Abstract
Objectives:
The aim of the study was to (1) assess the relationship between body mass index (BMI) and operative time during immediate postpartum tubal ligation procedures and to (2) determine whether operative time is non-inferior in women with BMI ≥30 versus women with BMI <30 and in women with BMI ≥40 versus women with BMI <40.
Study design:
We conducted a retrospective cohort study of women who received immediate postpartum tubal ligations following vaginal delivery from 2013 to 2017 at a university hospital. We abstracted demographic information, patient and procedural characteristics, and clinical outcomes. We assessed the relationship between BMI and operative time via linear regression. We also conducted non-inferiority analysis to determine whether the mean operative time in women with BMI ≥30 was non-inferior to the mean operative time in women with BMI <30, within a non-inferiority margin of 10 min. We compared intraoperative and postoperative complications in the two groups.
Results:
A total of 279 women were included for analysis, among whom N=79 (28%) had a BMI of 25–29.9 and N=171 (61%) had a BMI ≥30. Demographic characteristics were similar in both groups. We found that operative time increased by 35 s for each one-point increase in BMI (p<.01). Although mean operative time was 46.1 min (n=171; 95% CI 43.7, 48.6 min) for women with BMI ≥30 and 40.6 min (n=108; 95% CI 37.9 min, 43.4 min) for women with BMI <30, (p<.01), it was non-inferior within a 10-min margin. There was no difference in rates of intraoperative or postoperative complications, incision length, total anesthesia time, and median length of stay between women with BMI ≥30 and BMI <30.
Conclusion:
There is a small increase in postpartum tubal ligation operative time with increasing BMI. However, among women who received immediate postpartum tubal ligations at our institution, women with BMI ≥30 versus BMI <30 had operative times that were non-inferior within a 10-min margin.
Implications:
While increasing body mass index slightly increases the operative time for immediate postpartum tubal ligations, this increase in time does not appear to be clinically significant.
Keywords: Obesity, Tubal interruption, Postpartum, Contraception, Surgery, Surgical outcomes
1. Introduction
In the United States, more than one of three women are obese with a body mass index (BMI) of 30 or greater [1]. Obese women are less likely to have contraception uptake due to weight gain concerns and underestimation about their fertility potential [2]. Obese women experience unintended pregnancy at a higher rate than non-obese women, and have poorer access to contraceptive and family planning services [3]. Morbidly obese women, or those with a BMI of 40 or greater, have the highest risk of unintended pregnancy and are the least likely of all BMI groups to use contraception [4]. Unintended pregnancy in obese women is especially worrisome due to consequent maternal obstetrical risks and increased rates of neonatal morbidity [5,6].
The postpartum period is a critical time for contraception counseling, as uptake of contraception during this period results in improved birth spacing outcomes [7]. Tubal interruption is the most common contraceptive method in the country, with more than 10.3 million procedures performed annually [8–10]. Postpartum procedures account for half of all tubal interruption procedures and have benefits of a single infra-umbilical incision, option of regional anesthesia, and lower failure rate than interval laparoscopic tubal ligation [11]. Unfortunately, there are many unfulfilled postpartum tubal ligation requests in women, particularly in the obese population [12]. This may be due to providers’ perceived risks of longer operative time, greater surgical complications, and potential difficulty in successfully completing the procedure. Women who have unfulfilled requests for postpartum tubal interruption procedures are twice as likely to become pregnant in the 1 year following delivery than those who did not request these procedures [13]. The objectives of our study were twofold: (1) To assess the relationship between body mass index (BMI) and operative time during immediate postpartum tubal ligation procedures and (2) to determine whether operative time is non-inferior in women with BMI ≥30 versus BMI <30 and in women with BMI ≥40 versus BMI <40.
2. Material and methods
We obtained Institutional Review Board approval through the Hospital of the University of Pennsylvania. We conducted a retrospective cohort study of all women who received a postpartum tubal ligation following a vaginal delivery between April 2013 and March 2017 at our urban university hospital center. We identified eligible women through our electronic medical record database via procedural code 58605, indicating that a bilateral tubal ligation was performed as a surgical procedure following a vaginal delivery. Currently our institution does not perform immediate postpartum salpingectomies; therefore, these bilateral tubal ligations represented either Pomeroy or Parkland tubal ligations. We included women who had an inpatient postpartum tubal ligation following any non-Cesarean delivery. We excluded women who received a Cesarean section because they would have received their tubal ligation at the time of the delivery. We also excluded women who had no BMI recorded on admission, no operative note, or no anesthesia record in the chart.
