Summary
Background
Associations between parental asthma and prenatal exposure to asthma medications with offspring autism spectrum disorder (ASD) have been reported. However, the associations might be confounded by unmeasured (genetic and shared environmental) familial factors.
Objective
We investigated the association between (a) maternal/paternal asthma and offspring ASD, and (b) prenatal exposures to β2‐agonists, other asthma medications and offspring ASD using cases and controls selected from the population as well as biological relatives with different degrees of relatedness.
Methods
We included all children (N = 1 579 263) born in Sweden 1992‐2007. A nested case‐control design was used to compare 22 894 ASD cases identified from the National Patient Register to (a) 228 940 age‐, county‐ and sex‐matched controls randomly selected from the population, (b) their eligible full‐siblings (n = 1267), (c) half‐siblings (n = 1323), (d) full‐cousins (n = 11 477) and (e) half‐cousins (n = 3337). Conditional logistic regression was used to estimate the odds ratios (OR) and 95% confidence intervals (CI) for ASD in children differentially exposed to parental asthma or prenatal asthma medications.
Results
Maternal asthma was associated with increased risk of offspring ASD (OR 1.43, 95% CI 1.38‐1.49); there was a weaker association for paternal asthma (OR 1.17, 95% CI 1.11‐1.23). The risk of offspring ASD in mothers with asthma showed similar estimates when adjusting for shared familial factors among paternal half‐siblings (OR 1.20, 95% CI 0.80‐1.81), full‐cousins (OR 1.28, 95% CI 1.16‐1.41) and half‐cousins (OR 1.30, 95% CI 1.10‐1.54), albeit with wider confidence intervals. Prenatal exposure to asthma medications among subjects whose mothers had asthma was not associated with subsequent ASD.
Conclusions and Clinical Relevance
In this large observational study, parental asthma was associated with slightly elevated risk of ASD in offspring. More specifically, the increased risk by maternal asthma did not seem to be confounded by familial factors. There was no evidence of an association between asthma medications during pregnancy and offspring ASD.
Keywords: asthma, autism spectrum disorder, confounding, medications during pregnancy, nested case‐control
1. INTRODUCTION
Autism spectrum disorder (ASD) is a complex neurodevelopmental disorder defined by persistent deficits in social communication and social interaction alongside restricted, repetitive patterns of behaviour, interests, or activities.1, 2 The aetiology of ASD is unclear.3 In clinical care, comorbidity between maternal asthma and offspring neuropsychiatric disorders has been seen4, 5 and several studies have examined the possible contributions of parental asthma and allergy on ASD in offspring.6, 7, 8 Nevertheless, it is yet unknown if there is a causal link between asthma in parents and ASD in offspring.
First, the association could be explained by shared environmental and/or genetic factors within the family. Previous studies have focused on the increased risk of ASD by maternal asthma, but an association with paternal asthma has not been consistently found.6, 7, 8 Furthermore, the association of parental asthma and ASD in offspring has not been investigated across samples with genetic relatedness. If the risk of ASD in offspring to parents with asthma diminishes or is attenuated when comparing cases with their family members, it is more likely that shared familial factors confound the association. Conversely, if the association remains, then a potential causal explanation would be more likely.9 Therefore, comparing cases with extended family members, for example, siblings (on average sharing 50% genetic materials), half‐siblings (sharing 25%), full‐cousins (sharing 12.5%), half‐cousins (sharing 6.25%) and biologically unrelated controls from the general population is informative to further evaluate the potential role of shared environmental and/or genetic factors.9, 10, 11
Second, the potential association between parental asthma and offspring ASD may be due to unique environmental exposures related to asthma, such as exposure to medication use during pregnancy. Previous studies have suggested a linkage between prenatal exposure to β2‐agonists, subsequent ASD and other developmental disorders12, 13, 14, 15 caused by over‐stimulating the β2‐adrenergic receptor during gestation and altering foetal neurodevelopment.16, 17 Furthermore, a few studies have examined short‐term effects of prenatal exposure to other asthma medications, including inhaled corticosteroids, as well as leukotriene antagonists,18, 19, 20, 21 but none has focused on long‐term outcomes such as offspring ASD. Thus, there is reason to believe that the potential association between parental asthma and offspring ASD could be attributed to prenatal exposure to β2‐agonists and/or other asthma medications.
In this population‐based nested case‐control study, we investigated the association between parental asthma and use of asthma medication during pregnancy with the risk of ASD in offspring. First, the association between maternal/paternal asthma and offspring ASD was estimated using cases and various types of controls selected from the population and among biological relatives with different degrees of relatedness. Second, we investigated the association between prenatal exposures to β2‐agonists, other asthma medications and offspring ASD by comparing cases with unrelated and sibling controls.
2. METHODS
This nested case‐control study was based on data linkage of several Swedish registers via unique personal identity numbers.22 Detailed information on each register and variable used are found in Table E1. Briefly, we selected a birth cohort from the Medical Birth Register (MBR) including more than 99% of all singletons born in Sweden between 1 January 1992 and 31 December 2007 (N = 1 579 263) as index persons,23 and followed them until 31 December 2013 (due to in‐house data availability at the time of analysis), emigration or death. Through the Multi‐Generation Register (MGR), more than 95% of the index persons were linked to their biological mothers, fathers, siblings and cousins (Figure 1, for flow chart).24
Figure 1.
