Summary
Elevated programmed death‐1 (PD‐1) has been found in immune cells in viral infections and plays an important role in infection persistence. The soluble form of PD‐1 (sPD‐1) is involved in tumours and viral infections. The aim of this study was to investigate the role of sPD‐1 in chronic hepatitis B (CHB). A total of two hundred and eighteen CHB patients and sixty healthy controls (HC) were enrolled. Demographic data and clinical parameters were collected. An ELISA assay was used to measure serum sPD‐1 levels, and the relationships between sPD‐1 and clinical/virological characteristics was analysed. sPD‐1 levels in CHB patients were higher (median 4.409 IQR 3.435‐5.306 pg/mL) than those of HC individuals (median 0.3665 IQR 0.2425‐0.5010 pg/mL). Among patients at various disease stages, patients with immune activity showed the highest sPD‐1 levels (median 5.138 IQR 4.329‐5.406 pg/mL). sPD‐1 concentration was associated with HBV markers (HBsAg, HBV DNA and HBeAg) and biochemical parameters (serum aspartate aminotransferase [AST], alanine aminotransferase [ALT], total bilirubin [TBil] and gamma glutamyl transferase [γ‐GT] levels) (all P < 0.05). sPD‐1 levels were higher in CHB patients with moderate‐to‐severe inflammation or fibrosis than in those with mild inflammation or fibrosis, regardless of ALT levels. The association between sPD‐1 and disease progression of CHB suggests that sPD‐1 could serve as a new indicator in assessing liver fibrosis. These findings may further aid in determining the initiation of antiviral treatment in patients with normal ALT levels.
Keywords: antiviral treatment, hepatitis B surface antigen, hepatitis B virus
Abbreviation
- ALB
albumin
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BUN
blood urea nitrogen
- IQR
interquartile range
- TBil
total bilirubin
- γ‐GT
gamma glutamyl transferase
1. INTRODUCTION
Hepatitis B virus (HBV) infection is a serious global public health issue. There are approximately 240 million HBV carriers worldwide, of whom more than 686 000 die annually from HBV‐related liver disease.1 Chronic hepatitis B (CHB) can progress to cirrhosis, portal hypertension, liver decompensation and hepatocellular carcinoma (HCC).2
Programmed death‐1 (PD‐1) is a molecule expressed on various human immune cells, including T cells, B cells, myeloid cells, thymocytes and natural killer (NK) cells.3 The ligation of programmed death ligand‐1 (PD‐L1) and programmed death ligand‐2 (PD‐L2) to PD‐1 may have an inhibitory effect on target cells (eg T cells), playing an important role in the regulation of immune homeostasis and in maintenance of peripheral immune tolerance.4 PD‐1 expression correlates with the exhaustion of T cells in cancers and chronic infections.5 Blockade of PD‐1 using anti‐PD‐1 mAbs reverses T‐cell exhaustion (CD8 and CD4) and restores their anti‐tumour potential.6 Moreover, up‐regulated PD‐1 expression on immune cells has been detected in viral infections (eg HBV, HIV), indicating that PD‐1 may be a factor influencing the persistence of these infections.5
Recently, soluble forms of PD‐1 and PD‐L1 (sPD‐1 and sPD‐L1) have been detected in the blood of patients with tumours. Unlike PD‐1, the function of sPD‐1 is not fully understood. Some researchers hypothesized that binding of sPD‐1 to mPD‐L1/mPD‐L2 (membrane‐bound forms of PD‐1/PD‐L1) may prevent mPD‐1 from combining with its ligands, hence counteracting mPD‐1‐mediated inhibitory effects on immune cells.7 A study conducted in Taiwan indicated that sPD‐1 in serum was related to high viral load persistence and higher risk of progression to HCC in chronic HBV infection, indicating an immune‐inhibitory role for sPD‐1.8
In this study, we investigated serum sPD‐1 levels in CHB patients. We further explored the relationships between sPD‐1 and clinical parameters as well as histology on liver biopsies.