We abstracted data on demographic characteristics, medical comorbidities, and all procedural characteristics and complications from the electronic medical record. The primary exposure was obesity, defined by the woman’s BMI. In order to calculate BMI, we abstracted the weights of all women from the last prenatal visit and used a self-reported height. If no weight was available, women were either weighed at the time of hospital admission or self-reported their weight. We abstracted BMI at the time of hospital admission as we felt it was more relevant and applicable to the surgeon’s decision to perform tubal ligation and associated operative time, rather than the patient’s pre-pregnancy BMI. We analyzed BMI as a continuous and categorical variable, with BMI numerical categorization by similar definitions from the Centers of Disease Control and Prevention and the World Health Organization: normal or underweight (BMI <25 kg/m2), overweight (BMI 25–29.9 kg/m2), obese class I (BMI 30–34.9 kg/m2), obese class II (BMI 35–39.9 kg/m2), and obese class III (BMI 40 kg/m2 or greater, also referred to as morbid obesity). However, we only used these BMI numerical categorizations but not the labels of normal weight, underweight, overweight, or obese, since these terms are only applicable to pre-pregnancy BMI and not the BMI in the third trimester. We defined parity as prior to the delivery, therefore not including the birth outcome of the current pregnancy.
The primary outcome was operative time, defined as the difference between procedure start and end time, as recorded in the operating room record. We defined procedure start time as when the incision was first made and procedure end time as when the final suture was cut at the end of the incision closure. Secondary outcomes included total anesthesia time, incision length, hospital length of stay, intraoperative complications defined as any unintentional injury or adverse event during the procedure, and postoperative complications (which included emergency room visits, readmission, outpatient problem visits, wound infection, seroma, wound separation, or hematoma). We assigned estimated blood loss described as “minimal” in the operative note to equate to five milliliters. We assigned incision length described as “small” in the operative note to equate to three centimeters.
We conducted descriptive analysis evaluating the relationship between BMI and operative time, using multivariable linear regression, adjusting for confounders. We assessed the following potential confounders: parity, prior abdominal surgeries, age, time (in hours) from vaginal delivery to tubal ligation, quarter of the academic year, and race. We chose age, race, and parity as standard demographic factors; prior abdominal surgeries since prior surgeries may result in adhesions that may increase operative time; time from delivery to tubal ligation since there may be theoretical involution of the uterus the longer the wait time that may make the fundus more difficult to palpate; and finally, academic quarter since the expertise of the resident physicians involved with the surgery may improve throughout the course of the academic year. We reported on mean rather than median BMI in the population given its normal distribution. As a secondary analysis, we conducted non-inferiority testing within a margin of 10 min of operative time between women with a BMI ≥30 versus BMI <30 as well as women with a BMI ≥40 versus BMI <40, using the student t-test. We chose a margin of 10 min because data in the surgical literature shows that an increase in surgical duration greater than 15–30 min can significantly increase perioperative risks, and non-inferiority within a margin of 10 min is likely clinically insignificant [14–16].
For the sample size calculation for the non-inferiority analysis, we assumed a ratio of 3 to 2 of women with BMI ≥30 to women with BMI <30, based on pilot chart review. Assuming an alpha of 0.025, a standard deviation of 25 min, and an actual difference in mean procedural duration of 0 min, a sample size of 280 women would allow us to determine with 90% power that procedure duration was non-inferior within a margin of 10 min. This calculation was done using SAS v 9.4.
3. Results
We identified 281 women who underwent tubal ligation after a vaginal delivery between April 2013 and March 2017. All had accurate BMI information recorded in their chart. Two women (<1%) had no operative note or anesthesia record and were excluded from the study. A total of 279 women were included for analysis, including a wide variation of BMIs: BMI <25 (N=29, 10%), BMI of 25–29.9 (N=79, 28%), BMI 30–34.9 (N=103, 37%), BMI 35–39.9 (N=44, 16%), and BMI ≥40 (N=24, 9%).
