Abstract
Introduction: We compared the secretome of metastatic (non-small cell lung cancer (NSCLC)) and primary (mesothelioma) malignant pleural effusions, benign effusions and the published plasma profile of patients receiving chimeric antigen receptor T cells (CAR-T), to determine factors unique to neoplasia in pleural effusion (PE) and those accompanying an efficacious peripheral anti-tumor immune response.
Materials and Methods: Cryopreserved cell-free PE fluid from 101 NSCLC patients, 8 mesothelioma and 13 with benign PE was assayed for a panel of 40 cytokines/chemokines using the Luminex system.
Results: Profiles of benign and malignant PE were dominated by high concentrations of sIL-6Rα, CCL2/MCP1, CXCL10/IP10, IL-6, TGFβ1, CCL22/MDC, CXCL8/IL-8 and IL-10. Malignant PE contained significantly higher (p < 0.01, Bonferroni-corrected) concentrations of MIP1β, CCL22/MDC, CX3CL1/fractalkine, IFNα2, IFNγ, VEGF, IL-1α and FGF2. When grouped by function, mesothelioma PE had lower effector cytokines than NSCLC PE. Comparing NSCLC PE and published plasma levels of CAR-T recipients, both were dominated by sIL-6Rα and IL-6 but NSCLC PE had more VEGF, FGF2 and TNFα, and less IL-2, IL-4, IL-13, IL-15, MIP1α and IFNγ.
Conclusions: An immunosuppressive, wound-healing environment characterizes both benign and malignant PE. A dampened effector response (IFNα2, IFNγ, MIP1α, TNFα and TNFβ) was detected in NSCLC PE, but not mesothelioma or benign PE. The data indicate that immune effectors are present in NSCLC PE and suggest that the IL-6/sIL-6Rα axis is a central driver of the immunosuppressive, tumor-supportive pleural environment. A combination localized antibody-based immunotherapy with or without cellular therapy may be justified in this uniformly fatal condition.
Keywords: pleural effusion, non-small cell lung cancer, malignant mesothelioma, cytokines, IL-6
INTRODUCTION
The pleura represent a common site of metastasis for non-small cell lung cancer (NSCLC) and are the primary site of tumorigenesis in malignant mesothelioma. These conditions are accompanied by the development of pleural effusions, accumulations of serous fluid rich in tumor cells, mesothelial cells, immune cells, and the cytokines and chemokines which they secrete. The pleural space is a sequestered local environment formed by mesothelial cells joined by tight junctions [1]. The levels of endogenously produced cytokines and chemokines such as IL-6 and soluble tumor necrosis factor receptor can be orders of magnitude greater in the pleura than in the plasma [2, 3]. The movement of proteins from the plasma to the pleural space is impeded to a lesser extent and is inversely related to their molecular weight [4]. Pleural effusions are not limited to malignancies and can occur when the regulation of pleural fluid volume is disrupted by pathologic changes affecting the mechanical movement of the thoracic cage, the pulmonary or systemic circulations or lymphatic drainage [5]. Benign effusions, such as those accompanying congestive heart failure or asbestosis, provide an informative contrast to malignant effusions and may reveal both the factors conditioning a site for metastasis or tumorigenesis, and differences in the pleural secretome specific to malignancy or secreted by tumor cells themselves. In this report we compare levels of 40 cytokines and chemokines (hereafter referred to as cytokines) in non-small cell lung cancer (NSCLC), mesothelioma and benign pleural effusions, with the goal of identifying druggable targets and interventions that can change the pleural environment from one that suppresses immunity and promotes tumor invasiveness, to one conducive to anti-tumor effector responses [6].
RESULTS
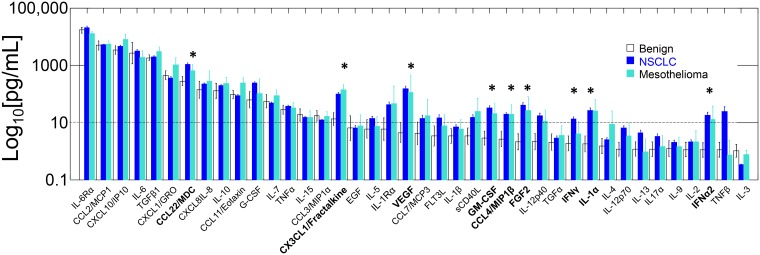
Figure 1 and Supplementary Table 1 show the cytokine profiles of pleural effusions from patients with malignant effusions (non-small cell lung cancer and mesothelioma) and benign effusions (heart failure, asbestos exposure without malignancy). There were no significant differences in single cytokine levels between NSCLC and mesothelioma samples (Figure 1, Student’s t-test, Bonferroni corrected for multiple comparisons), or between heart failure and asbestosis samples (not shown). The concentrations of 9 cytokines were significantly higher in malignant PE compared to benign (Supplementary Table 1). Three of the malignant PE-associated cytokines were chemoattractive (CCL4/MIP1β, CCL22/MDC, CX3CL1/ fractalkine), two were effector (IFNα2, IFNγ), one endothelial (VEGF), one inflammatory (IL-1α) and one mesenchymal (FGF2) (Table 1). Of the 6 cytokines present in concentrations greater than 1 ng/mL, only one (CCL22/MDC) was present at significantly higher concentration in malignant PE. The other cytokines highly concentrated in both malignant and benign PE were inflammatory (IL-6, IL-6Rα, CCL2/MCP1), regulatory (TGFβ1) and chemoattractive (CXCL10/IP10).
Figure 1. Geometric mean cytokine levels in benign, NSCLC and mesothelioma PE.
Cytokines are ordered left to right from the highest to the lowest concentration in benign PE. Error bars indicate standard errors of the geometric means. Significant differences (p < 0.01, Bonferroni-corrected 2-tailed t-test, benign vs neoplastic) are shown with asterisks and bold-text. A dashed line is provided at 10 pg/mL for reference.
