Abstract
Volatile organic compounds (VOCs) emitted by plant roots can influence the germination and growth of neighbouring plants. However, little is known about the effects of root VOCs on plant–herbivore interactions of neighbouring plants. The spotted knapweed (Centaurea stoebe) constitutively releases high amounts of sesquiterpenes into the rhizosphere. Here, we examine the impact of C. stoebe root VOCs on the primary and secondary metabolites of sympatric Taraxacum officinale plants and the resulting plant‐mediated effects on a generalist root herbivore, the white grub Melolontha melolontha. We show that exposure of T. officinale to C.stoebe root VOCs does not affect the accumulation of defensive secondary metabolites but modulates carbohydrate and total protein levels in T. officinale roots. Furthermore, VOC exposure increases M. melolontha growth on T. officinale plants. Exposure of T. officinale to a major C. stoebe root VOC, the sesquiterpene (E)‐β‐caryophyllene, partially mimics the effect of the full root VOC blend on M. melolontha growth. Thus, releasing root VOCs can modify plant–herbivore interactions of neighbouring plants. The release of VOCs to increase the susceptibility of other plants may be a form of plant offense.
Keywords: associational effects, belowground herbivory, neighbourhood effects, plant–herbivore interactions, plant–plant interactions, volatile priming
Short abstract
Root volatiles released by Centaurea stoebe modulate root growth and primary metabolism of Taraxacum officinale. These changes are associated with increased root herbivore growth on T. officinale, thus demonstrating that root volatiles can modify plant‐herbivore interactions in neighboring plants
1. INTRODUCTION
Plants emit a variety of volatile organic compounds (VOCs) that can affect the behaviour and performance of other organisms. VOCs induced by herbivory for instance can enhance defences and resistance of neighbouring plants (Arimura et al., 2000; Engelberth, Alborn, Schmelz, & Tumlinson, 2004; Erb et al., 2015; Frost, Mescher, Carlson, & De Moraes, 2008; Karban, Yang, & Edwards, 2014; Pearse, Hughes, Shiojiri, Ishizaki, & Karban, 2013; Sugimoto et al., 2014). As the benefit for the emitter plant is unclear, this phenomenon is commonly regarded as a form of “eavesdropping” by the receiver rather than a form of communication (Heil & Karban, 2010). From the perspective of an emitter plant, it would seem advantageous to use VOCs to suppress rather than enhance defences in neighbours (Heil & Karban, 2010). However, little is known about the capacity of VOCs to suppress defences and enhance herbivore attack rates in neighbouring plants. Broccoli plants were found to receive more oviposition by diamondback moths after exposure to VOCs from damaged conspecifics (Li & Blande, 2015). Furthermore, exposure to VOCs from damaged neighbours increases herbivore damage on blow wives (Achyrachaena mollis) and charlock (Sinapis arvensis) (Pearse et al., 2012). Finally, green leafy volatile (GLV) exposure suppresses several defence‐related genes in coyote tobacco (Nicotiana attenuata; Paschold, Halitschke, & Baldwin, 2006). Clearly, the capacity of VOCs to suppress rather than induce defences requires more attention in order to understand how VOCs influence plant–herbivore interactions of neighbouring plants (Erb, 2018a).
The majority of studies on the effects of VOCs on plant neighbours have focused on the phyllosphere. However, plants also release significant amounts of VOCs into the rhizosphere, which may affect plant defence and plant–herbivore interactions (Delory, Delaplace, Fauconnier, & du Jardin, 2016). Root chemicals, including VOCs, can affect the germination and growth of neighbouring plants (Ens, Bremner, French, & Korth, 2009; Jassbi, Zamanizadehnajari, & Baldwin, 2010) and the behaviour and performance of herbivores (Hu, Mateo, et al., 2018; Robert, Erb, et al., 2012; Robert, Veyrat, et al., 2012). Therefore, it is reasonable to assume that root VOCs may also affect plant–herbivore interactions of neighbouring plants. Root exudates and mycelial networks have been shown to alter plant defences and plant herbivore interactions in neighbouring plants (Babikova et al., 2013; Dicke & Dijkman, 2001), but the specific role of root VOCs in plant–plant interaction has, to the best of our knowledge, not been addressed (Delory et al., 2016).
In this study, we explored the influence of root VOCs on the common dandelion (Taraxacum officinale agg.) and its interaction with the common cockchafer Melolontha melolontha. In grasslands across Europe, T. officinale is often attacked by larvae of M. melolontha (Coleoptera, Scarabaeidae; Huber, Bont, et al., 2016), a highly polyphagous root feeder (Hauss & Schütte, 1976; Sukovata, Jaworski, Karolewski, & Kolk, 2015). Previous work found that the interaction between T. officinale and M. melolontha is modulated by the presence of sympatric plant species (Huang, Zwimpfer, Hervé, Bont, & Erb, 2018). Strong effects were for instance observed for Centaurea stoebe, a native European herb that is invasive in the United States. The M. melolontha larvae grew significantly better on T. officinale plants in the presence of C. stoebe, an effect that was found to be mediated through changes in T. officinale susceptibility rather than direct effects of C. stoebe on the herbivore (Huang et al., 2018). In a companion paper, we describe that C. stoebe constitutively produces and releases significant amounts of sesquiterpenes into the rhizosphere (Gfeller et al., 2019). Furthermore, we show that C. stoebe root VOCs have neutral to positive effects on the germination and growth of different neighbouring species (Gfeller et al., 2019). Based on these results, we hypothesized that C. stoebe root VOCs may play a role in increasing T. officinale susceptibility to M. melolontha. We tested this hypothesis by exposing T. officinale plants to root VOCs from C. stoebe and a major C. stoebe sesquiterpene and measuring changes in root primary and secondary metabolites and M. melolontha growth. This work provides evidence that root VOCs can influence plant–herbivore interactions on neighbouring plants.
2. METHODS AND MATERIALS
2.1. Study system
The study system consisted T. officinale (Genotype A34) as a receiver plant, C. stoebe as an emitter plant and M. melolontha as an herbivore of T. officinale. The T. officinale seeds were obtained from greenhouse‐grown A34 plants. The C. stoebe L. (diploid) seeds were obtained from a commercial vendor (UFA‐SAMEN, Winterthur, Switzerland). The M. melolontha larvae were collected from an apple tree yard in Sion, Switzerland (46.21°N, 7.38°E). The larvae were reared on carrot slices under controlled condition (12°C, 60% humidity and constant darkness) for several weeks until the start of the experiments.
