Abstract
Objective:
We had developed compact rotating gantry for carbon ion using superconducting magnets in 2015 which became clinically operational in 2017. The objective of this study was to assess the clinical feasibility and safety of using compact rotating gantry with three-dimensional active scanning in delivery of carbon-ion radiotherapy (C-ion RT) for relatively stationary tumours.
Methods:
A prospective feasibility study was conducted with 10 patients who had been treated with C-ion RT using compact rotating gantry between April 2017 and April 2018 at Hospital of the National Institute of Radiological Sciences (NIRS) for head and neck and prostate cancers. The primary end point was evaluation of acute toxicities within 3 months of starting C-ion RT.
Results:
Out of 10 cases 8 were of head and neck cancers and 2 were of prostate cancers. All of those eight head and neck cases were of locally advanced stages. Both of the prostate cancer patients belong to intermediate risk categories. None of the patients developed even Grade 2 or more severe skin reactions. Six out of eight cases with head and neck cancers experienced Grade 2 mucosal reactions; however, nobody developed Grade 3 or more severe mucosal reactions. There was no gastrointestinal reaction observed in prostate cancer patients. One patient developed Grade 2 genitourinary reaction.
Conclusion:
C-ion RT using compact rotating gantry and three-dimensional active scanning is a safe and feasible treatment for relatively less mobile tumours.
Advances in knowledge:
This study will be the first step to establish the use of superconducting rotating gantry in C-ionRT in clinical setting paving the way for treating large number of patients and make it a standard of practice in the future.
Introduction
Use of carbon-ion radiotherapy (C-ion RT) in treating deep seated hypoxic radioresistant tumours has been increasing owing to its unique physical profile and enhanced biological effectiveness because of its higher linear energy transfer (LET) and ability to cause complex clustered DNA double-stranded breakage. National Institute of Radiological Sciences (NIRS) had pioneered this technology since 1994 and treated more than 10,000 patients in last two decades using fixed beam ports.1 The Gesellschaft für Schwerionenforschung in Darmstadt, Germany treated their first patient in 1997. Currently there are 11 carbon ion centers operational in the world and actively treating the patients. Unlike protons, out of those 11 carbon ion centers only 2 centers Heidelberg Ion Therapy Center (HIT), Germany and NIRS Japan are using rotating gantry for clinical purpose. HIT in 2009 became the first C-ion facility with a 360degree rotating gantry. This rotational gantry built by HIT was huge in size with a weight of 600 ton, almost twice larger and five times heavier than standard proton gantry. Such a massive gantry was difficult to generate widely, operate and was too expensive.2 NIRS in collaboration with Toshiba (Toshiba Ltd, Tokyo, Japan) installed a compact rotational gantry of 300 tons and 13 m length in 2015 exploring the superconducting magnet technology as compared to HIT in Germany which was 600 tons and 25 m in length allowing a significant size and weight reduction.3–5 After undergoing several physical experiments the rotational gantry at NIRS became clinical for fixed and moving tumours in 2017 and 2018 respectively. The gantry allows to irradiate patients from multiple beam angles and its use enables to significantly reduce workload by eliminating the need for multiple setups, immobilization devices, and tilting couches. Here, we are going to report our clinical experience and feasibility of C-ion RT using lightweight compact rotational gantry at NIRS which is going to be the first of its kind.
Methods and materials
We conducted a prospective feasibility study of 10 patients treated with C-ion RT using rotating gantry between April 2017 and April 2018 at NIRS. The aim of this study was to evaluate the safety and tolerability of C-ion RT using rotating gantry instead of fixed beam ports in patients with head and neck cancer and prostate cancer. The primary end point was evaluation of acute toxicities within 3 months of starting C-ion RT. Enrollment was based on the following inclusion criteria: (1) Lesion located in the head and neck and prostate, (2) histologically proven malignancy, (3) treated with definitive intent, (4) non-metastatic disease, (5) ECOG performance status 0 or 1, (6) patient was able to comprehend the research methodology and of independent volition consent to the trial. Exclusion criteria were: (1) expected survival of less than 6 months. (2) Severe comorbidities. (3) Synchronous double primary malignancies. (4) Active infection in the irradiation site. Informed written consent for this safety and feasibility study was obtained from all the patients before starting treatment. The study was approved by the ethics committee and institutional review board (IRB) of NIRS. This trial is registered with UMINCTR with a registration number of UMIN000026933.
Pre-treatment work-up
Beside thorough clinical examination and baseline blood investigations all patients of histologically proven head and neck cancer patients underwent contrast-enhanced CT, MRI and whole body positron emission tomography-CT(PET-CT) for local staging and metastatic work up. Along with routine blood investigations prostate cancer patients underwent serum prostate specific antigen (PSA), MRI prostate and contrast-enhanced CT chest abdomen and pelvis for staging work-up and risk stratification.
