Abstract
MRI of articular cartilage (AC) integrity has potential to become a biomarker for osteoarthritis progression. Traditional MRI sequences evaluate AC morphology, allowing for the measurement of thickness and its change over time. In the last two decades, more advanced, dedicated MRI cartilage sequences have been developed aiming to assess AC matrix composition non-invasively and detect early changes in cartilage not captured on morphological sequences. T2-mapping and T1ρ sequences can be used to estimate the relaxation times of water inside the AC. These sequences have been introduced into clinical protocols and show promising results for cartilage assessment. Extracelullar matrix can also be assessed using diffusion-weighted imaging and diffusion tensor imaging as the movement of water is limited by the presence of extracellular matrix in AC. Specific techniques for glycosaminoglycans (GAG) evaluation, such as delayed gadolinium enhanced MRI of cartilage or Chemical Exchange Saturation Transfer imaging of GAG, as well as sodium imaging have also shown utility in the detection of AC damage. This manuscript provides an educational update on the physical principles behind advanced AC MRI techniques as well as a comprehensive review of the strengths and weaknesses of each approach. Current clinical applications and potential future applications of these techniques are also discussed.
Introduction
Osteoarthritis (OA) (i.e. degenerative joint disease) is the most common joint disease, with knee OA having a prevalence of 10%–13%.1 Articular cartilage (AC) changes are seen early in the pathogenesis of degenerative joint disease. Changes in the internal cartilage structure are among the first signs of OA; typically occurring before changes in cartilage thickness.2 The structure of AC is based on its two main compartments: cellular and extracellular matrix. The cellular compartment is composed by the cells responsible for the generation and preservation of extracellular matrix, the chondrocytes, which represent up to the 10% of the whole volume of normal AC. The extracellular matrix (ECM) compartment is mainly composed of water (up to 70%) and macromolecules, primarily collagen and proteoglycans (PGs)/GAGs (Figure 1).
Figure 1. .
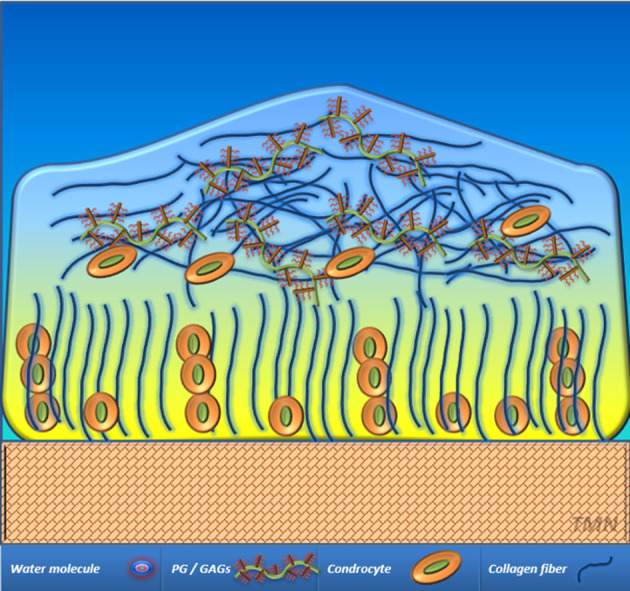
AC scheme. The classical layer-based structure of AC has three main zones. The deep zone is characterized by a dominance of collagen fibers that are oriented perpendicular to the surface of AC. Cellular components of AC are commonly identified at this zone, mainly fibroblasts and chondrocytes. The transitional zone usually shows a higher concentration of proteoglycans and glycosaminoglycans that are mixed with randomly orientated collagen fibers and fewer chondrocytes. The superficial zone characteristically shows a predominant orientation of collagen fiber parallel to AC surface in contact with synovial fluid. The line between the deep zona and subchondral bone is called tidemark zone where calcified cartilage can be identified. AC, articular cartilage; GAGs, glycosaminoglycans; PG, proteoglycan.