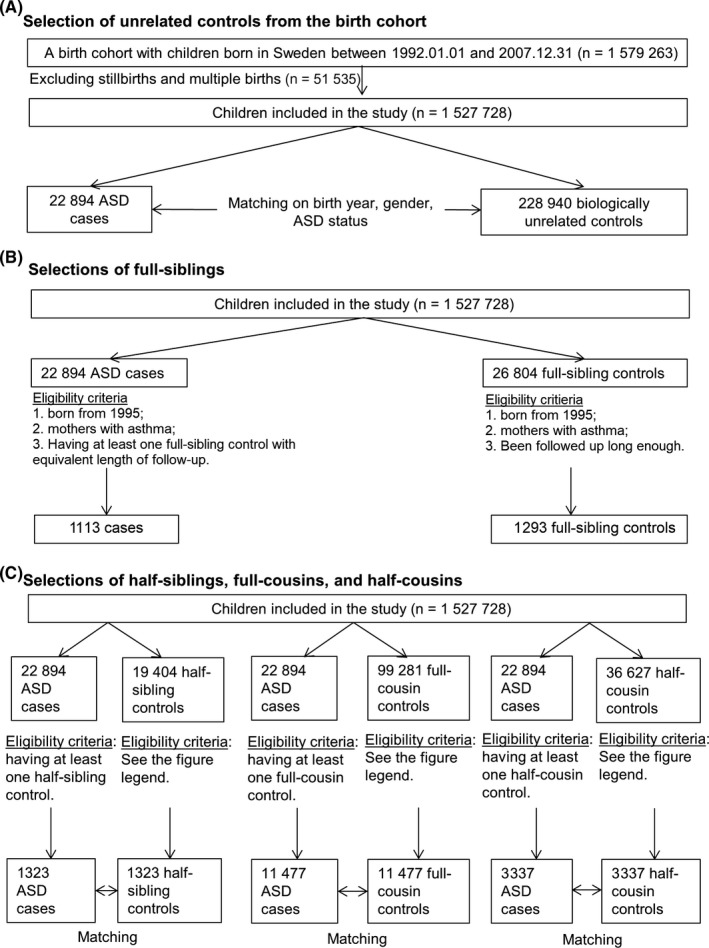
Overall representation of the study populations. The eligibility criteria of being at risk at the age when the case was diagnosed holds for all controls. Matching on sex and <5 years of age difference holds for all controls in panel (C)
2.1. Selection of cases
We identified ASD cases from the National Patient Register (NPR), where a primary or secondary diagnosis of ASD was recorded according to the International Classification of Diseases 9th and 10th revisions (299 for ICD‐9 and all F84s for ICD‐10, respectively). The National Patient Register (NPR) has complete coverage of inpatient diagnoses from 1987 and about 80% of the outpatient diagnosis from 2001 and onwards.25 In the NPR, a high positive predictive value has been noted for most diagnoses, including psychiatric ones.25 Furthermore, the ASD definitions using ICD codes have been validated in a previous study,26 where 96% of the ASD cases within Stockholm County identified from the national and regional registers could be verified by medical records.
2.2. Selection of controls
First, incidence density sampling was used to select 10 biologically unrelated controls for each ASD case. Controls were matched on birth year, sex and county of residence, which allowed us to increase the statistical efficiency without introducing selection bias, and to balance the distribution of such characteristics in both group.
Second, half‐siblings, full‐cousins and half‐cousins of each case as potential controls were identified through the MGR. Controls were eligible if they had the same sex, <5 years of age difference to the case (in order to have complete follow‐up from birth in the NPR), were singletons, and still at risk (ie, ASD free and still under follow‐up) at the age the case received an ASD diagnosis. In case of multiple eligible controls from the same extended family, we randomly selected one half‐sibling, full‐cousin and half‐cousin for each case.
Third, we used the MGR to identify all full‐siblings of each case born from January 1995 due to the data availability on medication use. The full siblings with maternal asthma still at risk at the age the case received an ASD diagnosis were eligible as controls for the analysis on asthma medication use during pregnancy (but not for the parental asthma comparisons as they share the same exposure).
2.3. Exposure assessment
We used records from any of the three registers, that is, MBR, NPR and the Swedish Prescribed Drug Register (SPDR) to identify asthma ever, as an indicator of chronic disease, for biological parents of cases and controls. In MBR, a tick‐box for asthma/lung diseases ever for the mother was indicated at her first antenatal visit from 1992 onwards. In NPR, we used any primary diagnosis of asthma from outpatient visits since 2001 or hospitalization records since 1961 (see Table E2 for diagnostic codes). In SPDR, we defined asthma as having ≥2 dispensed packages of inhaled corticosteroids (ICS), leukotriene antagonists (LTRA), fixed‐dose β2‐ICS combinations from July 2005, or having ≥3 packages of ICS, LTRA, fixed‐dose β2‐ICS combinations, or inhaled β2‐agonists within 12 months from July 2005 for individuals (see Table E3 for all Anatomical Therapeutic Chemical codes), based on a previous validation study.27 The reason we chose to define parental “asthma ever” as exposure was twofold. First, the NPR does not include diagnostic information from primary healthcare centres whereas the SPDR allows us to identify most prevalent cases since 2005 through the validated measure,27 reducing the possibility of exposure misclassification. Second, some asthma cases can be intermittent and may have been present at an earlier age or in later adulthood. Thus, the “asthma ever” definition of exposure would capture more intermittent asthma cases than the “asthma before child birth” definition of exposure over the study period. Parental asthma ever was categorized as asthma from either parent (yes/no), maternal asthma (yes/no) and paternal asthma (yes/no) for analysis.