Carbon-Ion radiotherapy
All head and neck and prostate cancer patients were immobilized in customized cradle (Moldcare, Alcare, Tokyo, Japan) with a relatively thicker shell made of low temperature thermoplastic mask (Shellfitter, kuraray, Osaka, Japan) in supine position. A set of non-contrast CT images with 2 mm slice thickness were obtained for treatment planning and was fused with contrast-enhanced CT and gadolinium-enhanced MRI images for accurate target volume delineation.
For head and neck cancer gross tumour volume (GTV) was contoured based on macroscopic tumour visible on radiological imaging, nasal endoscopy and clinical findings. A minimum of 5 mm margin was added to GTV to create clinical target volume (CTV). CTV was further modified respecting the biology, natural history and clinical course of the disease. Furthermore 2–3 mm margin for setup error was added around CTV to create final planning target volume (PTV). Three-dimensional treatment planning was performed with XIO-N2 software using the active spot scanning. C-ion RT was delivered from multiple angles using multiple field specific optimization(MFSO) technique according to the location of target and its proximity to critical structures. The dose prescription was at the isocenter and the prescribed doses for all head and neck cases were 57.6 Gy (RBE)(relative biological effectiveness)–64 Gy (RBE)/16 fractions.
For treatment planning of prostate cancer, the CTV was defined as the whole prostate and the proximal third or half of the seminal vesicle. The PTV 1 was defined as the CTV plus 5‐mm margins in the cranial, caudal and posterior directions and 10 mm margins in the right, left and anterior directions. The PTV 2 was created by adding 2–3 mm margins from the dorsal aspect of the CTV and was identical to the CTV in the cranial and caudal directions; PTV 2 was used for the latter half of the treatment course. The prescribed dose for prostate cancer patients was 51.6 Gy (RBE)/12 fractions at the isocenter. C-ion RT was planned with bilateral parallel opposed field with beam being delivered from 90° or 270° alternatively everyday with single field uniform dose(SFUD) technique.
After patient positioning with customized immobilization devices digital orthogonal radiographs were acquired and transferred to the computerized positioning system. The setup images were compared with the reference images digitally reconstructed from treatment planning CT before treatment execution.
The diagram of compact rotating gantry room and representative cases of head and neck and prostate cancer C-ion RT planning using rotating gantry are displayed in Figures 1–3.
Figure 1. .
Compact rotating gantry room of our carbon-ion facility.
Figure 2. .
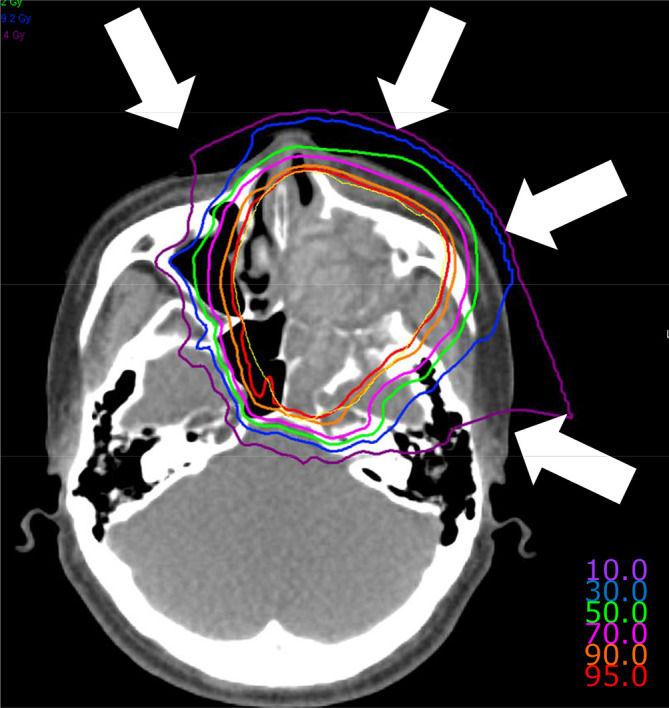
Composite dose distribution of carbon-ion radiotherapy in a case of maxillary sinus tumour treated from four different angles using compact rotating gantry and three-dimensional active scanning. The displayed isodose lines correspond to 95%, 90%, 70%, 50%, 30%, and 10% dose regions. The planning target volume is demarcated by yellow line.
Figure 3. .