Clinically, AC is evaluated either indirectly using radiographs or tomographically using MRI, computed tomography or ultrasound.3,4 Among these techniques, MRI has the highest sensitivity and specificity to detect AC damage and changes in tissue characteristics. Owing to its excelling soft tissue contrast, of the introduction of MRI represented a significant advance in the evaluation of AC allowing for more accurate morphologic characterization. Traditional morphologic MRI techniques have been widely used clinically for quantitative assessment of AC thickness, and can accurately detect focal or diffuse changes in cartilage shape as well as changes in its normal signal intensity.5 Changes in AC MR signal intensity on morphological sequences such as two-dimensional or three-dimensional T2-weighted sequences are interpreted as early signs of cartilage degeneration, typically occurring prior to changes in thickness.6 These signal changes can range from very subtle findings to deeper lesions that extended to the subchondral bone. Changes in signal intensity have been correlated with the degree of cartilage damage with chondromalacia severity staged using semi-quantitative scales.7 However, morphological MRI sequences lack specificity regarding the underlying pathophysiological changes in AC structure and composition. In most cases, modifications in the cartilage microstructure precede changes in cartilage thickness or signal intensity on morphological MRI sequences. There is a rising interest in the application of a newer MRI techniques to assess cartilage composition and structure.8–10 These techniques aim to reveal molecular changes to the cartilage ECM composition. With these techniques, focal or diffuse decreases in the collagen or PGs concentration can be assess allowing for an earlier evaluation of cartilage damage or pathology than with morphological MRI techniques.11 The detection of early changes in cartilage composition is critical to identify individuals at risk of OA progression who can benefit from specific treatment.
The pathophysiological assessment of cartilage structure using MRI techniques can be considered as the next step in comprehensive functional tissue characterization. Toward this aim, several MRI sequences have been developed for visualizing differences in the ECM of normal and abnormal AC. These techniques are based on specific physical or biological differences between normal and abnormal AC that can be assessed or enhanced by using specific technical adjustments to the MRI sequence focused on the different components of the ECM.12,13 These MRI techniques include T2-mapping, T1ρ, diffusion-weighted imaging (DWI), diffusion tensor imaging (DTI), delayed gadolinium-enhanced MRI cartilage (dGEMRIC), glycosaminoglycan chemical exchange saturation techniques (gagCEST), and sodium imaging.11,14 The main characteristics of these techniques are summarized in Table 1 and Figure 2.
Table 1. .
Summary of the main functional MRI techniques for cartilage assessment
| ECM target | Gadolinium | Exam time | Clinical use | |
|---|---|---|---|---|
| T2-mapping | Collagen | No | ++ | ++++ |
| T1ρ | PGs | No | +++ | + |
| dGEMRIC | GAGs/PGs | Yes | ++++ | ++ |
| DWI/DTI | Collagen | No | ++ | + |
| gagCEST | GAGs | No | +++ | + |
| Sodium imaging | GAGs | No | +++ | +/- |
DTI, diffusion tensor imaging; DWI, diffusion-weighted imaging; ECM, extracellular matrix; PGs, proteoglycans; dGEMRIC, delayed gadolinium enhanced MRI of cartilage; gagCEST, glycosaminoglycan chemical exchange saturation transfer.
Symbols range from (-) short exam time/limited clinical use to (+) large exam time/extended clinical use.
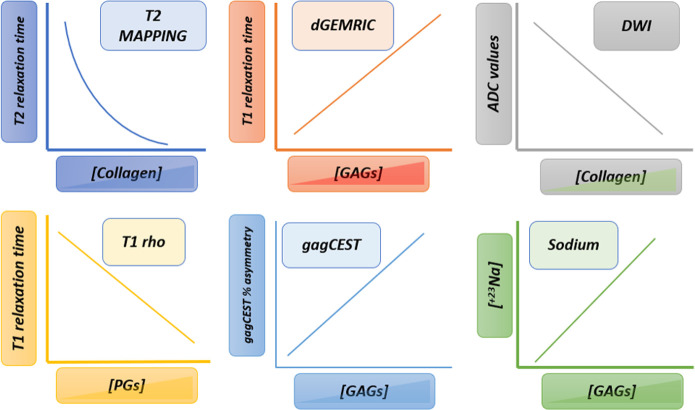
Figure 2. .