The mean BMI in our population of women was 31.5 (SD 5.6). The highest BMI in our population was 55. Most postpartum tubal ligations (71.3%) were performed on postpartum day 0 (day of delivery, IQR 0,1), 26.1% were performed on postpartum day 1, 2.1% were performed on postpartum day 2, and 0.36% were performed on postpartum day 3. All attempted procedures were completed. Similar patient and demographic characteristics were noted among all the different BMI numerical categories (Table 1).
Table 1.
Demographic and clinical characteristics of women undergoing postpartum tubal ligation stratified by BMI numerical category.
| Characteristic | BMI <25 | BMI 25–29.9 | BMI 30–34.9 | BMI 35–39.9 | BMI >40 | p |
|---|---|---|---|---|---|---|
| Age (y) | 31.7 | 32.0 | 31.3 | 30.6 | 31.9 | .61 |
| Race and ethnicity | N=29 | N=79 | N=103 | N=44 | N=24 | .57 |
| Black | 23 (79%) | 63 (80%) | 84 (81%) | 39 (89%) | 20 (83%) | |
| White | 2 (7%) | 8 (10%) | 5 (5%) | 1 (2%) | 3 (13%) | |
| Asian | 4 (14%) | 3 (4%) | 8 (8%) | 3 (7%) | 0 | |
| Hispanic | 0 | 4 (5%) | 4 (4%) | 0 | 1 (4%) | |
| Other | 0 | 1 (1%) | 2 (2%) | 1 (2%) | 0 | |
| Insurance status | N=29 | N=79 | N=103 | N=44 | N=24 | .52 |
| Public | 24 (83%) | 61 (77%) | 87 (85%) | 35 (80%) | 23 (96%) | |
| Private | 4 (14%) | 14 (18%) | 12 (12%) | 5 (11%) | 1 (4%) | |
| Other* | 1 (4%) | 4 (5%) | 4 (4%) | 4 (9%) | 0 | |
| Parity | N=29 | N=79 | N=103 | N=44 | N=24 | .95 |
| 0 | 0 | 0 | 1 (1%) | 0 | 0 | |
| 1 | 4 (14%) | 16 (20%) | 19 (19%) | 11 (25%) | 3 (13%) | |
| 2 | 6 (21%) | 20 (25%) | 30 (29%) | 14 (32%) | 6 (25%) | |
| 3 | 10 (35%) | 17 (22%) | 22 (21%) | 6 (14%) | 5 (21%) | |
| 4 | 5 (17%) | 11 (14%) | 18 (18%) | 6 (14%) | 5 (21%) | |
| 5 or more | 4 (14%) | 15 (19%) | 13 (13%) | 7 (6%) | 5 (21%) | |
| Mean BM1 (kg/m2) | 22.6 | 27.4 | 32.2 | 36.8 | 43.2 | <.01 |
| Mean weight (kg) | 62.3 | 74.6 | 86.5 | 98.6 | 111.3 | <.01 |
| Median Gestational age (wk) | 38.1 | 38.1 | 38.0 | 37.9 | 38.3 | .99 |
| Median time elapsed between delivery and tubal (hours) | 16.8 | 18.5 | 17.9 | 18.9 | 14.5 | .70 |
| History of prior abdominal surgery | N=29 | N=76 | N=102 | N=43 | N=24 | .87 |
| Yes | ||||||
| 21 (72%) | 62 (79%) | 84 (82%) | 35 (80%) | 21 (88%) |
Other insurance includes: military, charity, self-pay.
