Abstract
Objective:
To determine incidence of internal mammary nodes (IMN) at baseline CT of locally advanced breast cancer (LABC) and ascertain prognostic implication.
Methods and materials:
Retrospective review of all LABC patients from 1 January 2012 through 31 December 2014 was performed after approval from institutional review board. CTs of 182 patients enrolled were reviewed by two radiologists independently, and IMNs were documented based on size, location and relation with location of breast primary. 3-year follow-up was analysed and incidence of metastases was calculated as overall incidence, incidence in patients with and without discernible IMN at baseline imaging. Results are presented as numbers and percentages. Differences in metastases of two groups were compared using χ2 test. 95% CI was calculated and p < 0.05 was considered significant.
Results:
77 of 182 had identifiable IMN (42.3% incidence). Majority of identifiable nodes were on ipsilateral side of primary (incidence 90.90%) with higher incidence in patients with upper-outer quadrant tumours (55.9%). Majority were seen in second intercostal space (44.4%). 36 (19.7%) developed distant metastases within 3 years of therapy. Of these, 21 (27.3%) had IMN as compared with 15 (14.3 %) without IMN on baseline imaging. Patients with identifiable IMN on baseline CT had significantly higher incidence of distant metastases (p = 0.0321).
Conclusion:
Significant number LABC patients have identifiable IMN on baseline imaging with patients showing IMN on baseline CT showing significantly higher rate of metastatic disease following therapy.
Advances in knowledge:
Many LABC patients have identifiable IMNs on baseline imaging which show higher incidence of subsequent metastatic disease.
Introduction
Breast cancer is the most common cancer affecting females worldwide. With the growing emphasis on personalised medicine to deliver treatment according to biology of tumour, a precise staging of breast cancer is needed. The staging includes a comprehensive clinical examination and imaging.
Clinically, the breast cancers may be classified as1: operable breast cancer, locally advanced breast cancer (LABC) and metastatic breast cancer. AJCC-TNM (American Joint Committee on Cancer Tumor Node Metastasis) classification2 is widely employed in staging breast cancer. LABC includes stages IIB (T3N0M0), III and IV. Hence, the following are considered as locally advanced: (1), any tumour >5 cm in maximum dimensions; (2), tumour involving the chest wall; (3), tumour with skin involvement which includes skin oedema, peau d’ orange, ulceration of overlying skin and/or satellite nodules; and (4), clinically extensive nodal involvement: N2 or N3 disease. In addition, the following scenarios are also regarded as LABC: (a), T2 tumours but appearing large in proportion to size of the breasts3 and (b), inflammatory breast cancer.4 Different types of LABC have been tabulated in Table 1.5 Example of a LABC is shown in Figure 1.
Table 1.
Locally advanced breast cancer: different clinical presentations
| Operable breast cancer at initial presentation | Inoperable breast cancer at initial presentation |
|---|---|
| STAGE IIB | STAGE IIIB |
| T3N0M0 | T4a (skin), T4b (chest wall) or T4c (both skin and chest wall) stage. |
| STAGE IIIA | STAGE IIIC |
| T3 tumour with any N stage N2 stage with T1, T2 or T3 stage |
N3 stage with any T stage. |
| Also, may be considered as locally advanced breast cancer | |
| T2 tumours but appearing large in proportion to size of the breasts Inflammatory Ca breast – T4d. | |
Figure 1.
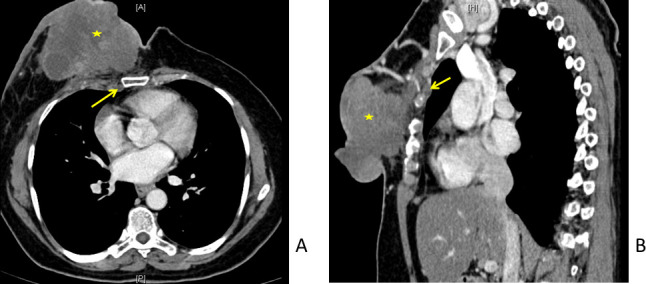
51-year-old female with locally advanced breast cancer of right breast. Contrast-enhanced CT scan in axial (A) and sagittal (B) planes. A large right breast malignancy (*) involving nearly all quadrants, with involvement of overlying skin and underlying muscle: T4c. Also seen is enlarged internal mammary node (arrows).