Summary techniques. Each functional technique is able to assess, in dominant manner, the integrity or depletion of a specific component of ECM. Thus, the relationship between collagen fiber concentration and T2 relaxation time is inverse, as well as the relationship between ADC values and collagen fibers. PGs and GAGs concentration have a positive correlation with the T1 relaxation times after gadolinium injection in dGEMRIC model as well as a positive lineal correlation with the percentage of gagCEST asymmetry and sodium concentration. T1 relaxation time in T1rho acquisition has inverse correlation with PGs concentration. ADC, apparent diffusion coefficient; dGEMRIC, delayedgadolinium enhanced MRI of cartilage; ECM, extracellular matrix; GAG, glycosaminoglycan; gagCEST, Chemical ExchangeSaturation Transfer imaging of GAG; PGs, proteoglycans.
In this manuscript, we review of these advanced MRI sequences for AC assessment focusing on how each technique evaluates the components of the ECM. In addition, we provide a brief discussion of the current state-of-the-art and clinical use of these techniques.
MRI approaches for functional MRI cartilage evaluation
T2-mapping
Physical principle
T2-mapping was one of the first quantitative techniques applied to AC evaluation. Sequences for T2 measurement acquire multiple images with varying TE, typically ranging from the lowest values that MRI system allows (~10 ms) to up to over 100 ms.15,16 A turbo spin echo (TSE) sequence is typically employed. The interaction of ECM, especially collagen fibers, with water protons results in a shortening of T2 relaxation time. Thus, T2 relaxation time depends on the amount of water protons within the cartilage as well as the integrity of ECM, which is mainly reflected by collagen fiber density. A direct correlation between T2 values and water content and an inverse correlation with collagen concentration within AC has been shown.17 Although color T2 maps can be directly visualized for qualitative interpretations, there are dedicated software packages available to extract the quantitative information; some of these packages permit semi-automated segmentation of the region of interest.Supplementary Video 1 .
Ex vivo/in vivo validation
Several studies have demonstrated that collagenase-degraded cartilage samples have a higher T2 value than healthy cartilage due to the loss of normal ECM composition and loss of collagen integrity and concentration.18,19 The disruption of the matrix decreases the collagen concentration and increases the overall water content within AC; both factors contribute to higher T2 relaxation times.19 While T2-mapping is influenced by loss of all ECM, including GAGs (and PGs), most of studies have demonstrated a greater specificity for collagen fiber assessment.
T2 values are less reliable in the deep and calcified cartilage layers, which in most cases are only evaluated by using ultrashort TE approaches.20,21 In addition, there is some spatial variation with superficial layers of AC having higher T2 relaxation times than deeper layers.18 T2 values may also be affected by the magic angle effect, which may introduce regional variation based collagen fiber orientation and can make longitudinal comparisons challenging.22 T2-mapping studies do not require high magnetic fields and thus can performed at 1.5 and 3 T. An additional benefit is that T2-mapping does not require administration of exogenous contrast agents.