2.2. Impact of C. stoebe root VOCs on the interaction between T. officinale and M. melolontha
To examine whether root VOCs emitted by C. stoebe affect the interaction between T. officinale and M. melolontha, the larvae were restricted to feed on T. officinale in the vicinity of C. stoebe, another T. officinale plant or soil only (combinations = 3, n = 16 per combination). Furthermore, to test how C. stoebe influences T. officinale growth and chemistry through root VOCs, T. officinale plants were grown in the vicinity of C. stoebe, another T. officinale plant or soil only in the absence of M. melolontha (combinations = 3, n = 8 per combination). Seeds of T. officinale and C. stoebe were germinated in the greenhouse at 50–70% relative humidity, 16/8‐hr light/dark cycle, and 24°C at day and 18°C at night. Ten days later, two seedlings of each species were transplanted into a mesh cage (12 × 9 × 10 cm, length × width × height) filled with a mixture of 1/3 landerde (Ricoter, Switzerland) and 2/3 seedling substrate (Klasmann‐Deilmann, Switzerland). The mesh cage was made of geotex fleece (Windhager, Austria). Then, two mesh cages were put into a 2‐L rectangular pot (18 × 12 × 10 cm, length × width × height). To reduce the interaction between focal and neighbouring plants through root exudates, the mesh cages in each pot were separated by two plastic angles (0.8‐cm width), and the pot was cut to produce a gap (12 × 0.5 cm, length × width) in the centre of the bottom paralleling to the longest side of mesh cage. Finally, the gap in the top between two mesh cages was covered by a plastic sheet. A schematic drawing of the setup is shown in Figure 2a. The setup is identical to the one used in the companion paper (Gfeller et al., 2019). Seven weeks after transplantation, a preweighted M. melolontha larva was added into the mesh cage with the focal plants (T. officinale). The larvae had been starved for 3 days prior to the experiment. After 18 days of infestation, the larvae were removed and reweighted. Then, roots of focal plants were harvested, weighted, flash‐frozen in liquid nitrogen, and stored in −80°C for further chemical analyses, including soluble protein and sugars as well as the defensive metabolite sesquiterpene lactone taraxinic acid β‐D glucopyranosyl ester (TA‐G). Soluble protein was estimated using the Bradford method (Bradford, 1976). Soluble sugars including glucose, fructose and sucrose were measured as described by Velterop and Vos (2001) and Machado et al. (2013). TA‐G was analysed as described by Huber et al. (2015) and Bont et al. (2017). During the experiment, pots were watered daily. Care was taken not to overwater the plants to avoid leachate to cross the air gap between the inner mesh cages. The plant pairs were arranged randomly on a greenhouse table, with distances between pairs equal to distances within pairs. The positions of the pots on the table were rearranged weekly. These two measures resulted in randomized above ground pairings between the two plant species, thus allowing us to exclude systematic effects of above ground interactions on root physiology and resistance.
Figure 2.
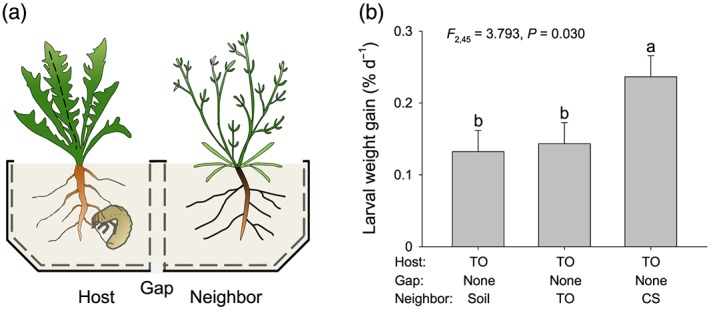
Root volatile organic compounds emitted by Centaurea stoebe increase Melolontha melolontha performance on neighbouring plants. (a) Experimental setup: Individual M. melolontha larvae were allowed to feed on Taraxacum officinale plants growing in the vicinity of empty soil compartments (soil), T. officinale (TO), or C. stoebe (CS) for 18 days. (b) Larval performance: Average larval weight gain was calculated as percentage increase in larval weight per day and is shown as mean ± 1 SE (n = 16). Differences between treatments were determined by one‐way ANOVAs followed by post hoc multiple comparisons (different letters indicate P < 0.05, least square mean) [Colour figure can be viewed at http://wileyonlinelibrary.com]
2.3. Analysis of root VOC profiles in the gap
To characterize the VOCs that accumulate in the gap when T. officinale is exposed to C. stoebe, another T. officinale or soil only (combinations = 3, n = 8 per combination), we collected and analysed VOCs using solid phase microextraction (SPME) and gas chromatography mass spectrometry (GC‐MS). A schematic drawing of the setup is shown in Figure 1a. After 7 weeks of transplantation, VOCs were collected from two randomly selected pots of each combination for one biological replicate (n = 4 per combination). An SPME fibre (coated with 100‐μm polydimethylsiloxane; Supelco, Bellefonte, PA, USA) was inserted into the gap of a pot and exposed to VOCs for 60 min at room temperature and then transferred to another pot for 60 min for collection. The incubated fibre was immediately analysed by GC‐MS using an Agilent 7820A GC interfaced with an Agilent 5977E MSD (source 230°C, quadrupole 150°C, ionization potential 70 eV, scan range 30–550, Palo Alto, CA, USA). Briefly, the fibre was inserted into the injector port at 250°C and desorbed for 2 min. VOCs were chromatographed on a capillary GC‐MS column (HP5‐MS, 30 m, 250 μm ID, 2.5 μm film, Agilent Technologies, Palo Alto, CA, USA) with He as carrier gas at a flow rate of 1 mL min−1. The GC temperature program was 60°C for 1 min, increased to 250 at 5°C min−1 and followed by 4 min at 250°C. The chromatograms were processed using default settings for spectral alignment and peak picking of PROGENESIS QI (Nonlinear Dynamics, Newcastle, UK). Features were assigned to individual compounds by retention time and peak shape matching and all VOCs were tentatively identified by the use of the NIST search 2.2 Mass Spectral Library (Gaithersburg, MD, USA) as well as retention time and spectral comparison with pure compounds as described (Gfeller et al., 2019). During the experiment, the pots were watered every day and rearranged every week. Plants were not infested by M. melolontha.
Figure 1.