Table 1. Classification of measured cytokines by function.
| Chemoattractive | Recruit immune cells to tumor site | CXCL10/IP10, CCL4/MIP1β, CCL22/MDC, CXCL1/GRO, CCL11/Eotaxin, CX3CL1/Fractalkine, CCL7/MCP3 |
| Effector | Anti-tumor immunity or cytotoxic functions | IFNα2, IFNγ, CCL3/MIP1α, TNFα, TNFβ |
| Endothelial | Promote angiogenesis | VEGF |
| Epithelial | Favors epithelial tumor phenotype | EGF |
| CCL3/Inflammatory | Elicit systematic inflammation and autoimmunity | IL-1α, IL-1β, IL-6, IL-6Rα, IL-17α, CCL2/MCP1 |
| Mesenchymal | Favors mesenchymal tumor phenotype | FGF2 |
| Regulatory | Dampen anti-tumor immune response | IL-4, IL-10, IL-13, TGFβ1, sCD40L, IL-1Rα |
| Stimulatory | Stimulation/proliferation of immune cells | GM-CSF, TGFα, G-CSF, Flt3L, IL-2, IL-5, IL-7, CXCL8/IL-8, IL-9, IL-12p40, IL-12p70, IL-15, IL-3 |
Cytokines significantly different between malignant and benign PE (Supplementary Table 1) are shown in bold. Cytokine classifications are modified from Rossi et al. [7].
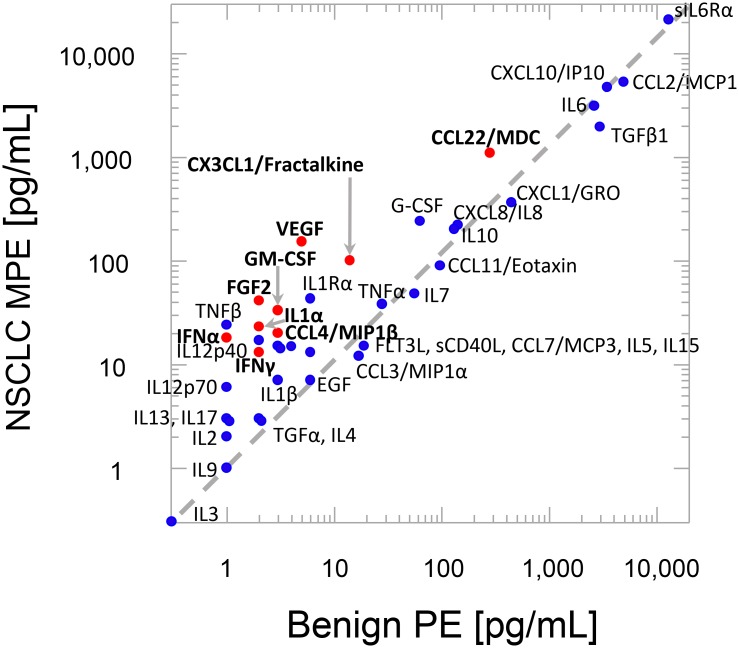
Figure 2 plots the geometric mean cytokine content of NSCLC PE versus the cytokine content of benign PE. Cytokines above the dashed line are more concentrated in NSCLC PE. Those in which this difference attained statistical significance are shown as red symbols with bold text.
Figure 2. Geometric mean cytokine levels.
Red symbols, bold text indicates P < 0.01 Bonferroni corrected 2-tailed t-test of log analyte concentrations (see Supplementary Table 1). Diagonal line indicates equal analyte concentration in benign and malignant effusions.
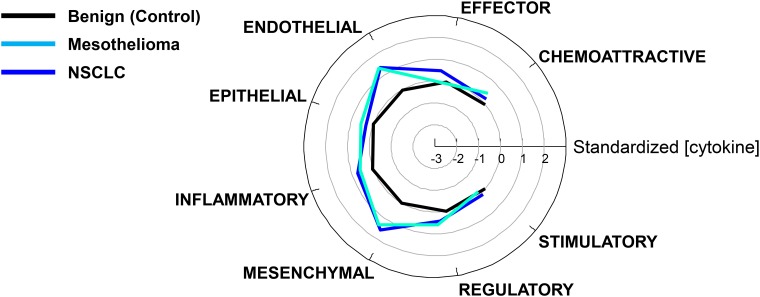
Table 1 classifies the cytokine analytes into 8 functional categories as modified from a scheme proposed by Rossi et al [7]. It should be noted that some cytokines are pleiotropic and therefore could have been placed into more than one category. For example, IL-6 [8, 9], CXCL8/IL-8 [10] and TGFβ1 [11] all promote the epithelial to mesenchymal transition (EMT). In Figure 3, log cytokine values were standardized to put them on a common scale relative to control (benign) values, where 0 is the mean value of benign pleural effusions (control), and each unit represents one standard deviation from the control mean. In most functional cytokine groups, NSCLC and mesothelial PE were indistinguishable from each other and highly significantly different from benign PE (p < 0.002, all comparisons, Bonferroni corrected). The exceptions were the epithelial cytokine group (F-test not significant) and the effector cytokine group, where NSCLC was higher than benign (p = 0.00001, by ANOVA Tukey’s honest significant difference test), and NSCLC was higher than mesothelioma (p = 0.013), but mesothelioma was indistinguishable from benign (p = 0.7).
Figure 3. Comparison of cytokine levels grouped by function.
Cytokine concentrations were standardized such that the geometric mean control value in the benign PE group was 0 with a standard deviation of 1 and grouped by function (Table 1).