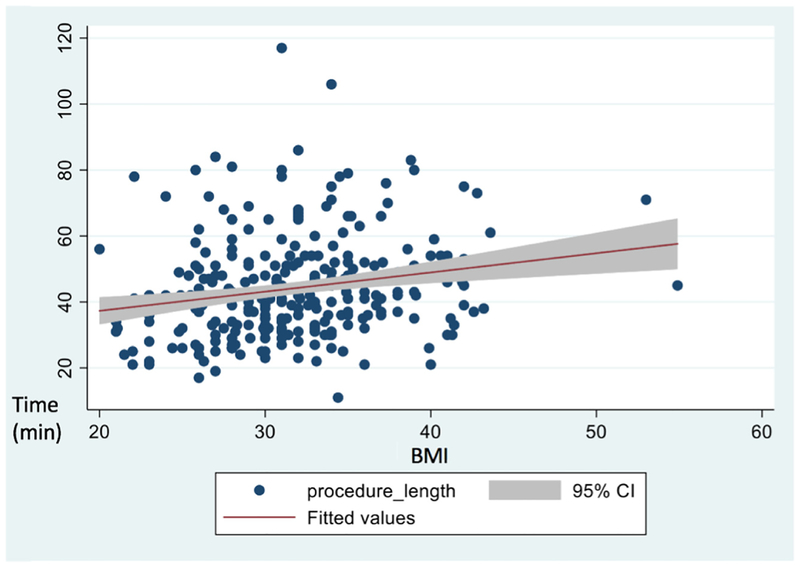
Across all BMI numerical categories, Pomeroy tubal ligations were the most prevalent and neuraxial anesthesia was the most common (Table 2). For two women for whom procedure start time was not recorded, we used anesthesia start time as a surrogate. Procedural characteristics were similar across all BMI numerical categories except the mean total operative time (Table 2). The mean total operative time was 46.1 min (n=171; 95% CI 43.7, 48.6 min) for women with a BMI ≥30 and 40.6 min (n=108; 95% CI 37.9 min, 43.4 min) for women with a BMI <30, (p<.01). We used linear regression to assess the relationship between BMI and operative time, and found a 35 s increase in procedure time per unit of BMI (Fig. 1). Procedure times between women with BMI ≥30 versus BMI <30 were non-inferior within a 10-min margin (p<.01). Incision length did not differ among BMI numerical categories. However, among the 279 women included in our analysis, 17 (6%) women did not have any mention of incision size in their operative note, 213 (76%) had “small incision” in their operative note, and 44 (16%) actually had a centimeter measure. Total hospital length of stay and estimated blood loss was also similar across all BMI numerical categories.
Table 2.
Procedural characteristics and surgical complications of women receiving postpartum tubal ligation stratified by BMI numerical category.
| BMI <25 | BMI 25–29.9 | BMI 30–34.9 | BMI 35–39.9 | BMI >40 | p | |
|---|---|---|---|---|---|---|
| Procedural characteristic | ||||||
| Type of tubal | N=29 | N=79 | N=103 | N=44 | N=24 | .42 |
| Pomeroy | 24 (83%) | 66 (84%) | 88 (85%) | 32 (73%) | 20 (83%) | |
| Parkland | 3 (10%) | 5 (6%) | 7 (7%) | 8 (18%) | 0 | |
| Pomeroy & Parkland | 0 | 2 (3%) | 2 (2%) | 0 | 1 (4%) | |
| Not specified | 2 (7%) | 6 (8%) | 6 (6%) | 4 (9%) | 3 (13%) | |
| Mean total operative time (min) | 37.7 (SD 13) | 41.7 (SD 15) | 44.8 (SD 17) | 48.2 (SD 15) | 48.2 (SD 14) | .02 |
| Mean total anesthesia time (min) | 85.0 | 87.7 | 94.5 | 95.7 | 96.1 | .07 |
| Type of anesthesia | N=29 | N=79 | N=103 | N=44 | N=24 | .89 |
| Epidural | 8 (26%) | 29 (37%) | 41 (40%) | 15 (34%) | 9 (38%) | |
| Spinal | 18 (62%) | 42 (53%) | 52 (51%) | 28 (64%) | 13 (54%) | |
| Combined spinal epidural | 0 | 0 | 1 (1%) | 0 | 0 | |
| General | 3 (10%) | 8 (10%) | 9 (9%) | 1 (2%) | 2 (8%) | |
| Median hospital Length of stay (days) | 1 (IQR1) | 1 (IQR1) | 1 (IQR1) | 1 (IQR1) | 1 (IQR1) | .78 |
| Mean length of incision (cm) | 3.0 | 3.07 | 3.06 | 3.03 | 3.02 | .69 |
| Adhesions present at outset Yes | N=29 | N=79 | N=103 | N=44 | N=24 | .83 |
| Total estimated blood loss (median mL) | 2 (7%) | 2 (3%) | 6 (6%) | 2 (5%) | 1 (4%) | |
| 13.3 | 9.8 | 9.8 | 13.2 | 8.7 | .49 | |
| Complication | N=29 | N=79 | N=103 | N=44 | N=24 | |
| Intraoperative complication | 1 (3%) | 0 | 3 (3%) | 0 | 0 | .33 |
| Postoperative complication+ | 7 (24%) | 8 (10%) | 10 (10%) | 4 (9%) | 4 (17%) | .22 |
| Emergency room visit | 2 | 4 | 3 | 2 | 1 | |
| Readmission | 3 | 2 | 3 | 0 | 1 | |
| Outpatient problem visit | 0 | 3 | 4 | 0 | 2 | |
| Wound infection | 1 | 1 | 0 | 1 | 0 | |
| Seroma | 1 | 0 | 0 | 0 | 0 | |
| Wound separation | 0 | 0 | 1 | 1 | 0 | |
| Hematoma | 0 | 0 | 0 | 0 | 0 |
Represents total number of patients with any postoperative complication, please note that one patient may have multiple different complications.