2. MATERIALS AND METHODS
2.1. Subjects and samples
Consecutive adult patients with CHB were recruited for this study from the liver clinic of the Third Affiliated Hospital of Sun Yat‐sen University during January 2016 and October 2016, as were healthy volunteers (healthy controls). Patients infected with HAV (hepatitis A virus), HCV (hepatitis C virus), HDV (hepatitis D virus), HEV (hepatitis E virus), HIV (human immunodeficiency virus) and patients with liver cirrhosis, HCC, cardiovascular disease, diabetes, kidney disease, pregnancy or autoimmune disease were excluded. All CHB patients had never previously received antiviral treatment (IFN‐α or nucleoside analogues). Blood samples were collected from each individual, centrifuged at 4000 × g for 10 minutes to obtain serum and stored at −80°C until use. Written informed consent was obtained from each individual. The study was approved by the Institutional Review Board of the Third Affiliated Hospital of Sun Yat‐sen University.
2.2. Virological assay
Serum HBV DNA was quantified by COBAS TaqMan (Roche Molecular Diagnostics, Indianapolis, IN). HBsAg and HBeAg were measured using chemiluminescence assay kits (Roche Diagnostics Gmbh, Mannheim, Germany).
2.3. Routine blood and biochemistry assay
White blood cell (WBC), red blood cell (RBC) and platelet (PLT) counts were measured by XN2000 (SYSMEX, Kobe, Japan). Levels of serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin (TBIL) and albumin (ALB) were measured on a 7600‐020 (ISE) Automatic Analyzer (HITACHI, Tokyo, Japan). The APRI (AST, platelet ratio index) was calculated using routine laboratory values [APRI = (AST/upper limit of normal) × 100/platelet count],9 and FIB‐4 using the formula age (years) × AST (IU/L)/platelet count (109/L) × (ALT (IU/L)1/2.10
2.4. Liver biopsy
Liver biopsy was conducted following standard procedures.11, 12 The histodiagnosis of liver tissues was determined by two pathologists blinded to clinical data according to the severity of the inflammation (activity or grade) and the degree of fibrosis (stage).13
2.5. Measurement of sPD‐1 in serum
Levels of sPD‐1 in serum of CHB patients and healthy volunteers were measured using an ELISA kit (DuoSet Human PD‐1, R&D systems, Minneapolis, MN,USA) in accordance with the manufacturer's instructions. Absorbance was measured at 450 nm, and the concentration of sPD‐1 was calculated by a standard curve.
2.6. Statistical analysis
Continuous variables were expressed as the median (interquartile range). Categorical variables were expressed as numbers and percentages. Continuous variables were compared with a 2‐sided Student's t test or a Mann‐Whitney U test, depending on the distribution. The correlations between sPD‐1 and detection markers were assessed by Spearman's rank correlation test. Binary logistic regression was conducted using the forward (conditional) method. The data were analysed using SPSS software, version 22 (SPSS Inc. Chicago, IL, USA). A P value <0.05 was considered statistically significant. P values in figures are shown as *P < 0.05; ** P < 0.01; and *** P < 0.001.
3. RESULTS
3.1. Demographic and clinical characteristics of the subjects in this study
A total of two hundred and eighteen CHB patients and sixty healthy controls were included for analysis. All patients were Chinese. Demographic characteristics are shown in Table 1.
Table 1.
Demographics and disease characteristics of subjects
| Chronic hepatitis B (n = 218) | Healthy controls (n = 60) | |
|---|---|---|
| Median (IQR)† | ||
| Gender (M/F) | 172/46 | 29/31 |
| Age (Years old) | 32 (26‐40) | 30.5 (27.0‐38.0) |
| AST (U/L) | 50.0 (29.0‐112.0)* | 18.0 (16.0‐21.0) |
| ALT (U/L) | 66.0 (33.0‐167.8)* | 15 (12‐18) |
| ALB (g/L) | 44.55 (41.08‐46.40)* | 46.85 (45.17‐48.83) |
| GLB (g/L) | 28.25 (25.93‐30.80) | 28.10 (26.00‐29.90) |
| TBiL (umol/L) | 14.60 (11.10‐21.30)* | 11.60 (8.60‐13.10) |
| γGT (U/L) | 50.00 (24.00‐92.00)* | 18.00 (14.50‐22.00) |
| BUN (mmol/L) | 4.280 (3.450‐5.115) | 4.040 (3.422‐4.785) |
| HBV DNA | 6.22 (3.70‐8.12)a | ‐ |
| HBsAg | 3.55 (2.95‐4.41)a | ‐ |
| HBeAg | ||
| Positive | 120 | ‐ |
| Negative | 95 | |
| Missing | 3 | |
ALB, albumin; AST, aspartate aminotransferase; ALT, alanine aminotransferase;TBil, total bilirubin; γ‐GT, gamma glutamyl transferase; BUN, blood urea nitrogen; IQR, interquartile range.
