SUMMARY
African swine fever (ASF) is a notifiable infectious disease, caused by the ASF virus (ASFV), which is a DNA virus belonging to the family Asfarviridae, genus Asfivirus. This disease has gained importance in the last decade after its spread in several countries in Eastern and Central Europe, and more recently, in China. Despite the efforts made to eradicate it, ASF is still present on the Mediterranean island of Sardinia (Italy) and has been since 1978. ASF risk factors on the island have been analysed in previous studies; the role of free‐ranging pigs in virus persistence has been suggested, but has not been fully elucidated. The most recent eradication plan provides more stringent measures to combat free‐ranging pigs and any kind of illegality in the pig sector. From December 2017 to June 2018, a total of 29 depopulation actions were performed in 13 municipalities in central Sardinia, during which 2,281 free‐ranging pigs were culled and more than 50% of them were tested for ASFV and antibody presence (1,218 and 1,416, respectively). A total of 651 pigs were seropositive, with a mean seroprevalence of 53.4% (CI 95% = 50.6–56.3), and 38 were ASFV positive (virus prevalence = 2.6%; CI 95% = 2.1–3.0). To the best of our knowledge, the present study is the first to provide a complete evaluation of this millennial system of pig farming and ASFV prevalence in free‐ranging pigs. Furthermore, it has emphasised the necessity of combining the maintenance of an epidemiological surveillance program with continuous education of farmers and other people involved in pig husbandry, based on cultural and economic aspects.
Keywords: African swine fever, disease control, eradication plan, free‐ranging pigs, new intervention strategies, Sardinia
1. INTRODUCTION
African swine fever (ASF) is one of the most important and complex viral infectious diseases affecting swine species (Dixon et al., 2005) and is notifiable to the World Organisation for Animal Health (OIE). ASF has been endemic in Sardinia (Italy) for 40 years, probably due to the introduction of food wastes containing ASF contaminated meat from the Iberian Peninsula (Mannelli et al., 1997). The first scientific papers on ASF in Sardinia in the early 1980s (Contini, Cossu, Rutili, & Firinu, 1982; Wilkinson, 1984), described the most likely factors involved in disease spread. The presence of pigs kept at free‐range in some mountainous areas of inner Sardinia, uncontrolled pig movements, and feeding of contaminated meat were identified as the most likely causes of ASF persistence in Sardinia (Cappai, Rolesu, Coccollone, Laddomada, & Loi, 2018; Jurado et al., 2017; Mur et al., 2014). Swine husbandry has been practised in Sardinia since at least the sixth century BC (Albarella, Manconi, Rowley‐Conwy, & Vigne, 2006). It is still, at least to a large extent, oriented for individual family needs, while sheep farming, which also has a long history, has gradually become the first Sardinian economic source (RAS, 2004). The culture of breeding one or a few pigs is still a very common practice, in which the pig is fattened in a small backyard for several months before being slaughtered during a family festival, usually in early winter, or a few sows are retained to produce piglets that are slaughtered at the end of the feeding period. Furthermore, pigs can be kept in free‐ranging conditions. Wilkinson (1984) estimated that two‐thirds of the pigs in the affected area were kept under an extensive, traditional husbandry system in common land, while the other one‐third were confined to restricted areas near houses and bred solely for domestic consumption. In the early 2000s, it was estimated (Pozio et al., 2006) that although keeping pigs at a free‐range was forbidden by law because of the presence of ASF, thousands of pigs could still be found living in the wild in central Sardinia. In these territories, free‐ranging pigs consume natural food, such as acorns, chestnuts, and hazelnuts. Therefore, the farming cost of these pigs, that are only occasionally gathered to be fed or slaughtered, is near zero. The link between ASF persistence and the presence of free‐ranging pigs in particular territories has been extensively studied by various authors (Feliziani et al., 2010; Jurado et al., 2017; Mannelli et al., 1997, 1998; Mur et al., 2014). The role of free‐ranging pigs as the primary cause for the endemic persistence of ASF on the island has been suggested (Cappai et al., 2018). Conversely, several studies have suggested that wild boars are not permanent virus reservoirs in Sardinia (Laddomada et al., 1994). In Cappai et al. (2018) concluded that the presence of free‐ranging pigs caused a sevenfold increase in the risk of ASF outbreaks in each municipality. Finally, Jurado et al. (2018) in their review and expert opinion consensus found that one of the most important preventive measures is the containment of pigs, as this prevents contact with pigs from other farms, feral pigs or wild boars/their products. However, none of these studies provide detailed information on the actual occurrence of ASF in the free‐ranging pigs, as they escaped the veterinary authorities and were not subjected to ASF testing. Specific measures have been implemented in the ASF eradication plan 2015–2018 that aim to quickly eradicate ASF outbreaks when they occur in registered holdings, eliminate free‐ranging pigs, and incentivise good practices of swine breeding (PE‐ASF15‐18; Regional Decree Number 5/6, 6 February 2015). In this context, for the first time since the disease has been first detected in Sardinia, strong measures against illegal, free‐ranging pigs have been devised. The present study is focused on the description of these measures, organisation required for the depopulation of free‐ranging pigs, and ASF statuses of these pigs.
2. MATERIALS AND METHODS
In the framework of the PE‐ASF15‐18, the Sardinian government set up a special unit in charge of the overall coordination of ASF eradication activities (Unità di Progetto), which includes the Italian Chief Veterinary Officer, who issues the orders of depopulation actions against free‐ranging pigs. All these actions were coordinated on‐the‐spot by the veterinarian responsible for the ASF eradication at the Animal Health Services (AHS) of the Local Health Authority of Sardinia and by the head of the forest guards (Corpo Forestale e di Vigilianza Ambientale, CFVA) of Nuoro. A highly specialised task force (Gruppo di Intervento Veterinario, GIV) of veterinary and auxiliary personnel was also established, to provide support to the AHS in its eradication activities. The main tasks of the CFVA were to: (a) sight free‐ranging pigs, (b) record the number and geographical location of animals spotted, and thus prepare for the subsequent depopulation actions, and (c) guarantee the personal security of all staff during the culling operations with the police, mobilized by the local Prefecture. The AHS and CFVA were also supported by the regional body for forest maintenance (Forestas), which handled gathering free‐ranging pigs, properly disposing the carcasses, and the cleaning and disinfection procedures in the zones where culling occurred and in the official laboratory, Istituto Zooprofilattico Sperimentale della Sardegna (IZS), where the tests for the ASF virus and antibody detection were carried out. Up to 200 people had to be deployed in the field in a single day of depopulation actions. Pigs were culled after being stunned, in accordance with the methods and specific requirements EU legislation on animal welfare (Council Regulation N°1099/2009). Given the free‐ranging nature of the pigs, their gathering was highly cumbersome and chemical immobilization (tele‐narcosis) was often necessary (Figure 1). Around 50% of the culled animals were randomly chosen, from those older than 3 months, to be sampled (i.e., serum, blood, spleen, kidney, tonsils and lymph nodes) immediately after culling and tested for ASFV and antibody presence. Sampling was conducted in accordance with the ELISA test (INgezim PPA Compac – Ingenasa), immunoblotting test and real‐time PCR techniques (King et al., 2003), as described in the EU Diagnostic Manual for ASF (Commission Decision 2003/422/EC). Serum samples were considered positive when they scored positive in both the ELISA and immunoblotting tests (Ib). The culling actions were carried out in 13 municipalities, all located in the “wild boar infected zone” (Infected Zone, IZ), which covers approximately 40% of the Sardinian territory. In this zone, ASF had occurred in the wild boars in previous years and highly intensive disease surveillance/control measures are implemented, in accordance with the EU Directive 2002/60/EC and PE‐ASF15‐18. Culling was carried out, prioritizing the areas with the highest density of free‐ranging pigs, where the culling operations were less cumbersome.