The main draining group of lymph nodes for breast is the axillary group. The internal mammary group constitutes an important pathway of lymphatic drainage which lies along the internal mammary vessels (shown in Figure 2). These get involved by the disease when there is obstruction of lymphatics to the axillary group by tumour cells. It is very rare to find isolated internal mammary node (IMN) metastases in absence of enlarged axillary nodes.6 This pathway is also important in cases of breast cancer that have undergone surgery with axillary node clearance. The postoperative status results in blockade in axillary drainage and opens up alternate pathways such as IMN. Hence, recurrent breast cancers in IMN are quite common. Metastases to IMN have been well-documented as site of metastases and also as an important independent prognostic factor.7
Figure 2.
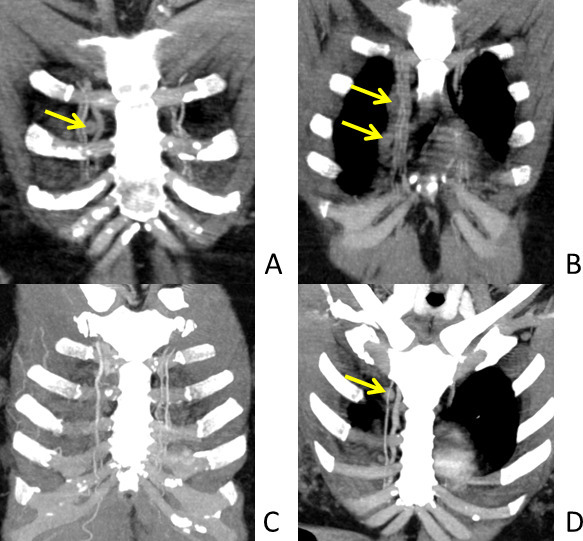
Location of internal mammary nodes (IMN). Oblique coronal views (A & B) and oblique coronal views with maximum intensity projection (MIP) (C and D). The above images show course of internal mammary arteries in parasternal region. The vessels are better visualised on MIP images. IMN are located along these vessels as shown by the arrows.
Few studies have demonstrated that PET-CT has higher sensitivity as compared with contrast-enhanced CT (CECT) in detecting IMN. An 85% sensitivity and 90% specificity have been reported for PET by Eubank et al.8 Another study by Segaert et al compared sensitivity of PET-CT with that of CECT.9 They reported 100% sensitivity and 85% specificity of PET-CT as opposed to sensitivity of 67% for CECT. Another large study by Wang et al10 retrospectively analysed baseline PET/CT imaging of patients and correlated the findings with fine needle aspiration cytology (FNAC) (ultrasound-guided) of IMN. They concluded that high-grade tumours, tumours with evidence of lympohovascular spread and triple negative breast cancers (cancers which are negative for all hormonal receptors: estrogen receptor (ER), progesterone receptor (PR) and HER2/Neu) were high-risk factors for internal mammary nodal metastasis.
The purpose of our study was to determine the incidence IMN on baseline CT in patients with LABC. We also correlated its association with metastatic disease by detecting the incidence of metastatic disease at other sites in cohorts with and without enlarged IMN at baseline imaging over follow-up.
Methods and materials
This is a single institute retrospective study performed after approval from institute review board. Patients with clinical LABC undergo CECT of thorax, abdomen and pelvis along with bone scan at our institute as a part of staging. Waiver of consent was obtained from the ethics committee for this retrospective study to audit the scans and review the medical records of these patients.
Patients
A retrospective review of our database of all consecutive LABC patients presenting from 1 January 2012 through 31 December 2014 was performed. All consecutive patients with clinical and operable LABC were screened. Patients found to have distant metastases at baseline imaging were excluded from the study. A total of 196 consecutive patients of LABC were screened. Of these, 14 patients had metastatic disease on baseline study and hence were excluded. A total of 182 patients were enrolled in the study. All patients were females and the age ranged from 25 to 82 years. All underwent treatment with curative intent: 131 patients underwent breast conservative surgery and 51 patients underwent mastectomy.