Data expressed in log(IU/mL)
P < 0.05 when compared with healthy controls.
3.2. Distribution of sPD‐1 titres in various disease stages
We first compared sPD‐1 levels between CHB patients and healthy controls. As shown in Figure 1A, sPD‐1 levels were significantly higher in the CHB patients (median 4.409 IQR 3.435‐5.306 pg/mL) than in the healthy controls (median 0.3665 IQR 0.2425‐0.5010 pg/mL). Then, patients were classified into four disease stage groups: immune active (IA), inactive CHB (IC), immune tolerance (IT) and gray zone (GZ) (“gray zone” here refers to those patients who could not be classified as IA, IC or IT), according to the criteria shown in Table 2 (ULN of ALT: 30 U/L for males and 19 U/L for females; the ULN used here is different from our laboratory reference range).14 IA patients had the highest sPD‐1 levels (median 5.138 IQR 4.329‐5.406 pg/mL), while patients in IC and IT stages had lower and similar sPD‐1 levels, respectively (Figure 1B).
Figure 1.
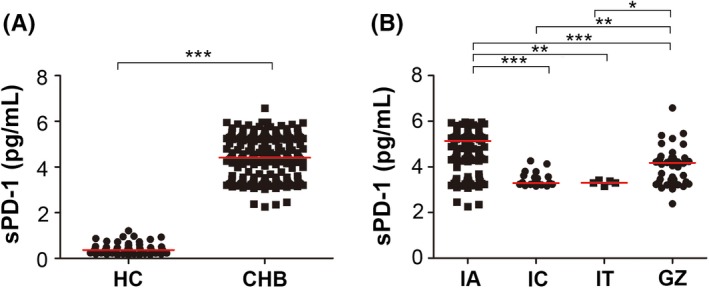
Serum sPD‐1 levels in various groups of CHB patients. sPD‐1 levels in healthy controls (HC) and chronic hepatitis B patients (CHB) (A) and sPD‐1 levels in CHB patients in various disease stages (B). CHB, chronic hepatitis B; HC, healthy control; IA, immune active; IC, inactive CHB; IT, immune tolerance; and GZ, gray zone (here, the gray zone refers to those patients who cannot be classified as IA, IC or IT)
Table 2.
Disease stage classification criteria
| Classification | ALT | HBV DNA | HBeAg |
|---|---|---|---|
| Immune active (IA) | Elevated | >20 000 IU/mL | Positive |
| >2000 IU/mL | Negative | ||
| Inactive CHB (IC) | Normal | low HBV DNA level | Negative |
| Immune tolerance (IT) | Normal | >1 million IU/mL | Positive |
| Gray zone (GZ) | Not classified as IC, IT or IA | ||
Notes: Upper limit of normal (ULN) of ALT: 30 U/L for males and 19 U/L for females
3.3. High sPD‐1 levels correlated with viral replication
HBV markers are important indicators of viral replication and activity. Hepatitis B e antigen (HBeAg) seroconversion (from positive to negative) is associated with clinical remission and transition to inactive liver disease,15 making it an important parameter in clinical use. We found that HBeAg‐positive patients had higher sPD‐1 levels than did HBeAg‐negative patients (Figure 2A). Moreover, serum HBsAg and HBV DNA levels positively correlated with sPD‐1 levels (Figure 2B,C). The association between these two HBV markers and sPD‐1 showed a similar tendency as that of HBeAg status. These results indicate that sPD‐1 levels correlate with HBV replication.
Figure 2.