Information on asthma medication use during pregnancy was retrieved from two sources, that is, midwife‐reported medication use during pregnancy in MBR since 1995 or dispensed medications at pharmacies recorded in SPDR since July 2005. Two forms of β2‐agonists were particularly addressed in the study, as systemic β2‐agonists (ie, oral and injection) used to be mainly administered to suppress premature labour, while inhaled β2‐agonists were primarily indicated for asthma. We categorized the exposure to asthma, β2‐agonists and other asthma medications into four mutually exclusive groups: with asthma but no medications, systemic β2‐agonists only, inhaled β2‐agonists with or without other asthma medications, and other asthma medications without any β2‐agonists.
2.4. Covariates
Socio‐economic characteristics, including maternal civil status, parental country of birth and highest education of parents by the end of the year of child birth, were retrieved from the longitudinal integration database for health insurance and labour market studies. Other background characteristics (maternal body mass index (BMI) at first antenatal visit, smoking during pregnancy and maternal age at delivery, mode of delivery, parity, gestational age and birth weight) were retrieved from MBR. Paternal age at child birth came from the Total Population Register. Maternal diagnoses during pregnancy and delivery (pre‐eclampsia, pre‐gestational and gestational diabetes, premature rupture of membranes, premature contraction, placental abruption and haemorrhage) were retrieved from MBR and NPR. Family history of psychiatric disorders was defined as any psychiatric diagnosis (yes/no) from NPR for each parent. Information on maternal asthma exacerbation history was retrieved from NPR and PDR and defined as having ≥1 emergency visit or hospital admission for asthma, or having dispensed ≥1 package of oral corticosteroid during the 12‐month period prior to the pregnancy.
2.5. Statistical analyses
Statistical Analysis Software 9.4 (SAS Institute, Cary, NC) was used to conduct conditional logistic regression analyses and estimate odds ratios (OR) with 95% confidence intervals (CIs) of all analyses on parental asthma, use of asthma medication during pregnancy and offspring ASD. In addition to crude models, we also provide estimates adjusted for parity, maternal smoking, BMI, and civil status, parental age, country of birth, and education. A directed acyclic graph was used to identify potential confounders and mediators based on prior knowledge and a priori assumptions on causal relationship (Figure E1 and eMethods for detailed information). In the analyses with the unrelated matched controls, the conditional analysis automatically gave adjustment for the matching variables (age, sex and county).
First, the association between parental asthma and offspring ASD was estimated using cases and unrelated controls. We further tested if the association was moderated by sex, county of residence, maternal smoking during pregnancy, family history of psychiatric disorders and parental education by introducing an interaction term in the model. The independent effect and effect modification of maternal and paternal asthma were analyzed by mutual adjustment and interaction test. Sensitivity analyses were performed to explore the result robustness by gestational age, birth weight, maternal BMI, information sources of asthma and timing of maternal asthma, that is, before or after pregnancy (see eMethods for detailed descriptions).
Second, we performed separate analyses by comparing cases with (a) maternal half‐sibling controls to estimate the effect of paternal asthma ever (ie, case and control exposed to different biological fathers w/without asthma); (b) paternal half‐sibling controls to estimate the effect of maternal asthma ever (ie, case and control exposed to different biological mothers w/without asthma); and (c) full‐cousin and half‐cousin controls to estimate the effects of maternal and paternal asthma, respectively. These comparisons allowed us to account for unmeasured familial confounding factors shared by cases and their relatives, as well as the measured confounders mentioned above (full‐sibling controls sharing the same parental asthma ever with cases were not used here). If the association remains when comparing cases to sibling and cousin controls, this indicates that parental asthma is a potential causal risk factor for offspring ASD. In contrast, if the risk of ASD diminishes or is further attenuated, this indicates that familial factors shared between siblings and/or cousins confound the association.
Third, to investigate the association between prenatal exposures to β2‐agonists, other asthma medications during pregnancy and ASD, different subsets of cases were compared to matched unrelated and full‐sibling controls if their mothers had asthma or took asthma medications during pregnancy.
The Regional Ethical Review Board in Stockholm, Sweden granted permission for the study, Dnr 2013/862‐31/5, and individual information was de‐identified by the register holders.
3. RESULTS
Characteristics of all study populations are presented in Table E4 and Table E5 and described in eResults section of the online repository text.
3.1. Parental asthma and offspring ASD
Among 22 894 cases compared to 228 940 unrelated controls from the birth cohort, more mothers were single or with other civil status at the year of child birth (15% among cases vs 9.3% among controls), smoked during pregnancy (19.5% vs 13.7%), and had pregnancy‐ and delivery‐related complications (see Table E4). Maternal asthma was associated with 43% increased odds of ASD overall when compared to unrelated controls (adjusted OR 1.43, 95% CI 1.38‐1.49, Figure 2 and Table E6). In the comparisons within extended families, point estimates for the association between maternal asthma and offspring ASD were similar for half‐siblings (adjusted OR 1.20, 95% CI 0.80‐1.81), full‐cousins (adjusted OR 1.28, 95% CI 1.16‐1.41) and half‐cousins (adjusted OR 1.30, 95% CI 1.10‐1.54) although with wide confidence intervals and not all being statistically significant (Figure 2 and Table E7). The results were similar when mutually adjusting for paternal asthma (Table E8).
Figure 2.