Clinical value
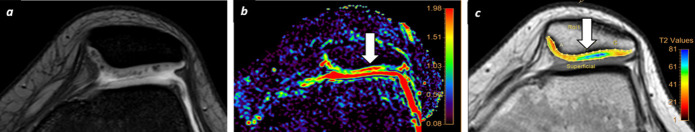
T2 mapping has been applied for evaluation of OA with promising results. Focal or diffuse areas of increased T2 relaxation times have been identified in patients with OA even prior to changes on morphological sequences Figure 3. Moreover, a positive correlation between T2 values and cartilage damage has been demonstrated. As T2-mapping is less sensitive to PGs concentration, it can provide complementary information to other techniques such as dGEMRIC, sodium imaging or T1ρ mapping that are mainly affected by the loss of GAGs/PGs. T2-mapping has also been used for follow up after knee cartilage repair surgery. These studies have shown decreased T2 relaxation times of repair tissue after microfracture compared to healthy cartilage, likely owing to its more fibrocartilaginous construct.23 However, on longer term follow-up in patient treated with microfracture, T2 relaxation values in the cartilage repair zone tend to normalize with respect to the rest of cartilage.24 Other repair approaches such as chondrocyte grafts, tend to form hyaline-like cartilage showing T2 relaxation values similar to healthy AC (Figure 4).25
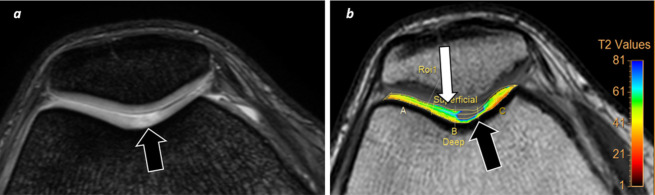
Figure 3. .
T2-mapping cartilage assessment. A 32-year-old female runner with knee pain. (a) A focal area of signal intensity increase is identified (arrow) at trochlea on morphological axial FFE T2W sequence. (b) T2-mapping confirms a focal defect of signal with severe increase of T2 relaxation times at trochlear sulcus as well as early cartilage damage changes at superficial cartilage layer (white arrow), which not clearly seen on morphological sequence. FFE, fast field echo; T2W, T2 weighted.
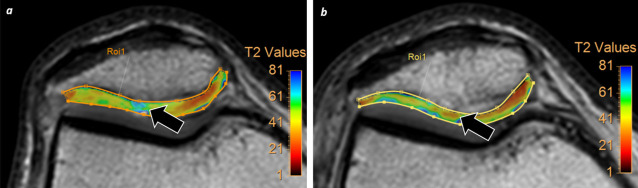
Figure 4. .
Treatment monitoring. A 46-year-old male with knee pain. (a) First study was performed on January 2018 whereas a focal area of increase of T2 relaxation values was identified at lateral aspect of articular surface of patella (white arrow) on T2-mapping sequences consistent with chondromalacia. Patient undergone surgery (chondrocyte graft) and a new MRI was performed on March 2019. (b) This new study shows decrease of T2 values at surgical area (white arrow) which suggest presence of new hyaline cartilage.
T1ρ
Physical principle
T1ρ imaging assesses the relaxation of spin under the influence of a constant radiofrequency (RF) pulse that is set just when the magnetization is tipped into the transverse plane. The role of this RF pulse is to spin-lock the magnetization. T1ρ is the constant that reflects the spin-lattice relaxation time in the rotating frame after application of the RF spin-lock pulse as there is a relationship between T1ρ and the interchange of energy between water molecules and macro-molecules.26,27 Several studies have demonstrated that T1ρ values depend on the proton exchange with amide and hydroxyl groups present on the PGs/GAGs side-chains.28,29 Thus, the slow interaction of water molecules and ECM can be accurately assessed in a way similar to the interaction of water molecules and collagen in T2-mapping sequences.17 This technique does not require gadolinium-based contrast agents (GBCAs). However, T1ρ mapping does require an MRI system able to create a specific RF pulse for achieve a proper spin-locking. The time required for T1ρ acquistition is considerably longer than for T2-mapping (Supplementary Video 210,30,Figure 5a and b).
Figure 5. .