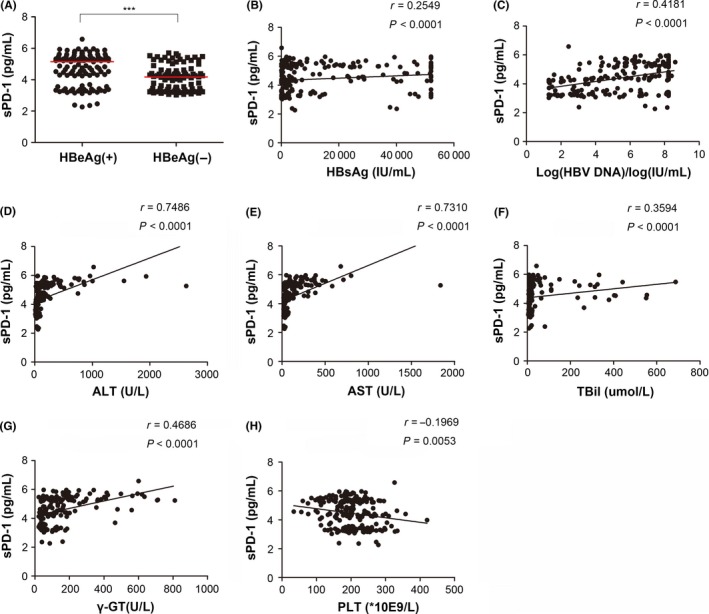
Association between serum sPD‐1 levels and HBV markers as well as clinical parameters in chronic hepatitis B (CHB) patients. Serum sPD‐1 levels in HBeAg‐positive or HBeAg‐negative CHB patients (A). Correlations of serum sPD‐1 levels with quantitative HBV surface antigen (HBsAg) (B), HBV DNA (C), alanine aminotransferase (ALT) (D), aspartate aminotransferase (AST) (E), total bilirubin (TBil) (F), gamma glutamyl transferase (γ‐GT) (G) and platelet (PLT) (H). Correlations were tested by Spearman's rho (r), and the level of significance was expressed as the P‐value (P)
3.4. Serum levels of sPD‐1 were associated with disease fluctuation
Correlation analysis indicated that sPD1 levels were significantly positively associated with levels of ALT and AST, both being widely used biochemical parameters indicating inflammatory fluctuation in hepatitis (Figure 2D, E). Serum TBiL and γ‐GT are usually elevated in patients suffering from liver disease,16 and these two parameters were also positively correlated with sPD‐1 levels (Figure 2F,G). Furthermore, there was a negative correlation between sPD‐1 and PLT (Figure 2H). The serum sPD1 titre was not associated with age (Figure S1), gender or other biochemical parameters (data not shown).
3.5. Correlation of sPD1 with fibrosis and liver histology
APRI and FIB‐4 are two convenient estimators of hepatic fibrosis, as has been validated in several studies.10, 17 As shown in Figure 3A,B, sPD‐1 positively correlated with APRI and FIB‐4. We further analysed the relationship between sPD‐1 and histology data. The liver histology assessment via biopsy is the gold standard for determining exact inflammatory and fibrosis levels. The liver histology results were available in thirty‐three out of our two hundred and eighteen CHB patients. Four patients had G3‐4 (moderate‐to‐severe) inflammation, and twenty of them had S2‐4 (moderate‐to‐severe) fibrosis. sPD1 levels were higher in patients with moderate‐to‐severe inflammation or fibrosis than in those with mild inflammation or fibrosis (Figure 3C,D).
Figure 3.