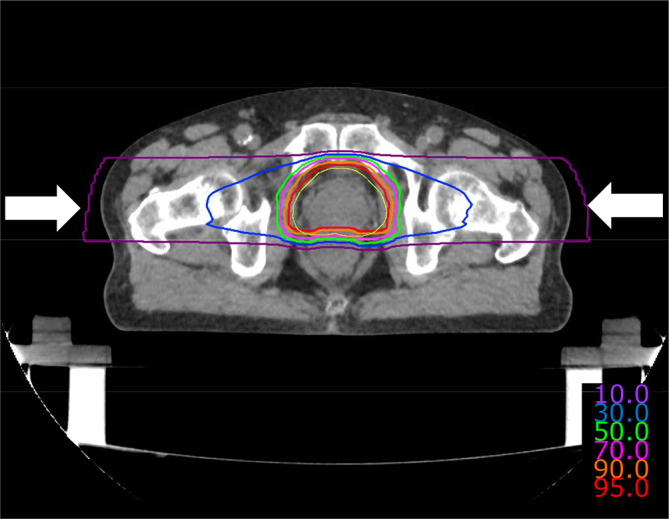
Composite dose distribution of carbon-ion radiotherapy in a case of prostate cancer treated with two lateral fields using compact rotating gantry and three-dimensional active scanning. The displayed isodose lines correspond to 95%, 90%, 70%, 50%, 30%, and 10% dose regions. The planning target volume is demarcated by yellow line.
Chemotherapy and hormonal therapy
Malignant melanoma patients received one cycle of concurrent and four cycles of DAV-based adjuvant chemotherapy with a dose of Vincristine 0.7 mg/m2 day 1, Nimustine Hydrochloride 70 mg/m2 day 1 and DTIC 120 mg/m2 day 1 to day 5 repeated every four weekly.
Prostate cancer patients received neoadjuvant and concomitant androgen deprivation therapy (ADT) with leutinizing hormone releasing hormone analogue for 6 months.
Endpoint/analyses
The primary end point acute toxicity was evaluated using the National Cancer Institute Common Terminology Criteria for Adverse Events, v. 4.0. Secondary end point was tumour response at 6 months after C-ion RT for head and neck cancer and biochemical control for prostate cancer after the same duration. Outcomes of this study were summarized using standard descriptive analysis.
Follow up
The duration of follow up for this study was 6 months. All head and neck cancer patients underwent follow up during C-ion RT at 2 weeks and on the last day of treatment, 3 months and 6 months after completion of C-ion RT. They were evaluated with contrast-enhanced CT head neck, chest and abdomen and MRI head neck at 3 and 6 months. Prostate cancer patients were evaluated weekly for acute toxicity assessment and serum PSA was checked every 3 monthly.
Results
10 cases were recruited for this study. Out of 10 cases, 8 were of head and neck cancers and 2 were of prostate cancers. Five out of eight head and neck cases belong to nasal cavity and paranasal sinuses. All of those eight cases were of locally advanced stages. Dose to head and neck patients ranged from 57.6 Gy (RBE) to 64 Gy (RBE)/16 fractions. Both of the prostate cancer patients belong to intermediate risk category and received 51.6 Gy (RBE)/12 fractions. The patient characteristics are summarised in Tables 1 and 2.
Table 1. .
Summary of head and neck cancer patients
| Age (years) |
Sex | Subsites | Histology | T-classification | Dose Gy (RBE) |
Number of fractions | Skin reaction Maximum/3 months |
Mucosal reaction Maximum/3 months |
|---|---|---|---|---|---|---|---|---|
| 66 | Female | Nasal cavity | Melanoma | T3 | 57.6 | 16 | Grade 1/0 | Grade 2/1 |
| 43 | Male | Maxillary Sinus | ACC | T4b | 64 | 16 | Grade 1/0 | Grade 2/0 |
| 69 | Male | Maxillary Sinus | ACC | T4a | 64 | 16 | Grade 1/0 | Grade 2/0 |
| 74 | Female | Maxillary sinus | ACC | T4b | 64 | 16 | Grade 1/0 | Grade 2/0 |
| 75 | Male | Oropharynx | PDC | T4a | 57.6 | 16 | Grade 0/0 | Grade 2/1 |
| 74 | Female | Ethmoid sinus | Melanoma | T3 | 57.6 | 16 | Grade 1/1 | Grade 1/0 |
| 74 | Male | Nasopharynx | Adenocarcinoma | T3 | 64 | 16 | Grade 0/0 | Grade 2/0 |
| 51 | Female | Parotid | Carcinoma | T4b | 64 | 16 | Grade 1/0 | Grade 0/0 |
ACC, adenoid cystic carcinoma; PDC, poorly differentiated carcinoma; RBE, relative biological effectiveness.