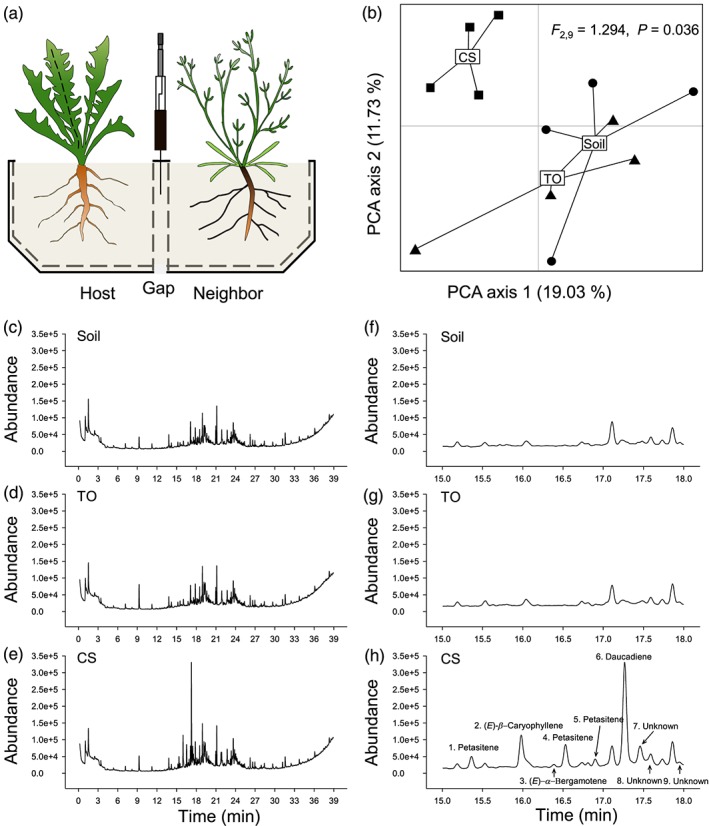
Sesquiterpene VOCs from Centaurea stoebe diffuse through the rhizosphere. (a) Experimental setup: Taraxacum officinale plants were grown in the vicinity of empty soil compartments (soil), T. officinale plants (TO), or C. stoebe plants (CS), and volatiles were collected in the gap between the plants. (b) The results of a principal component analysis of the volatile organic compound profiles in the gap are shown: The first two axes explained 19.03% and 11.73% of the total variation, respectively. Differences between treatments were visualized by principal component analysis (PCA). Data points represent biological replicates (n = 4). Circles, http://www.baidu.com/link?url=VsyTNqpQEzvCHtnzvlozV5VEDn_x09pEoTLtC-8ztmOJgBf7gzPQ_0GupRIKHUCFqVrM43nFIeQPfrZuok2O8l9-ek_vrQyHdPxTvg5DDpl9kUaCvrmAnUnhdnKGSFnfs, and squares indicate neighbour identities. Typical total‐ion count gas chromatography mass spectrometry chromatograms of volatiles collected from gap between focal and neighbouring plants from 0 to 39 min (c–e) and from 15 to 18 mins (f–h) are shown
2.4. Contribution of (E)‐β‐caryophyllene to plant–plant interactions
(E)‐β‐caryophyllene is one of the major sesquiterpenes released by C. stoebe roots and is produced by the root‐expressed terpene synthase CsTPS4 (Gfeller et al., 2019). To test whether (E)‐β‐caryophyllene is sufficient to account for the increased growth of M. melolontha on T. officinale plants, we determined concentration of (E)‐β‐caryophyllene in the air gap between the rhizosphere of C. stoebe and T. officinale (see above) and then used corresponding synthetic doses to investigate its impact on the interaction between T. officinale and M. melolontha. A schematic drawing of the setup is shown in Figure 4a.
Figure 4.
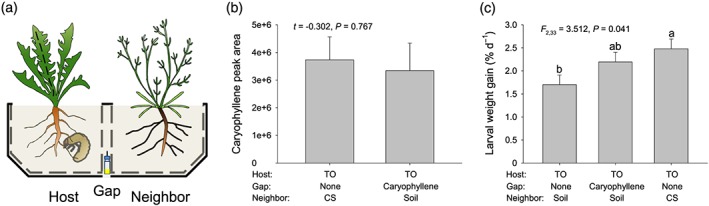
(E)‐β‐caryophyllene contributes to increased Melolontha melolontha growth on neighboring plants. (a) Experimental setup: Taraxacum officinale plants were growing in the vicinity of empty soil compartment (soil) or Centaurea stoebe (CS) and supplemented with or without synthetic (E)‐β‐caryophyllene in the gap. Physiological concentration of (E)‐β‐caryophyllene in gap (b): Control and (E)‐β‐caryophyllene dispensers were put in the gap for 2 days before measurements. Values were mean ± 1 SE (n = 8). Differences between treatments were determined by independent sample t tests. Impact of (E)‐β‐caryophyllene on M. melolontha larval growth (c): The M. melolontha larva was allowed to feed on T. officinale for 18 days. Values were mean ± 1 SE (n = 12). Differences between treatments were determined by one‐way ANOVA followed by post hoc multiple comparisons (different letters indicate P < 0.05, least square mean) [Colour figure can be viewed at http://wileyonlinelibrary.com]
To check whether we can mimick the (E)‐β‐caryophyllene release of C. stoebe with a dispenser containing synthetic (E)‐β‐caryophyllene, we measured (E)‐β‐caryophyllene in the air gap of T. officinale plants growing with C. stoebe or T. officinale plants growing without C. stoebe but with an (E)‐β‐caryophyllene dispenser in the air gap (n = 16). Both plant species were 7 weeks old. Dispensers were constructed from 1.5‐ml glass vials (VWR) that were pierced by a 1‐ul micro‐pipette (Drummond) and sealed with parafilm (Bemis). Dispensers were filled with with 100‐ul (E)‐β‐caryophyllene (˃98.5%, GC, Sigma‐Aldrich). This device allowed for constant release rates of (E)‐β‐caryophyllene. Two days after the dispensers were added, (E)‐β‐caryophyllene concentrations were determined by SPME‐GC‐MS as described above, resulting in eight biological replicates (two pooled setups per replicate).
To test the effect of (E)‐β‐caryophyllene on the interaction between T. officinale and M. melolontha, we conducted an experiment within which T. officinale plants were exposed to (a) control dispensers without neighbouring plant, (b) (E)‐β‐caryophyllene dispensers without neighboring plant, and (c) control dispensers with C. stoebe as a neighbouring plant (n = 12 per combination). The experimental setup was as described above. Seven weeks after the transplantation of C. stoebe and the addition of the dispensers, one preweighted and starved M. melolontha larva was added to the mesh cage in which the T. officinale plants were growing. After 18 days, all larvae were recovered from mesh cages and reweighted. During the experiment, the dispensers were replaced every 10 days and pots were rearranged every week.