DISCUSSION
IL-6 as a key driver of the pleural immune environment
The secretome of benign pleural effusions has important implications for the biology of NSCLC cancer metastatic to the pleura and malignant mesothelioma originating in the pleural space. Little is known concerning the constitutive cytokine content of normal pleural fluid, the volume of which is small (8.4 ± 4.3 mL per side) and tightly regulated [12]. The present study demonstrates that when the homeostatic balance between fluid filtration and removal is mechanically perturbed by cardiac insufficiency or by inflammation associated with asbestosis, the resulting effusions contain a rich mixture of highly concentrated cytokines (Figures 1 and 2, Supplementary Table 1). We identified sixteen cytokines present in benign effusions at concentrations > 10 pg/mL and five exceeding 1 ng/mL. These highly concentrated cytokines are dominated by inflammatory factors (IL-6, soluble IL-6Rα/CD126, CCL2/MCP1) but also include the chemotactic factor CXCL10/IP10 and the regulatory cytokine TGFβ1. Of these, the most striking are IL-6 (mean = 2.6 ng/mL, Supplementary Table 1) and sIL-6Rα (13.0 ng/mL). IL-6 has been termed a pleiotropic cytokine because it can mediate a wide variety of pro- and anti-inflammatory effects, including stimulation or inhibition of cell growth and differentiation depending on the target cell and the environment [13, 14]. Our results agree with those of Dore et al., who reported high levels of IL-6 and sIL-6Rα in patients with malignant, benign, and infectious PE [3]. Interestingly, IL-6 and sIL-6Rα levels were higher in effusions than in plasma [3]. This argues for local production of IL-6 by mesothelial cells [15], mesenchymal cells [16], and infiltrating lymphocytes, neutrophils and macrophages. The large difference between intrapleural and systemic cytokine levels [3] reinforces the notion that the pleural space forms a distinct immune environment in which locally secreted large molecules are concentrated in isolation from the peripheral circulation.
Although the signal transducing portion of the IL-6 receptor (IL-6Rβ/CD130) is widely expressed, only leukocytes, skeletal muscle and hepatocytes normally express the complete receptor (the ligand binding protein IL-6Rα plus the signal transducing protein IL-6Rβ) and are therefore constitutively responsive to IL-6 [17, 18]. T cell receptor-activated CD4+ T cells [19], neutrophils and macrophages [20] are capable of shedding soluble IL-6Rα and are its likely source in benign PE. In the presence of sIL-6Rα, IL-6 is transformed from a highly restricted to a highly promiscuous cytokine, capable of interacting with all cell-types that express IL-6Rβ—this process has been termed trans-signaling [21]. In acute responses IL-6 participates in the resolution of neutrophil infiltration and initiation of immune effector responses, but IL-6 also increases the mononuclear cell infiltrate and participates in the pathogenesis of chronic inflammation [17].
IL-6 in malignant PE
The importance of the IL-6/IL-6Rα axis in the pathogenesis of MPE comes from its key role as an upstream cytokine in a wound healing cascade that amplifies macrophage recruitment and promotes angiogenesis and macrophage polarization. IL-6 downregulates macrophage secretion of IL-1β, TNFα, and IL-12. It also amplifies recruitment of macrophages, T cells and dendritic cells (DC) by promoting secretion of the chemoattractant CCL2/MCP-1 by macrophages [17]. IL-6 also facilitates trans-signaling to a wide range of target cells by inducing sIL-1Rα secretion by neutrophils and macrophages. For example, endothelial and mesenchymal cells, secrete CCL2/MCP-1 in the presence of IL-6 and sIL-1Rα [17]. IL-6 may also trans-signal to epithelial cells, as it does in the thymus, upregulating CXCL1/GRO, CCL2/MCP-1, and CXCL8/IL-8 [22], three abundant cytokines in both malignant and benign PE (Figure 1). CXCL1/GRO upregulation is of particular importance in malignant PE because it is implicated in angiogenesis and tumorigenesis [23]. The central role of IL-6 trans-signaling in pathologic processes mediated by multi-cytokine cascades is further supported by the efficacy of the anti-IL-6Rα antibody tocilizumab in the treatment of rheumatoid arthritis [24] and cytokine release syndrome (CRS) induced by administration of therapeutic chimeric antigen receptor T cells (CAR-T) [25].
The present study confirms earlier reports of high IL-6 in mesothelioma PE [26] and of IL-6 plus IL-6Rα in NSCLC [27, 28] PE. The combination of IL-6 and IL-6Rα has been shown to drive VEGF production in mesothelioma cells [29]; in the present study VEGF was significantly elevated in malignant PE as compared to benign PE (Supplementary Table 1, Figure 2), suggesting a contribution by tumor cells. In NSCLC, elevated EGFR activity is associated with induction of IL-6 transcription [30], which in turn drives autocrine IL-6 signaling and tumor proliferation [31]. Interestingly, despite its tissue restriction in normal tissue, IL-6Rα has been detected in lung cancer cells and is associated with the EMT/cancer stem cell phenotype [32]. In primary adenocarcinoma of the colon, IL-6R expression has been positively correlated with EMT-associated mesenchymal markers SNAIL, SLUG, VIM, ZEB1, and ZEB2 [33]. Similarly CXCL8/IL-8, which is expressed in benign and malignant PE is associated with the mesenchymal phenotype in lung cancer [34]. We and other have shown that IL-6 and CXCL8/IL-8 play a critical role in the epithelial to mesenchymal transition of human carcinoma cells [9, 10, 35]. Taken together, the secretomes of both benign and malignant effusions are comprised of a complex mixture of cytokines dominated by IL-6 and sIL-6R. Such an environment would predictably present a protected and fertile setting for the growth and EMT of neoplastic cells.
Other cytokines contributing to a wound-healing, immunosuppressive environment in malignant PE
We identified high levels of the regulatory cytokine TGFβ1 in both malignant and benign PE. TGFβ1 is secreted by activated platelets and is an early player in the wound healing response, directly promoting angiogenesis [36] and EMT [37]. The ability of TGFβ to amplify its own production by inducing expression in its target cells may explain why this wound healing cytokine is deleterious in chronic injury settings like cancer [38]. Indeed, high plasma levels of TGFβ1 is predictive of poor prognosis in NSCLC [39]. Further, as an immunoregulatory cytokine, TGFβ has been shown to drive naïve CD4+ T cells to differentiate into immunosuppressive regulatory T cells (T-regs) [40], an effect that may synergize with CCL4/MIP1β, which has been shown to recruit T-regs [41]. CCL22/MDC (significantly higher in MPE than benign PE, Supplementary Table 1, Bonferroni corrected p = 0.0028) is secreted by macrophages and DC; also attracts T-regs [42], further contributing to the suppressive immune environment of malignant PE. Like TGFβ1, high plasma levels of FGF2 are associated with shorter survival time in NSCLC [43]. This may be due to FGF2-mediated promotion of an undifferentiated state in neoplastic cells, as it does for cultured embryonic stem cells [44]. FGF2 also promotes angiogenesis indirectly by induction of VEGF secretion [45]. In NSCLC, G-CSF has been shown to enhance myeloid-derived suppressor cell function and may contribute to tumor progression [46]. G-CSF is produced by mesothelial cells in response to EGF or TNF [47], both of which are present at low levels in PE. Similarly, high levels of GM-CSF (significantly higher in MPE than benign PE, Bonferroni corrected p = 0.0002) are associated with lung cancer progression and invasion [48].
