Abstract
Objective:
To evaluate safety and efficacy of image guided-hypofractionated radiation therapy (IG-HRT) in patients with thoracic nodes oligometastases
Methods:
The present study is a multicenter analysis. Oligometastatic patients, affected by a maximum of five active lesions in three or less different organs, treated with IG-HRT to thoracic nodes metastases between 2012 and 2017 were included in the analysis. Primary end point was local control (LC), secondary end points were overall survival (OS), progression-free survival, acute and late toxicity. Univariate and multivariate analysis were performed to identify possible prognostic factors for the survival end points.
Results:
76 patients were included in the analysis. Different RT dose and fractionation schedules were prescribed according to site, number, size of the lymph node(s) and to respect dose constraints for relevant organs at risk. Median biologically effective dose delivered was 75 Gy (interquartile range: 59–86 Gy). Treatment was optimal; one G1 acute toxicity and seven G1 late toxicities of any grade were recorded. Median follow-up time was 23.16 months. 16 patients (21.05%) had a local progression, while 52 patients progressed in distant sites (68.42 %).
Median local relapse free survival was not reached, LC at 6, 12 and 24 months was 96.05% [confidence interval (CI) 88.26–98.71%], 86.68% (CI 75.86–92.87) and 68.21% (CI 51.89–80.00%), respectively. Median OS was 28.3 months (interquartile range 16.1–47.2). Median progression-freesurvival was 9.2 months (interquartile range 4.1–17.93).
At multivariate analysis, RT dose, colorectal histology, systemic therapies were correlated with LC. Performance status and the presence of metastatic sites other than the thoracic nodes were correlated with OS. Local response was a predictor of OS.
Conclusion:
IG-HRT for thoracic nodes was safe and feasible. Higher RT doses were correlated to better LC and should be taken in consideration at least in patients with isolated nodal metastases and colorectal histology.
Advances in knowledge:
Radiotherapy is safe and effective treatment for thoracic nodes metastases, higher radiotherapy doses are correlated to better LC. Oligometastatic patients can receive IG-HRT also for thoracic nodes metastases.
Introduction
Lymph nodes are a common site of relapse for various solid tumors. Recurrence in the mediastinal nodes are mostly due to non-small-cell lung carcinoma (NSCLC) dissemination, considering that approximately 20% of patients with Stage I disease1 and up to 50% of patients with Stage III disease will develop locoregional relapse.2 However, also other type of solid tumors, such as renal cancer, breast cancer, colorectal cancer, etc. could colonize thoracic nodes. Although often part of a widespread dissemination, in a proportion of patients nodal metastases occur as an isolated site of recurrence, questioning the role of local ablative therapies to improve the prognosis of these patients.
In many different organ sites, in order to ablate all visible metastatic deposits, local therapies are current part of standard treatment, as well as, for instance, in colorectal cancer3 or NSCLC.4 Stereotactic body radiation therapy (SBRT) is one of the most commonly used local ablative therapy, since it is a technique that allows the delivery of very high ablative doses with an excellent sparing of healthy surrounding tissues and a low toxicity. Moreover, SBRT is deliverable in almost all kind of patients and all organ sites.5 In literature, various experiences about SBRT for macroscopic lymphadenopathies have been published. Considering them altogether, these studies report promising results in terms of local control (LC) (1 year LC >90%), symptom control, systemic therapy-free interval and also toxicity.6–10
However, almost all of these published series focused on abdominal and pelvic nodes metastases. Very few and limited data are available on thoracic node metastases, due to their challenging position. Indeed, in the mediastinum various critical structures (e.g. esophagus, great vessels, heart, bronchi and trachea) represent a concrete limitation not only for surgeons but also for radiation oncologist. Severe bronchial stenosis, hemoptysis or fistulas after SBRT for central tumors have been reported by different authors.11–14 Only recently, with the use of more conservative RT schemes, although still delivering a sufficiently high biologically effective dose (BED), safe and effective SBRT came back in the standard treatment of the central lung lesions.15–20
The debate is now moving from central lesions to the so called “ultracentral” lesions. Although no clear definition has been reached, lung lesions with target volumes overlapping the central structures or mediastinal nodes should be included in this new category. The ideal risk-adapted fractionation regimen for treatment of these tumors and whether they can be safely treated with fractionated SBRT is unknown.
We previously reviewed our clinical experience in the treatment of mediastinal nodes oligometastases with SBRT, with encouraging results.21 Now, we combined the data from our patients with those treated for the same indication in two other Italian institutions, with large experience, due to the high volume of patients treated with extracranial SBRT. The aim of the present study is to verify with a larger number of patients the safety and efficacy of image guided-hypofractionated radiation therapy (IG-HRT) for patients with oligometastases in the mediastinal nodes.