Figure 1.

Picture of illegal free‐ranging pigs culled during eradication activities
3. RESULTS AND DISCUSSION
From December 2017 to June 2018, a total of 29 depopulation actions have been carried out, in 13 different Sardinian municipalities, on the 3,000–5,000 free‐ranging pigs estimated to be present in central Sardinia (areas of Nuoro and Ogliastra (Figure 2b)). A total of 2,281 free‐ranging pigs were culled (mean = 79, SD = 68, median = 59), with a minimum of 10 pigs culled in Loculi (12 May 2018) and a maximum of 268 pigs culled in the Orgosolo Municipality (8 January 2018). Figures 2a–b show the density of free‐ranging pigs, during 2013–2015 and 2016–2017, estimated using the sightings of forest guards. Table 1 presents the data on each of the conducted slaughtering actions. The first culling action was performed on 8 December 2017 and the last was on 19 June 2018. About one‐third of the actions were carried out in the Orgosolo municipality alone, with nine interventions. As shown in Table 2, of the 2,281 culled pigs, 1,218 (53.4%) were tested for ASF antibody presence and 1,416 (62.1%) for ASF virus presence. A total of 651 pigs were seropositive, with a mean of seroprevalence (SP) of 53.4% (CI 95% = 50.6–56.3), calculated as the number of seropositive animals (positive ELISA and Ib) over the total number of animals serologically tested. Seropositive pigs were found in 21 of the 29 culling actions, with negative results mostly found during the last culling period (May–June 2018). The highest seroprevalence was detected in Orgosolo (SP = 72.3%, CI 95% = 68.9–76.1), where 566 were serologically tested,of a total 1092 pigs culled, and 409 of them were found ASF positive. Of the 1461 pigs tested using real‐time PCR (King et al., 2003), 38 were ASFV positive. The mean virus prevalence (VP) was 2.6% (CI 95% = 2.1–3.0), calculated as the number of ASFV positive animals (positive PCRs) over the total number of animals virologically tested. The highest virus prevalence was found in Desulo (VP = 17.1%, CI 95% = 15.5–18.8), where a total of 211 animals were culled, 111 samples were tested by PCR, and 19 of them were ASFV positive.
Figure 2.
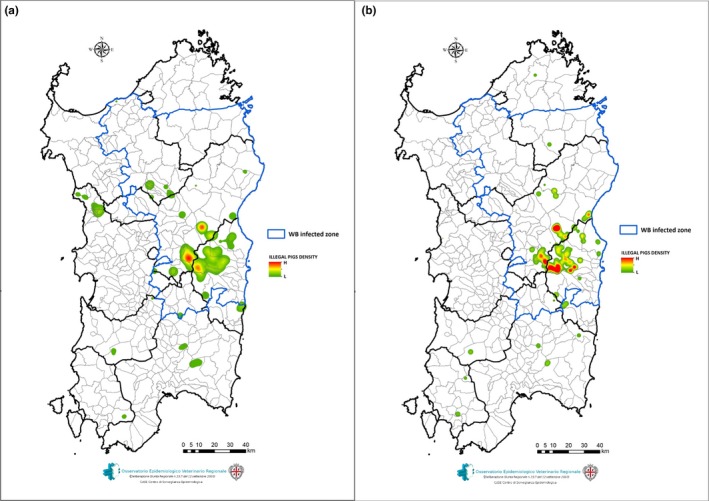
Map representing the density of free‐ranging pigs in Sardinia, during 2013–2015 (a) and 2016–2017 (b), obtained using the Kernel density method
Table 1.