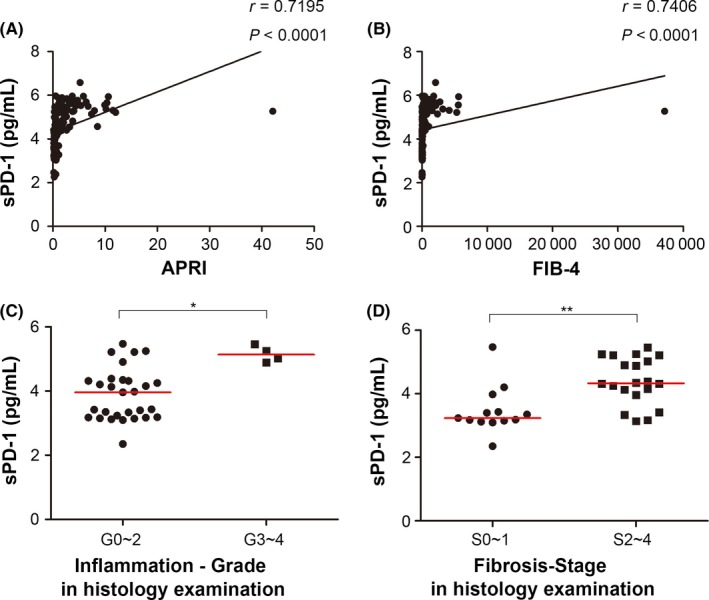
Serum sPD‐1 level was an indicator of liver fibrosis in CHB. Serum sPD‐1 levels in CHB patients positively correlated with AST, platelet ratio index (APRI) (A) and FIB‐4 (B). Serum sPD‐1 levels were higher in patients with moderate‐to‐severe inflammation or fibrosis than in those with mild change (C for inflammation and D for fibrosis)
3.6. sPD‐1 was an independent factor predicting eligibility for antiviral therapy
We classified CHB patients into two groups according to current treatment guidelines14: The CA group was recommended to start antiviral treatment (ALT >70 U/L and HBV DNA >20 000 IU [HBeAg‐positive]/ HBV DNA> 2000 IU [HBeAg‐negative]), and the CAN group was recommended to monitor disease progression rather than to receive immediate treatment (patients who did not meet the above‐mentioned criteria). As shown in Figure 4A, sPD‐1 levels were higher in the CA patients. Multivariate analysis using logistic regression showed that sPD‐1 level was an independent factor for discrimination of the CA from CAN patients (Table S1). To evaluate performance of serum sPD‐1 in predicting whether patients need treatment, receiver operating characteristic (ROC) curves were employed. The area under the ROC curve (AUROC) value and 95% CI for discriminating CAN from CA in CHB patients overall was 0.877 (0.827‐0.927), and the optimal cut‐off value was 4.351 ng/mL (Figure 4B).
Figure 4.
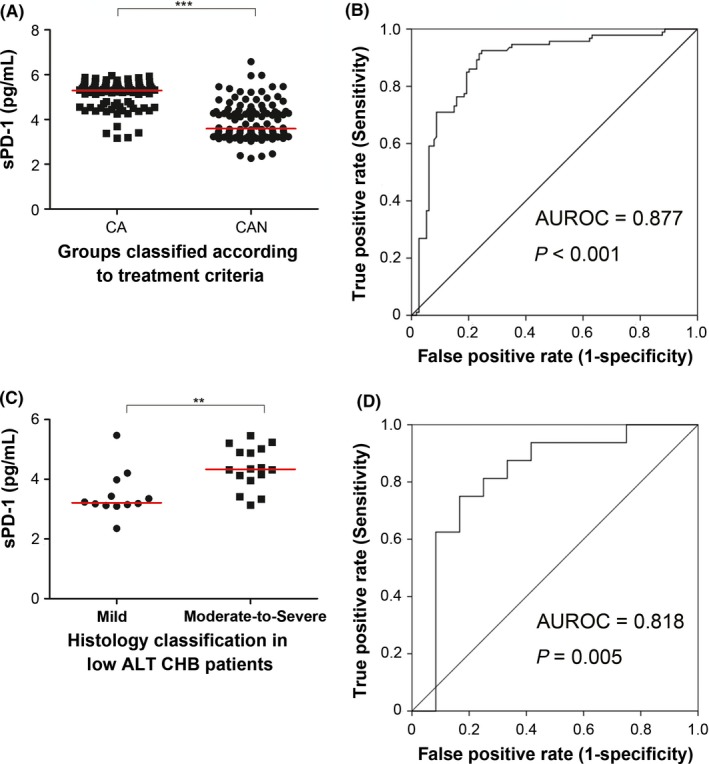
sPD‐1 was an indicator of initiating antiviral treatment in chronic hepatitis B (CHB). Patients in the CA group had higher serum sPD‐1 levels than did their counterparts in the CAN group (A). Receiver operating characteristic (ROC) curve for predicting CA group patients from CAN group patients (B). Among CHB patients with alanine aminotransferase (ALT) <2 ULN, those whose histology condition met the treatment criteria had higher serum sPD‐1 levels than did those who did not meet the criteria (C). ROC curve for predicting patients with ALT <2 ULN whose histology met the treatment criteria from those whose histology did not meet the treatment criteria (D)
3.7. sPD‐1 could be used as an indicator for initiating treatment in addition to ALT level
We further investigated sPD‐1 levels in patients whose ALT levels were less than 2 ULN (70 U/L); these patients usually need liver biopsy to determine whether antiviral therapy should be initiated.14 Treatment was recommended for patients with moderate‐to‐severe inflammation and/or fibrosis (G3‐4 and/or S2‐4). Our data showed that such patients had higher sPD‐1 levels than did those with minor inflammation or fibrosis (Figure 4C). To identify patients needing therapy among the CHB patients who did not have ALT <70 U/L, the AUROC and 95% CI were 0.818 and (0.640‐0.996), respectively, for discriminating patients needing therapy from those not, and the optimal cut‐off sPD‐1 level value was 3.516 ng/mL (Figure 4D).