Methods and Materials
Institutional databases of three Italian institutions were retrospectively reviewed to collect data about patients treated with IG-HRT for oligometastases in the mediastinal nodes. Eligible patients were defined as oligometastatic if affected by a maximum of five active lesions in three or less different organs. Concomitant, previous or “adjuvant” systemic therapy was allowed and registered. Medical charts were reviewed and the following variables were collected: age, site of the primary tumor, histology of the primary tumor, disease-free interval, performance status at IG-HRT, timing of metastases occurrence (synchronous or metachronous), previous local or systemic therapies, number of disease progressions before the nodal progression, number of the irradiated nodes, dose and fractionation of IG-HRT, presence or absence of “inactive” extra target disease (i.e. metastatic lesions under control after previous local or systemic therapies, not directly irradiated), concomitant or adjuvant systemic therapies to IG-HRT.
BED was calculated assuming an α–β ratio of 10 Gy.
In case different metastases were treated with different doses in the same patient, the lowest BED per patient was used for this analysis.
Equation 1 biologically effective dose (BED). N = number of treatment fractions, d = dose per fraction in Gray (Gy), α/β = dose at which the linear and quadratic components of cell kill are equal.
All patients underwent contrast-enhanced four-dimensional CT scan for target definition and treatment simulation. All patients were immobilized in supine position with thermoplastic masks. The clinical target volume (CTV) was considered equal to the gross tumor volume (GTV), i.e. the metastatic lymph node (s). The internal target volume was obtained through the delineation of the CTV on all four-dimensional CT images. A margin of 5 mm was added to CTV in all directions to generate planning target volume (PTV). Critical structures were: lungs, esophagus/stomach, heart, large vessels, main bronchus/trachea and spinal cord. Patients were treated with SBRT with different fractionation scheme according to nodal size, site and number, organs at risk proximity and previous mediastinal irradiation. Regarding prescription, treatment plan was performed in order to assure 95% of the whole PTV to receive at least 95% of the prescribed dose.
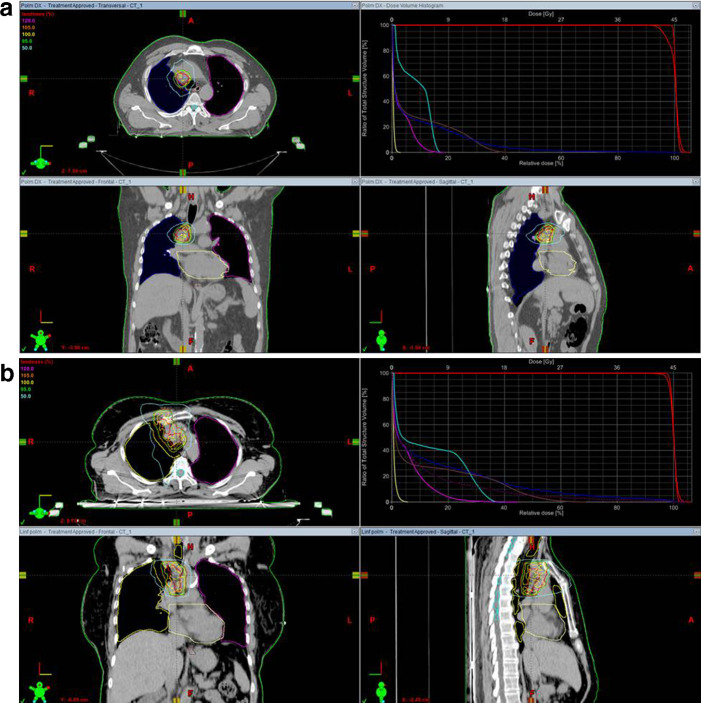
Two examples of treatment plans for single and multiple nodal metastases with isodose curves and dose–volume histogram (DVH) are shown in Figure 1.
Figure 1. .
Examples of isodose coverage and dose–volume histogram in single (a) and multiple (b) nodal metastases.
Treatment image guidance to ensure accurate patient positioning was performed by means of cone beam CT (CBCT) at every session. Patients were evaluated for toxicity halfway during treatment and on the last day of SBRT. As per institutional protocols, patients were followed up every 3 months for the first 2 years, usually with thorax and abdomen CT, routine blood tests and clinical examination, then every 6 months until 5 years after SBRT. Toxicity was scored at each follow-up examination. Patients were included in the analysis if they had at least 6 months follow-up.