A nascent immune effector response in NSCLC PE
Despite the extreme immunosuppressive, angiogenic and tumor promoting environment of the injured pleural space, there is evidence of a nascent but thwarted effector response in NSCLC, but not in malignant mesothelioma (Figure 3). This difference may be due to the fact that mesothelioma has one of the lowest mutational burdens (~3 mutations/Mb) of any cancer [49]. CXCL10, also known as IFNγ-inducible protein 10 (IP10) plays a role in the generation of effector T cells and T cell trafficking [50] and is a potent inhibitor of angiogenesis [51]. CCL2/MCP1 promotes effector responses by recruiting monocytes, memory T cells, and DC to sites of inflammation produced by tissue injury and infection [52, 53]. Both CXCL10 and CCL2 were present at high concentration in malignant and benign effusions (Figure 1, Supplementary Table 1), supporting the recruitment of potential effector cells to the pleural space. CXC3CL1/fractalkine is produced by endothelial cells and can also promote effector responses by attracting and capturing CD4+ T cells [54]. CXC3CL1 was significantly higher in NSCLC PE compared to benign PE and has been proposed as an amplification circuit of polarized Th1 responses [55]. This effect may be dampened by sIL-6Rα [56]. Most importantly, the potent effector cytokines IFNα2, IFNγ, MIP1α, TNFα and TNFβ were all detected in NSCLC PE. IFNα2 and IFNγ levels were relatively low, but significantly higher than in benign PE (Bonferroni corrected p = 0.0092 and 0.027, respectively), indicating a weak, yet detectable effector response. Indeed, in patients with primary NSCLC, increased plasma levels of effector cytokines within the first 3 months of diagnosis are significantly correlated with improved response to anti-PD-1 therapy and prolonged overall survival [57].
Comparison with the cytokine environment of patients receiving CAR-T
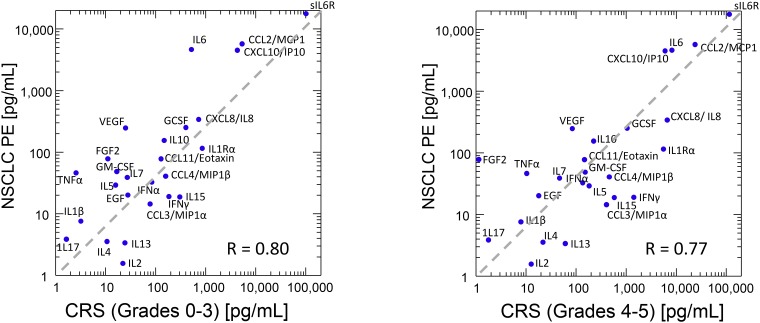
The plasma cytokine profile elicited during successful CAR-T therapy provides a paradigm for efficacious anti-tumor effector response. Comparison of our findings with those of Teachey et al. [58], who used similar methods in patients receiving CAR-T for B-cell malignancies, is instructive. Twenty-six cytokines were shared by our respective data sets and are plotted in Figure 4. Overall, the cytokines profiles of NSCLC PE and plasma from CAR-T recipients were remarkably similar and dominated by sIL-6Rα, CCL2/MCP1, CXCL10/IP10, IL-6 and CXCL8/IL-8 (R = 0.80 and 0.77 compared to patients with CRS grades 0–3 and CRS 4–5, respectively). Compared to plasma from patients with no or minimal CRS (Figure 4, left panel), the NSCLC PE had lower concentrations of IL-2, IL-4, IL-13, IL-15, MIP1α, and IFNγ, including higher concentrations of IL-6, VEGF, FGF2, and TNFα. A similar pattern was noted when our data were compared to results from patients with clinically significant CRS (Figure 4, right panel), for which the anti-IL-6R antibody tocilizumab is an effective treatment. Importantly, despite its efficacy against CRS, tocilizumab does not appear to interfere with the expansion or efficacy of CAR-T effector cells [59]. Taken together, our findings suggest that the IL-6/sIL-6Rα axis, which is the principal driver of CRS, may also be central to the immunosuppressive, growth promoting, wound-healing milieu common to injured pleura. In malignant PE, tumor cells, whether primary or metastatic, participate in and amplify the cytokine cascade responsible for these effects.
Figure 4. Median values of 26 cytokines common to our dataset and the median one-month-peak values published as an online supplement by Teachey et al.
(2016). Cytokine Release Syndrome (CRS) grades 0–3, N = 9, CRS grades 4 and 5, N = 3. Pearson product-moment correlation coefficients (R), are shown. Diagonal line indicates equal analyte concentration in the plasma of CAR-T recipients and in malignant effusions.
Toward localized combinatorial immunotherapy
Despite this hostile environment, there is evidence in NSCLC PE of a nascent immune effector response that may be harnessed therapeutically by modifying the local pleural immune environment with antibody-based therapeutics, by ex vivo activation and reinstillation of pleural T cells or engineered T cells [60]. We speculate that conditioning the immune environment of the pleura will greatly increase the chances of success [6]. Adusumilli et al. recently reported that local administration of anti-mesothelin CAR-T in combination with systemic administration of anti-PD1 agents yielded encouraging clinical results in a proportion of patients with malignant pleural disease [61]. We suggest that such results could be enhanced by conditioning the local environment using combinatorial immunotherapies in which several mechanisms of tumor-mediated immune suppression are simultaneously targeted. Our study suggests that, in addition to PD1, such combination therapies might target IL-6Rα and VEGF, for which therapeutic antibodies are available. The routine use of tunneled pleural catheters for PE drainage [62] provides the opportunity for local administration of antibody and cellular therapeutics as well as for sequential sampling and monitoring. Local delivery of combination antibody therapy to the pleural space also has the potential to decrease total delivered dose and reduce systemic toxicities.