Imaging
All LABC patients undergo CECT scan of thorax, abdomen and pelvis along with Technetium methylene diphosphonate (99mTc-MDP) bone scan as part of metastatic work-up at our institute. These patients underwent CECT on a 16-slice multidetector CT scanner (Siemens Somatom Sensation 16, Seimens Healthineers, Erlangen, Germany) after administration of 80–100 ml intravenous contrast medium. The parameters employed were:150 kVp, 150–300 mA, 25 mm collimation, 1.5 mm slice thickness and 70 cm display FOV. The patients were scanned in supine position. Soft copy reconstructions were performed on dedicated workstations. The radiological reports and images were reviewed by two radiologists independently on a dedicated workstation after obtaining multiplanar reformats (Figure 3). One radiologist has experience of 30 years in breast radiology and another 3 years of experience in radiology. In cases of discrepancy, a consensus meeting was carried out. Nodes were not classified as benign or pathological based on imaging characteristics. All identifiable IMNs were documented in terms of size in short axis, number of nodes and their location with respect to the intercostal space. They were also documented as to whether they were ipsilateral or contralateral. The incidence of IMN with respect to location of breast primary quadrant location was also calculated. Correlation of these patients with tumour biology was carried out. Follow-up medical records of these patients up to 31 December 2017 were reviewed to look for development of distant metastases to sites like liver, lung, non-regional nodes, bones, brain etc. All patients’ follow-up notes by treating physicians are documented digitally on our electronic medical records system. No patient was lost to follow-up or succumbed during follow-up. Metastases were considered when the patients had unequivocal imaging findings of metastases, subsequently proven on FNAC and/or fluid cytology.
Figure 3.
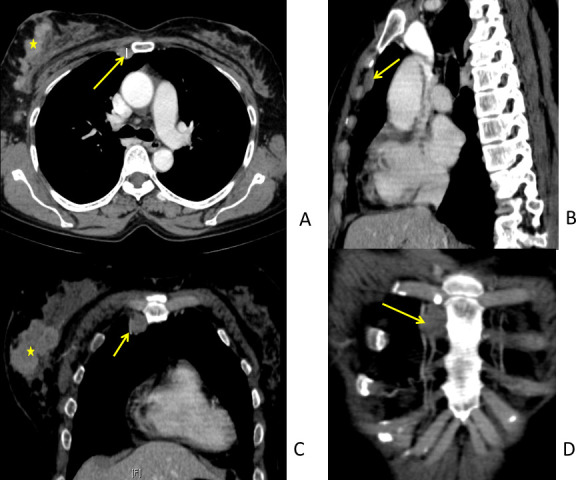
43-year-old female with right LABC. Role of multiplanar imaging. Axial (A), Sagittal (B), Coronal (C) and Oblique Coronal images to visualise the internal mammary nodes (IMN). Sagittal images are useful to locate the IMN (arrows) with respect to the intercostal space. Oblique coronals define the relation with internal mammary vessels well. The method of measuring IMN in short axis (A) is demonstrated. Note of enhancing large tumour (>5 cm) (*) in right breast made.
Statistical analysis
Descriptive statistical analysis was performed. The incidence of identifiable IMN on imaging of these patients was determined. The distribution of these nodes with respect to their size and location in intercostal space was assessed along with incidence with respect to quadrant location of breast primary and incidence of nodes present on the ipsilateral or contralateral side. Incidence of distant metastases over follow-up in these patients was calculated. Incident rates of distant metastases in patients with and without identifiable IMNs on follow-up were also calculated. The differences in rates of metastases of two groups were compared using χ2 test and 95% confidence interval (CI) with null hypothesis that there is no difference in metastatic rates between patients showing IMN and patients not showing IMN on baseline imaging. A p-value of less than 0.05 was considered significant. Medcalc statistical software was used to calculate the p-value.
Results
Of 182 patients enrolled, IMNs were identified in a total of 77 patients (age range from 25 to 82 years). All patients were females. The incidence of IMN in LABC in our study was 77/182 (42.3%). The demographics and follow-up analysis have been tabulated in Table 2. A total of 115 nodes were identified in these 77 females with an average of 1.5 nodes per female. The average size of these 115 nodes was 6.7 mm. The distribution of internal mammary with respect to size, intercostal spaces, relation with breast primary quadrant and laterality with respect to primary is presented in Table 3.
Table 2.
Patient demographics of the study
| Patient Subset | Total Number | Percentage (%) |
|---|---|---|
| Consecutive LABC patients presenting from 1 January 2012 to 31 December 2014 | 196 | |
| Distant metastases on baseline imaging (excluded from the study) | 14 | 7.1 |
| Number enrolled | 182 | 92.9 |
| Identifiable IMN on CT scan | 77 | 42.3 |
| Without any identifiable nodes on CT scan | 105 | 57.7 |
| Lost to follow-up | 0 | 0 |
| Distant metastases on follow-up | 36/182 | 19.8 |
| Distant metastases on follow-up and identifiable IMN at baseline imaging | 21/77 | 27.3 |
| Distant metastases on follow-up and without any identifiable IMN at baseline imaging | 15/105 | 14.3 |
Table 3.