Information on the culling actions, including date of slaughter, municipality number of culled pigs and number of culled pigs by municipality
| Municipality | Date | Culled pigs | Total culled pigs, by municipality |
|---|---|---|---|
| Aritzo | 27/04/2018 | 15 | 15 |
| Arzana | 11/12/2017 | 50 | 50 |
| Baunei | 24/01/2018 | 37 | 37 |
| Desulo | 08/12/2017 | 90 | 211 |
| Desulo | 21/12/2017 | 35 | |
| Desulo | 10/05/2018 | 27 | |
| Desulo | 19/06/2018 | 59 | |
| Irgoli | 12/05/2018 | 179 | 179 |
| Loculi | 12/05/2018 | 7 | 7 |
| Nuoro | 26/02/2018 | 139 | 196 |
| Nuoro | 05/05/2018 | 57 | |
| Orgosolo | 11/12/2017 | 70 | 1,092 |
| Orgosolo | 21/12/2017 | 200 | |
| Orgosolo | 03/01/2018 | 215 | |
| Orgosolo | 09/01/2018 | 268 | |
| Orgosolo | 02/02/2018 | 81 | |
| Orgosolo | 09/03/2018 | 72 | |
| Orgosolo | 23/03/2018 | 10 | |
| Orgosolo | 20/04/2018 | 57 | |
| Orgosolo | 13/05/2018 | 119 | |
| Orosei | 12/05/2018 | 19 | 19 |
| Ovodda | 10/05/2018 | 30 | 30 |
| Talana | 30/12/2017 | 60 | 60 |
| Urzulei | 24/01/2018 | 64 | 234 |
| Urzulei | 16/02/2018 | 170 | |
| Villagrande Strisaili | 18/12/2017 | 60 | 151 |
| Villagrande Strisaili | 30/12/2017 | 51 | |
| Villagrande Strisaili | 08/03/2018 | 20 | |
| Villagrande Strisaili | 09/03/2018 | 20 | |
| Total | 2,281 | ||
Table 2.
Information on the animals tested, including the municipality, number of samples tested for ASF antibody detection, number of samples positive to antibody presence from the ELISA and immunoblotting tests, the consequential seroprevalence, number of tested organs for ASFV, number of samples positive to ASFV presence using real‐time PCR, and consequential virus prevalence
| Municipality | Number serum samples tested | Samples positive to antibody presence (ELISA + Ib) | Seroprevalence (%) | Tested organs | Samples positive to virus presence (PCR) | Virus prevalence (%) |
|---|---|---|---|---|---|---|
| Aritzo | 15 | 9 | 60 | 15 | 0 | 0 |
| Arzana | 46 | 32 | 69.6 | 46 | 4 | 8.7 |
| Baunei | 37 | 5 | 13.5 | 35 | 0 | 0 |
| Desulo | 147 | 104 | 70.7 | 111 | 19 | 17.1 |
| Irgoli | 61 | 0 | 0 | 61 | 0 | 0 |
| Loculi | 6 | 0 | 0 | 6 | 0 | 0 |
| Nuoro | 33 | 0 | 0 | 59 | 0 | 0 |
| Orgosolo | 566 | 409 | 72.3 | 690 | 11 | 1.6 |
| Orosei | 17 | 0 | 0 | 17 | 0 | 0 |
| Ovodda | 13 | 0 | 0 | 13 | 0 | 0 |
| Talana | 39 | 2 | 5.1 | 39 | 0 | 0 |
| Urzulei | 139 | 60 | 43.2 | 265 | 0 | 0 |
| Villagrande Strisaili | 99 | 30 | 30.3 | 104 | 4 | 3.8 |
| Total | 1,218 | 651 | 53.4 | 1,461 | 38 | 2.6 |
Several changes have been observed since the first occurrence of ASF in Sardinia in 1978. Since 1978, different regional plans that aimed to eradicate the disease have been implemented, which have focused on both pigs and wild boars. From the first eradication plan in 1982 (Regional Decree, no 6/82, PE‐ASF82‐87), many others were conducted, with highly variable results. Some of these plans were able to get close to the eradication of ASF (i.e., the eradication plan of 1994–1998), but none have solved the problem posed by the free‐ranging pigs. Many studies have highlighted that disease persistence on the island is significantly associated with the socio‐economic, cultural and traditional practices, which are identified in free‐ranging pigs, even though this way of keeping pigs is less common than that in the past decades (Firinu, Ruiu, Cossu, & Patta, 1988; Laddomada et