2.5. Data analysis
All data analyses were performed with the statistical analysis software R 3.2.0 (R Foundation for Statistical Computing, Vienna, Austria) using “CAR,” “LME4,” “LSMEANS,” “VEGAN,” and “RVAIDEMEMOIRE” packages (Bates, Mächler, Bolker, & Walker, 2015; Fox & Weisberg, 2011; Hervé, 2016; Lenth, 2016; Oksanen et al., 2016). Larval weight gain and plant variables were analysed using one‐ or two‐way analyses of variance (ANOVAs using Type II Sums of Squares). ANOVA assumptions were verified by inspecting residuals and variance. Multiple comparisons were carried out using least square mean post‐hoc tests (LSM) for significant terms. P values were corrected using the false discovery rate method (Benjamini & Hochberg, 1995). To examine the linear associations between larval weight gain and root parameters, Pearson's product–moment correlations were carried out. To examine the overall differences in VOC profiles among different combinations, the relative abundance of the detected features was subjected to principal component analysis (PCA). Monte Carlo tests with 999 permutations were then used to test for significant differences between combinations.
3. RESULTS
3.1. Neighbour identity determines VOC profiles in the rhizosphere
PCA analysis revealed that VOC profiles in the air gap between the T. officinale rhizosphere and the rhizospheres of the neighbouring treatments differed significantly (F 2,9 = 1.294, P = 0.036, Figure 1b). VOC profiles of T.officinale plants exposed to bare soil or T. officinale plants were indistinguishable (P = 0.516, Figure 1b). By contrast, profiles were strongly altered by the presence of C. stoebe (P = 0.040, Figure 1b). VOC profiles in the air gap between T. officinale and C. stoebe were dominated by sesquiterpenes that are released by C. stoebe roots (Gfeller et al., 2019), including petasitenes, (E)‐β‐caryophyllene and daucadiene (peak area, P < 0.05, Figure 1c–h).
3.2. Root VOCs of C. stoebe increase M. melolontha growth on T. officinale
The growth of M. melolontha was similar on T. officinale plants that received below ground VOCs from bare soil or T. officinale neighbours (P = 0.791, Figure 2b). By contrast, M. melolontha weight gain was significantly higher on T. officinale plants that were exposed to root VOCs of C. stoebe (P = 0.045, Figure 2b). Thus, C. stoebe root VOCs increase M. melolontha growth on T. officinale.
3.3. Root VOCs of C. stoebe change primary metabolites in T. offinicale roots
The T. officinale root biomass was significantly affected by the different VOC exposure treatments (F 2,66 = 5.571, P = 0.006) but not by M. melolontha attack (F 1,66 = 1.761, P = 0.189) or the interaction (F 2,66 = 0.253, P = 0.777). Root biomass was higher in C. stoebe exposed plants compared to plants exposed to T. officinale and bare soil (Figure 3a). Root VOC exposure also influenced the concentration of root primary and secondary metabolites (Figure 3b–f). Total root protein concentrations were significantly affected by the VOC source (F 2,66 = 50.383, P < 0.001), M. melolontha attack (F 1,66 = 16.351, P < 0.001) and their interaction (F 2,66 = 12.506, P < 0.001). Root protein was the highest in C. stoebe exposed plants and lowest in plants exposed to bare soil when M. melolontha was present (Figure 3b). Root glucose levels were significantly affected by the VOC source (F 2,66 = 6.841, P = 0.002) but not by M. melolontha attack (F 1,66 = 0.023, P = 0.880) or their interaction (F 2,66 = 3.118, P = 0.051). Root glucose levels were higher in C. stoebe and T. officinale exposed plants compared with plants exposed to bare soil (Figure 3c). Root fructose and sucrose were significantly affected by neighbour identify (fructose: F 2,66 = 3.810, P = 0.027; sucrose: F 2,66 = 4.595, P = 0.014) and M. melolontha attack (fructose: F 1,66 = 10.346, P = 0.002; sucrose: F 1,66 = 5.659, P = 0.020) but not by their interaction (fructose: F 2,66 = 2.661, P = 0.077; sucrose: F 2,66 = 0.699, P = 0.501). Both root fructose and sucrose levels were higher in plants exposed to T. officinale, whereas root fructose levels were lower in plants exposed to bare soil and root sucrose levels were lower in plants exposed to C. stoebe (Figure 3d–e). Root fructose levels were higher when M. melolontha was absent, whereas root sucrose levels were higher when M. melolontha was present (Figure 3d–e). The secondary metabolite TA‐G was significantly decreased when T. officinale was attacked by M. melolontha larvae (F 1,66 = 4.339, P = 0.041) but was not affected by the VOC source (F 2,66 = 2.741, P = 0.072) or their interaction (F 2,66 = 0.157, P = 0.855, Figure 3f). Across treatments, M. melolontha larval weight gain was positively correlated with T. officinale root biomass (P = 0.007, R 2 = 0.186, Figure 3g) and soluble protein (P = 0.001, R 2 = 0.272, Figure 3h) but not significantly correlated with soluble sugars (glucose, P = 0.255, R 2 = 0.035; fructose, P = 0.507, R 2 = 0.010; sucrose, P = 0.233, R 2 = 0.053; Figure 3i–k) or TA‐G (P = 0.255, R 2 = 0.034, Figure 3l).
Figure 3.