Size, location and laterality distribution of identifiable internal mammary nodes (total nodes identified in 77 patients were 115)
| Number of Nodes |
Percentage (%) |
|
|---|---|---|
| Size (measured in short axis) distribution of identifiable internal mammary nodes (IMN) | ||
| <4 mm | 23 | 20.0 |
| 4–6 mm | 28 | 24.4 |
| 6–8 mm | 29 | 25.2 |
| 8–10 mm | 16 | 13.9 |
| >10 mm | 19 | 16.5 |
| Distribution of identifiable IMN with respect to intercostal space | ||
| first Intercostal space | 40 | 34.8 |
| second Intercostal space | 51 | 44.4 |
| third Intercostal space | 19 | 16.5 |
| fourth Intercostal space | 5 | 4.3 |
| Location of identifiable IMN with respect to breast primary quadrant location | ||
| Upper outer quadrant | 61 | 53.0 |
| Upper inner quadrant | 17 | 14.8 |
| Lower outer quadrant | 11 | 9.6 |
| Lower inner quadrant | 10 | 8.7 |
| Multicentric or tumour involving all quadrants | 16 | 13.9 |
| Laterality of identifiable IMN with respect to breast primary | ||
| Ipsilateral | 70/77 | 90.9 |
| Contralateral | 1/77 | 1.3 |
| Bilateral | 6/77 | 7.8 |
55.7% (64/115) of identifiable IMNs were greater than 6 mm in size. Majority of the IMNs were localised to the second intercostal space [51/115 (44.4%)]. In our study, incidence of IMN was higher with upper-outer quadrant of breast tumours [61/115 (53.0%)]. 70 out of 77 patients had ipsilateral internal mammary adenopathy (90.9%), whereas only one patient had contralateral internal mammary adenopathy (1.3%). Six patients had bilateral internal mammary adenopathy (7.8%). Histopathological grading and hormonal receptor status were also reviewed and correlated with these patients. Details of same has been tabulated in Table 4.
Table 4.
IMN and tumour biology
| IMN and Correlation with Tumour Grade | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiable IMN on Baseline CT (n = 77) | No Identifiable IMN on Baseline CT (n = 105) | ||||||||||
| Grade 1 | 0 | 0% | Grade 1 | 01 | 0.01% | ||||||
| Grade 2 | 6 | 7.80% | Grade 2 | 13 | 12.38% | ||||||
| Grade 3 | 71 | 92.20% | Grade 3 | 91 | 87.61% | ||||||
| Identifiable IMN with subsequent Metastases (n = 21) | No Identifiable IMN with subsequent Metastases (n = 15) | ||||||||||
| Grade 1 | 0 | 0% | Grade 1 | 0 | 0% | ||||||
| Grade 2 | 2 | 9.52% | Grade 2 | 0 | 0% | ||||||
| Grade 3 | 19 | 90.48% | Grade 3 | 15 | 100% | ||||||
| IMN and Correlation with Hormonal Receptor Status | |||||||||||
| Identifiable IMN on Baseline CT (n = 77) | No Identifiable IMN on Baseline CT (n = 105) | ||||||||||
| ER | PR | Her2/Neu | ER | PR | Her2/Neu | ||||||
| + | + | + | 10 | 12.99% | + | + | + | 10 | 9.52% | ||
| + | + | - | 19 | 24.66% | + | + | - | 35 | 33.33% | ||
| + | - | - | 9 | 11.69% | + | - | - | 8 | 7.62% | ||
| - | - | + | 13 | 16.88% | - | - | + | 15 | 14.29% | ||
| - | + | + | 0 | 0% | - | + | + | 1 | 0.10% | ||
| + | - | + | 3 | 3.90% | + | - | + | 3 | 2.86% | ||
| - | + | - | 0 | 0% | - | + | - | 0 | 0% | ||
| - | - | - | 23 | 29.88% | - | - | - | 33 | 32.28% | ||
| Identifiable IMN with subsequent Metastases (n = 21) | No Identifiable IMN with subsequent Metastases (n = 15) | ||||||||||
| ER | PR | Her2/Neu | ER | PR | Her2/Neu | ||||||
| + | + | + | 1 | 4.76% | + | + | + | 2 | 13.34% | ||
| + | + | - | 6 | 28.57% | + | + | - | 5 | 33.33% | ||
| + | - | - | 3 | 14.29% | + | - | - | 0 | 0% | ||
| - | - | + | 6 | 28.57% | - | - | + | 2 | 13.34% | ||
| - | + | + | 0 | 0% | - | + | + | 0 | 0% | ||
| + | - | + | 1 | 4.76% | + | - | + | 1 | 6.66% | ||
| - | + | - | 0 | 0% | - | + | - | 0 | 0% | ||
| - | - | - | 4 | 19.05% | - | - | - | 5 | 33.33% | ||
Follow-up records of all these patients upto 31 December 2017 were reviewed to look for occurrence of distant metastases. All 182 patients underwent treatment with curative intent: 131 patients underwent breast conservative surgery and 51 patients underwent mastectomy. All patients received adjuvant radiotherapy and chemotherapy. None were lost to follow-up. 36/182 (19.8%) of patients developed distant metastases. Of these, 21/77 (27.3%) of patients had baseline identifiable IMN as compared with 15/105 (14.3%) without identifiable IMN on baseline CT. Patients with identifiable IMN on baseline CT had significantly higher incidence of distant metastases (p = 0.0321). The odds ratio and relative risk (Table 5) were calculated using Medcalc Software. The relative risk for development of distant metastases in patients with identifiable internal nodes on baseline imaging was found to be 2.2 with 95% CI of 1.2003 to 3.9660. The p-value was found to be 0.0321 with 95% CI of 1.0715 to 4.7245 (statistically significant).