al., 1994; Mannelli et al., 1998, 1997; Feliziani et al., 2010; Mur et al., 2014; Jurado et al., 2017; Cappai et al., 2018). A possible explanation for the difficulties associated with removing free‐ranging pigs, is that this is part of the cultural identity of their “owners”, and thus, they refuse to change their habits because this would mean losing their cultural identity (Cappai et al., 2018; Firinu et al., 1988; Mannelli et al., 1997). However, it has become clear to experts, regional authorities and local authorities that these unregistered pigs, living in groups of tens or hundreds, play a very important role in the virus reservoir and may act as a virus‐link between domestic pigs kept in backyards and wild boar populations, where ASF has been found consistently in the last 40 years (Cappai et al., 2018; Costard et al., 2009; EFSA, 2014; Jurado et al., 2017; Mur et al., 2017). Free‐ranging pigs share the same habitat as the wild boar, facilitating the spread of the ASF virus and hindering its control (Costard, Mur, Lubroth, Sánchez‐Vizcaíno, & Pfeiffer, 2013; FAO, 2013; Fasina et al., 2010; Iglesias, Rodríguez, Feliziani, Rolesu, & de la Torre, 2017; Jori et al., 2016). The above depopulation actions against the free‐ranging pigs are the first effective actions taken against them. The present study also shows that the level of virus prevalence and seroprevalence found in the culled pigs, is extremely high compared to the prevalence of ASFV found in wild boars and domestic pigs, during the last six years (Cappai et al., 2018). The data presented here confirm the paramount role of free‐ranging pigs as a main source of the ASF virus in Sardinia. The highest ASF seroprevalence was found in the Orgosolo Municipality, where a significant proportion of the overall population of free‐ranging pigs used to live. These findings suggest that this area could be defined as the centre of the disease in Sardinia. The elimination of 1092 free‐ranging pigs in Orgosolo and the subsequent reduction in the number and density of ASF positive free‐ranging pigs are likely to have a very strong, positive impact on the eradication of ASF in Sardinia. This objective will probably be achieved, provided that the ongoing eradication measures are implemented continuously. However, it is necessary to underline that besides the maintenance of the present epidemiological surveillance program and focus on the three swine populations (domestic pigs, wild boars and free‐ranging pigs), the confrontation of the cultural and economic aspects of this millennial system of pig farming requires continuous education of farmers and other people involved in pig husbandry. Furthermore, all measures must take cultural and economic aspects into consideration.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Laddomada A, Rolesu S, Loi F, et al. Surveillance and control of African Swine Fever in free‐ranging pigs in Sardinia. Transbound Emerg Dis. 2019;66:1114–1119. 10.1111/tbed.13138
REFERENCES
- Albarella, U. , Manconi, F. , Rowley‐Conwy, P. , & Vigne, J. D. (2006). Pigs of Corsica and Sardinia: a biometrical re‐evaluation of their status and history In Tecchiati U., & Sala B. (Eds.), Archaeozoological Studies in Honour of Alfredo Riedel (pp. 285–302). Bolzano, Province of Bolzano: Ufficio Beni Archeology. [Google Scholar]