MATERIALS AND METHODS
Patients and samples
Pleural effusions (PE) were collected as anonymized medical waste under an IRB exemption (No. 0503126), or IRB approved protocol No. 16110093, under which patients consented to use of the sample and access to medical records. Pleural effusions were collected from 101 NSCLC patients, 8 mesothelioma patients and 13 patients without pleural malignancy (11 with heart failure, 2 with asbestosis). Pleural fluids were picked up from the operating room or patient hospital room by laboratory staff, transported immediately to the laboratory where they were held on wet ice, and processed within 30 minutes of receipt. Cells were first removed by centrifugation (10 min at 600 x g, 4°C), and then further clarified (10 min 1880 x g, 4°C) prior to storage at –86°C. Immediately prior to analysis for cytokines and chemokines, samples were thawed and clarified by high-speed centrifugation (3 min at 16,000 x g, Beckman Microfuge E, Cat No. 348720, Beckman Coulter) in a coldroom environment (4°C).
Quantification of cytokines
Cytokines were quantified on the Luminex platform (Hillman Cancer Center Luminex Facility, Dr. Anna Lokshin, Director), using the Curiox LT-MX plate washer, Curiox DA-96 plates, the Luminex 200 System analyzer and xPonent data acquisition and analysis software. Six-point standard curves were run for each cytokine with each of 2 sample batches. Cytokines were measured in 5 µL of neat clarified effusion using the MILLIPLEX MAP Human Cytokine/Chemokine Magnetic Bead Panel, Premixed 38 Plex (Cat. No. HCYTMAG-60K-PX38), MILLIPLEX MAP Human TGFβ (Cat. No. TGFBMAG-64K-01) and IL-6Rα from the Human Angiogenesis/Growth Factor Panel 2 (Cat. No. HANG2MAG-12K-01) kits. The first 33 samples that we assayed were run in duplicate or triplicate and had a mean difference of 5.8% (all cytokines, Supplementary Figure 1), after which samples were run without replicates. Determinations that were designated “Out of Range Below” (i.e., below the limit of quantification) by the analytical software were arbitrarily filled with a value 1/10 the lowest valid measurement for that cytokine. Values designated “Out of Range Above” (i.e., above the limit of quantification) were assigned the value of the highest valid measurement for that cytokine.
Statistical analysis
Descriptive statistics and statistical comparisons (Student’s t-test, 2-tailed, ANOVA) were performed on log10-transformed data. P-values were Bonferroni-corrected for multiple comparisons. For analysis of functional characteristics of cytokines unique to malignant pleural effusions, log cytokine concentrations were expressed on a common scale (standardized) relative to control values (benign effusions). This was accomplished by subtracting the mean control value from each cytokine determination and dividing by the standard deviation of the control value. SYSTAT 13 software (San Jose, CA) was used for data analysis and graphics.
SUPPLEMENTARY MATERIALS
ACKNOWLEDGMENTS
The authors would like to thank Bosko Popov and Denise Prosser for their excellent technical assistance and Drs. Rodney Landreneau, Neil Christie, Rajeev Dhupar and Inderpal Sarkaria for patient recruitment and tissue collection.
Abbreviations
- ANOVA
Analysis of variance
- CAR-T
Chimeric antigen receptor T cell
- CC, CXC, CX3C
Chemokine motif subfamilies
- EGF
Epidermal growth factor
- EMT
Epithelial to mesenchymal transition
- FGF2
Basic fibroblast growth factor
- Flt3L
Fms-related tyrosine kinase 3 ligand
- G-CSF
Granulocyte colony stimulating factor
- GM-CSF
Granulocyte macrophage colony stimulating factor
- GRO
Growth regulated oncogene (CXCL1)
- IFN
Interferon
- IL
Interleukin
- IP10
IFNγ-inducible protein 10 (CXCL10)
- IRB
Institutional review board
- L1, L2, L7, L10, L22
Chemokine ligand subfamilies
- MCP1
Monocyte chemotactic protein-1 (CCL2)
- MCP3
Monocyte chemotactic protein-3 (CCL7)
- MDC
Macrophage-derived chemokine (CCL22)
- MIP1β
Macrophage inflammatory protein-1β (CCL4)
- MPE
Malignant pleural effusion
- NSCLC
Non-small cell lung cancer
- PE
Pleural Effusion
- sCD40L
Soluble CD40 ligand
- TGFα
Transforming growth factor alpha
- TGFβ1
Transforming growth factor beta 1
- TNFβ
Tumor necrosis factor beta
- VEGF
Vascular endothelial growth factor
Author contributions
Albert and Vera Donnenberg designed the study, analyzed the data and wrote the manuscript, contributing equally. James Luketich provided the clinical impetus for this study and the infrastructure that made it possible.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to report.
FUNDING
This work was supported by BC032981, BC044784, W81XWH-12-1-0415 and BC132245_W81XWH-14-0258 from the Department of Defense, National Cancer Institute grant R21 CA191647, the Glimmer of Hope Foundation, and the David Downing Fund. The Hillman Cancer Center Luminex Facility is supported by Cancer Center Support Grant P30CA047904.