4. DISCUSSION
A previous study showed that sPD‐1 levels in chronic viral hepatitis patients correlated with higher HBV DNA level persistence and higher possibility of HCC morbidity.8 In the present study, we studied the role of sPD‐1 in chronic HBV infection; we found that sPD‐1 could be employed as an indicator for determining the initiation of treatment in CHB patients with low levels of ALT (<70 U/L).
Our results showed that sPD1 correlated with HBV chronic infection, demonstrated by higher sPD‐1 levels in CHB patients than in HC individuals. Our previous studies showed that immune cell statuses varied depending on disease phases of CHB.18, 19 When taking disease phases into consideration, IA patients had the highest sPD‐1 levels compared with those of the other phases. Since sPD‐1 is thought to prevent mPD‐1 from combining with its ligands, thereby counteracting mPD‐1‐mediated inhibitory effects on immune cells,7 the elevated sPD‐1 level in IA patients supported our hypothesis. In other studies, the functional effect of sPD‐1 was also observed in autoimmune disease and chronic viral infection.20, 21 Our result is consistent with that of Li et al's study,22 in which a similar trend was reported.
The relationship between serum sPD‐1 and other clinical parameters provided additional cues regarding the role of sPD‐1 in CHB disease progression. Our data demonstrated that sPD‐1 level positively correlated with ALT, TBiL and γ‐GT levels. These results support the proposal that sPD‐1 is an indicator of liver inflammation. In addition to an association with liver inflammation, we also found that sPD‐1 level was associated with HBV markers (HBsAg, HBeAg and HBV DNA). These positive correlations between HBV DNA or HBsAg and sPD‐1 are consistent with results of a previous study.8 Combining these results, it appears that sPD‐1 is an important factor related to liver inflammation and viral replication.
Recurrent liver inflammation increases the risk of developing cirrhosis and hepatocellular carcinoma in CHB patients. A previous study also revealed a relationship between sPD‐1 and HCC.8 In our study, the higher levels of sPD‐1 in patients with greater APRI and FIB‐4 scores suggest that sPD‐1 also reflects the level of liver fibrosis in chronic hepatitis B patients. This result was further supported by liver biopsy data, although these data were only available in some cases.
Previous studies of membrane PD‐1 indicated that the engagement of PD‐L1 and PD‐1 may cause T‐cell apoptosis, anergy, exhaustion and interleukin‐10 (IL‐10) expression.23 As the soluble form of PD‐1, sPD‐1 is believed to be an antagonist of the PD‐1/PD‐L1 pathway by its combination with PD‐L1, thereby preventing its activation of PD‐1. Elevations of sPD‐1 have been reported in autoimmune diseases such as arthritis 24 and aplastic anaemia.25 Due to the blocking effect of sPD‐1 against PD‐L1, T cells from these patients showed increased proliferation after culture with recombinant sPD‐1.21 Similar results have been reported in chronic infection.20 These results explained why serum sPD‐1 correlated with liver inflammation and fibrosis, while PD‐1 has been recognized as an exhaustion marker on T cells. Overall, our results further support the role of sPD‐1 as antagonist of the PD‐1/PD‐L1 pathway.
There was a distinct separation between CA and CAN patients in terms of sPD‐1 levels. Multivariate logistic regression also revealed that sPD‐1 was an independent predictor of patients meeting the treatment criteria (CA) as opposed to other patients (CAN). The AUROC of using sPD‐1 alone to discriminate CA patients from CAN patients was 0.883, also supporting sPD‐1 as a good index for making treatment decisions.