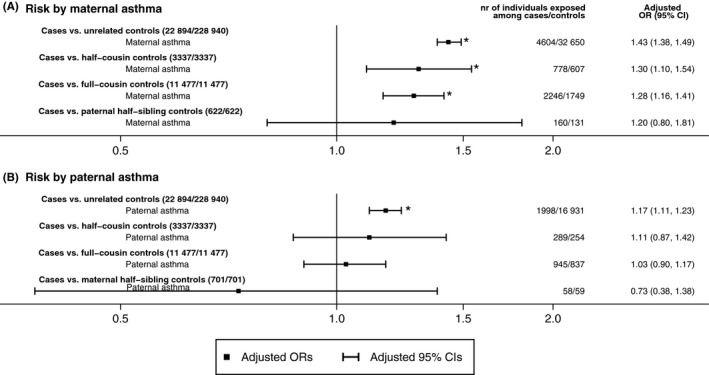
Adjusted odds ratios (OR) and 95% confidence intervals (CI) of offspring autism spectrum disorders (ASD) by parental asthma when comparing cases with unrelated controls, half‐cousin, full‐cousin, and maternal/paternal half‐sibling controls nested in the birth cohort. Panels (A and B) show the estimates for maternal asthma and paternal asthma separately, after adjusting for parity, maternal smoking during pregnancy and civil status at year of child birth, country of birth and age at child birth for mothers and fathers, highest education between parents, and maternal body mass index at first antenatal visit. The asterisks were used to indicate statistically significant associations
We observed an association between paternal asthma and offspring ASD (adjusted OR 1.17, 95% CI 1.11‐1.23) when compared to unrelated controls (Figure 2 and Table E6). However, the estimate was attenuated in comparisons for cases and their extended family members [(adjusted OR 0.73 (95% CI 0.38‐1.38) for half‐siblings, OR 1.03 (95% CI 0.90‐1.17) for full‐cousins, and OR 1.11 (95% CI 0.87‐1.42) for half‐cousins, Figure 2 and Table E7]. No interaction was observed between maternal and paternal asthma (Table E8).
The estimates were similar when further adjusted for potential mediators (Table E6). Moreover, there was no evidence that these associations were modified by child sex, maternal smoking during pregnancy, county of residence, family history of psychiatric disorders, or parental education (Figure E2, all interaction P‐values >0.05). Results of sensitivity analyses showed similar estimates for maternal and paternal asthma in subgroups with normal birth weight and gestational age, maternal BMI, and when asthma was identified from different information sources. When limiting the analysis to children born 2006 to 2007 (ie, with full exposure information from all three registers), we found an association of maternal but not paternal asthma with offspring ASD. There were no differences by parental asthma identified before and after pregnancy (Tables E9 and E10).
3.2. Use of asthma medication during pregnancy and offspring ASD
Table E5 shows the characteristics of 3507 cases and 5350 unrelated controls eligible for the analyses of prenatal exposure to asthma medications and subsequent ASD. In comparison with the previously described characteristics of the study population, similar patterns of maternal background factors were observed among eligible cases and unrelated controls for the medication analysis. Among mothers with asthma, offspring prenatally exposed to different types of asthma medications, for example, inhaled β2‐agonists did not have increased risk of subsequent ASD (adjusted OR 1.02, 95% CI 0.85‐1.24) compared to offspring of asthmatic mothers without any medications. Furthermore, using full‐sibling controls, adjusting for pregnancy‐ and delivery‐related factors measured in the study, as well as for maternal exacerbation history did not seem to change the result (Table 1, Tables E11 and E12).
Table 1.
Association between use of asthma medication during pregnancy and offspring ASD
| No. of cases/controls | OR (95% CI) | ||
|---|---|---|---|
| Model 1a | Model 2b | ||
| Comparing ASD cases to unrelated controls | 3507/5350 | ||
| With asthma but no medications | 2747/4208 | Ref | Ref |
| Systemic β2‐agonists only | 225/336 | 1.03 (0.85, 1.25) | 0.96 (0.76, 1.22) |
| Inhaled β2‐agonists with or without other asthma medications | 380/536 | 1.04 (0.89, 1.22) | 1.02 (0.85, 1.24) |
| Other asthma medications without any type of β2‐agonists | 155/270 | 0.84 (0.67, 1.06) | 0.75 (0.57, 0.99) |
| Model 3 a | Model 4 c | ||
| Comparing ASD cases to their full‐sibling controlsd | 1133/1293 | ||
| With asthma but no medications | 930/1066 | Ref | Ref |
| Systemic β2‐agonists only | 49/52 | 1.24 (0.75, 2.05) | 0.88 (0.50, 1.55) |
| Inhaled β2‐agonists with or without other asthma medications | 105/118 | 1.00 (0.70, 1.42) | 1.03 (0.67, 1.58) |
| Other asthma medications without any type of β2‐agonists | 49/57 | 1.01 (0.64, 1.59) | 0.87 (0.50, 1.51) |
ASD, autism spectrum disorders; CI, confidence interval; OR, odds ratio.
Model 1 and 3 did not adjust for any covariates.
Model 2 adjusted for birth year, parity, maternal smoking during pregnancy and civil status, country of birth and age for mothers and fathers, highest education between parents, and maternal BMI at first antenatal visit.
Model 4 adjusted for birth year, parity, maternal smoking during pregnancy, age for mothers and fathers, highest education between parents, and maternal BMI at first antenatal visit.
Analyses using half‐sibling, and cousin controls are available on request.