REFERENCES
- 1. Amasheh S, Markov AG, Volgin GN, Voronkova MA, Yablonsky PK, Fromm M. Barrier function of human pleura mesothelium is constituted by tight junctions. FASEB J. 2011; 25:10363. Accessed from: https://www.fasebj.org/toc/fasebj/25/1_supplement. [DOI] [PubMed] [Google Scholar]
- 2. Marie C, Losser MR, Fitting C, Kermarrec N, Payen D, Cavaillon JM. Cytokines and Soluble Cytokine Receptors in Pleural Effusions from Septic and Nonseptic Patients. Am J Respir Crit Care Med. 1997; 156:1515–22. 10.1164/ajrccm.156.5.9702108. [DOI] [PubMed] [Google Scholar]
- 3. Dore P, Lelievre E, Morel F, Brizard A, Fourcin M, Clement C, Ingrand P, Daneski L, Gascan H, Wijdenes J, Gombert J, Preud’homme JL, Lecron JC. IL-6 and soluble IL-6 receptors (sIL-6R and sgp130) in human pleural effusions: massive IL-6 production independently of underlying diseases. Clin Exp Immunol. 1997; 107:182–8. 10.1046/j.1365-2249.1997.d01-889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Telvi L, Jaubert F, Eyquem A, Andreux JP, Labrousse F, Chretien J. Study of immunoglobulins in pleura and pleural effusions. Thorax. 1979; 34:389–92. 10.1136/thx.34.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pistolesi M, Miniati M, Giuntini C. Pleural Liquid and Solute Exchange. Am Rev Respir Dis. 1989; 140:825–47. 10.1164/ajrccm/140.3.825. [DOI] [PubMed] [Google Scholar]
- 6. Donnenberg AD, Luketich JD, Dhupar R, Donnenberg VS. Treatment of malignant pleural effusions: the case for localized immunotherapy. J Immunother Cancer. 2019; 7:110. 10.1186/s40425-019-0590-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rossi J, Paczkowski P, Shen YW, Morse K, Flynn B, Kaiser A, Ng C, Gallatin K, Cain T, Fan R, Mackay S, Heath JR, Rosenberg SA, et al. Preinfusion polyfunctional anti-CD19 chimeric antigen receptor T cells are associated with clinical outcomes in NHL. Blood. 2018; 132:804–14. 10.1182/blood-2018-01-828343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee SO, Yang X, Duan S, Tsai Y, Strojny LR, Keng P, Chen Y. IL-6 promotes growth and epithelial-mesenchymal transition of CD133+ cells of non-small cell lung cancer. Oncotarget. 2016; 7:6626–38. 10.18632/oncotarget.6570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lu H, Clauser KR, Tam WL, Frose J, Ye X, Eaton EN, Reinhardt F, Donnenberg VS, Bhargava R, Carr SA, Weinberg RA. A breast cancer stem cell niche supported by juxtacrine signalling from monocytes and macrophages. Nat Cell Biol. 2014; 16:1105–17. 10.1038/ncb3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fernando RI, Castillo MD, Litzinger M, Hamilton DH, Palena C. IL-8 Signaling Plays a Critical Role in the Epithelial–Mesenchymal Transition of Human Carcinoma Cells. Cancer Res. 2011; 71:5296–306. 10.1158/0008-5472.can-11-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009; 19:156–72. 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Noppen M, De Waele M, Li R, Gucht KV, D’Haese J, Gerlo E, Vincken W. Volume and cellular content of normal pleural fluid in humans examined by pleural lavage. Am J Respir Crit Care Med. 2000; 162:1023–26. 10.1164/ajrccm.162.3.9910050. [DOI] [PubMed] [Google Scholar]
- 13. Kishimoto T. The biology of interleukin-6. Blood. 1989; 74:1–10. 10.1182/blood.V74.1.1.1. [DOI] [PubMed] [Google Scholar]
- 14. Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011; 1813:878–88. 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 15. Topley N, Jörres A, Luttmann W, Petersen MM, Lang MJ, Thierauch KH, Müller C, Coles GA, Davies M, Williams JD. Human peritoneal mesothelial cells synthesize interleukin-6: Induction by IL-1β and TNFα. Kidney Int. 1993; 43:226–33. 10.1038/ki.1993.36. [DOI] [PubMed] [Google Scholar]
- 16. Anton K, Banerjee D, Glod J. Macrophage-Associated Mesenchymal Stem Cells Assume an Activated, Migratory, Pro-Inflammatory Phenotype with Increased IL-6 and CXCL10 Secretion. PLoS One. 2012; 7:e35036. 10.1371/journal.pone.0035036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kaplanski G, Marin V, Montero-Julian F, Mantovani A, Farnarier C. IL-6: a regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends Immunol. 2003; 24:25–9. 10.1016/S1471-4906(02)00013-3. [DOI] [PubMed] [Google Scholar]
- 18. The Genotype-Tissue Expression (GTEx) Project. Office of the Director of the National Institutes of Health). 2019.
- 19. Briso EM, Dienz O, Rincon M. Cutting edge: soluble IL-6R is produced by IL-6R ectodomain shedding in activated CD4 T cells. J Immunol. 2008; 180:7102–6. 10.4049/jimmunol.180.11.7102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Horiuchi S, Koyanagi Y, Zhou Y, Miyamoto H, Tanaka Y, Waki M, Matsumoto A, Yamamoto M, Yamamoto N. Soluble interleukin-6 receptors released from T cell or granulocyte/macrophage cell lines and human peripheral blood mononuclear cells are generated through an alternative splicing mechanism. Eur J Immunol. 1994; 24:1945–48. 10.1002/eji.1830240837. [DOI] [PubMed] [Google Scholar]
- 21. Lo CW, Chen MW, Hsiao M, Wang S, Chen CA, Hsiao SM, Chang JS, Lai TC, Rose-John S, Kuo ML, Wei LH. IL-6 trans-signaling information and progression of malignant ascites in ovarian cancer. Cancer Res. 2011; 71:424–34. 10.1158/0008-5472.CAN-10-1496. [DOI] [PubMed] [Google Scholar]