As opposed to patients whose ALT levels were elevated > 2 ULN, patients with ALT levels < 2 ULN usually need liver biopsy or noninvasive tests to measure the degrees of inflammation or fibrosis inside the liver in order to determine whether antiviral treatment should be initiated. We found that the relationships between sPD‐1 and liver inflammation and/or fibrosis remain in such patients. sPD‐1 levels in patients with moderate‐to‐severe inflammation and/or fibrosis (patients who should start antiviral treatment as soon as possible 2) were higher than those with mild inflammation and/or fibrosis. This finding suggests that sPD‐1 might serve as a convenient and less invasive marker to indicate histological status. Therefore, sPD‐1 is a potential marker to aid in the decision to initiation of treatment, possibly benefiting patients without significant ALT elevation. It was reported that untreated patients in the IT phase with normal ALT had higher risk of HCC and death/transplantation than did treated IA‐phase patients.26 Of course, a larger cohort is needed to validate this finding in the future, and a specific cut‐off value is also needed to help on deciding treatment.
The strengths of our study include the following. 1) It demonstrated the practical value of sPD‐1 in identifying patients requiring antiviral treatment. Previous studies showed that sPD‐1 is a good predictor for HCC risk and is an important immune‐related marker that could be used to identify disease phase in CHB,8, 22 while our study focused on the role of sPD‐1 in discriminating CHB patients needing antiviral treatment immediately from those who do not; it would be a less invasive option than liver biopsy for CHB patients with low ALT levels. 2) Our study provided the novel finding that sPD‐1 was associated with various clinical parameters, including HBV DNA and HBsAg (viral replication), γ‐GT and TBil (liver damage) and ALT as well as AST (inflammation); such characteristics partially explain why sPD‐1 alone showed good discrimination between patients needing immediate antiviral treatment and those not needing it.
It should be noted that only 33 patients with biopsy data were included in our study, and the degrees of other patients’ liver damage were assessed through noninvasive methods (APRI and FIB‐4). According to a previous study,10 the AUROCs for differentiating none‐to‐minimal fibrosis (F0‐F1) from significant (F2‐F4) liver fibrosis were 0.81 for APRI and 0.81 for FIB‐4; the optimal cut‐off values were 1.59 for APRI and 2.08 for FIB‐4. These alternative methods are less accurate than are liver biopsies. The number of immune‐tolerant patients included in this study was relatively small, which is another limitation that should not be neglected. Due to the retrospective and cross‐sectional nature of our study, classification bias and diagnostic bias are unavoidable. Ideally, a larger‐sized, longitudinal cohort including various type of patients would be desirable to further confirm the findings in this study.
In conclusion, our study demonstrated that serum sPD‐1 level correlated with various clinical parameters, reflecting inflammation and viral replication in CHB patients. sPD‐1 could serve as a new indicator for assessing liver fibrosis and may further aid in deciding on antiviral treatment, especially for patients whose ALTs are elevated to a lesser extent.
CONFLICTS OF INTEREST
All authors claim no conflicts of interest.
Supporting information
ACKNOWLEDGEMENTS
This study is sponsored by International Cooperation Project of Guangzhou Science and Technology Program (2016201604030021) and Guangdong Provincial Medical Science Research Foundation (A2017419).
Zhou L, Li X, Huang X, Chen L, Gu L, Huang Y. Soluble programmed death‐1 is a useful indicator for inflammatory and fibrosis severity in chronic hepatitis B. J Viral Hepat. 2019;26:795–802. 10.1111/jvh.13055
Liang Zhou and Xiaoyan Li contributed equally to this work.
Contributor Information
Lin Gu, Email: gulin@mail.sysu.edu.cn.
Yuehua Huang, Email: huangyh53@mail.sysu.edu.cn.