4. DISCUSSION
We found an increased risk of offspring ASD with maternal asthma and a weaker, but significantly increased risk with paternal asthma. The association between maternal asthma remained similar in magnitude within extended families, suggesting that genetic and environmental factors shared by half‐siblings and cousins did not seem to confound the association with maternal asthma. Comparing to the maternal effect, the observed association between paternal asthma and offspring ASD, however, seems to be confounded by shared familial factors. Moreover, we did not observe any statistically significant difference in offspring ASD risk for asthmatic women medicated with β2‐agonists or other asthma medications during pregnancy compared to those without medications, which suggests a possible biological effect related to the maternal asthma, not mediated by medications.
4.1. Risk of offspring ASD by parental asthma
Our findings are consistent with the results from a case‐control study by Croen et al,6 which reported a moderately positive association between maternal asthma and ASD in offspring, particularly in asthma diagnosed prior to and during pregnancy (OR 1.6, 95% CI 1.2‐2.2). However, Lyall and colleagues found no association between maternal asthma or allergies overall and offspring ASD in another case‐control study.8 Characteristics of cases, study time periods, and the prevalence of maternal asthma before and after pregnancy may all contribute to differences between studies. For example, a cohort study by Langridge et al7 showed a strikingly similar finding as ours for ASD without intellectual disability, with 40% increased risk with maternal asthma. Yet, they observed no association on ASD with intellectual disability, which could be due to either the relatively lower reported rates of ASD in Australia, or exclusion criteria from their cohort. Therefore, we speculate that children of mothers with asthma have increased susceptibility to develop ASD. Furthermore, Croen et al. observed more than doubled risk of offspring ASD by maternal asthma and allergies identified in early‐ and mid‐pregnancy6 but this was not confirmed by Lyall et al.8 Unfortunately, we were not able to provide trimester‐specific OR estimates due to limitations in exposure data regarding timing. Additional studies with proper comparisons on the timing and severity of asthma will still be required to elucidate the possibility of a causal association between maternal asthma and offspring ASD.28
There have been few publications on the association between paternal asthma ever and offspring ASD. Two studies by Mouridsen et al and Larsson et al did not find any evidence of increased risk of offspring ASD neither by maternal nor paternal asthma29, 30 although both had limited statistical power. The current result showed a difference in the effect of maternal and paternal asthma. The significant association of paternal asthma and offspring ASD was attenuated when adjusting for familial factors, potentially indicating a familial confounding between paternal asthma and ASD. However, maternal asthma had similar effect estimates on offspring ASD when comparing cases to unrelated individuals as when controlling for some degree of genetic relatedness and other measured and unmeasured familial characteristics, by comparing cases to relatives of different degrees of relatedness. Two further scenarios may be inferred: first, the association could be due to an observed differences in health‐seeking behaviours of asthmatic mothers compared with those of non‐asthmatic mothers, that is, an increased proportion of ASD cases were incorrectly classified as controls among offspring of non‐asthmatic mothers, however this seems unlikely since the estimates remained among biological relatives with different degrees of relatedness; second, the result can also indicate that maternal asthma is independently associated with offspring ASD, albeit the absolute risk changes related to maternal asthma being small.
Although we cannot completely rule out a genetic association via maternal inheritance of mitochondrial DNA,31 our results helped to identify a possible causal association between maternal asthma and offspring ASD. A possible biological mechanism is through immune system dysregulation during pregnancy and the ensuing development in offspring.32, 33 Recent animal studies have confirmed possible epigenetic modifications in the offspring's behavioural and cognitive abnormalities due to maternal immune activation.34, 35 Studies on immune mediators including cytokines,36 T cells37 and auto‐antibodies38 suggest several pathways to ASD through immune dysfunction, but these findings are inconclusive. Furthermore, asthma, especially uncontrolled asthma, during pregnancy has also been associated with intermittent hypoxia39, 40 and foetal growth restriction,41, 42, 43 which are common risk factors for several psychiatric and neurodevelopmental disorders including schizophrenia,44, 45 bipolar disorder,46, 47 ASD48, 49 and intellectual disability.40 However, adjusting for birth weight, premature delivery, and other pregnancy and delivery‐related factors as mediators, did not change the results. There may nevertheless be other potential mediators that deserve further investigation. For example, it has been proposed that asthma‐associated intermittent hypoxia might be a mediator in ASD aetiology.50, 51
4.2. Risk of offspring ASD by use of asthma medication during pregnancy
Another potential mediator for the association between maternal asthma and subsequent offspring ASD is the use of asthma medication during pregnancy. A few reports on long‐term effects on offspring's neurodevelopmental outcomes showed inconsistent results for β2‐agonists12, 14, 16 and no effect for corticosteroids.21, 52 Animal studies suggest a link with β2‐agonists (mainly terbutaline) to cerebellar abnormalities and behavioural impairment.14 A clinical study reported a higher ASD concordance rate in dizygotic twins in mothers treated with terbutaline due to preterm labour,16 which was partly replicated in a case‐control study.12 The twin sample, strict timing, long durations and high doses of terbutaline exposure may explain the positive findings in previous studies. However, comparisons from animals to humans should also be drawn with caution. Our results did not suggest an association between prenatal exposure to asthma medication and ASD when comparing cases to unrelated and full‐sibling controls. Neither is there an evidence on confounding by a history of asthma exacerbation before pregnancy. One possible explanation could be that level of asthma severity and symptom control during pregnancy confounded the association between medication use and ASD. The alternative explanation could be that a true causal effect has been diluted by a non‐differential misclassification of asthma medication usage, as we observed a relatively low percentage of asthmatic women with medications during pregnancy (21%‐22% in cases and unrelated controls).