Table 5.
Odds ratio and relative risk
| Internal mammary nodes seen on baseline imaging | No internal mammary nodes seen on baseline imaging | Total | |
|---|---|---|---|
| Distant metastases on 3 years follow-up | 21 | 15 | 36 |
| No distant metastases on 3 years follow-up | 56 | 90 | 146 |
| Total | 77 | 105 | 182 |
| Odds ratio results | Relative risk results | ||
| Odds ratio 2.3 95% Confidence interval 1.0715 to 4.7245 Z statistic 2.143 Significance level p = 0.0321 Effect Size 2.25 |
Relative risk 2.2 95% Confidence interval 1.2003 to 3.9660 Z statistic 2.559 Significance level p = 0.0105 NNT (Harm) 6.769 |
||
Discussion
LABCs are clinically advanced tumours and have a greater probability of distant metastases. Breast cancers are treated with a combination of surgery, adjuvant chemotherapy and radiotherapy depending on the stage at presentation.1,5,11 LABCs are offered multimodality/multidisciplinary treatment, which include neoadjuvant chemotherapy, surgery, loco regional radiotherapy and adjuvant chemotherapy. More and more advanced tumours are being offered breast conservation surgeries after instituting adequate neoadjuvant chemotherapy. Hence, these require a comprehensive metastatic workup. The various imaging modalities employed include chest radiograph, ultrasound of abdomen and pelvis, Technetium methylene diphosphonate (99mTc-MDP) bone scan, CECT scan of thorax, abdomen and pelvis and seldom PET-fluoride for skeletal metastases. CT is recommended for clinically advanced breast cancers. It serves to assess the locoregional extent (primary, axillary, supraclavicular adenopathy etc.) and for evaluating enlarged IMN and distant metastases involving lungs, liver, adrenals, bones etc. Fluorodeoxyglucose (18F-FDG) PET/CT is also useful and is more sensitive to detect suspicious IMN.10
Lymphatic drainage of breast12,13
IMN are an important accessory pathway of lymphatic drainage in breast tumours. These are important site of tumour spread both at the time of presentation and as a site of disease recurrence. These lymphatics may be involved in a retrograde manner from blockage of axillary lymphatics due to obstruction by tumour cells. Postoperatively, the axillary lymphatics may also be blocked and result in drainage to the IMN. There is intricate anastomosis between the lymphatics of breast and overlying dermal lymphatics. This was first described by Oelsner and proved by Rouvie`re. Locoregional spread of breast cancers has largely been studied by either injecting dyes or radioactive substances. These have demonstrated that there are no rigid compartmentalisation of the breast lymphatics. Breast lymphatics accompany the ducts of breast tissue. From here on, they drain into the plexus of Sappey in subareolar region14 and then into the axillary group mainly via two lymphatic trunks, the lymphatica mammaria magna.15
Some studies have validated IMN as an independent prognostic factor in breast cancers.16 These studies have shown decreased survival and increased rate of distant metastases. In another large series of patients, 10-year survival rates for patients with histologically documented internal mammary nodal metastases were calculated, which revealed that survival rates in axillary nodal metastases to that of internal mammary nodal metastases were identical.17 In addition, it was found that there was considerable reduction in 10-year survival rate with concomitant metastases to axillary and internal mammary group nodes. In another study, the survival was significantly lower in patients presenting with internal mammary nodal metastasis as compared with those presenting with axillary nodal metastases.18