Radiological response was evaluated according to RECIST criteria. Acute and late toxicities were scored according to Common Terminology Criteria for Adverse Events v. 4.03.
Primary end point of this analysis was LC, secondary end points were acute and late toxicity, progression-free survival (PFS) and overall survival (OS). Survival times were all calculated from the last day of SBRT.
Statistics were performed in Stata v. 15. The distribution of clinical and demographic characteristics was summarized using percentiles for continuous variables, and percentages and frequencies for categorical variables. All statistical tests were two-sided with significance at p < 0.05.
OS, PFS and LC were assessed with the Kaplan–Meier method and assessment of variables impacting OS, PFS, and LC was performed with univariate and multivariate Cox proportional hazards models. Multivariate Cox proportional hazards models for each end point were constructed based on hypothesized clinical relevance and results of univariate analysis (p < 0.3).
Results
Between January 2012 and December 2017, 76 patients were treated with IG-HRT to mediastinal nodes. Main patients and treatment characteristics are reported in Table 1.
Table 1. .
Baseline demographics and clinical characteristics
| Variable | Median (Interquartile range)/Patients (%) |
|---|---|
| Age at diagnosis of Stage IV | 62.6 years (47.0–71.5) |
| Sex | |
| Male | 37 (48.68%) |
| Female | 39 (51.32%) |
| Primary tumor site | |
| Colon–Rectum | 10 (13.16%) |
| Lung | 35 (46.05%) |
| Upper GI | 6 (7.89%) |
| Breast | 10 (13.16%) |
| Kidney | 4 (5.26%) |
| Othera | 11 (14.47%) |
| Histology of the primary tumor | |
| Adenocarcinoma Squamous cell carcinoma Infiltrating ductal carcinoma |
43 (56.58%) 12 (15.79%) 10 (13.16%) |
| Otherb | 11 (14.47%) |
| Disease free interval | 15.7 months (7.8–25.6) |
| ECOG Performance status | |
| 0 | 43 (56.58%) |
| 1 | 31 (40.79%) |
| 2 | 2 (2.63%) |
| Type of metastases | |
| Synchronous Metachronous |
10 (13.16%) 66 (86.84%) |
| Type of nodal progression | |
| First progression Second or more progression |
37 (48.68%) 39 (51.32%) |
| Previous medical therapies | |
| No | 19 (25.0%) |
| One line | 31 (40.79%) |
| Two lines | 26 (34.21%) |
| Number of treated metastatic nodes | |
| 1 | 61 (80.26%) |
| 2 | 11 (14.47%) |
| 3 | 4 (5.26%) |
| Previous medical therapies for nodal metastases | |
| No | 46 (60.53%) |
| Yes | 30 (39.47%) |
| Previous mediastinal RT No Yes |
65 (85.53%) 11 (14.47%) |
| Presence of extra target disease | |
| No | 41 (53.95%) |
| Yes | 35 (46.05%) |
| BED | 75 Gy (59-86) |
| Adjuvant medical therapies | |
| No | 55 (72.37%) |
| Yes | 21 (27.63%) |
ECOG, Eastern Cooperative Oncology Group; GI, gastrointestinal; RT, radiation therapy.
Other primary tumor sites: ovary, salivary gland, endometrium, prostate, soft tissues, oropharynx, thymus and pleura
Other primary tumor histologies: clear cell carcinoma, neuroendocrine tumor, mesothelioma, thymoma, sarcoma and adenoid cystic carcinoma
Median age at diagnosis of Stage IV was 62.6 years (interquartile range 47.0–71.5). Almost half patients were affected by primary lung cancer, other common histologies were breast cancer, colorectal cancer and renal cancer. Median disease free interval was 15.7 months (interquartile range 7.8–25.6), in 10 patients metastatic nodes diagnosis was synchronous to primary diagnosis. 11 patients had already received RT in the mediastinal region (ranging from 36 Gy in 12 fractions to 66 Gy in 30 fractions).
Different RT dose and fractionation schedules prescribed are summarized in Table 2.
Table 2. .