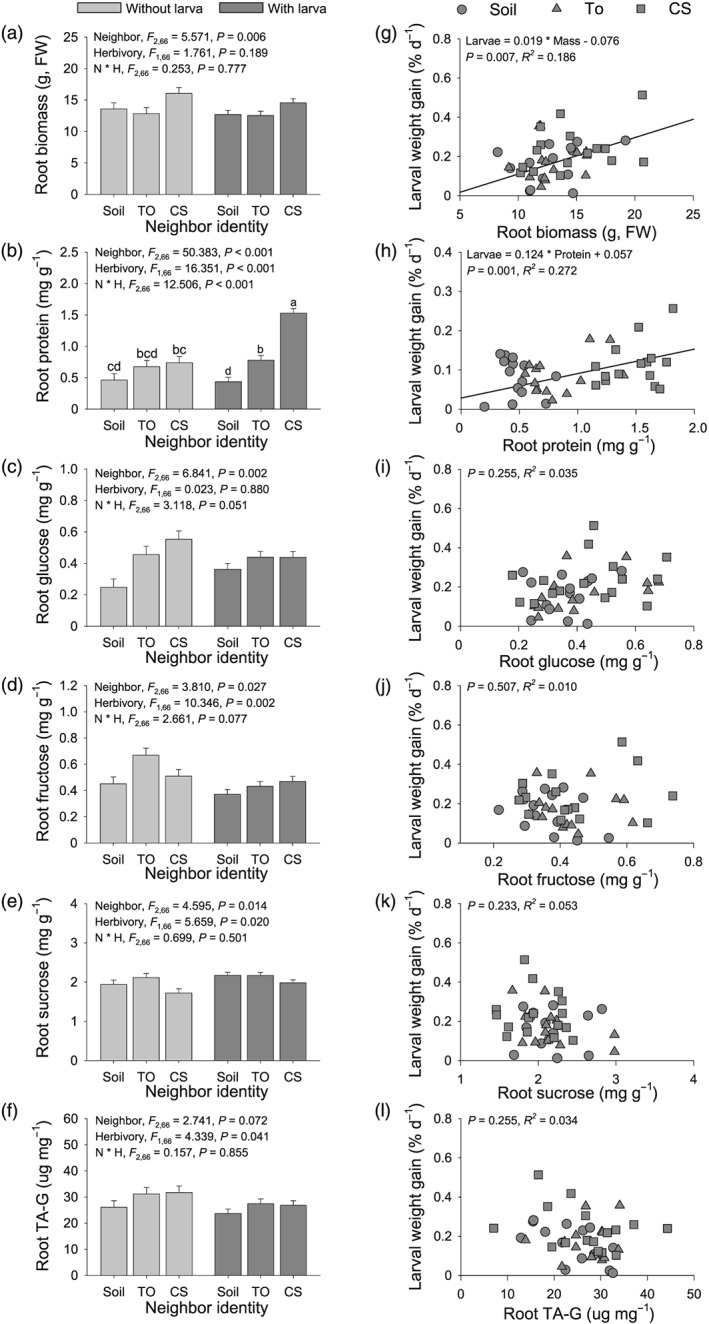
Root volatile organic compounds emitted by neighbouring plant influence growth and chemistry of Taraxacum officinale. (a) Root biomass, (b) soluble protein, (c) glucose, (d) fructose, (e) sucrose, and (f) taraxinic acid β‐D glucopyranosyl ester (TA‐G) of T. officinale growing in the vicinity of empty soil compartment (soil), T. officinale (TO), or Centaurea stoebe (CS) are shown on the left. The T. officinale plants were not attacked (light grey bars, n = 8) or attacked by Melolontha melolontha larvae (dark grey bars, n = 16). Values are means ± 1 SE. Differences between treatments were determined by two‐way ANOVAs followed by post hoc multiple comparisons (different letters indicate P < 0.05, least square mean). The relationships between larval weight gain and (g) root biomass, (h) soluble protein, (i) glucose, (j) fructose, (k) sucrose, and (l) TA‐G of T. officinale are shown on the right. Circles, http://www.baidu.com/link?url=VsyTNqpQEzvCHtnzvlozV5VEDn_x09pEoTLtC-8ztmOJgBf7gzPQ_0GupRIKHUCFqVrM43nFIeQPfrZuok2O8l9-ek_vrQyHdPxTvg5DDpl9kUaCvrmAnUnhdnKGSFnfs, and squares indicate T. officinale growing in the vicinity of soil, TO, or CS, respectively. Pearson coefficients, and R 2 values are shown in the top of the figures. Regression lines and equations are shown for significant correlations
3.4. Synthetic (E)‐β‐caryophyllene partially mimics C. stoebe root VOC effects
The amount of (E)‐β‐caryophyllene that accumulated in the air gap supplied with a dispenser was similar to the emission of (E)‐β‐caryophyllene into the gap by C. stoebe (t = −0.302, P = 0.767, Figure 4b). Similar to the previous experiment, the presence of C. stoebe increased M. melolontha weight gain compared with bare soil (Figure 4c). The M. melolontha growth in the presence of (E)‐β‐caryophyllene dispensers was intermediate and not statistically different from the control treatment or the C. stoebe treatment (Figure 4c). Thus, (E)‐β‐caryophyllene partially mimics C. stoebe root VOC effects on M. melolontha growth on neighbouring plants.
4. DISCUSSION
Associational effects triggered by plant VOCs play important roles in determining plant–herbivore interactions in the field (Barbosa et al., 2009; Underwood, 2014). However, to date, most studies focused on above ground interactions through airborne signals and most studies document that leaf VOCs trigger associational resistance in neighbours (Arimura et al., 2000; Engelberth et al., 2004; Erb et al., 2015; Frost et al., 2008; Pearse et al., 2013; Sugimoto et al., 2014). Our results show that root VOCs modulate plant–herbivore interactions and that VOCs may lead to associational susceptibility.
In an earlier study, we found that the presence of C. stoebe enhanced the performance of M. melolontha larvae feeding on T. officinale roots (Huang et al., 2018). In general, physical (e.g., light and contact), chemical (e.g., volatile and exudates), and biological (e.g., arbuscular mycorrhizal fungi) factors may trigger neighbourhood effects and affect plant growth and defence (Babikova et al., 2013; Crepy & Casal, 2015; Erb et al., 2015; Hu, Robert, et al., 2018; Kong et al., 2018; Semchenko, Saar, & Lepik, 2014; Yang, Callaway, & Atwater, 2015). As C. stoebe constitutively releases large amounts of sesquiterpenes into the rhizosphere (Gfeller et al., 2019), we hypothesized that root VOCs may be responsible for the plant‐mediated changes in M. melolontha growth. Using an experimental setup that effectively randomizes above ground cues and eliminates root contact and the exchange of soluble exudates, we found that C. stoebe root volatiles diffuse through the rhizosphere and are sufficient to increase the growth of M. melolontha on neighbouring T. officinale. Thus, this study provides experimental evidence that root VOCs play an important role in below ground associational effects impacting plant–herbivore interactions. Future experiments could for instance address the interactions between VOCs and soluble exudates in below ground associational effects and determine distance‐activity relationships of root VOCs.
Plant VOCs can influence herbivore performance directly or indirectly by changing the chemistry of receiver plants (Engelberth et al., 2004; Erb et al., 2015; Huang et al., 2018; Sugimoto et al., 2014; Veyrat, Robert, Turlings, & Erb, 2016; Ye et al., 2018). In our earlier work, we excluded the possibility that M. melolontha is directly affected by C. stoebe root VOCs or exudates, suggesting that C. stoebe increases M. melolontha growth through plant‐mediated effects. In line with this hypothesis, we demonstrate here that growth and primary metabolism of T. officinale roots changes upon exposure to root VOCs of C. stoebe. Some of these effects are even stronger when the plants are attacked by M. melolontha, suggesting an interaction between root VOC exposure and herbivory. For instance, exposure to C. stoebe root VOCs increases root protein content and root growth of T. officinale plants. Both parameters are positively correlated with larval performance, indicating that M. melolontha growth may be stimulated by enhanced root growth and nutrient levels. Previous studies demonstrated that secondary metabolites such as TA‐G protect T. officinale against M. melolontha (Bont et al., 2017; Huber, Bont, et al., 2016; Huber, Epping, et al., 2016). We found on clear effects of C. stoebe VOCs on root TA‐G concentrations, implying that C. stoebe VOCs do not act by suppressing this plant defence.