Adverse effects and outcomes
Six out of eight cases with head and neck cancers developed maximum skin reactions of Grade 1 only. None of the patients developed even Grade 2 or more severe skin reactions. Six out of eight cases experienced Grade 2 mucosal reactions however nobody developed Grade 3 or more severe mucosal reactions. There was no gastrointestinal reaction observed in carcinoma prostate patients. One patient developed Grade 2 genitourinary reaction and was managed conservatively. The acute reaction profile is provided in Tables 1 and 2.
Table 2. .
Summary of prostate cancer patients
| Age (years) |
T classification | PSA ng/ml | Gleason score | Dose Gy (RBE) |
No of fractions | Gastrointestinal reaction Maximum/3 months |
Genitourinary reaction Maximum/3 months |
|---|---|---|---|---|---|---|---|
| 75 | T1c | 5.85 | 3 + 4 | 51.6 | 12 | Grade 0/0 | Grade 0/0 |
| 55 | T2a | 5.19 | 3 + 4 | 51.6 | 12 | Grade 0/0 | Grade 2/2 |
PSA, prostate specific antigen; RBE, relative biological effectiveness.
After 6 months of follow-up, out of eight head and neck cancer cases seven patients had partial response and one patient had stable disease. In both the prostate cancer patients, there was rapid decline in PSA levels; however, ADT in addition to C-ion RT might have enhanced it.
Discussion
Experience of C-ion RT in last 20 years at NIRS was based on fixed beam ports. With increasing experience of exploring C-ion RT in different malignancies, we felt the need to develop rotating gantry to enhance the clinical benefits. There are several clinical advantages of rotating gantry as compared to fixed beam ports. It provides more flexibility of beam angles and also allows multiport irradiation per day which enable us to conform the dose maximally to the target sparing the critical structures. Hence, major physical advantages of carbon-ion Bragg peak and sharp lateral penumbra can be optimally utilized. Intensity modulated carbon-ion radiotherapy (IMCT) optimizing LET in the target has an excellent potential to overcome tumour hypoxia. Though carbon-ion is considered as a high LET radiation but the average LET of the target is around 40–50 Kev/micron in current clinical situations. LET-painting attempts to restrict high-LET radiation to compartments that are found to be hypoxic, while applying lower LET radiation to normoxic tissues.6,7 LET painting using IMCT and three-dimensional scanning will be available soon in the clinic with the use of rotating gantry. Lack of beam angle flexibility in fixed ports restricts the patient positions for treatment planning and execution. Patients are planned and treated in unusual positions such as oblique prone or lateral which are quite uncomfortable especially for patients who have huge tumours or have severe pain. Those patients can be treated in a single comfortable position with optimum beam angle flexibility allowing improved setup reproducibility, reducing setup uncertainties and setup time. Use of rotating gantry has also made the carbon-ion treatment planning more efficient, accurate and less time consuming. It has reduced the contouring time significantly by avoiding the target and organ at risk delineation in different positions. Patients getting treatment in single position with rotating gantry also reduces the planning time in multiple positions which is quite labour intensive as well. Rotating gantry facilitates the evaluation of composite dose distribution in a single position which is more accurate and precise as compared to combined virtual dose distribution used in fixed beam planning.
Head and neck cancer has been selected for this feasibility study as it is a classic example where flexibility of multiple beam angles using a single patient position is extremely important because of its critical location and proximity to critical structures enabling efficiency and accuracy in contouring planning and execution of C-ion RT. At present, we are trying to find out optimum angles of beam delivery in head and neck and other malignancies and will conduct dosimetric and clinical comparison studies between fixed ports and rotating gantry in the near future exploring angle flexibility. Other than the head and neck cancers, two prostate carcinoma cases were also included in this study and were treated in single supine position using bilateral parallel opposed fields similar to fixed beam. A significant proportion of patients treated with C-ion RT in Japan are prostate carcinomas. We believe that fixed ports in C-ion RT will be obsolete in the near future as proton and establishment of feasibility in treating prostate cancer with rotating gantry is important and relevant. Recently, more hypofractionated form of IMCT regimen is being investigated in our institute for which establishing clinical feasibility of rotating gantry in prostate cancer was of utmost importance.
The initial follow-up in our study has demonstrated minimal adverse effects from treatment, appearing comparable to those adverse effect profiles seen with the fixed beams as we have experienced over last two decades. This study suggests C-ion RT using rotating gantry is a safe and feasible treatment for less mobile tumours and it has an excellent role especially in head and neck where target is very close to critical organs at risk. With increasing experience adverse effects may be reduced further. As rotating gantry can irradiate from any angle with a single patient setup, allowing use of a single CT data set in treatment planning and a single patient setup procedure in treatment, the use of the rotating gantry also has the potential to improve the throughput. Feasibility study for moving tumours using the rotating gantry and phase controlled rescanning has already been conducted though the result is not yet published.