RT dose and fractionation schedules
| Dose and fractionation | Number of patients | BED |
|---|---|---|
| 5 Gy × five fractions | 3 (4%) | 37.5 Gy |
| 5 Gy × six fractions | 3 (4%) | 45 Gy |
| 6 Gy × five fractions | 8 (10.5%) | 48 Gy |
| 6 Gy × six fractions | 3 (4%) | 57.6 Gy |
| 7 Gy × five fractions | 8 (10.5%) | 59.5 Gy |
| 7.5 Gy × five fractions | 2 (2.6%) | 65.63 Gy |
| 8 Gy five fractions | 11 (14.5%) | 72 Gy |
| 6 Gy × eight fractions | 1 (1.3%) | 76.8 Gy |
| 7.5 Gy × six fractions | 20 (26.3%) | 78.75 Gy |
| 9 Gy × five fractions | 1 (1.3%) | 85.5 |
| 10 Gy × five fractions | 1 (1.3%) | 100 Gy |
| 7.5 Gy × eight fractions | 15 (19.7%) | 105 Gy |
Treatment was well tolerated, just one patient complained about asthenia G1 during SBRT. During follow-up, toxicity of any grade was recorded in seven cases, just one G4. This latter adverse event occurred 6 months after the end of SBRT in a patient previously irradiated on the same area. No G5 toxicity was recorded. Toxicities are summarized in Table 3.
Table 3. .
Acute and late toxicities
| Acute toxicities | Any grade | G0 | G1 | G2 | G3 | G4 | G5 |
|---|---|---|---|---|---|---|---|
| Pneumonitis | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Chest pain | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cough | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dyspnea | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Asthenia | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| Esophagitis | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Late Toxicities | |||||||
| Pneumonitis | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| Cough | 2 | 0 | 2 | 0 | 0 | 0 | 0 |
| Dyspnea | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| Esophagitis | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Chest pain | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fistula | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cardiac toxicity | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| Esophageal ulceration | 1 | 0 | 0 | 2 | 0 | 0 | 0 |
| Bronchial stricture | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
Median follow-up time was 23.16 months (interquartile range 11.96–35.46).
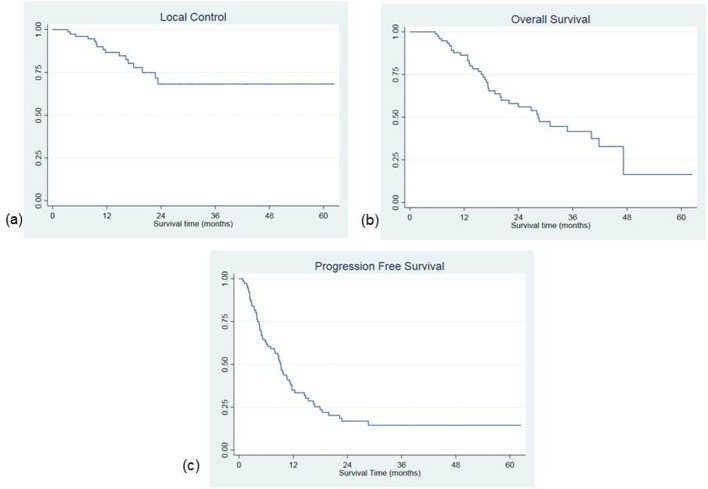
During follow-up, a complete response was recorded in 47 patients (61.84%), partial response in 22 (28.95%) and stable disease in 5 cases (6.58%). Two patients (2.63%) experienced local progression at first radiological evaluation. In other 14 patients, a local progression was recorded during follow-up after an initial response. Most patients progressed in distant sites (52 patients 68.42%) during follow-up. Combining local and distant progression 60 patients (78.95%) experienced a disease relapse. At last follow-up examination, 15 patients were free of disease (19.74%), 3 patients were alive with nodal persisting disease (3.95%), 21 were alive with distant metastases 21 (27.63%). During follow-up, 35 patients (46.05%) died for disease progression, 2 patients died for other non-oncological causes (2.63%). Median local relapse free survival was not reached, LC at 6, 12 and 24 months was 96.05% [confidence interval (CI) 88.26–98.71%], 86.68% (CI 75.86–92.87) and 68.21% (CI 51.89–80.00%), respectively. No more local progression was recorded after 24 months in patients with sufficient follow-up (Figure 2a).
Figure 2. .