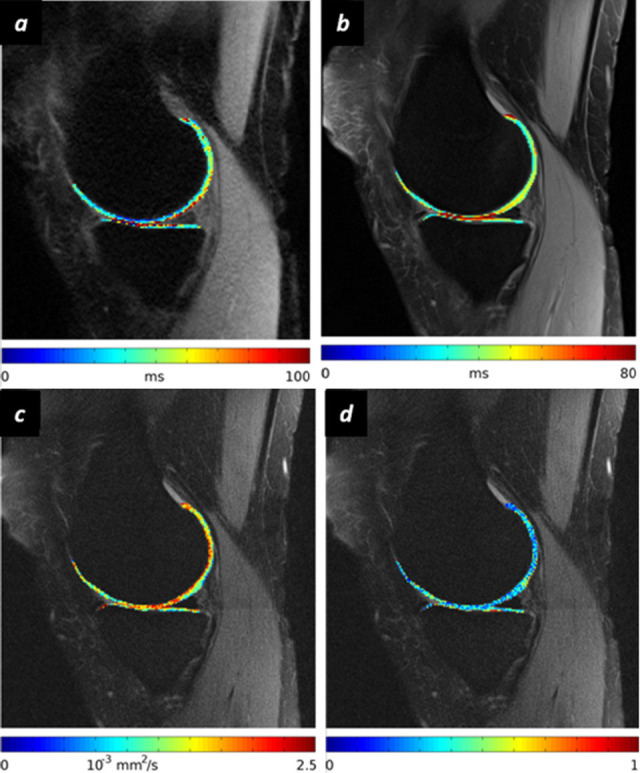
Examples of MRI parameter acquisition on a 54-year-old subject with knee OA (KL Grade 1). (a) T1ρ map of cartilage acquired with a spin lock frequency of 500 Hz and spin lock times of 10, 20, 40, 60 ms. Background image corresponds to 10 ms. (b) T2 parameter map of cartilage acquired with a multiecho turbo spin echo sequence (TEs from 10 to 100 ms). (c, d) Diffusion tensor imaging of the knee (RAISED pulse sequence). Diffusion images are acquired with a b-value of 300 s/mm2 and a TE of 35 ms. MD (c) and FA (d) overlaid over the b0-image. FA, fractional anisotropy; MD, mean diffusivity; OA, osteoarthritis; RAISED, radial imaging spin echo diffusion; TE, echo time.
Ex vivo/in vivo validation
The evaluation of T1ρ imaging using ex vivo models allows for the assessment of variations in PGs concentration in AC. The use of trypsin enzymes for PGs degradation in bovine articular specimens demonstrated that the T1ρ approach was more sensitive for detection of changes in PGs concentration than conventional T1 or T2 weighted images.31 After collagenases administration, these studies did not reveal significant sensitivity of T1ρ for collagen depletion assessment within AC.32,33 Ex vivo human specimens have demonstrated similar results showing that the dominant contribution to T1ρ AC imaging is from PGs degradation rather than collagen degradation.34
Clinical value
T1ρ is able to detect changes in the composition of ECM related to PGs concentration as occurs in osteoarthritis. T1ρ values are higher in healthy volunteers. In the early stages of the OA, T1ρ is more accurate than T2-mapping.35 Moreover, T1ρ values are able to discriminate between intermediate and advanced cartilage degeneration. However, since T2-mapping and T1ρ evaluate two different relaxation mechanisms within cartilage, the information provided should be considered complementary, not exclusionary.36 T1ρ has also been applied for assessment of early cartilage degeneration in patients with meniscal lesions. The loss of PGs in the femoral condyles in these patients correlates with increase of T1ρ values.28 Patient with anterior cruciate ligament injuries, even years after reconstruction, may also show changes in AC composition that can be accurately assessed by T1ρ.37,38 T1ρ has been measured in conjunction with synovial fluid biomarkers such as GAGs concentration in order to evaluate, in a non-invasive manner, the characteristics and integrity of ECM. These studies show a negative correlation between T1ρ relaxation times and GAGs concentration in both cartilage and synovial fluid.27,36
Physical principle
dGEMRIC is a non-invasive functional technique to study cartilage GAGs content in vivo. The dGEMRIC technique uses the negatively charged contrast agent gadolinium-diethylene triamine pentaacetic acid (Gd-DTPA2-).39 GBCAs can be administrated either intra-articularly or intravenously. However, the intravenous injection (usually with double dose) is preferred over the intra-articular injection as there is low risk of septic arthritis and the higher rate of gadolinium penetration (from both articular surface and subchondral bone).40 After intravenous injection and systemic circulation, Gd-DTPA2- distributes within cartilage in a manner inversely related to the negatively charged GAGs