdominal lymphangiomatosis with GI bleeding and hypoproteinemia. It has been suggested that hemorrhage into cystic lymphatic spaces resulting in GI bleed could be due to trauma, development of a venolymphatic communication or a developing hemangioma as a tumour component. Hypoproteinemia results from an associated protein-losing enteropathy due to impaired mucosal integrity. Although our patient presented in a similar manner, the association of his bleeding D1 ulcer and abdominal lymphangiomatosis was likely coincidental.
Investigation
Plain abdominal radiography cannot diagnose abdominal lymphangiomatosis per se but may detect complications such as intestinal obstruction or bowel displacement from mass effect. Ultrasonography may reveal cystic lesions with internal echoes. Occasionally, the lesions can be complicated by haemorrhage or infection, resulting in echogenic content within. Cross sectional imaging can provide information pertaining to size, anatomical location, degree of organ involvement and presence of complications. Lymphangiomatosis can be differentiated from ascites by its retroperitoneal location, presence of septations, and lack of fluid in the mesenteric leaves as well as the dependent portions of the abdomen and pelvis. On CT, they are seen as uni- or multilocular low density lesions with thin, imperceptible walls. This is seen in our patient's case as well (see Fig. 3). Lesions are typically of fluid (near 0 HU) or chylous (around -20 HU) density depending on its contents. Rarely, haemorrhage can increase the density of the internal content and be confused with solid masses. Calcifications within the lesions are rare [13]. Lymphangiomatosis have low signal on T1-weighted imaging and high signal on T2-weighted imaging on MRI. With a large amount of chyle, the lesions will demonstrate high signal on T1-weighted images and intermediate signal on T2-weighted images [14].
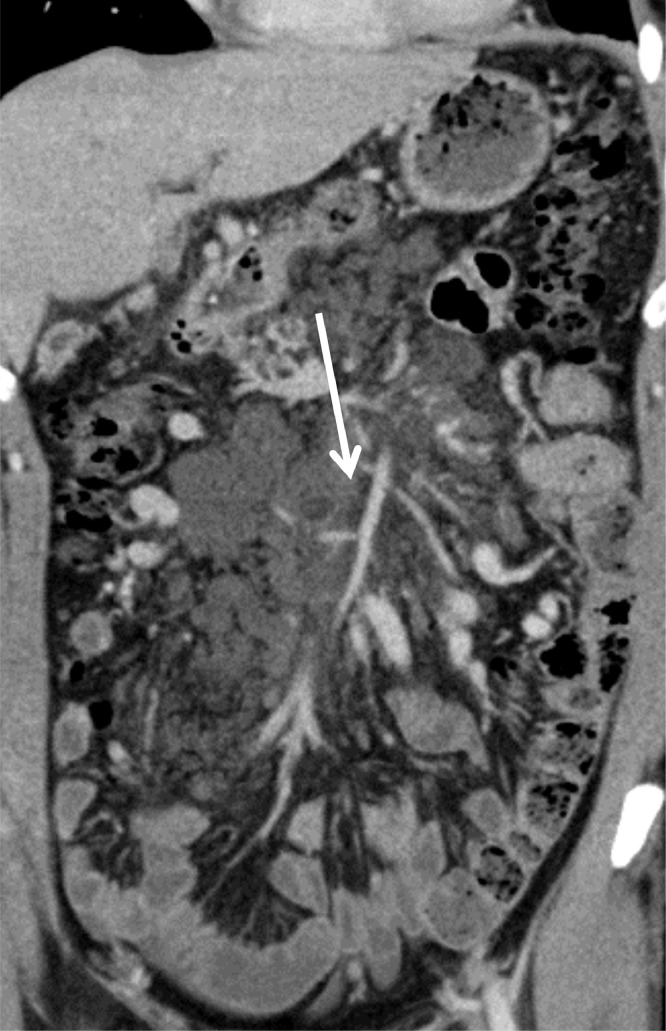
Fig. 3.
Contrast-enhanced coronal sections of the abdomen demonstrating a multilocular cystic lesion at the root of the mesentery (arrow). Note the absence of a perceptible wall
Occasionally, abdominal lymphangiomatosis incite a marked inflammatory reaction that can mimic acute appendicitis or even a malignant tumour. As such, a definitive diagnosis is usually made postoperatively based on histopathological or immunochemical findings. Lining mesothelial cells are immunoreactive for cytokeratin and negative for factor VIIIs. Double staining with CD31 and Proxim-1 is the most reliable method to demonstrate lymphangioma endothelial cells [7], [15].
Treatment
Some authors have suggested that the ideal treatment for abdominal lymphangiomatosis is radical surgical excision, even for asymptomatic patients, due to its potential for invasion and/or growth to large sizes [10]. However, surgery may be too risky or technically impossible for disease involving major abdominal structures. Furthermore, Kochman et al advocated that aggressive surgery be avoided in these asymptomatic patients as these lesions are inherently benign [16]. Other reported treatments in the literature include: marsupialization, percutaneous drainage, injection sclerotherapy and biologic response modifiers [17], [18], [19], [20]. Owing to the rarity of this disease and the paucity of evidence in the literature, patients should be managed on an individual basis – taking into account their symptoms, extent of disease and presence of complications.
The extensive involvement of the small bowel mesentery and retroperitoneum in our patient rendered radical excision impossible. Furthermore, as the condition was incidentally diagnosed, the procedure was limited to an open incisional biopsy for histologic diagnosis. As he remained asymptomatic on follow-up, no further treatment of the condition was offered.
Conclusion
Abdominal lymphangiomatosis is an uncommon disease entity seldom encountered in adult surgical practice. Its presentation may be subtle and varied. CT or MRI is the recommended diagnostic investigation of choice. As the majority of patients are asymptomatic, this condition should be conservatively managed. However, patients presenting with acute complications may require urgent surgical consult and intervention.