- 22. Tseng YL, Wu MH, Yang HC, Wang CY, Lin CF. Autocrine IL-6 regulates GRO-α production in thymic epithelial cells. Cytokine. 2010; 51:195–201. 10.1016/j.cyto.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 23. Haghnegahdar H, Du J, Wang D, Strieter RM, Burdick MD, Nanney LB, Cardwell N, Luan J, Shattuck-Brandt R, Richmond A. The tumorigenic and angiogenic effects of MGSA/GRO proteins in melanoma. J Leukoc Biol. 2000; 67:53–62. 10.1002/jlb.67.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smolen JS, Beaulieu A, Rubbert-Roth A, Ramos-Remus C, Rovensky J, Alecock E, Woodworth T, Alten R, and OPTION Investigators. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet. 2008; 371:987–97. 10.1016/S0140-6736(08)60453-5. [DOI] [PubMed] [Google Scholar]
- 25. Maude SL, Barrett D, Teachey DT, Grupp SA. Managing Cytokine Release Syndrome Associated With Novel T Cell-Engaging Therapies. Cancer J. 2014; 20:119–22. 10.1097/ppo.0000000000000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Abdul Rahim SN, Ho GY, Coward JIG. The role of interleukin-6 in malignant mesothelioma. Transl Lung Cancer Res. 2015; 4:55–66. 10.3978/j.issn.2218-6751.2014.07.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yanagawa H, Sone S, Munekata M, Atagi S, Nii A, Ogura T. IL-6 in malignant pleural effusions and its augmentation by intrapleural instillation of IL-2. Clin Exp Immunol. 1992; 88:207–12. 10.1111/j.1365-2249.1992.tb03063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hoheisel G, Izbicki G, Roth M, Chan CHS, Reichenberger F, Schauer J, Perruchoud AP. Proinflammatory Cytokine Levels in Patients with Lung Cancer and Carcinomatous Pleurisy. Respiration. 1998; 65:183–6. 10.1159/000029256. [DOI] [PubMed] [Google Scholar]
- 29. Adachi Y, Aoki C, Yoshio-Hoshino N, Takayama K, Curiel DT, Nishimoto N. Interleukin-6 induces both cell growth and VEGF production in malignant mesotheliomas. Int J Cancer. 2006; 119:1303–11. 10.1002/ijc.22006. [DOI] [PubMed] [Google Scholar]
- 30. Gao SP, Mark KG, Leslie K, Pao W, Motoi N, Gerald WL, Travis WD, Bornmann W, Veach D, Clarkson B, Bromberg JF. Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomas. J Clin Invest. 2007; 117:3846–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Grivennikov S, Karin M. Autocrine IL-6 Signaling: A Key Event in Tumorigenesis? Cancer Cell. 2008; 13:7–9. 10.1016/j.ccr.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 32. Yi H, Cho HJ, Cho SM, Jo K, Park JA, Kim NH, Amidon GL, Kim JS, Shin HC. Blockade of interleukin-6 receptor suppresses the proliferation of H460 lung cancer stem cells. Int J Oncol. 2012; 41:310–6. 10.3892/ijo.2012.1447. [DOI] [PubMed] [Google Scholar]
- 33. Rokavec M, Öner MG, Li H, Jackstadt R, Jiang L, Lodygin D, Kaller M, Horst D, Ziegler PK, Schwitalla S, Slotta-Huspenina J, Bader FG, Greten FR, Hermeking H. IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated colorectal cancer invasion and metastasis. J Clin Invest. 2014; 124:1853–67. 10.1172/JCI73531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chae YK, Chang S, Ko T, Anker J, Agte S, Iams W, Choi WM, Lee K, Cruz M. Epithelial-mesenchymal transition (EMT) signature is inversely associated with T-cell infiltration in non-small cell lung cancer (NSCLC). Sci Rep. 2018; 8:2918. 10.1038/s41598-018-21061-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dehai C, Bo P, Qiang T, Lihua S, Fang L, Shi J, Jingyan C, Yan Y, Guangbin W, Zhenjun Y. Enhanced invasion of lung adenocarcinoma cells after co-culture with THP-1-derived macrophages via the induction of EMT by IL-6. Immunol Lett. 2014; 160:1–10. 10.1016/j.imlet.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 36. Ueki N, Nakazato M, Ohkawa T, Ikeda T, Amuro Y, Hada T, Higashino K. Excessive production of transforming growth-factor β 1 can play an important role in the development of tumorigenesis by its action for angiogenesis: validity of neutralizing antibodies to block tumor growth. Biochim Biophys Acta. 1992; 1137:189–96. 10.1016/0167-4889(92)90201-L. [DOI] [PubMed] [Google Scholar]
- 37. Kasai H, Allen JT, Mason RM, Kamimura T, Zhang Z. TGF-β1 induces human alveolar epithelial to mesenchymal cell transition (EMT). Respir Res. 2005; 6:56. 10.1186/1465-9921-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Border WA, Ruoslahti E. Transforming growth factor-beta in disease: the dark side of tissue repair. J Clin Invest. 1992; 90:1–7. 10.1172/JCI115821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhao L, Ji W, Zhang L, Ou G, Feng Q, Zhou Z, Lei M, Yang W, Wang L. Changes of circulating transforming growth factor-beta1 level during radiation therapy are correlated with the prognosis of locally advanced non-small cell lung cancer. J Thorac Oncol. 2010; 5:521–5. 10.1097/JTO.0b013e3181cbf761. [DOI] [PubMed] [Google Scholar]
- 40. Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of Peripheral CD4+ CD25− Naive T Cells to CD4+ CD25+Regulatory T Cells by TGF-β Induction of Transcription Factor Foxp3. J Exp Med. 2003; 198:1875–86. 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bystry RS, Aluvihare V, Welch KA, Kallikourdis M, Betz AG. B cells and professional APCs recruit regulatory T cells via CCL4. Nat Immunol. 2001; 2:1126–32. 10.1038/ni735. [DOI] [PubMed] [Google Scholar]
- 42. Iellem A, Mariani M, Lang R, Recalde H, Panina-Bordignon P, Sinigaglia F, D’Ambrosio D. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2001; 194:847–53. 10.1084/jem.194.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Naumnik W, Ossolińska M, Płońska I, Chyczewska E, Nikliński J. Circulating Thrombospondin-2 and FGF-2 in Patients with Advanced Non-small Cell Lung Cancer: correlation with Survival. Adv Exp Med Biol. 2015; 833:9–14. 10.1007/5584_2014_78. [DOI] [PubMed] [Google Scholar]