content. Gd-DTPA2- shortens the T1 relaxation time of cartilage. In addition, the need for exogenous contrast agent administration, the dGEMRIC technique has other drawbacks. A delay of over 60 min after gadolinium administration is mandatory to ensure its filtration through the synovial membrane into synovial fluid and its diffusion into the cartilage. Moreover, a specific MRI sequence with multiple inversion times (TIs) has to be acquired for a proper quantification of the T1 shortening of the cartilage in a manner similar to the T1-mapping technique. An intermediate approach is a semi-quantitative assessment by acquiring heavily weighted T1 sequences that allow the radiologist to evaluate the presence or absence of gadolinium uptake at AC Figure 6. Long T1 relaxation time values are found in healthy cartilage whereas short T1 relaxation times values are found in degenerated cartilage due to the high amount of infiltrated Gd-DTPA2- (Supplementary Video 3).
Figure 6. .
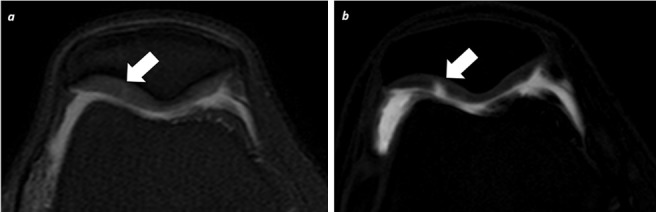
dGEMRIC assessment. A 41-year-old male tennis player, who refers knee pain with flexion and extension movements. (a) Conventional axial FFE T2W shows non-significative increase of signal intensity at lateral patellar articular surface (arrow). (b) Qualitative dGEMRIC sequence after intraarticular gadolinium injection, demonstrates intense uptake of gadolinium at this area (arrow) consistent with loss of GAGs (focal lineal ulcer with deep cartilage involvement). dGEMRIC, delayed gadolinium enhanced MRI of cartilage; FFE, fast field echo; GAG, glycosaminoglycan; T2W, T2 weighted.
Ex vivo/in vivo validation
The dGEMRIC approach has been tested using in vitro and ex vivo models with robust results for both animal and human AC assessment.41 In vivo and ex vivo studies for GAGs concentration and distribution within AC have demonstrated good histological and biochemical correlation.42 These kinds of studies have been necessary in order to determine the type of GBCA needed to obtain the highest tissue contrast within AC. In this respect, there are studies with contradictory results with regard the penetration rate of non-ionic GBCAs and ionic GBCAs, however, the global recommendation is to use ionic GBCAs.39
Clinical value
The dGEMRIC technique is a useful clinical imaging method for evaluating cartilage biochemical composition and to monitor the effects of therapies for osteoarthritis and cartilage injury. Those areas with GAGs concentration preserved will not uptake gadolinium as the negative charged hydroxyl group prevents significant gadolinium diffusion into cartilage (Figure 7). On the other hand, those areas with loss of GAGs (and their negative charges) will allow gadolinium molecules to penetrate the cartilage surface. Several studies have demonstrated the utility of the dGEMRIC approach for detecting early signs of OA related to the loss of GAGs in knee.43 In these patients, lower T1 relaxation times are identified in areas with cartilage damage compared with areas of normal cartilage. dGEMRIC has also be applied in other anatomical regions and with different purposes than evaluation of OA. For example, in hip dysplasia, dGEMRIC has been tested with good correlation with patient symptoms and the severity of dysplasia.44
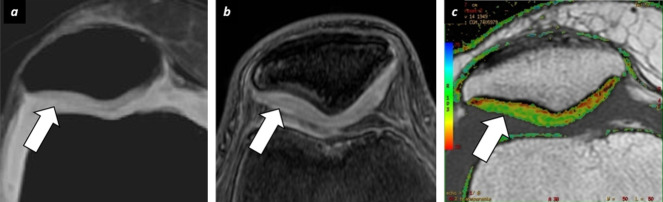
Figure 7. .