- Cappai, S. , Rolesu, S. , Coccollone, A. , Laddomada, A. , & Loi, F . (2018). Evaluation of biological and socio‐economic factors related to persistence of African swine fever in Sardinia. Accepted in Preventive Veterinary Medicine. [DOI] [PubMed]
- Contini, A. , Cossu, P. , Rutili, D. , & Firinu, A . (1982). African swine fever in Sardinia In Wilkinson P. J. (Ed.), African Swine Fever (pp. 1–6). EUR 8466 EN: Proceedings of CEC/FAO Research Seminar, Sardinia. [Google Scholar]
- Costard, S. , Mur, L. , Lubroth, J. , Sánchez‐Vizcaíno, J. M. , & Pfeiffer, D. U. (2013). Epidemiology of African Swine Fever Virus. Virus Research, 173, 191–197. 10.1016/j.virusres.2012.10.030 [DOI] [PubMed] [Google Scholar]
- Costard, S. , Wieland, B. , de Glanville, W. , Jori, F. , Rowlands, R. , Vosloo, W. , … Dixon, L. K. (2009). African swine fever: How can global spread be prevented? Philosophical Transactions of the Royal Society London B: Biological Science, 364, 2683–2696. 10.1098/rstb.2009.0098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon, L. K. , Escribano, J. M. , Martins, C. , Rock, D. L. , Salas, M. L. , Wilkinson, P. J. , … Ball, L. A . (2005). Asfarviridae Virus Taxonomy. 8th ed London, UK. [Google Scholar]
- EFSA (European Food Safety Authority). (2014). Evaluation of possible migration measures to prevent introduction and spread of African Swine fever virus through wild boar. EFSA Journal 2014;12(3): 23 pp. [Google Scholar]
- FAO . (2013) EMPRESS watch, vol. 28. Contributors: Khomenko S., Beltrán‐Alcrudo D., Rozstalnyy A., Gogin A., Kolbasov D., Pinto J., Lubroth J., Martin V. African swine fever in the Russian Federation: risk factors for Europe and beyond. Available online: http://www.fao.org/docrep/018/aq240e/aq240e.pdf
- Fasina, F. O. , Shamaki, D. , Makinde, A. A. , Lombin, L. H. , Lazarus, D. D. , & Rufai, S. A. (2010). Surveillance for African swine fever in Nigeria, 2006–2009. Transbounding and Emerging Diseases, 57(4), 244–253. [DOI] [PubMed] [Google Scholar]
- Feliziani, F. , Rolesu, S. , Aloi, D. , Panichi, G. , Marongiu, D. , & De Mia, G.M . (2010). Validation analysis of risk factors conditioning the persistence and the diffusion of African Swine Fever (ASF) infection in Sardinia Region (Italy)”. Webzine Sanità Pubblica Veterinaria, 60, 1581–1592. [Google Scholar]
- Firinu, A. , Ruiu, A. , Cossu, P. , & Patta, C . (1988). Indagine socio economica sulla peste suina africana in provincia di Nuoro. Proceeding of the International Meeting on African Swine Fever. Cala Gonone, Italy 5‐7 October 1988. Quaderni dell'Istituto Zooprofilattico Sperimentale della Sardegna 5, 179:199.
- Iglesias, I. , Rodríguez, A. , Feliziani, F. , Rolesu, S. , & de la Torre, A. (2017). Spatio‐temporal Analysis of African Swine Fever in Sardinia (2012–2014): Trends in Domestic Pigs and Wild Boar. Transbounding and Emerging Diseases, 64, 656–662. 10.1111/tbed.12408 [DOI] [PubMed] [Google Scholar]
- Jori, F. , Laval, M. , Maestrini, O. , Casabianca, F. , Charrier, F. , & Pavio, N. (2016). Assessment of domestic pigs, wild boars and feral hybrid pigs as reservoirs of Hepatitis E virus in Corsica, France. Viruses, 8(8), 236 10.3390/v8080236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurado, C. , Fernandez‐Carrion, E. , Mur, L. , Rolesu, S. , Laddomada, A. , & Sanchez Vizcaino, J. M. (2017). Why is African swine fever still present in Sardinia? Transbounding and Emerging Diseases, 66, 1–10. [DOI] [PubMed] [Google Scholar]