Fig. 1.
Scatter plot of operative time in minutes by body mass index. Values are unadjusted for confounders.
We conducted a secondary analysis comparing operative time between women with a BMI ≥40 and women with a BMI <40. Women with a BMI ≥40 had a mean operative time of 48.2 min (n=24; 95% CI 42.3–54.0 min) and women with a BMI <40 had a mean operative time of 43.6 min (n=255; 95% CI of 41.7–45.6 min), p=.17. The mean operative time for women with a BMI ≥40 was non-inferior to the mean operative time for women with a BMI <40, within a non-inferiority margin of 10 min (p=.04).
The overall intraoperative surgical complication rate, defined as an incidence of an unintended adverse surgical injury requiring repair, was low (N=4, 1%, Table 2), and did not differ across all BMI numerical categories (p=.33). In one woman with a BMI of 24.8, there was an unintentional entry into the skin that occurred 1 cm below the original incision. In a woman with a BMI 32, there was an unintentional laceration of the left fallopian tube that was repaired. One woman with a BMI of 32.9 had unintentional omental injury requiring a partial omentectomy. Finally, one woman with a BMI of 34 had moderate bleeding from the tubal stump due to a bleeding artery requiring ligation.
The postoperative complication was also low and similar across all BMI numerical categories (Table 2). A total of 12 (4%) women presented to the emergency room for reasons including abdominal pain, fevers, and urinary symptoms. There were 9 (3%) women readmitted postoperatively for reasons that were not related to the tubal ligation surgery, such as endometritis, pyelonephritis, urinary tract infection, and a tubo-ovarian abscess. There was a small proportion of women who presented for outpatient problem visits (N=9, 3%) related to the tubal ligation for concerns about the incision including drainage, soreness, skin separation, and rash. There were 3 (1%) women with superficial wound infections, 1 (<1%) woman with a postoperative seroma, 2 (<1%) women with a superficial wound separation, and no one with a postoperative hematoma.
Our linear regression modeling allowed us to assess for other variables that may have had an effect on operative time. Both BMI numerical category (p=.029) and first (p<.01) and second (p=.026) academic quarter were associated with increased operative time (p<.01). Including academic quarter in the model resulted in the estimated difference in operative time between women with BMI ≥30 versus BMI <30 to be 4.1 min, a smaller difference than the unadjusted 5.5-min difference in operative time between women with BMI ≥30 versus BMI <30.
4. Discussion
In this single-institution retrospective cohort study, we demonstrate that there is a small increase in operative time with increasing BMI. Our study showed that although there was an approximately 6-min increase in operative time for women with BMI ≥30 compared with women with BMI <30, operative times in the two groups were non-inferior within a 10-min margin, suggesting that any increase in operative time is not clinically significant. Women with BMI ≥30 and BMI ≥40 did not have greater intraoperative or postoperative complications related to postpartum tubal ligations, as compared respectively to women with BMI <30 or BMI <40.
We chose to measure operative time as a proxy for operative complexity and difficulty. Studies evaluating operative time during abdominal surgery, including both open and laparoscopic cases, have shown that a surgical duration as short as 15–30 min can significantly increase perioperative risks, including increased rate of surgical site infection and increased hospital stay [14–16]. Prior studies show that many factors can influence a provider’s decision to forgo a desired tubal ligation, and perceived surgical complexity, BMI, and limited time appear to drive these decisions [12,17] [18]. In our secondary analysis comparing operative times between women with BMI ≥40 versus BMI <40, we found no significant difference in operative time or anesthesia time. While our study did not specifically investigate obesity as a factor associated with denied postpartum tubal ligations, we found that obesity and BMI do not have a clinically significant impact on operative time or complications in women who receive tubal ligations, and therefore, obesity should not be used as a reason for tubal ligation denial.