Multimodality functional MRI cartilage evaluation. A 29-year-old male which refers knee pain after running. (a) Axial fat suppression PD sequence shows slight increase of signal intensity within the lateral aspect of patellar cartilage (arrow). (b) Qualitative assessment of axial dGEMRIC acquisition does not reveal any abnormal gadolinium uptake at this area (arrow). (c) Axial T2-mapping shows increase of T2 relaxation time values at this level (arrow). The combination of findings described suggest hyaline cartilage damage due to loss of collagen fibers with intact GAGs levels. dGEMRIC, delayed gadolinium enhanced MRI of cartilage; GAG, glycosaminoglycan.
DWI/DTI
Physical principle
Water molecules in biological tissues are in constant and random motion due to the thermal energy and interactions with macromolecules or membranes.45 In DWI, the microscopic random displacements of water molecules is measured to extract information about tissues.46 DWI measures the average displacements of all water molecules in a voxel along a direction (diffusion direction) during a time called diffusion time.47 Using the measurements of average water displacement in different directions, we can infer the organization of the underlying tissue microstructure using diffusion models. The ECM of AC constrains free diffusion of water molecules. DWI allows one to estimate the integrity of ECM based on the motion of water molecules.48 The apparent diffusion coefficient (ADC) can be used to measure the average movement of these water molecules in mm2/s (Supplementary Video 4).49 AC has a water content of approximately 75% and physiologically displays ADC values in the range between 1.4 and 1.6 × 10−3 mm2/s.6 Cartilage damage results in less restriction to the motion of water and thus increased ADC values (Figure 8).
Figure 8. .
Evaluation of AC with DWI in a 45-year-old male with knee instability. (a) Conventional axial FFE T2W sequence does not show clear abnormalities at patellar cartilage surface. (b) DWI acquisition demonstrates increase of ADC values at lateral patellar surface (arrow) revealing loss of collagen fibers. (c) T2- mapping shows proper correlation with DWI demonstrating also increase of T2 values at the same area (arrow). Both functional techniques reflect collagen loss with different physical basis and show early cartilage damage changes before morphological imaging. AC, articular cartilage; ADC, apparent diffusioncoefficient; DWI, diffusion-weighted imaging; FFE, fast field echo; T2W, T2 weighted.