Imaging has come to play an important role in detection of suspicious IMN. The gold standard for ascertaining metastatic status of IMN is surgical sampling. However, majority of surgeons do not practice sampling of IMN during breast surgery. Two large studies by European group performing sampling of IMN have reported overall improved survival in these patients: due to realisation of more tailored therapy and more accurate staging.19,20 It has also been shown that undetected metastatic IMNs, especially in inner quadrant tumours, have worse prognosis.21
Imaging for IMN is also largely unexplored. However, to the best of our knowledge, there are no large prospective trials establishing size criteria for abnormal IMN. However, a recent study showed average size of IMN to be 4.5 mm in patients who underwent high-risk breast MRI screening without any proven malignancy.22 Similarly, there is need to compare the various imaging modalities (CT, PET-CT, MRI) for sensitivity and specificity in detection of IMN. It must be noted that normal appearing or non-visualisable IMNs may still be sites of micrometastases.
The management of IMN is controversial. In early 19th century, these were routinely sampled along with radical breast surgeries. However, subsequently, randomised controlled trials demonstrated no significant difference in survival rates of patients’ undergoing IMN sampling versus those undergoing no sampling of these nodes. Three major randomised control trials demonstrated no benefit in terms of survival rates.23–25 Following this, the practice of IMN sampling was given up as these procedures involved considerable morbidity.26 Later, suspicious IMNs were offered radiation. However, this is associated with considerable cardiac morbidity. Hence, even in patients with visualised primary IMN drainage, the potential benefit of treatment should be balanced against the risk of added morbidity.
Recent studies have shown that suspicious IMN is a significant prognostic factor for long-term survival in patients with breast cancer. Presence of IMN is associated with a worse prognosis. In addition, the incidence of IMN is more commonly seen in inner quadrant tumours27 and with ER/PR positive status. IMNs are also an important site for recurrent breast cancer.28 A recent study evaluated locoregional failures involving IMN and found that patients with isolated IMN recurrences had longer survival if treated with aggressive therapies.29 Another study found that radiologically abnormally appearing IMN were more often associated with aggressive tumour biology (higher tumour grade, ER negative status) and advanced stage.30 These patients responded well to radiation therapy without any need for surgical intervention.
Out study revealed that nearly half the patients with LABC show identifiable IMN on baseline imaging and that these patients showed statistically significant higher rates of metastases as compared with patients without any identifiable IMN on baseline imaging (p = 0.0321).
The incidence of IMN was highest in second intercostal space, followed by first intercostal space. Majority of IMN were identified on the same side of primary. In our study, the incidence of IMN was higher in upper-outer quadrant tumours. However, this finding may have been confounded by the fact that upper outer quadrant tumours have a considerably higher incidence in general.
The limitation of our study was less sample size for analysis of calculation of incident rates. In addition, the study was limited to a single institute. Also, no similar study calculating incident rates of IMN has been published so far to our knowledge. Further prospective studies are needed for ascertaining same.
Conclusion
We conclude that IMNs are an important prognostic factor in LABC and these should be reported (whether present or absent) in all cases of breast cancer undergoing cross-sectional imaging for appropriate management.
Future directions
A synoptic reporting of breast cancer baseline imaging with reporting of IMN is suggested with emphasis on size, location and number of IMN.
Contributor Information
Somesh Singh, Email: someshsingh88@gmail.com.
Subhash K Ramani, Email: dr.ramani@gmail.com.
Ashita Rastogi, Email: ashitarastogi@hotmail.com.
Meenakshi H Thakur, Email: thakurmh@yahoo.co.in.