After Loma Linda University installed rotating gantry for proton therapy in 1991, almost all the proton centers in the world started using it and became the standard practice.8 At the time of writing, no reports regarding the clinical results of carbon-ion using compact rotating gantry with superconducting magnets exist in the literature. This clinical report is the first of its kind and NIRS as a pioneer of C-ion RT will change the landscape of C-ion RT using compact rotating gantry exploring superconducting magnets in different clinical settings. Once we establish the clinical feasibility of rotating gantry and able to find out the optimum beam angles in different malignancies, dosimetric and clinical studies comparing doses, tumour control and toxicity profiles between superconducting rotating gantry and fixed beams are warranted.
Conclusion
Our early results suggest that C-ion RT using compact rotating gantry and three-dimensional active scanning is a safe and feasible treatment for relatively less mobile tumours such as head and neck cancers and carcinoma prostate. Future studies using rotating gantry in different subsites with large sample size is warranted. With increasing experience and the development of IMCT, LET painting the results will improve further.
Footnotes
Acknowledgment: We sincerely acknowledge our rotational gantry development working group members; Hiroshi Tsuji, Naoyoshi Yamamoto, Masashi Koto, Toshiyuki Shirai, Yousuke Hara, Taku Inaniwa, Shinichiro Mori, Osami Saito, Minoru Tajiri for their support.
Contributor Information
Tapesh Bhattacharyya, Email: tapesh27@gmail.com.
Masashi Koto, Email: koto.masashi@qst.go.jp.
Hiroaki Ikawa, Email: ikawa.hiroaki@qst.go.jp.
Kazuhiko Hayashi, Email: hayashi.kazuhiko@qst.go.jp.
Yasuhito Hagiwara, Email: hagiwara.yasuhito@gmail.com.
Hirokazu Makishima, Email: makishima.hirokazu@qst.go.jp.
Goro Kasuya, Email: kasuya.goro@qst.go.jp.
Naoyoshi Yamamoto, Email: yamamoto.naoyoshi@qst.go.jp.
Tadashi Kamada, Email: kamada.tadashi@qst.go.jp.
Hiroshi Tsuji, Email: tsuji.hiroshi@qst.go.jp.
REFERENCES
- 1.Mohamad O, Makishima H, Kamada T. Evolution of carbon ion radiotherapy at the National Institute of radiological sciences in Japan. Cancers 2018; 10: 66. doi: 10.3390/cancers10030066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fuchs R, Weinrich U, Sust E. Assembly of the carbon ion beam gantry at the Heidelberg ion therapy (HIT) accelerator. Proceedings of EPAC08,Genoa, Italy. [Google Scholar]
- 3.Iwata Y, Noda K, Murakami T, Shirai T, Furukawa T, Fujita T, et al. . Development of a superconducting rotating-gantry for heavy-ion therapy. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms 2013; 317: 793–7. doi: 10.1016/j.nimb.2013.03.050 [DOI] [Google Scholar]
- 4.Iwata Y, Fujimoto T, Matsuba S, Fujita T, Sato S, Furukawa T, et al. . Beam commissioning of a superconducting rotating-gantry for carbon-ion radiotherapy. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment 2016; 834: 71–80. doi: 10.1016/j.nima.2016.07.050 [DOI] [Google Scholar]
- 5.Kim J, Yoon M. Design study of a superconducting gantry for carbon beam therapy. Journal of the Korean Physical Society 2016; 69: 1048–52. doi: 10.3938/jkps.69.1048 [DOI] [Google Scholar]
- 6.Bassler N, Toftegaard J, Lühr A, Sørensen BS, Scifoni E, Krämer M, et al. . LET-painting increases tumour control probability in hypoxic tumours. Acta Oncol 2014; 53: 25–32. doi: 10.3109/0284186X.2013.832835 [DOI] [PubMed] [Google Scholar]
- 7.Tinganelli W, Durante M, Hirayama R, Krämer M, Maier A, Kraft-Weyrather W, et al. . Kill-painting of hypoxic tumours in charged particle therapy. Sci Rep 2015; 5: 17016. doi: 10.1038/srep17016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slater JD. Development and operation of the Loma Linda University medical center proton facility. Technol Cancer Res Treat 2007; 6(4 Supplement.): 67–72. doi: 10.1177/15330346070060S411 [DOI] [PubMed] [Google Scholar]