The identification of bioactive VOCs from plant‐derived blends remains an important bottleneck in chemical ecology. We show that C. stoebe releases a complex blend of sesquiterpenes as well as other minor unidentified VOCs from its roots (Gfeller et al., 2019), all of which may be associated with the observed effects on M. melolontha growth. Here, we tested whether (E)‐β‐caryophyllene, one of the major sesquiterpenes emitted by C. stoebe, is sufficient to increase the growth of M. melolontha on T. officinale in comparison with the full VOC blend of C. stoebe. (E)‐β‐caryophyllene is a widespread sesquiterpene in nature that can influence the physiology and behaviour of pathogen, nematodes, and insects (Fantaye, Köpke, Gershenzon, & Degenhardt, 2015; Huang et al., 2012; Rasmann et al., 2005; Robert et al., 2013) and may act as an antioxidant in plants (Palmer‐Young, Veit, Gershenzon, & Schuman, 2015). We demonstrate that (E)‐β‐caryophyllene exposure leads to M. melolontha growth that is intermediate between non‐exposed and C. stoebe exposed T. officinale plants, suggesting that it can partially account for the VOC effects of C. stoebe. We propose that other sesquiterpenes emitted by C. stoebe such as daucadiene and petasitene may also contribute to enhanced M. melolontha growth. More work is needed to test this hypothesis. The identification of TPSs that are likely responsible for sesquiterpene production in C. stoebe (Gfeller et al., 2019) represents the first step towards the manipulation and functional assessment of C. stoebe root VOCs in vivo (Vaughan et al., 2013).
VOCs of neighbouring plants are well known to increase defences and resistance of neighbouring plants (Arimura et al., 2000; Erb et al., 2015; Sugimoto et al., 2014), and only few documented examples exist where VOCs decrease the resistance of neighbouring plants (Li & Blande, 2015). From the perspective of the sender, inducing susceptibility to herbivores in neighbouring plants may be an advantage, as it may reduce their competitiveness. VOC‐induced susceptibility may thus be a form of plant offense. However, several caveats need to be considered. First, many herbivores are mobile, and increasing herbivore growth on neighbouring plants may lead to accelerated migration to the sender plant. Second, herbivore growth, as measured here, is not synonymous with plant damage and may be the result of an increase in performance of the receiver plant, in which case their competitiveness would not be reduced, and the benefit for the emitter would be less evident (Erb, 2018b; Veyrat et al., 2016). Third, the benefits of inducing susceptibility in neighbouring plants may be offset in the absence of herbivores. Indeed, we show that C. stoebe VOCs can increase germination and growth of heterospecific neighbouring plants in the absence of herbivores (Gfeller et al., 2019). Therefore, more research is needed to understand the evolutionary and ecological context of the present findings.
In conclusion, the present study shows that root VOCs can influence plant–herbivore interactions on neighbouring plants through plant‐mediated effects. Thus, associational effects mediated by below ground VOCs need to be included into models on plant interaction ecology.
AUTHOR CONTRIBUTIONS
W.H. and M.E. designed the experiments. W.H. carried out greenhouse research. W.H., V.G., and M.E. performed chemical analyses, analysed data, and wrote the manuscript.
ACKNOWLEDGEMENTS
We are grateful to Noelle Schenk for insect rearing and the gardeners of the IPS for plant cultivation. This study was supported by The National Key Research and Development Program of China (2017YFC1200100 to WH), the Swiss National Science Foundation (grants nos' 153517 and 157884 to M.E.), the European Commission (MC‐CIG no. 629134 to M.E., MC‐IEF no. 704334 to W.H.), and the National Natural Science Foundation of China (31470447 and 31822007 to W.H.). The authors declare that they have no conflict of interest.
Huang W, Gfeller V, Erb M. Root volatiles in plant–plant interactions II: Root volatiles alter root chemistry and plant–herbivore interactions of neighbouring plants. Plant Cell Environ. 2019;42:1964–1973. 10.1111/pce.13534
Contributor Information
Wei Huang, Email: huangwei0519@wbgcas.cn.
Matthias Erb, Email: matthias.erb@ips.unibe.ch.
Data Accessibility
Raw data associated with this study can be downloaded from Dryad (to be inserted at a later stage).
REFERENCES
- Arimura, G.‐I. , Ozawa, R. , Shimoda, T. , Nishioka, T. , Boland, W. , & Takabayashi, J. (2000). Herbivory‐induced volatiles elicit defence genes in lima bean leaves. Nature, 406, 512–515. [DOI] [PubMed] [Google Scholar]
- Babikova, Z. , Gilbert, L. , Bruce, T. J. A. , Birkett, M. , Caulfield, J. C. , Woodcock, C. , … Johnson, D. (2013). Underground signals carried through common mycelial networks warn neighbouring plants of aphid attack. Ecology Letters, 16, 835–843. 10.1111/ele.12115 [DOI] [PubMed] [Google Scholar]
- Barbosa, P. , Hines, J. , Kaplan, I. , Martinson, H. , Szczepaniec, A. , & Szendrei, Z. (2009). Associational resistance and associational susceptibility: Having right or wrong neighbors. Annual Review of Ecology, Evolution, and Systematics, 40, 1–20. 10.1146/annurev.ecolsys.110308.120242 [DOI] [Google Scholar]
- Bates, D. , Mächler, M. , Bolker, B. , & Walker, S. (2015). Fitting linear mixed‐effects models using lme4. Journal of Statistical Software, 67, 1–48. [Google Scholar]
- Benjamini, Y. , & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B‐Statistical Methodology, 57, 289–300. [Google Scholar]
- Bont, Z. , Arce, C. , Huber, M. , Huang, W. , Mestrot, A. , Sturrock, C. J. , & Erb, M. (2017). A herbivore tag‐and‐trace system reveals contact‐ and density‐dependent repellence of a root toxin. Journal of Chemical Ecology, 43, 295–306. 10.1007/s10886-017-0830-3 [DOI] [PubMed] [Google Scholar]
- Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein‐dye binding. Analytical Biochemistry, 72, 248–254. 10.1016/0003-2697(76)90527-3 [DOI] [PubMed] [Google Scholar]
- Crepy, M. A. , & Casal, J. J. (2015). Photoreceptor‐mediated kin recognition in plants. New Phytologist, 205, 329–338. 10.1111/nph.13040 [DOI] [PubMed] [Google Scholar]
- Delory, B. M. , Delaplace, P. , Fauconnier, M.‐L. , & du Jardin, P. (2016). Root‐emitted volatile organic compounds: Can they mediate belowground plant‐plant interactions? Plant and Soil, 402, 1–26. 10.1007/s11104-016-2823-3 [DOI] [Google Scholar]
- Dicke, M. , & Dijkman, H. (2001). Within‐plant circulation of systemic elicitor of induced defence and release from roots of elicitor that affects neighbouring plants. Biochemical Systematics and Ecology, 29, 1075–1087. 10.1016/S0305-1978(01)00051-5 [DOI] [Google Scholar]