Local control (a), overall survival (b) and progression-free survival curves (c)
Median OS was 28.3 months (interquartile range 16.1–47.2). OS at 6, 12, 24 and 36 months was 98.68% (CI 91.03–99.81%), 86.34% (CI 76.07–92.42%), 55.98% (CI 42.61–67.40%) and 41.61% (CI 27.85–54.80%), respectively (Figure 2b). Median PFS was 9.2 months (interquartile range 4.1–17.93). PFS at 6, 12 and 24 months was 63,16% (CI 51.29–72.89%), 34.99% (CI 24.22–45.94%) and 16.84% (CI 8.91–26.92%), respectively (Figure 2c). On multivariate analysis, colorectal primary tumor was correlated with a higher risk of local recurrence [hazard ration (HR) 5.58 (CI 1.43–21.65), p = 0.013]. LC at 1 and 2 years in colorectal cancer patients was 78.75 and 39.37% compared with 87.14 and 71.25% in lung cancer patients. On the contrary, the administration of medical therapies prior to SBRT [HR 0.45 (0.21–0.97), p = 0.043] and a BED >75 Gy [HR 0.30 (0.09–0.94), p = 0.039] were found to be correlated with higher LC rates. In patients receiving a BED > 75 Gy, median LC was not reached, while in patients receiving a BED ≤ 75 Gy it was 23.3 months. 1 and 2 years LC rates according to BED were 91.18 vs 82.3 and 87.67 vs 47.68% respectively (Figure 3).
Figure 3. .
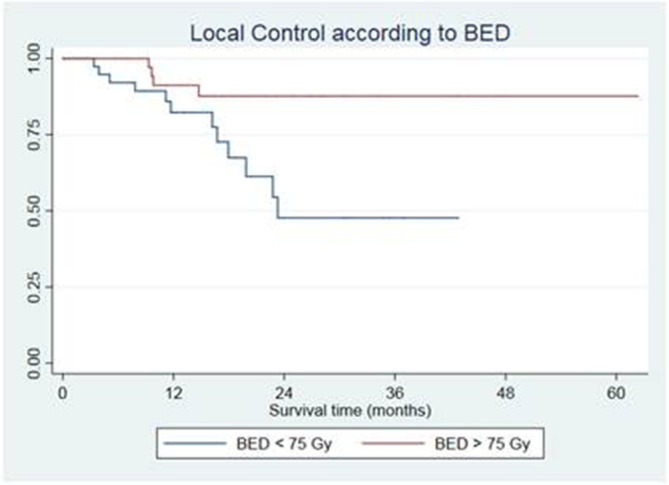
Local control according to BED
On multivariate analysis, the number of metastatic nodes was correlated with longer OS [HR 0.24 (0.09–0.60), p = 0.002]. PS 1 or 2 [HR 2.50 (1.20–5.21), p = 0.014], the number of disease progressions prior to thoracic nodal involvement [2.31 (1.09–4.87), p = 0.028] and the presence of “extratarget” disease [HR 2.93 (1.38–6.21) 0.005] were statistically correlated with OS. Median OS was 41.8 months in patients with isolated nodal relapse vs 17.5 months in patients with other sites of “inactive” disease. The type of local response to IG-HRT also resulted a significant predictor of OS, particularly patients obtaining a partial response had a significantly worse OS [2.15 (1.01–4.54), p = 0.046] if compared with those obtaining a complete response. In patients obtaining a complete response, the median OS was 34.8 months, compared with 16.9 months in patients with partial response, stable or progressive disease (Figure 4).
Figure 4. .
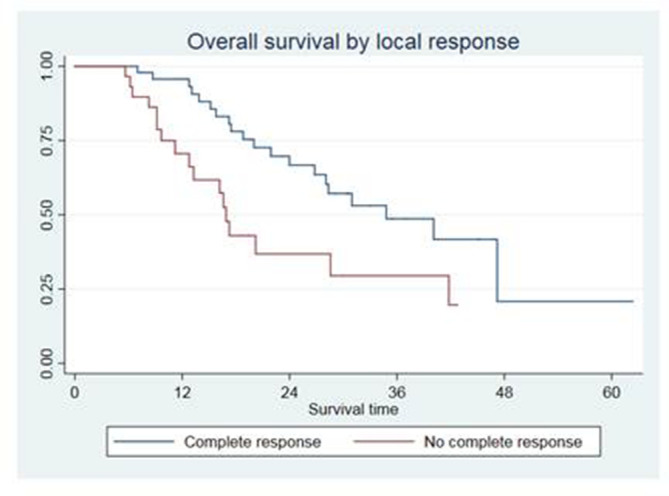
Overall survival according to local response
Discussion
We report a multi-institutional experience on the use of IG-HRT for oligometastatic patients with thoracic nodes metastases. The ideal treatment in this subset of patients still remains unclear. Systemic therapies aimed to prolong survival and prevent/control symptoms are still the standard treatment, since these patients are affected by Stage IV disease. Whether a local ablative therapy, such as surgery or IG-HRT, could improve the OS of patients, delay the progression of the disease, delay the use of systemic therapies (the so called “drug holiday”), similarly to what has been already demonstrated in other clinical situations,11,22,23 is still unclear. Combining the good results coming from the SBRT experiences on abdominal nodes and the steadily increasing use of local ablative therapies in the treatment of oligometastatic patients,5,24 we conducted this retrospective analysis to better clarify if IG-HRT could be safely and effectively prescribed also in patients with thoracic nodes metastases.