Author contributions
Dr Shaun Chan – First author. Collected data, literature search and drafted manuscript.
Dr Kenneth Kwan – Contributing author. Reviewed CT images and provided comments.
Dr Teo Li-Tserng - Supervising Author. Responsible for final edits to the manuscript.
Footnotes
Competing Interests: The authors declare no conflict of interest regarding the publication of this paper.
References
- 1.Lin R.Y., Zou H., Chen T.Z. Abdominal lymphangiomatosis in a 38-year-old female: case report and literature review. World J Gastroenterol. 2014;20(25):8320–8324. doi: 10.3748/wjg.v20.i25.8320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alqahtani A., Nguyen L.T., Flageole H. 25 Years’ experience with lymphangiomas in children. J Pediatr Surg. 1999;34:1164–1168. doi: 10.1016/s0022-3468(99)90590-0. [DOI] [PubMed] [Google Scholar]
- 3.Hanagiri T., Baba M., Shimabukuro T. Lymphangioma in the small intestine: report of a case and review of the Japanese literature. Surg Today. 1992;22:363–367. doi: 10.1007/BF00308747. [DOI] [PubMed] [Google Scholar]
- 4.Suthiwartnarueput W., Kiatipunsodsai S., Kwankua A. Lymphangioma of the small bowel mesentery: a case report and review of the literature. World J Gastroenterol. 2012;18(43):6328–6338. doi: 10.3748/wjg.v18.i43.6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stopinski J., Stephan S., Staib I. Intra-abdominal cystic lymphangioma and mesenteric cysts as a cause of abdominal discomfort. Langenbecks Arch Chir. 1994;379:182–187. doi: 10.1007/BF00680116. [DOI] [PubMed] [Google Scholar]
- 6.Roisman I., Manny J., Fields S. Intra-abdominal lymphangioma. Br J Surg. 1989;76:485–489. doi: 10.1002/bjs.1800760519. [DOI] [PubMed] [Google Scholar]
- 7.Aprea G., Guida F., Canfora A. Mesenteric cystic lymphangioma in adults: a case series and review of the literature. BMC Surg. 2013;13(Suppl 1):A4. [Google Scholar]
- 8.Takiff H., Calabria R., Yin L., Stabile B.E. Mesenteric cysts and intra-abdominal cystic lymphangiomas. Arch Surg. 1985;120:1266–1269. doi: 10.1001/archsurg.1985.01390350048010. [DOI] [PubMed] [Google Scholar]
- 9.Giuliani A., Romano L., Coletti G. Lymphangiomatosis of the ileum with perforation: a case report and literature review. Ann Med Surg (Lond) 2019;41:6–10. doi: 10.1016/j.amsu.2019.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jang J.H., Lee S.L., Ku Y.M. Small bowel volvulus induced by mesenteric lymphangioma in an adult: a case report. Korean J Radiol. 2009;10(3):319–322. doi: 10.3348/kjr.2009.10.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amadori G., Micciolo R., Poletti A. A case of intra-abdominal multiple lymphangiomas in an adult in whom the immunological evaluation supported the diagnosis. Eur J Gastroenterol Hepatol. 1999;11:347–351. doi: 10.1097/00042737-199903000-00022. [DOI] [PubMed] [Google Scholar]
- 12.Takami A., Nakao S., Sugimori N. Management of disseminated intra-abdominal lymphangiomatosis with protein-losing enteropathy and intestinal bleeding. South Med J. 1995;88:1156–1158. doi: 10.1097/00007611-199511000-00016. [DOI] [PubMed] [Google Scholar]
- 13.Cutillo D.P., Swayne L.C., Cucco J. CT and MR imaging in cystic abdominal lymphangiomatosis. J Comput Assist Tomogr. 1989;13(3):534–536. doi: 10.1097/00004728-198905000-00038. [DOI] [PubMed] [Google Scholar]
- 14.Rajiah P., Sinha R., Cuevas C. Imaging of uncommon retroperitoneal masses. Radiographics. 2011;31(4):949–976. doi: 10.1148/rg.314095132. [DOI] [PubMed] [Google Scholar]
- 15.Hornick J.L., Fletcher C.D. Intraabdominal cystic lymphangiomas obscured by marked superimposed reactive changes: clinicopathological analysis of a series. Hum Pathol. 2005;36(4):426–432. doi: 10.1016/j.humpath.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Kochman M.L., Wiersema M.J., Hawes R.H. Preoperative diagnosis of cystic lymphangioma of the colon by endoscopic ultrasound. Gastrointest Endosc. 1997;45:204–206. doi: 10.1016/s0016-5107(97)70253-0. [DOI] [PubMed] [Google Scholar]
- 17.Chung W.C., Kim H.K., Yoo J.Y. Colonic lymphangiomatosis associated with anemia. World J Gastroenterol. 2008;14:5760–5762. doi: 10.3748/wjg.14.5760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rostom A.Y. Treatment of thoracic lymphangiomatosis. Arch Dis Child. 2000;83:138–139. doi: 10.1136/adc.83.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grunewald T.G., Damke L., Maschan M. First report of effective and feasible treatment of multifocal lymphangiomatosis (Gorham-Stout) with bevacizumab in a child. Ann Oncol. 2010;21(8):1733–1734. doi: 10.1093/annonc/mdq331. [DOI] [PubMed] [Google Scholar]
- 20.Valerio M., Meuwly J.Y., Tawadros C. Percutaneous drainage and sclerotherapy as definitive treatment of renal lymphangiomatosis. Can Urol Assoc J. 2012;6(1):E3–E7. doi: 10.5489/cuaj.11034. [DOI] [PMC free article] [PubMed] [Google Scholar]