- 44. Levenstein ME, Ludwig TE, Xu RH, Llanas RA, VanDenHeuvel-Kramer K, Manning D, Thomson JA. Basic fibroblast growth factor support of human embryonic stem cell self-renewal. Stem Cells. 2006; 24:568–74. 10.1634/stemcells.2005-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Seghezzi G, Patel S, Ren CJ, Gualandris A, Pintucci G, Robbins ES, Shapiro RL, Galloway AC, Rifkin DB, Mignatti P. Fibroblast Growth Factor-2 (FGF-2) Induces Vascular Endothelial Growth Factor (VEGF) Expression in the Endothelial Cells of Forming Capillaries: An Autocrine Mechanism Contributing to Angiogenesis. J Cell Biol. 1998; 141:1659. 10.1083/jcb.141.7.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tavakkoli M, Wilkins CR, Mones JV, Mauro MJ. A Novel Paradigm Between Leukocytosis, G-CSF Secretion, Neutrophil-to-Lymphocyte Ratio, Myeloid-Derived Suppressor Cells, and Prognosis in Non-small Cell Lung Cancer. Front Oncol. 2019; 9:295. 10.3389/fonc.2019.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Demetri GD, Zenzie BW, Rheinwald JG, Griffin JD. Expression of colony-stimulating factor genes by normal human mesothelial cells and human malignant mesothelioma cells lines in vitro . Blood. 1989; 74:940–6. [PubMed] [Google Scholar]
- 48. Uemura Y, Kobayashi M, Nakata H, Kubota T, Bandobashi K, Saito T, Taguchi H. Effects of GM-CSF and M-CSF on tumor progression of lung cancer: roles of MEK1/ERK and AKT/PKB pathways. Int J Mol Med. 2006; 18:365–73. [PubMed] [Google Scholar]
- 49. Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, Schrock A, Campbell B, Shlien A, Chmielecki J, Huang F, He Y, Sun J, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017; 9:34. 10.1186/s13073-017-0424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dufour JH, Dziejman M, Liu MT, Leung JH, Lane TE, Luster AD. IFN-γ-Inducible Protein 10 (IP-10; CXCL10)-Deficient Mice Reveal a Role for IP-10 in Effector T Cell Generation and Trafficking. J Immunol. 2002; 168:3195–204. 10.4049/jimmunol.168.7.3195. [DOI] [PubMed] [Google Scholar]
- 51. Angiolillo AL, Sgadari C, Taub DD, Liao F, Farber JM, Maheshwari S, Kleinman HK, Reaman GH, Tosato G. Human interferon-inducible protein 10 is a potent inhibitor of angiogenesis in vivo . J Exp Med. 1995; 182:155–62. 10.1084/jem.182.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Carr MW, Roth SJ, Luther E, Rose SS, Springer TA. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci U S A. 1994; 91:3652–6. 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Xu LL, Warren MK, Rose WL, Gong W, Wang JM. Human recombinant monocyte chemotactic protein and other c-c chemokines bind and induce directional migration of dendritic cells in vitro . J Leukoc Biol. 1996; 60:365–71. 10.1002/jlb.60.3.365. [DOI] [PubMed] [Google Scholar]
- 54. Fong AM, Robinson LA, Steeber DA, Tedder TF, Yoshie O, Imai T, Patel DD. Fractalkine and CX3CR1 Mediate a Novel Mechanism of Leukocyte Capture, Firm Adhesion, and Activation under Physiologic Flow. J Exp Med. 1998; 188:1413. 10.1084/jem.188.8.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fraticelli P, Sironi M, Bianchi G, D’Ambrosio D, Albanesi C, Stoppacciaro A, Chieppa M, Allavena P, Ruco L, Girolomoni G, Sinigaglia F, Vecchi A, Mantovani A. Fractalkine (CX3CL1) as an amplification circuit of polarized Th1 responses. J Clin Invest. 2001; 107:1173–81. 10.1172/JCI11517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Matsumiya T, Imaizumi T, Fujimoto K, Cui X, Shibata T, Tamo W, Kumagai M, Tanji K, Yoshida H, Kimura H, Satoh K. Soluble Interleukin-6 Receptor α Inhibits the Cytokine-Induced Fractalkine/CX3CL1 Expression in Human Vascular Endothelial Cells in Culture. Exp Cell Res. 2001; 269:35–41. 10.1006/excr.2001.5300. [DOI] [PubMed] [Google Scholar]
- 57. Boutsikou E, Domvri K, Hardavella G, Tsiouda D, Zarogoulidis K, Kontakiotis T. Tumour necrosis factor, interferon-gamma and interleukins as predictive markers of antiprogrammed cell-death protein-1 treatment in advanced non-small cell lung cancer: a pragmatic approach in clinical practice. Ther Adv Med Oncol. 2018; 10:1758835918768238. 10.1177/1758835918768238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Teachey DT, Lacey SF, Shaw PA, Melenhorst JJ, Maude SL, Frey N, Pequignot E, Gonzalez VE, Chen F, Finklestein J, Barrett DM, Weiss SL, Fitzgerald JC, et al. Identification of Predictive Biomarkers for Cytokine Release Syndrome after Chimeric Antigen Receptor T-cell Therapy for Acute Lymphoblastic Leukemia. Cancer Discov. 2016; 6:664–79. 10.1158/2159-8290.CD-16-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, Chung SS, Stefanski J, Borquez-Ojeda O, Olszewska M, Qu J, Wasielewska T, He Q, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014; 6:224ra25. 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kiesgen S, Chicaybam L, Chintala NK, Adusumilli PS. Chimeric Antigen Receptor (CAR) T-Cell Therapy for Thoracic Malignancies. J Thorac Oncol. 2018; 13:16–26. 10.1016/j.jtho.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Adusumilli PS, Zauderer MG, Rusch VW, O’Cearbhaill RE, Zhu A, Ngai DA, McGee E, Chintala NK, Messinger JC, Vincent A, Halton EF, Diamonte C, Pineda J, et al. Abstract CT036: A phase I clinical trial of malignant pleural disease treated with regionally delivered autologous mesothelin-targeted CAR T cells: Safety and efficacy. Cancer Res. 2019; 79:CT036 10.1158/1538-7445.AM2019-CT036. [DOI] [Google Scholar]
- 62. Suzuki K, Servais EL, Rizk NP, Solomon SB, Sima CS, Park BJ, Kachala SS, Zlobinsky M, Rusch VW, Adusumilli PS. Palliation and Pleurodesis in Malignant Pleural Effusion: The Role for Tunneled Pleural Catheters. J Thorac Oncol. 2011; 6:762–7. 10.1097/JTO.0b013e31820d614f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.