The hypothesis tested was that IG-HRT in oligometastatic patients experiencing limited nodal relapse in the mediastinal nodes could impact on the spread of the disease, at least in a proportion of patients, with an acceptable toxicity rate, despite the unfavorable anatomic localization. Secondarily, we also tried to identify possible prognostic factors for LC and OS, which could drive the selection of patients ideally suitable for an aggressive local approach.
According to our experience, we can confirm with a larger number of patients and longer follow-up that IG-HRT for thoracic nodes is safe and feasible. In our experience, we did not find any limiting toxicity. These results also confirm the good tolerability of this treatment showed by Meng et al.25 In their experience, three patients (9%) experienced Grade 3 acute toxicities including esophagitis and tracheitis, particularly in patients who were already irradiated in the mediastinal area. Authors also reported four patients (12%) with late toxicities and two G5 toxicities during follow-up. Noteworthy, in our patients, also those already irradiated in the same area well tolerated the treatment. Although mostly treated with lower doses (30 Gy in five fractions or 36 Gy in six fractions in the majority of cases), we recorded in this subgroup of patients only one G4 adverse event, no other toxicity was recorded.
Apart from the feasibility, IG-HRT seems also effective. In our experience, we found a LC rate of 86.68% at 1 year and 68.21% at 2 and 3 years. These results are in line with similar reports of 90% LC at 1 year for abdominal lymph nodes.5–10 However, it is inferior to the results reported by Meng et al, with 1 year and 3 year actuarial LC rates of 100 and 85.5%.25 Apart from the longer follow-up in our series, the higher number of patients treated and a different patients selection compared to Meng et al that included only patients affected by primary NSCLC, a partial explanation for this observation could derive from the different median BED delivered (75 Gy in our experience vs 83 in the experience by Meng et al). Indeed, we found that BED is a significant predictor for LC, patients treated with a BED >75 Gy had a higher LC when compared to patients treated with a lower dose (1 and 2 years LC rates: 91.18 vs 82.3 and 87.67 vs 47.68% respectively).
In our experience, another parameter able to influence LC was the primary histology. Specifically, colorectal cancer patients had a higher risk of local recurrence, with a median LC time of 22.8 months, while it was still not reached for other primary cancers. This result confirms the already well-recognized radioresistance of colorectal cancer metastases. Indeed, in many experiences, for instance on SBRT for lung or liver metastases, a primary colorectal histology is often correlated with lower LC rates.26–28 Therefore, a higher RT dose in case of primary colorectal histology should be taken in consideration to improve the LC rates. In lung metastases, a similar approach proved effective.29 We are aware of the limitations of the present study, mostly the retrospective nature and the heterogeneity of patients and treatments. However, to our knowledge, this is the largest series of SBRT on thoracic nodes.
Conclusion
In the present large multicenter experience on IG-HRT for thoracic nodes oligometastases, we show that the safety and feasibility of the approach, also in patients already irradiated in the mediastinal region. LC rates achieved are satisfactory. Nevertheless, considering that local response will influence OS of these patients, a slight increase of the delivered BED could further maximize results.
Contributor Information
Davide Franceschini, Email: davide.franceschini@humanitas.it.
Federico Bianciardi, Email: davide.franceschini@humanitas.it.
Rosario Mazzola, Email: davide.franceschini@humanitas.it.
Fiorenza De Rose, Email: davide.franceschini@humanitas.it.
Piercarlo Gentile, Email: davide.franceschini@humanitas.it.
Filippo Alongi, Email: davide.franceschini@humanitas.it.
Marta Scorsetti, Email: davide.franceschini@humanitas.it.