REFERENCES
- 1.Cancer Information Tata Memorial centre. India Web site. [Google Scholar]
- 2. American Joint Committee on Cancer Breast cancer staging, 7th Edition. https://cancerstaging.org/references-tools/quickreferences/Documents/BreastMedium.pdf. Accessed February 15, 2018.. [Google Scholar]
- 3.Buzdar AU, Hortobagyi G. Locally advanced breast cancer. The Oncologist 996(1(1 & 2)): 8–17. [PubMed] [Google Scholar]
- 4.Jaiyesimi IA, Buzdar AU, Hortobagyi G. Inflammatory breast cancer: a review. JCO 1992; 10: 1014–24. doi: 10.1200/JCO.1992.10.6.1014 [DOI] [PubMed] [Google Scholar]
- 5.El Saghir Nagi S, Eniu Alexandru W, Robert C, Zeba A, Daniel V, Hortobagyi Gabriel N.on behalf of the Breast Health Global Initiative Systemic Therapy Focus Group . Locally Advanced Breast Cancer - Treatment Guideline Implementation with Particular Attention to Low- and Middle-Income Countries. Cancer Supplement 2008; 113(Number 8). [DOI] [PubMed] [Google Scholar]
- 6.Veronesi U, Cascinelli N, Bufalino R, Morabito A, Greco M, Galluzzo D, et al. . Risk of internal mammary lymph node metastases and its relevance on prognosis of breast cancer patients. Ann Surg 1983; 198: 681–4. doi: 10.1097/00000658-198312000-00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cody HS, Urban JA. Internal mammary node status: a major prognosticator in axillary node-negative breast cancer. Ann Surg Oncol 1995; 2: 32–7. doi: 10.1007/BF02303699 [DOI] [PubMed] [Google Scholar]
- 8.Eubank WB, Mankoff DA, Takasugi J, Vesselle H, Eary JF, Shanley TJ, et al. . 18 Fluorodeoxyglucose Positron Emission Tomography to Detect Mediastinal or Internal Mammary Metastases in Breast Cancer. JCO 2001; 19: 3516–23. doi: 10.1200/JCO.2001.19.15.3516 [DOI] [PubMed] [Google Scholar]
- 9.Isabelle S, Felix M, Sarah C, Walter WD, Sigrid S, Van OC, et al. . Additional value of PET-CT in staging of clinical stage IIb and III breast cancer. Breast J 2010; 2010: 617–24. [DOI] [PubMed] [Google Scholar]
- 10.Wang Carolyn L, Eissa Marna J, Rogers James V, Aravkin Aleksandr Y, Porter Bruce A, Beatty David J. 18F-Fdg PET/CT–Positive internal mammary lymph nodes: pathologic correlation by ultrasound-guided fine-needle aspiration and assessment of associated risk factors. American Journal of Roentgenology 2013; 2013: 1138–44. [DOI] [PubMed] [Google Scholar]
- 11.Hortobagyi GN. Comprehensive management of locally advanced breast cancer. Cancer 1990; 66(S14): 1387–91. doi: [DOI] [PubMed] [Google Scholar]
- 12.Eubank William B, David Mankoff A, Vesselle Hubert J, Eary Janet F, Schubert Erin K, Dunnwald Lisa K, et al. . Detection of locoregional and distant recurrences in breast cancer patients by using FDG PET. RadioGraphics 2002; 1(5-17): 2002–22. [DOI] [PubMed] [Google Scholar]
- 13.Borgstein PJ, Meijer S, Pijpers RJ, van Diest PJ. Functional lymphatic anatomy for sentinel node biopsy in breast cancer: echoes from the past and the periareolar blue method. Ann Surg 2000; 232: 81–9. doi: 10.1097/00000658-200007000-00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanis PJ, van Rijk MC, Nieweg OE, Tanis Pieter J, van Rijk Maartje C. The posterior lymphatic network of the breast rediscovered. J Surg Oncol 2005; 91: 195–8. doi: 10.1002/jso.20299 [DOI] [PubMed] [Google Scholar]
- 15.Borgstein PJ, Meijer S, Pijpers RJ, van Diest PJ. Functional lymphatic anatomy for sentinel node biopsy in breast cancer: echoes from the past and the periareolar blue method. Ann Surg 2000; 232: 81–9. doi: 10.1097/00000658-200007000-00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen RC, Lin NU, Golshan M, Harris JR, Bellon JR. Internal mammary nodes in breast cancer: diagnosis and implications for patient Management—A systematic review. JCO 2008; 26: 4981–9. doi: 10.1200/JCO.2008.17.4862 [DOI] [PubMed] [Google Scholar]