- Jurado, C. , Martínez‐Avilés, M. , De La Torre, A. , Štukelj, M. , de Carvalho Ferreira, H. C. , Cerioli, M. , … Bellini, S. (2018). Relevant measures to prevent the spread of African Swine Fever in the European Union Domestic Pig Sector. Frontiers in Veterinary Science, 5, 77 10.3389/fvets.2018.00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, D. P. , Reid, S. M. , Hutchings, G. H. , Grierson, S. S. , Wilkinson, P. J. , Dixon, L. K. , … Drew, T. W. (2003). Development of a TaqMan® PCR assay with internal amplification control for the detection of African swine fever virus. Journal of Virological Methods, 107, 53–61. 10.1016/S0166-0934(02)00189-1 [DOI] [PubMed] [Google Scholar]
- Laddomada, A. , Patta, C. , Oggiano, A. , Caccia, A. , Ruiu, A. , Cossu, P. , & Firinu, A. (1994). Epidemiology of classical swine fever in Sardinia: a serological survey of wild boar and comparison with African swine fever. Veterinary Record, 134, 183–187. 10.1136/vr.134.8.183 [DOI] [PubMed] [Google Scholar]
- Mannelli, A. , Sotgia, S. , Patta, C. , Oggiano, A. , Carboni, A. , Cossu, P. , & Laddomada, A. (1998). Temporal and spatial patterns of African swine fever in Sardinia. Preventive Veterinary Medicine, 35, 297–306. 10.1016/S0167-5877(98)00063-4 [DOI] [PubMed] [Google Scholar]
- Mannelli, A. , Sotgia, S. , Patta, C. , Sarria, A. , Madrau, P. , Sanna, L. , … Laddomada, A. (1997). Effect of husbandry methods on seropositivity to African swine fever virus in Sardinian swine herds. Preventive Veterinary Medicine, 32, 235–241. 10.1016/S0167-5877(97)00026-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mur, L. , Atzeni, M. , Martínez‐López, B. , Feliziani, F. , Rolesu, S. , & Sanchez‐Vizcaino, J.M . (2014). 35‐Year presence of African Swine Fever in Sardinia: History, evolution and risk factors for disease maintenance. Transboundary and Emerging Diseases, 3, 113. [DOI] [PubMed] [Google Scholar]
- Mur, L. , Sánchez‐Vizcaíno, J. M. , Fernandez‐Carriòn, E. , Jurado, C. , Rolesu, S. , Feliziani, F. , … Martínez‐López, B . (2017). Understanding African Swiner Fever infection dynamics in Sardinia using a spatially explicit transmission model in domestic pig farms. Transboundary and Emerging Diseases. 10.1111/tbed.12636 [DOI] [PubMed] [Google Scholar]
- PE‐ASF15‐18 , Regional Decree Number 50/17, 16 December 2014, Retrieved from http://www.assindnu.it/attachments/article/3219/Piano_eradicazione_dicembre_2014.pdf (accessed date 10/04/2017).
- Pozio, E. , Mesina, P. , Sechi, F. , Pira, M. , Liciardi, M. , Cossu, P. , … Firinu, A . (2006). Human outbreak of trichinellosis in the Mediterranean island of Sardinia, Italy. Veterinary Parasitology, 140 (1–2), 177–180. 10.1016/j.vetpar.2006.03.012 [DOI] [PubMed] [Google Scholar]
- Regione Autonoma de la Sardegna (RAS) (2004). Analisi degli sbocchi di mercato delle produzione agricole e agroalimentari della Regione Sardegna. Allegato alle Misure 4.9. pp. e4.10. Retrieved from http://www.dps.tesoro.it/documentazione/qcs/POR_rmp/POR_per_capitoli/POR_Sardegna_per_capitoli/Allegato_POR_Sbocchi_di_mercato.pdf (last accessed 31/06/2018).
- Wilkinson, P. J. (1984). The persistence of African Swine fever in Africa and the Mediterranean. Preventive Veterinary Medicine, 2, 71–82. 10.1016/0167-5877(84)90050-3 [DOI] [Google Scholar]