4.3. Strengths and limitations
In addition to the population‐based nested case‐control design, the comparisons within extended families helped us control for unmeasured genetic and environmental factors shared by cases and their family members.10 The study base identified from data linkage between reliable registers and up to 21 years of follow‐up is advantageous for low selection bias and no recall bias.22 The data sources for case ascertainment have been previously validated showing generally high quality.26, 53 No earlier study has investigated the association of interest using more than 20 000 ASD cases, which allowed us to report group‐specific effects while accounting for measured covariates including socio‐demographic characteristics and family history of psychiatric disorders.
Some limitations of this study need to be addressed. First, the coverage of some registers was incomplete during parts of the follow‐up. For example, maternal asthma during pregnancy might not be identifiable in the registers if symptoms were not severe enough, because diagnostic records at primary care centres during the whole study period or mothers’ medication dispensing records before pregnancy and after delivery among older children were not retrieved. Nevertheless, the prevalence of ASD and asthma in our study was similar to other publications using Swedish data26, 54, 55 and sensitivity analyses based on children born during 2006‐2007, shortly after the SPDR was initiated, verified our main findings. Second, it is difficult to draw conclusions on causal relationships and biological mechanisms from observational data, although using sibling and cousin controls allowed us to adjust for varying amounts of shared familial factors as a way of exploring the importance of confounding from such factors. Still, it is unknown whether we were able to control for all possible confounders, and there are assumptions and limitations of the family‐based designs, for example, no carry‐over effect and the way of choosing discordant pairs, power issues and limited control on shared postnatal environment for half‐siblings, which can influence both the internal and external validity of the results.11 Third, reverse causality cannot be ruled out as our exposure measurement was parental asthma ever. However, sensitivity analyses based on maternal asthma before child birth gave results in line with our main findings. Fourth, because no trimester‐specific medication use was recorded in MBR, our risk estimates for medication exposures were only based on medication use anytime during pregnancy, and the proportion of asthmatic women with medications during pregnancy might be underestimated due to the incomplete information in the registers.43 Fifth, some cases not captured in the NPR could be misclassified as controls, which might have resulted in an attenuation of OR if we assume the misclassification was non‐differential by their parental asthma status. However, if cases and controls were differentially misclassified (eg, different health‐seeking behaviours among parent with or without asthma), it would be difficult to predict the direction of the estimates. Finally, results on full‐siblings prenatally exposed to asthma medications may have limited power to detect an association due to the low number of asthmatic women with different asthma medication between pregnancies. Future studies are still needed to evaluate on the role of different medication uses during pregnancy.
In conclusion, we found an association between parental asthma and offspring ASD, which might be crucial for the understanding of the ASD aetiology in a subgroup of cases. The association between maternal asthma could not be fully explained by measured confounders such as socio‐economic and demographic factors, mediators including pregnancy and delivery‐related factors, or unmeasured familial factors such as genetics and shared environment, which indicate the importance of investigating other maternal environmental factors. For pregnant women with asthma, use of asthma medications during pregnancy did not seem to increase the risk of offspring ASD, which further highlights the importance of surveillance and monitoring of asthma in pregnancy. In comparison to the maternal effect, the association between paternal asthma and offspring ASD seemed to be confounded by unmeasured familial factors.
Supporting information
ACKNOWLEDGEMENTS
This work was supported by the Swedish Research Council [2011‐3060, 523‐2009‐7054] and the Swedish Initiative for research on Microdata in the Social And Medical Sciences (SIMSAM) framework grant [340‐2013‐5867]; the Stockholm County Council (ALF projects); the Strategic Research Programme in Epidemiology at Karolinska Institutet; the Swedish Heart‐Lung Foundation; FORTE (grant no 2015‐00289); and the HKH Kronprinsessan Lovisas förening för barnasjukvård. The funders had no role in study design, data collection and analyses, decision to publish or preparation of the manuscript.
Gong T, Lundholm C, Rejnö G, et al. Parental asthma and risk of autism spectrum disorder in offspring: A population and family‐based case‐control study. Clin Exp Allergy. 2019;49:883–891. 10.1111/cea.13353
REFERENCES
- 1. American Psychiatric Association . The Diagnostic and Statistical Manual of Mental Disorders. 5th ed 2013.
- 2. World Health Organization . The ICD‐10 Classification of Mental and Behavioural Disorders: Diagnostic Criteria for Research: World Health Organization; 1993.