- Engelberth, J. , Alborn, H. T. , Schmelz, E. A. , & Tumlinson, J. H. (2004). Airborne signals prime plants against insect herbivore attack. Proceedings of the National Academy of Sciences of the United States of America, 101, 1781–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ens, E. J. , Bremner, J. B. , French, K. , & Korth, J. (2009). Identification of volatile compounds released by roots of an invasive plant, bitou bush (Chrysanthemoides monilifera spp. rotundata), and their inhibition of native seedling growth. Biological Invasions, 11, 275–287. 10.1007/s10530-008-9232-3 [DOI] [Google Scholar]
- Erb, M. (2018a). Volatiles as inducers and suppressors of plant defense and immunity‐origins, specificity, perception and signaling. Current Opinion in Plant Biology, 44, 117–121. 10.1016/j.pbi.2018.03.008 [DOI] [PubMed] [Google Scholar]
- Erb, M. (2018b). Plant defenses against herbivory: Closing the fitness gap. Trends in Plant Science, 23, 187–194. 10.1016/j.tplants.2017.11.005 [DOI] [PubMed] [Google Scholar]
- Erb, M. , Veyrat, N. , Robert, C. A. M. , Xu, H. , Frey, M. , Ton, J. , & Turlings, T. C. J. (2015). Indole is an essential herbivore‐induced volatile priming signal in maize. Nature Communications, 6, 6273 10.1038/ncomms7273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantaye, C. A. , Köpke, D. , Gershenzon, J. , & Degenhardt, J. (2015). Restoring (E)‐β‐caryophyllene production in a non‐producing maize line compromises its resistance against the fungus Colletotrichum graminicola . Journal of Chemical Ecology, 41, 213–223. 10.1007/s10886-015-0556-z [DOI] [PubMed] [Google Scholar]
- Fox, J. , & Weisberg, S. (2011). An R companion to applied regression (Second ed.). Thousand Oaks CA, USA: Sage. [Google Scholar]
- Frost, C. J. , Mescher, M. C. , Carlson, J. E. , & De Moraes, C. M. (2008). Plant defense priming against herbivores: Getting ready for a different battle. Plant Physiology, 146, 818–824. 10.1104/pp.107.113027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gfeller, V. , Huber, M. , Förster, C. , Huang, W. , Köllner, T. G. , & Erb, M. (2019). Root volatiles in plant‐plant interactions I: High root sesquiterpene release is associated with increased germination and growth of plant neighbours. Plant, Cell & Environment, 1–14. 10.1111/pce.13532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauss, R. , & Schütte, F. (1976). Experiments on polyphagous habits of white grubs Melolontha melolontha on plants of grassland. Anz Schädk Pfl Umw, 49, 129–132. [Google Scholar]
- Heil, M. , & Karban, R. (2010). Explaining evolution of plant communication by airborne signals. Trends in Ecology and Evolution, 25, 137–144. 10.1016/j.tree.2009.09.010 [DOI] [PubMed] [Google Scholar]
- Hervé, M. (2016) RVAideMemoire: Diverse basic statistical and graphical functions. R package version 0.9‐56, URL https://CRAN.R-project.org/package=RVAideMemoire.
- Hu, L. , Mateo, P. , Ye, M. , Zhang, X. , Berset, J. D. , Handrick, V. , … Erb, M. (2018). Plant iron acquisition strategy exploited by an insect herbivore. Science, 361, 694–697. 10.1126/science.aat4082 [DOI] [PubMed] [Google Scholar]
- Hu, L. , Robert, C. A. M. , Cadot, S. , Zhang, X. , Ye, M. , Li, B. , … Erb, M. (2018). Root exudate metabolites drive plant‐soil feedbacks on growth and defense by shaping the rhizosphere microbiota. Nature Communications, 9, 2738 10.1038/s41467-018-05122-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, M. , Sanchez‐Moreiras, A. M. , Abel, C. , Sohrabi, R. , Lee, S. , Gershenzon, J. , & Tholl, D. (2012). The major volatile organic compound emitted from Arabidopsis thaliana flowers, the sesquiterpene (E)‐β‐caryophyllene, is a defense against a bacterial pathogen. New Phytologist, 193, 997–1008. 10.1111/j.1469-8137.2011.04001.x [DOI] [PubMed] [Google Scholar]
- Huang, W. , Zwimpfer, E. , Hervé, M. R. , Bont, Z. , & Erb, M. (2018). Neighbourhood effects determine plant‐herbivore interactions below‐ground. Journal of Ecology, 106, 347–356. 10.1111/1365-2745.12805 [DOI] [Google Scholar]
- Huber, M. , Bont, Z. , Fricke, J. , Brillatz, T. , Aziz, Z. , Gershenzon, J. , & Erb, M. (2016). A below‐ground herbivore shapes root defensive chemistry in natural plant populations. Proceedings of the Royal Society B: Biological Sciences, 283, 20160285 10.1098/rspb.2016.0285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber, M. , Epping, J. , Schulze, G. C. , Fricke, J. , Aziz, Z. , Brillatz, T. , … Erb, M. (2016). A latex metabolite benefits plant fitness under root herbivore attack. PLoS Biology, 14, e1002332 10.1371/journal.pbio.1002332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber, M. , Triebwasser‐Freese, D. , Reichelt, M. , Heiling, S. , Paetz, C. , Chandran, J. N. , … Erb, M. (2015). Identification, quantification, spatiotemporal distribution and genetic variation of major latex secondary metabolites in the common dandelion (Taraxacum officinale agg.). Phytochemistry, 115, 89–98. 10.1016/j.phytochem.2015.01.003 [DOI] [PubMed] [Google Scholar]
- Jassbi, A. R. , Zamanizadehnajari, S. , & Baldwin, I. T. (2010). Phytotoxic volatiles in the roots and shoots of Artemisia tridentata as detected by headspace solid‐phase microextraction and gas chromatographic‐mass spectrometry analysis. Journal of Chemical Ecology, 36, 1398–1407. 10.1007/s10886-010-9885-0 [DOI] [PubMed] [Google Scholar]
- Karban, R. , Yang, L. H. , & Edwards, K. F. (2014). Volatile communication between plants that affects herbivory: A meta‐analysis. Ecology Letters, 17, 44–52. 10.1111/ele.12205 [DOI] [PubMed] [Google Scholar]
- Kong, C. H. , Zhang, S. Z. , Li, Y. H. , Xia, Z. C. , Yang, X. F. , Meiners, S. J. , & Wang, P. (2018). Plant neighbor detection and allelochemical response are driven by root‐secreted signaling chemicals. Nature Communications, 9, 3867 10.1038/s41467-018-06429-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenth, R. V. (2016). Least‐squares means: The R package lsmeans. Journal of Statistical Software, 69, 1–33. [Google Scholar]
- Li, T. , & Blande, J. D. (2015). Associational susceptibility in broccoli: Mediated by plant volatiles, impeded by ozone. Global Change Biology, 21, 1993–2004. 10.1111/gcb.12835 [DOI] [PubMed] [Google Scholar]
- Machado, R. A. R. , Ferrieri, A. P. , Robert, C. A. M. , Glauser, G. , Kallenbach, M. , Baldwin, I. T. , & Erb, M. (2013). Leaf‐herbivore attack reduces carbon reserves and regrowth from the roots via jasmonate and auxin signaling. New Phytologist, 200, 1234–1246. 10.1111/nph.12438 [DOI] [PubMed] [Google Scholar]
- Oksanen, J. , Blanchet, F. G. , Kindt, R. , Legendre, P. , Minchin, P. R. , O'Hara, R. B. , . . . Wagner, H. (2016). Vegan: Community ecology package. R package version 2.4‐0, URL https://CRAN.R-project.org/package=vegan.