Strengths of our study included our diverse population of women and relatively large sample size at an institution that performs many immediate postpartum tubal ligations on women with BMI ≥30. Our data include women from only one site, but the multitude of residents, fellows and attendings who participate in the care of our patients and the diversity of these care providers increases the generalizability of our results.
Our study has some limitations. Our primary outcome was operative time, defined by when incision was first made and the final suture was cut at the end of an incision closure. We relied on accurate nursing and anesthesia documentation to determine the operative and anesthesia time. Similarly, if not directly measured, weight and height were often self-reported which could influence the calculated BMI of the woman. We also do not have accurate data on pre-pre-pregnancy weight or BMI or total weight gain during pregnancy. Another limitation is inherent selection bias by surgeons selecting operative candidates on subjective and objective factors (body fat distribution, comorbidities, abdominal and fundal examination). We have no data on provider preferences, on how women were counseled and possibly dissuaded or denied these procedures, or on women who were never offered these procedures due to their obesity. Our study sample may represent women who had inherently lower risk than those who were denied requests for tubal ligation, and our data collection methods did not allow for a comparison to those women who were denied tubal ligations requests. Thus, these findings may not be generalizable to an unselected population of women with BMI ≥30 or BMI ≥40. Tubal ligation procedures have an overall low complication rate; thus, our comparisons between the complication rates in women with BMI ≥30 versus BMI <30 are underpowered. In our comparison of women with BMI ≥40 versus BMI <40, we are limited in making inferences due to a small sample of women with BMI ≥40. While we are able to report our postoperative complication rates based on women who presented to our own institution, we do not have data on whether women may have presented to other institutions. Similarly, we do not have long-term data regarding the efficacy of these postpartum tubal ligations and how many women subsequently became pregnant; based upon historic data, pregnancy contraceptive efficacy is expected to be high [22]. Finally, operative time is a proxy rather than a direct measure of surgical complexity. We are unable to determine whether increased operative time was a product of a difficult surgery or an inexperienced surgeon. However, including quarter of academic year in our linear regression analysis resulted in a shorter estimated difference in procedure time between women with BMI ≥30 versus BMI <30.
Women specifically requesting a tubal ligation, who have an unfulfilled request, are at the highest risk of becoming pregnant in the one-year postpartum period.12 Obese women also have poorer access to contraception and family planning services, and the stakes of unintended pregnancy in obese women are higher due to delayed recognition of pregnancy and pregnancy complications [19]. At time of unfulfilled tubal ligation request, obese women may be less likely to accept alternative contraceptive methods due to perceived effects on weight gain [2]. There are also risks of interval tubal ligation in obese women, mainly the risk of general anesthesia, intubation, and airway management; thus, postpartum tubal under regional anesthesia may be more beneficial than interval laparoscopic tubal interruption for the obese woman [20].
In our study, we found that women with BMI ≥30 undergoing postpartum tubal ligation do not have clinically significant longer operative time when compared to women with BMI <30; likewise, women with BMI ≥30 do not face greater surgical or post-operative risks. Furthermore, there is no need for larger incision length and there is low risk of inability to perform the procedure.
In conclusion, a perceived fear that obesity lengthens the operative time for a requested and desired tubal ligation, should not prevent a woman from obtaining this procedure. Our data provide evidence that there is no clinically significant increase in operative time or rates of complications in immediate postpartum tubal ligations in women with BMI ≥30. Postpartum tubal ligations are a safe procedure for women, regardless of weight or BMI numerical category.