REFERENCES
- 1.Trodella L, Granone P, Valente S, Valentini V, Balducci M, Mantini G, et al. . Adjuvant radiotherapy in non-small cell lung cancer with pathological stage I: definitive results of a phase III randomized trial. Radiother Oncol 2002; 62: 11–19. doi: 10.1016/S0167-8140(01)00478-9 [DOI] [PubMed] [Google Scholar]
- 2.Dautzenberg B, Arriagada R, Chammard AB, Jarema A, Mezzetti M, Mattson K, et al. . A controlled study of postoperative radiotherapy for patients with completely resected nonsmall cell lung carcinoma. Groupe d'Etude et de Traitement des cancers Bronchiques. Cancer 1999; 86: 265–73. doi: [DOI] [PubMed] [Google Scholar]
- 3.Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, et al. . ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 2016; 27: 1386–422. doi: 10.1093/annonc/mdw235 [DOI] [PubMed] [Google Scholar]
- 4.NCCN Guidelines version. 2018; 5. [Google Scholar]
- 5.Alongi F, Arcangeli S, Filippi AR, Ricardi U, Scorsetti M. Review and uses of stereotactic body radiation therapy for oligometastases. Oncologist 2012; 17: 1100–7. doi: 10.1634/theoncologist.2012-0092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Detti B, Bonomo P, Masi L, Doro R, Cipressi S, Iermano C, et al. . Stereotactic radiotherapy for isolated nodal recurrence of prostate cancer. World J Urol 2015; 33: 1197–203. doi: 10.1007/s00345-014-1427-x [DOI] [PubMed] [Google Scholar]
- 7.Bignardi M, Navarria P, Mancosu P, Cozzi L, Fogliata A, Tozzi A, et al. . Clinical outcome of hypofractionated stereotactic radiotherapy for abdominal lymph node metastases. Int J Radiat Oncol Biol Phys 2011; 81: 831–8. doi: 10.1016/j.ijrobp.2010.05.032 [DOI] [PubMed] [Google Scholar]
- 8.Jereczek-Fossa BA, Piperno G, Ronchi S, Catalano G, Fodor C, Cambria R, et al. . Linac-based stereotactic body radiotherapy for oligometastatic patients with single abdominal lymph node recurrent cancer. Am J Clin Oncol 2014; 37: 227–33. doi: 10.1097/COC.0b013e3182610878 [DOI] [PubMed] [Google Scholar]
- 9.Alongi F, Fogliata A, Clerici E, Navarria P, Tozzi A, Comito T, et al. . Volumetric modulated Arc therapy with flattening filter free beams for isolated abdominal/pelvic lymph nodes: report of dosimetric and early clinical results in oligometastatic patients. Radiat Oncol 2012; 7: 204. doi: 10.1186/1748-717X-7-204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ost P, Jereczek-Fossa BA, Van As N, Zilli T, Tree A, Henderson D, et al. . Pattern of progression after stereotactic body radiotherapy for oligometastatic prostate cancer nodal recurrences. Clin Oncol 2016; 28: e115–20. doi: 10.1016/j.clon.2016.04.040 [DOI] [PubMed] [Google Scholar]
- 11.Timmerman R, McGarry R, Yiannoutsos C, Papiez L, Tudor K, DeLuca J, et al. . Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. JCO 2006; 24: 4833–9. doi: 10.1200/JCO.2006.07.5937 [DOI] [PubMed] [Google Scholar]
- 12.Milano MT, Chen Y, Katz AW, Philip A, Schell MC, Okunieff P, et al. . Central thoracic lesions treated with hypofractionated stereotactic body radiotherapy. Radiother Oncol 2009; 91: 301–6. doi: 10.1016/j.radonc.2009.03.005 [DOI] [PubMed] [Google Scholar]
- 13.Song SY, Choi W, Shin SS, Lee S-W, Ahn SD, Kim JH, et al. . Fractionated stereotactic body radiation therapy for medically inoperable stage I lung cancer adjacent to central large bronchus. Lung Cancer 2009; 66: 89–93. doi: 10.1016/j.lungcan.2008.12.016 [DOI] [PubMed] [Google Scholar]
- 14.Corradetti MN, Haas AR, Rengan R. Central-airway necrosis after stereotactic body-radiation therapy. N Engl J Med 2012; 366: 2327–9. doi: 10.1056/NEJMc1203770 [DOI] [PubMed] [Google Scholar]
- 15.Senthi S, Haasbeek CJA, Slotman BJ, Senan S. Outcomes of stereotactic ablative radiotherapy for central lung tumours: a systematic review. Radiother Oncol 2013; 106: 276–82. doi: 10.1016/j.radonc.2013.01.004 [DOI] [PubMed] [Google Scholar]