- Palmer‐Young, E. C. , Veit, D. , Gershenzon, J. , & Schuman, M. C. (2015). The sesquiterpenes(E)‐ß‐farnesene and (E)‐α‐bergamotene quench ozone but fail to protect the wild tobacco Nicotiana attenuata from ozone, UVB, and drought stresses. PLoS ONE, 10, e0127296 10.1371/journal.pone.0127296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschold, A. , Halitschke, R. , & Baldwin, I. T. (2006). Using ‘mute’ plants to translate volatile signals. The Plant Journal, 45, 275–291. 10.1111/j.1365-313X.2005.02623.x [DOI] [PubMed] [Google Scholar]
- Pearse, I. S. , Hughes, K. , Shiojiri, K. , Ishizaki, S. , & Karban, R. (2013). Interplant volatile signaling in willows: Revisiting the original talking trees. Oecologia, 172, 869–875. 10.1007/s00442-013-2610-2 [DOI] [PubMed] [Google Scholar]
- Pearse, I. S. , Porensky, L. M. , Yang, L. H. , Stanton, M. L. , Karban, R. , Bhattacharyya, L. , … Tanner, K. (2012). Complex consequences of herbivory and interplant cues in three annual plants. PLoS ONE, 7, e38105 10.1371/journal.pone.0038105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmann, S. , Kollner, T. G. , Degenhardt, J. , Hiltpold, I. , Toepfer, S. , Kuhlmann, U. , … Turlings, T. C. J. (2005). Recruitment of entomopathogenic nematodes by insect‐damaged maize roots. Nature, 434, 732–737. 10.1038/nature03451 [DOI] [PubMed] [Google Scholar]
- Robert, C. A. M. , Erb, M. , Duployer, M. , Zwahlen, C. , Doyen, G. R. , & Turlings, T. C. J. (2012). Herbivore‐induced plant volatiles mediate host selection by a root herbivore. New Phytologist, 194, 1061–1069. 10.1111/j.1469-8137.2012.04127.x [DOI] [PubMed] [Google Scholar]
- Robert, C. A. M. , Erb, M. , Hiltpold, I. , Hibbard, B. E. , Gaillard, M. D. P. , Bilat, J. , … Zwahlen, C. (2013). Genetically engineered maize plants reveal distinct costs and benefits of constitutive volatile emissions in the field. Plant Biotechnology Journal, 11, 628–639. 10.1111/pbi.12053 [DOI] [PubMed] [Google Scholar]
- Robert, C. A. M. , Veyrat, N. , Glauser, G. , Marti, G. , Doyen, G. R. , Villard, N. , … Erb, M. (2012). A specialist root herbivore exploits defensive metabolites to locate nutritious tissues. Ecology Letters, 15, 55–64. 10.1111/j.1461-0248.2011.01708.x [DOI] [PubMed] [Google Scholar]
- Semchenko, M. , Saar, S. , & Lepik, A. (2014). Plant root exudates mediate neighbour recognition and trigger complex behavioural changes. New Phytologist, 204, 631–637. 10.1111/nph.12930 [DOI] [PubMed] [Google Scholar]
- Sugimoto, K. , Matsui, K. , Iijima, Y. , Akakabe, Y. , Muramoto, S. , Ozawa, R. , … Takabayashi, J. (2014). Intake and transformation to a glycoside of (Z)‐3‐hexenol from infested neighbors reveals a mode of plant odor reception and defense. Proceedings of the National Academy of Sciences of the United States of America, 111, 7144–7149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukovata, L. , Jaworski, T. , Karolewski, P. , & Kolk, A. (2015). The performance of Melolontha grubs on the roots of various plant species. Turkish Journal of Agriculture and Forestry, 39, 107–116. 10.3906/tar-1405-60 [DOI] [Google Scholar]
- Underwood, N. (2014). A conceptual framework for associational effects: When do neighbors matter and how would we know? Quarterly Review of Biology, 89, 1–19. 10.1086/674991 [DOI] [PubMed] [Google Scholar]
- Vaughan, M. M. , Wang, Q. , Webster, F. X. , Kiemle, D. , Hong, Y. J. , Tantillo, D. J. , … Tholl, D. (2013). Formation of the unusual semivolatile diterpene rhizathalene by the Arabidopsis class I terpene synthase TPS08 in the root stele is involved in defense against belowground herbivory. The Plant Cell, 25, 1108–1125. 10.1105/tpc.112.100057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velterop, J. S. , & Vos, F. (2001). A rapid and inexpensive microplate assay for the enzymatic determination of glucose, fructose, sucrose, L‐malate and citrate in tomato (Lycopersicon esculentum) extracts and in orange juice. Phytochemical Analysis, 12, 299–304. 10.1002/pca.598 [DOI] [PubMed] [Google Scholar]
- Veyrat, N. , Robert, C. A. M. , Turlings, T. C. J. , & Erb, M. (2016). Herbivore intoxication as a potential primary function of an inducible volatile plant signal. Journal of Ecology, 104, 591–600. 10.1111/1365-2745.12526 [DOI] [Google Scholar]
- Yang, L. , Callaway, R. M. , & Atwater, D. Z. (2015). Root contact responses and the positive relationship between intraspecific diversity and ecosystem productivity. AoB PLANTS, 7, plv053–plv053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye, M. , Veyrat, N. , Xu, H. , Hu, L. , Turlings, T. C. J. , & Erb, M. (2018). An herbivore‐induced plant volatile reduces parasitoid attraction by changing the smell of caterpillars. Science Advances, 4, eaar4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data associated with this study can be downloaded from Dryad (to be inserted at a later stage).