- 3. Lai MC, Lombardo MV, Baron‐Cohen S. Autism. Lancet. 2014;383(9920):896‐910. [DOI] [PubMed] [Google Scholar]
- 4. Instanes JT, Halmøy A, Engeland A, Haavik J, Furu K, Klungsøyr K. Attention‐deficit/hyperactivity disorder in offspring of mothers with inflammatory and immune system diseases. Biol Psychiat. 2017;81(5):452‐459. [DOI] [PubMed] [Google Scholar]
- 5. van der Schans J, Pleiter JC, de Vries TW, et al. Association between medication prescription for atopic diseases and attention‐deficit/hyperactivity disorder. Ann Allergy Asthma Immunol. 2016;117(2):186‐191. [DOI] [PubMed] [Google Scholar]
- 6. Croen LA, Grether JK, Yoshida CK, Odouli R, de Van Water J. Maternal autoimmune diseases, asthma and allergies, and childhood autism spectrum disorders: a case‐control study. Arch Pediatr Adolesc Med. 2005;159(2):151‐157. [DOI] [PubMed] [Google Scholar]
- 7. Langridge AT, Glasson EJ, Nassar N, et al. Maternal conditions and perinatal characteristics associated with autism spectrum disorder and intellectual disability. PLoS ONE. 2013;8(1):e50963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lyall K, Ashwood P, Van de Water J, Hertz‐Picciotto I. Maternal immune‐mediated conditions, autism spectrum disorders, and developmental delay. J Autism Dev Disord. 2014;44(7):1546‐1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Plomin R, DeFries JC, Knopik VS, Neiderhiser JM. Behavioral Genetics: A Primer, 6th edn New York: Worth Publishers; 2013:xix, 503, 19. [Google Scholar]
- 10. D'Onofrio BM, Lahey BB, Turkheimer E, Lichtenstein P. Critical need for family‐based, quasi‐experimental designs in integrating genetic and social science research. Am J Public Health. 2013;103(Suppl 1):S46‐S55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. D'Onofrio BM, Class QA, Lahey BB, Larsson H. Testing the Developmental Origins of Health and Disease Hypothesis for Psychopathology Using Family‐Based Quasi‐Experimental Designs. Child Dev Perspect. 2014;8(3):151‐157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Croen LA, Connors SL, Matevia M, Qian Y, Newschaffer C, Zimmerman AW. Prenatal exposure to beta2‐adrenergic receptor agonists and risk of autism spectrum disorders. J Neurodev Disord. 2011;3(4):307‐315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pitzer M, Schmidt MH, Esser G, Laucht M. Child development after maternal tocolysis with beta‐sympathomimetic drugs. Child Psychiatry Hum Dev. 2001;31(3):165‐182. [DOI] [PubMed] [Google Scholar]
- 14. Witter FR, Zimmerman AW, Reichmann JP, Connors SL. In utero beta 2 adrenergic agonist exposure and adverse neurophysiologic and behavioral outcomes. Am J Obstet Gynecol. 2009;201(6):553‐559. [DOI] [PubMed] [Google Scholar]
- 15. Gidaya NB, Lee BK, Burstyn I, Michael Y, Newschaffer CJ, Mortensen EL. In utero exposure to β‐2‐adrenergic receptor agonist drugs and risk for autism spectrum disorders. Pediatrics. 2016;137:e20151316. [DOI] [PubMed] [Google Scholar]
- 16. Connors SL, Crowell DE, Eberhart CG, et al. beta2‐adrenergic receptor activation and genetic polymorphisms in autism: data from dizygotic twins. J Child Neurol. 2005;20(11):876‐884. [DOI] [PubMed] [Google Scholar]
- 17. Cheslack‐Postava K, Fallin MD, Avramopoulos D, et al. beta2‐Adrenergic receptor gene variants and risk for autism in the AGRE cohort. Mol Psychiatry. 2007;12(3):283‐291. [DOI] [PubMed] [Google Scholar]
- 18. Blais L, Beauchesne MF, Lemiere C, Elftouh N. High doses of inhaled corticosteroids during the first trimester of pregnancy and congenital malformations. J Allergy Clin Immunol. 2009;124(6):1229‐1234. e4. [DOI] [PubMed] [Google Scholar]
- 19. Lin S, Herdt‐Losavio M, Gensburg L, Marshall E, Druschel C. Maternal asthma, asthma medication use, and the risk of congenital heart defects. Birth Defects Res A Clin Mol Teratol. 2009;85(2):161‐168. [DOI] [PubMed] [Google Scholar]
- 20. Bakhireva LN, Jones KL, Schatz M, et al. Safety of leukotriene receptor antagonists in pregnancy. J Allergy Clin Immunol. 2007;119(3):618‐625. [DOI] [PubMed] [Google Scholar]
- 21. Tegethoff M, Greene N, Olsen J, Schaffner E, Meinlschmidt G. Inhaled glucocorticoids during pregnancy and offspring pediatric diseases: a national cohort study. Am J Respir Crit Care Med. 2012;185(5):557‐563. [DOI] [PubMed] [Google Scholar]
- 22. Ludvigsson JF, Otterblad‐Olausson P, Pettersson BU, Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24(11):659‐667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Axelsson O. The Swedish medical birth register. Acta Obstet Gynecol Scand. 2003;82(6):491‐492. [DOI] [PubMed] [Google Scholar]
- 24. Ludvigsson JF, Almqvist C, Bonamy AK, et al. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol. 2016;31(2):125‐136. [DOI] [PubMed] [Google Scholar]
- 25. Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Idring S, Rai D, Dal H, et al. Autism spectrum disorders in the Stockholm Youth Cohort: design, prevalence and validity. PLoS ONE. 2012;7(7):e41280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ortqvist AK, Lundholm C, Wettermark B, Ludvigsson JF, Ye W, Almqvist C. Validation of asthma and eczema in population‐based Swedish drug and patient registers. Pharmacoepidemiol Drug Saf. 2013;22(8):850‐860. [DOI] [PubMed] [Google Scholar]
- 28. Hill AB. The environment and disease: association or causation? 1965. J R Soc Med. 2015;108(1):32‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Larsson M, Weiss B, Janson S, Sundell J, Bornehag CG. Associations between indoor environmental factors and parental‐reported autistic spectrum disorders in children 6‐8 years of age. Neurotoxicology. 2009;30(5):822‐831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mouridsen SE, Rich B, Isager T, Nedergaard NJ. Autoimmune diseases in parents of children with infantile autism: a case‐control study. Dev Med Child Neurol. 2007;49(6):429‐432. [DOI] [PubMed] [Google Scholar]