- 16.Modh A, Rimner A, Williams E, Foster A, Shah M, Shi W, et al. . Local control and toxicity in a large cohort of central lung tumors treated with stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2014; 90: 1168–76. doi: 10.1016/j.ijrobp.2014.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mangona VS, Aneese AM, Marina O, Hymas RV, Stromberg D, Ionascu D, et al. . Clinical outcomes after central versus peripheral stereotactic lung radiation therapy (SBRT): a propensity score matched-pair analysis. Int J Radiat Oncol Biol Phys 2013; 87: S34. doi: 10.1016/j.ijrobp.2013.06.1356 [DOI] [PubMed] [Google Scholar]
- 18.Nishimura S, Takeda A, Sanuki N, Ishikura S, Oku Y, Aoki Y, et al. . Toxicities of organs at risk in the mediastinal and hilar regions following stereotactic body radiotherapy for centrally located lung tumors. J Thorac Oncol 2014; 9: 1370–6. doi: 10.1097/JTO.0000000000000260 [DOI] [PubMed] [Google Scholar]
- 19.Schanne DH, Nestle U, Allgäuer M, Andratschke N, Appold S, Dieckmann U, et al. . Stereotactic body radiotherapy for centrally located stage I NSCLC: a multicenter analysis. Strahlenther Onkol 2015; 191: 125–32. doi: 10.1007/s00066-014-0739-5 [DOI] [PubMed] [Google Scholar]
- 20.Chang JY, Li Q-Q, Xu Q-Y, Allen PK, Rebueno N, Gomez DR, et al. . Stereotactic ablative radiation therapy for centrally located early stage or isolated parenchymal recurrences of non-small cell lung cancer: how to fly in a "no fly zone". Int J Radiat Oncol Biol Phys 2014; 88: 1120–8. doi: 10.1016/j.ijrobp.2014.01.022 [DOI] [PubMed] [Google Scholar]
- 21.Franceschini D, De Rose F, Fogliata A, Navarria P, Ascolese AM, Franzese C, et al. . Volumetric modulated Arc therapy for thoracic node metastases: a safe and effective treatment for a neglected disease. Oncotarget 2016; 7: 53321–9. doi: 10.18632/oncotarget.10826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong JC, Ayala-Peacock DN, Lee J, Blackstock AW, Okunieff P, Sung MW, et al. . Classification for long-term survival in oligometastatic patients treated with ablative radiotherapy: a multi-institutional pooled analysis. PLoS One 2018; 13: e0195149. doi: 10.1371/journal.pone.0195149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong AC, Watson SP, Pitroda SP, Son CH, Das LC, Stack ME, et al. . Clinical and molecular markers of long-term survival after oligometastasis-directed stereotactic body radiotherapy (SBRT. Cancer 2016; 122: 2242–50. doi: 10.1002/cncr.30058 [DOI] [PubMed] [Google Scholar]
- 24.De Bari B, Alongi F, Buglione M, Campostrini F, Briganti A, Berardi G, et al. . Salvage therapy of small volume prostate cancer nodal failures: a review of the literature. Crit Rev Oncol Hematol 2014; 90: 24–35. doi: 10.1016/j.critrevonc.2013.11.003 [DOI] [PubMed] [Google Scholar]
- 25.Meng M-B, Wang H-H, Zaorsky NG, Zhao X-Z, Wu Z-Q, Jiang B, et al. . Clinical evaluation of stereotactic radiation therapy for recurrent or second primary mediastinal lymph node metastases originating from non-small cell lung cancer. Oncotarget 2015; 6: 15690–703. doi: 10.18632/oncotarget.3704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmed KA, Caudell JJ, El-Haddad G, Berglund AE, Welsh EA, Yue B, et al. . Radiosensitivity differences between liver metastases based on primary histology suggest implications for clinical outcomes after stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2016; 95: 1399–404. doi: 10.1016/j.ijrobp.2016.03.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takeda A, Kunieda E, Ohashi T, Aoki Y, Koike N, Takeda T, et al. . Stereotactic body radiotherapy (SBRT) for oligometastatic lung tumors from colorectal cancer and other primary cancers in comparison with primary lung cancer. Radiother Oncol 2011; 101: 255–9. doi: 10.1016/j.radonc.2011.05.033 [DOI] [PubMed] [Google Scholar]
- 28.Franceschini D, Cozzi L, De Rose F, Navarria P, Franzese C, Comito T, et al. . Role of stereotactic body radiation therapy for lung metastases from radio-resistant primary tumours. J Cancer Res Clin Oncol 2017; 143: 1293–9. doi: 10.1007/s00432-017-2373-y [DOI] [PubMed] [Google Scholar]
- 29.Helou J, Thibault I, Poon I, Chiang A, Jain S, Soliman H, et al. . Stereotactic ablative radiation therapy for pulmonary metastases: histology, dose, and indication matter. Int J Radiat Oncol Biol Phys 2017; 98: 419–27. doi: 10.1016/j.ijrobp.2017.02.093 [DOI] [PubMed] [Google Scholar]