REFERENCES
- 1. EASL . Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;2017(67):370‐398. [DOI] [PubMed] [Google Scholar]
- 2. Trepo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet. 2014;384:2053‐2063. [DOI] [PubMed] [Google Scholar]
- 3. Dai S, Jia R, Zhang X. The PD‐1/PD‐Ls pathway and autoimmune diseases. Cell Immunol. 2014;290:72‐79. [DOI] [PubMed] [Google Scholar]
- 4. Fukasawa T, Yoshizaki A, Ebata S. Contribution of soluble forms of programmed death 1 and programmed death ligand 2 to disease severity and progression in systemic sclerosis. Arthritis Rheumatol. 2017;69:1879‐1890. [DOI] [PubMed] [Google Scholar]
- 5. Rao M, Valentini D, Dodoo E. Anti‐PD‐1/PD‐L1 therapy for infectious diseases: learning from the cancer paradigm. Int J Infect Dis. 2017;56:221‐228. [DOI] [PubMed] [Google Scholar]
- 6. Tumeh PC, Harview CL, Yearley JH. PD‐1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568‐571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen Y, Wang Q, Shi B. Development of a sandwich ELISA for evaluating soluble PD‐L1 (CD274) in human sera of different ages as well as supernatants of PD‐L1+ cell lines. Cytokine. 2011;56:231‐238. [DOI] [PubMed] [Google Scholar]
- 8. Cheng HY, Kang PJ, Chuang YH. Circulating programmed death‐1 as a marker for sustained high hepatitis B viral load and risk of hepatocellular carcinoma. PLoS ONE. 2014;9:e95870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Petersen JR, Stevenson HL, Kasturi KS. Evaluation of the aspartate aminotransferase/platelet ratio index and enhanced liver fibrosis tests to detect significant fibrosis due to chronic hepatitis C. J Clin Gastroenterol. 2014;48:370‐376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Teshale E, Lu M, Rupp LB. APRI and FIB‐4 are good predictors of the stage of liver fibrosis in chronic hepatitis B: the Chronic Hepatitis Cohort Study (CHeCS). J Viral Hepat. 2014;21:917‐920. [DOI] [PubMed] [Google Scholar]
- 11. Rockey DC, Caldwell SH, Goodman ZD. Liver biopsy. Hepatology. 2009;49:1017‐1044. [DOI] [PubMed] [Google Scholar]
- 12. Tapper EB, Lok AS. Use of liver imaging and biopsy in clinical practice. N Engl J Med. 2017;377:756‐768. [DOI] [PubMed] [Google Scholar]
- 13. Desmet VJ, Gerber M, Hoofnagle JH. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513‐1520. [PubMed] [Google Scholar]
- 14. Terrault NA, Bzowej NH, Chang KM. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63:261‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48:335‐352. [DOI] [PubMed] [Google Scholar]
- 16. Whitfield JB. Gamma glutamyl transferase. Crit Rev Clin Lab Sci. 2001;38:263‐355. [DOI] [PubMed] [Google Scholar]
- 17. Wai CT, Greenson JK, Fontana RJ. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518‐526. [DOI] [PubMed] [Google Scholar]
- 18. Li X, Zhou L, Gu L. Veritable antiviral capacity of natural killer cells in chronic HBV infection: an argument for an earlier anti‐virus treatment. J Transl Med. 2017;15:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li X, Gu Y, Guo X. A practical model evaluating antiviral cytokines by natural killer cells in treatment naive patients with chronic hepatitis B virus infection. Sci Rep. 2017;7:5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Onlamoon N, Rogers K, Mayne AE. Soluble PD‐1 rescues the proliferative response of simian immunodeficiency virus‐specific CD4 and CD8 T cells during chronic infection. Immunology. 2008;124:277‐293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu C, Jiang J, Gao L. Soluble PD‐1 aggravates progression of collagen‐induced arthritis through Th1 and Th17 pathways. Arthritis Res Ther. 2015;17:340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li N, Zhou Z, Li F. Circulating soluble programmed death‐1 levels may differentiate immune‐tolerant phase from other phases and hepatocellular carcinoma from other clinical diseases in chronic hepatitis B virus infection. Oncotarget. 2017;8:46020‐46033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zou W, Wolchok JD, Chen L. PD‐L1 (B7‐H1) and PD‐1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med 2016;8:328rv4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wan B, Nie H, Liu A. Aberrant regulation of synovial T cell activation by soluble costimulatory molecules in rheumatoid arthritis. J Immunol. 2006;177:8844‐8850. [DOI] [PubMed] [Google Scholar]
- 25. Wu H, Miao M, Zhang G. Soluble PD‐1 is associated with aberrant regulation of T cells activation in aplastic anemia. Immunol Invest. 2009;38:408‐421. [DOI] [PubMed] [Google Scholar]
- 26. Kim GA, Lim YS, Han S. High risk of hepatocellular carcinoma and death in patients with immune‐tolerant‐phase chronic hepatitis B. Gut 2018;67:945‐952. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