References
- [1].Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. J Am Med Assoc 2016;315(21): 2284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chuang CH, Chase GA, Bensyl DM, Weisman CS. Contraceptive use by diabetic and obese women. Women’s Heal Issues 2005;15:167–73. [DOI] [PubMed] [Google Scholar]
- [3].Callegari LS, Nelson KM, Arterburn DE, Prager SW, Schiff MA, Schwarz EB. Factors associated with lack of effective contraception among obese women in the United States. Contraception 2014;90:265–71. [DOI] [PubMed] [Google Scholar]
- [4].Nguyen BT, Elia JL, Ha CY, Kaneshiro BE. Pregnancy intention and contraceptive use among women by class of obesity: results from the 2006–2010 and 2011–2013 National Survey of family growth. Women’s Heal Issues 2018;28(1):51–8 [DOI] [PubMed] [Google Scholar]
- [5].Heslehurst N, Simpson H, Ells LJ, Rankin J, Wilkinson J, Lang R, et al. The impact of maternal BMI status on pregnancy outcomes with immediate short-term obstetric resource implications: a meta-analysis. Obes Rev 2008;9:635–83. [DOI] [PubMed] [Google Scholar]
- [6].Mills JL, Troendle J, Conley MR, Carter T, Druschel CM. Maternal obesity and congenital heart defects: a population-based study 1–3. Am J Clin Nutr 2010; 91:1543–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Stuebe A, Auguste T, Gulati M. Presidential task force on redefining the postpartum visit committee on obstetric practice optimizing postpartum care, 131; 2018. [Google Scholar]
- [8].Daniels K, Abma JC. Current Contraceptive Status Among Women Aged 15–49: United States, 2015–2017 2018:1–7. [PubMed] [Google Scholar]
- [9].Chandra A, Martinez GM, Mosher WD, Abma JC, Jones J. Fertility, family planning, and reproductive health of U.S. women: data from the 2002 National Survey of family growth. Vital Health Stat 2005;23:1–160. [PubMed] [Google Scholar]
- [10].Mosher WD, Martinez GM, Chandra A, Abma JC, Willson SJ. Use of contraception and use of family planning services in the United States: 1982–2002. Adv Data 2004:1–36. [PubMed] [Google Scholar]
- [11].American College of Obstetrics and Gynecology. ACOG practice bulletin. Benefits and risks of sterilization. Number 46, September 2003 (replaces Technical Bulletin Number 222, April 1996), Int J Gynaecol Obstet 2003;83:339–50. [DOI] [PubMed] [Google Scholar]
- [12].Seibel-Seamon J, Visintine JF, Leiby BE, Weinstein L. Factors predictive for failure to perform postpartum tubal ligations following vaginal delivery. J Reprod Med 2009; 54:160–4. [PubMed] [Google Scholar]
- [13].Thurman AR, Janecek T. One-year follow-up of women with unfulfilled postpartum sterilization requests. Obstet Gynecol 2010;116:1071–7. [DOI] [PubMed] [Google Scholar]
- [14].Cheng H, Chen BP-H, Soleas IM, Ferko NC, Cameron CG, Hinoul P. Prolonged operative duration increases risk of surgical site infections: a systematic review. Surg Infect (Larchmt) 2017;18:722–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Procter LD, Davenport DL, Bernard AC, Zwischenberger JB. General surgical operative duration is associated with increased risk-adjusted infectious complication rates and length of hospital stay. J Am Coll Surg 2010;210. [DOI] [PubMed] [Google Scholar]
- [16].Mahdi H, Goodrich S, Lockhart D, DeBernardo R, Moslemi-Kebria M. Predictors of surgical site infection in women undergoing hysterectomy for benign gynecologic disease: a multicenter analysis using the national surgical quality improvement program data. J Minim Invasive Gynecol 2014;21:901–9. [DOI] [PubMed] [Google Scholar]
- [17].Boardman LA, DeSimone M, Allen RH. Barriers to completion of desired postpartum sterilization. R I Med J (2013) 2013;96:32–4. [PubMed] [Google Scholar]
- [18].Wolfe KK, Wilson MD, Hou MY, Creinin MD. An updated assessment of postpartum sterilization fulfillment after vaginal delivery. Contraception 2017;96:41–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Foster DG, Jackson RA, Cosby K, Weitz TA, Darney PD, Drey EA. Predictors of delay in each step leading to an abortion. Contraception 2008;77:289–93. [DOI] [PubMed] [Google Scholar]
- [20].Adams JP, Murphy PG. Obesity in anaesthesia and intensive care. Br J Anaesth 2000; 85:91–108. [DOI] [PubMed] [Google Scholar]
- [22].Peterson HB, Xia Z, Hughes JM, Wilcox LS, Tylor LR, Trussell J, et al. The risk of pregnancy after tubal sterilization: findings from the U.S. collaborative review of sterilization. Am J Obstet Gynecol 1996;174:1161–70. [DOI] [PubMed] [Google Scholar]