- 17.Ismail J. Internal mammary sentinel nodes in primary breast cancer. Curr Med Res Opin 2003; 19. [DOI] [PubMed] [Google Scholar]
- 18.Sachdev S, Kalakota K, Donnelly ED, Hayes JP, Prescott A, Strauss JB. Identification of Involved Internal Mammary Lymph Node(s) on Pretreatment Breast MRI Correlated With Aggressive Histology and Poorer Outcomes. Int J Radiat Oncol Biol Phys 2015; 93(Issue 3): E20. doi: 10.1016/j.ijrobp.2015.07.594 [DOI] [Google Scholar]
- 19.Veronesi U, Arnone P, Veronesi P, Galimberti V, Luini A, Rotmensz N, et al. . The value of radiotherapy on metastatic internal mammary nodes in breast cancer. results on a large series. Ann Oncol 2008; 19: 1553–60. doi: 10.1093/annonc/mdn183 [DOI] [PubMed] [Google Scholar]
- 20.Heuts EM, van der Ent FW, von Meyenfeldt MF, Voogd AC. Internal mammary lymph drainage and sentinel node biopsy in breast cancer: a study on 1008 patients. Eur J Surg Oncol 2008; 2009: 252–7. [DOI] [PubMed] [Google Scholar]
- 21.Noushi F, Spillane AJ, Uren RF, Gebski V. Internal mammary lymph node metastasis in breast cancer: predictive models to assist with prognostic influence. Breast 2011; 20: 278–83. doi: 10.1016/j.breast.2010.12.008 [DOI] [PubMed] [Google Scholar]
- 22.Mack M, Chetlen A, Liao J. Incidental internal mammary lymph nodes visualized on screening breast MRI. AJR Am J Roentgenol 2015; 205: 209–14. doi: 10.2214/AJR.14.13586 [DOI] [PubMed] [Google Scholar]
- 23.Donegan WL. The influence of untreated internal mammary metastases upon the course of mammary cancer. Cancer 1977; 39: 533–8. doi: [DOI] [PubMed] [Google Scholar]
- 24.Lacour J, Le M, Caceres E, Koszarowski T, Veronesi U, Hill C, Jean L, Le Monique CE, Tadeus K, Umberto V, Catherine H. Radical mastectomy versus radical mastectomy plus internal mammary dissection. ten year results of an international cooperative trial in breast cancer. Cancer 1983; 51: 1941–3. doi: [DOI] [PubMed] [Google Scholar]
- 25.Veronesi U, Valagussa P. Inefficacy of internal mammary nodes dissection in breast cancer surgery. Cancer 1981; 47: 170–5. doi: [DOI] [PubMed] [Google Scholar]
- 26.Veronesi U, Cascinelli N, Greco M, Bufalino R, Morabito A, Galluzzo D, et al. . Prognosis of breast cancer patients after mastectomy and dissection of internal mammary nodes. Ann Surg 1985; 202: 702–7. doi: 10.1097/00000658-198512000-00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shahar KH, Buchholz TA, Delpassand E, Sahin AA, Ross MI, Ames FC, et al. . Lower and central tumor location correlates with lymphoscintigraphy drainage to the internal mammary lymph nodes in breast carcinoma. Cancer 2005; 103: 1323–9. doi: 10.1002/cncr.20914 [DOI] [PubMed] [Google Scholar]
- 28.Chen L, Gu Y, Leaw S, Wang Z, Wang P, Hu X, et al. . Internal mammary lymph node recurrence: rare but characteristic metastasis site in breast cancer. BMC Cancer 2010; 10: 201010. doi: 10.1186/1471-2407-10-479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Amy J. Xu, Carl J.DeSelm, Alice Y.Ho, Erin F.Gillespie, Lior Z.Braunstein, Atif J.Khan, et al. Overall Survival of Breast Cancer Patients With Locoregional Failures Involving Internal Mammary Nodes. Advances in Radiation OncologyIn press. Accessed June 2, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sachdev S, Goodman CR, Neuschler E, Kalakota K, Cutright D, Donnelly ED, et al. . Radiotherapy of MRI-detected involved internal mammary lymph nodes in breast cancer. Radiat Oncol 2017; 12: 199. doi: 10.1186/s13014-017-0934-5 [DOI] [PMC free article] [PubMed] [Google Scholar]