- 31. Rossignol DA, Frye RE. Mitochondrial dysfunction in autism spectrum disorders: a systematic review and meta‐analysis. Mol Psychiatry. 2012;17(3):290‐314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hsiao EY, McBride SW, Chow J, Mazmanian SK, Patterson PH. Modeling an autism risk factor in mice leads to permanent immune dysregulation. Proc Natl Acad Sci USA. 2012;109(31):12776‐12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Knuesel I, Chicha L, Britschgi M, et al. Maternal immune activation and abnormal brain development across CNS disorders. Nat Rev Neurol. 2014;10(11):643‐660. [DOI] [PubMed] [Google Scholar]
- 34. Basil P, Li Q, Dempster EL, et al. Prenatal maternal immune activation causes epigenetic differences in adolescent mouse brain. Transl Psychiatry. 2014;4:e434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Labouesse MA, Dong E, Grayson D, Guidotti A, Meyer U. Maternal immune activation induces GAD1 and GAD2 promoter remodeling in the offspring prefrontal cortex. Epigenetics. 2015;10:1143‐1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ashwood P, Krakowiak P, Hertz‐Picciotto I, Hansen R, Pessah I, Van de Water J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun. 2011;25(1):40‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ashwood P, Krakowiak P, Hertz‐Picciotto I, Hansen R, Pessah IN, Van de Water J. Altered T cell responses in children with autism. Brain Behav Immun. 2011;25(5):840‐849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Croen LA, Braunschweig D, Haapanen L, et al. Maternal mid‐pregnancy autoantibodies to fetal brain protein: the early markers for autism study. Biol Psychiatry. 2008;64(7):583‐588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Murphy VE, Clifton VL, Gibson PG. Asthma exacerbations during pregnancy: incidence and association with adverse pregnancy outcomes. Thorax. 2006;61(2):169‐176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Modabbernia A, Mollon J, Boffetta P, Reichenberg A. Impaired Gas Exchange at Birth and Risk of Intellectual Disability and Autism: a Meta‐analysis. J Autism Dev Disord. 2016;46(5):1847‐1859. [DOI] [PubMed] [Google Scholar]
- 41. Luppi P. How immune mechanisms are affected by pregnancy. Vaccine. 2003;21(24):3352‐3357. [DOI] [PubMed] [Google Scholar]
- 42. Tamasi L, Horvath I, Bohacs A, Muller V, Losonczy G, Schatz M. Asthma in pregnancy–immunological changes and clinical management. Respir Med. 2011;105(2):159‐164. [DOI] [PubMed] [Google Scholar]
- 43. Rejno G, Lundholm C, Gong T, Larsson K, Saltvedt S, Almqvist C. Asthma during pregnancy in a population‐based study–pregnancy complications and adverse perinatal outcomes. PLoS ONE. 2014;9(8):e104755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cannon TD, Yolken R, Buka S, Torrey EF. Decreased neurotrophic response to birth hypoxia in the etiology of schizophrenia. Biol Psychiatry. 2008;64(9):797‐802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nilsson E, Stalberg G, Lichtenstein P, Cnattingius S, Olausson PO, Hultman CM. Fetal growth restriction and schizophrenia: a Swedish twin study. Twin Res Hum Genet. 2005;8(4):402‐408. [DOI] [PubMed] [Google Scholar]
- 46. Haukvik UK, McNeil T, Lange EH, et al. Pre‐ and perinatal hypoxia associated with hippocampus/amygdala volume in bipolar disorder. Psychol Med. 2014;44(5):975‐985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Class QA, Rickert ME, Larsson H, Lichtenstein P, D'Onofrio BM. Fetal growth and psychiatric and socioeconomic problems: population‐based sibling comparison. Br J Psychiatry. 2014;205(5):355‐361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gardener H, Spiegelman D, Buka SL. Perinatal and neonatal risk factors for autism: a comprehensive meta‐analysis. Pediatrics. 2011;128(2):344‐355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. D'Onofrio BM, Class QA, Rickert ME, Larsson H, Langstrom N, Lichtenstein P. Preterm birth and mortality and morbidity: a population‐based quasi‐experimental study. JAMA psychiatry. 2013;70(11):1231‐1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gardener H, Spiegelman D, Buka SL. Prenatal risk factors for autism: comprehensive meta‐analysis. Br J Psychiatry. 2009;195(1):7‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Murphy VE, Gibson PG, Smith R, Clifton VL. Asthma during pregnancy: mechanisms and treatment implications. Eur Respir J. 2005;25(4):731‐750. [DOI] [PubMed] [Google Scholar]
- 52. MacArthur BA, Howie RN, Dezoete JA, Elkins J. School progress and cognitive development of 6‐year‐old children whose mothers were treated antenatally with betamethasone. Pediatrics. 1982;70(1):99‐105. [PubMed] [Google Scholar]
- 53. Sandin S, Lichtenstein P, Kuja‐Halkola R, Larsson H, Hultman CM, Reichenberg A. The familial risk of autism. JAMA. 2014;311(17):1770‐1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lundback B, Ronmark E, Jonsson E, Larsson K, Sandstrom T. Incidence of physician‐diagnosed asthma in adults–a real incidence or a result of increased awareness? Report from The Obstructive Lung Disease in Northern Sweden Studies. Respir Med. 2001;95(8):685‐692. [DOI] [PubMed] [Google Scholar]
- 55. Bjerg A, Ekerljung L, Middelveld R, et al. Increased prevalence of symptoms of rhinitis but not of asthma between 1990 and 2008 in Swedish adults: comparisons of the ECRHS and GA(2)LEN surveys. PLoS ONE. 2011;6(2):e16082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