To extract information about the tissue structure we measure the diffusion in at least six non-collinear directions and use the diffusion tensor model.50 This model describes the diffusion in tissue as a symmetric tensor. Through measurements of diffusions in at least six non-collinear directions, all elements of the tensor can be calculated. Parameters derived from DTI such as fractional anisotropy (FA), which is an index of tissue organization, or the mean diffusivity (MD), which represents the average of the main three eigenvalues (the numeric representation of the magnitude of each direction of water molecules within the tissue). Applied to cartilage, these parameters provide a method to differentiate the contributions of collagen structure, as captured with the fractional anisotropy, from the PGs as captured with the mean diffusivity (Supplementary Video 5).49
Ex vivo/in vivo validation
Ex vivo studies have reported a 5─30% increase of diffusivity after enzymatic depletion of PGs that correlated with GAGs concentration.48,51–53 Diffusivity also increases with disease severity in osteochondral samples.54,55 Enzymatic cleavage of PGs resulted in increased MD but resulted in no change in FA or diffusion orientation, thus indicating the ability of DTI to track both collagen and PGs changes independently.56,57 MD has shown correlation with mechanical properties of cartilage.58 MD and FA also changed with OA.59 Indeed, MD and FA have shown the potential to grade histologic cartilage damage as measured with the Osteoarthritis Research Society International score with an area under the receiver operating curve between 0.7 and 0.9 for the detection of early degenerative changes (Figure 5C and d).59,60
Clinical diagnostic and prognostic value
Translation of diffusion measurements to the clinical scanners was hampered by the technical challenges of acquiring diffusion in a low-T2 (~30 ms) tissue with high resolution (<1 mm). Clinical echoplanar imaging sequences have not been able to provide satisfactory results due to their long TEs (>80 ms), their sensitivity to susceptibility artifacts, and their limited resolution (~2–3 mm).61 Thus, the first studies used only diffusion-weighted images with steady-state free precession sequences assessing changes semi-quantitatively.62 Spin-echo-based sequences provide excellent image quality.63,64 The first clinical studies of DTI were published at 7 T using a line scan sequence and showed potential to differentiate the patellar cartilage of healthy and early OA subjects (area under the receiver operating curve = 0.92, n = 26).63 DTI studies have shown significant increase in MD (10 to 20%) between healthy controls and OA subjects and a decrease in FA (−18 to −11%), both at 3 and 7 T. Reproducibility of DTI in vivo was high with variation below 4% for MD and 6% for FA on repeated measurements.63,65
In summary, diffusion imaging can provide insight in cartilage microstructure. DTI has the potential to track changes in both collagen and PGs. Clinical studies are still sparse but have consistently shown feasibility of these techniques and their potential for early diagnosis.
gagCEST
Physical principle
gagCEST can be considered a technical upgrade of conventional magnetization transfer (MT) sequences. MT allows for the evaluation of the contribution to MRI signal from the protons in unbound bulk water molecules that are present in certain tissues. The final goal of the MT, and gagCEST, techniques is to increase the contrast between free water and bound water, evaluating the exchange of energy between these two water pools. In the case of gagCEST, a selective RF pulse excites exchangeable GAGs protons.66 These excited protons experience a chemical exchange phenomenon with free water protons that results in a quantifiable decrease in the magnetization of the free water pool. The target in the case of GAGs is the hydroxyl and sulfate group, with allows one to measure the GAGs concentration in vivo within AC. The amount of the magnetization transfer between both water pools (free and bound to GAGs) can be expressed as the asymmetry of magnetization transfer ratio (MTRasym), which reflects the distribution of free protons around a central water peak in AC. This parameter is obtained after saturating protons linked to GAGs on either side-of the water peak. As occurs with other techniques that require the application of selective RF pulses, a high strength magnetic field (up to 3 T) and very homogeneous B0, are needed to saturate of the hydroxyl group, which has a resonance frequency very close to that of free water (Supplementary Video 6).
Ex vivo/in vivo validation
Several studies have evaluated the physical principle of chemical exchange saturation in ex vivo models (animal and phantoms) confirming the feasibility of this approach to characterize AC damage and for early damage detection. Phantom studies are required in order to properly adjust the RF pulse to GAGs exchangeable protons saturation as well as to center the CEST spectrum and fitting it with water resonance frequency. The fitting of the whole spectrum of gagCEST is time consuming in presence of B0 inhomogeneities, so strategies such as the acquisition of a dual gradient echo B0 map have been proposed to reduce scan time.67 Regarding the need for high magnetic field systems, some authors have evaluated the differences in gagCEST asymmetry in vivo between 3 and 7 T. After B0 inhomogeneities correction, the gagCEST asymmetry at 3 T is scarce, so 7 T is recommended in order to increase gagCEST asymmetry and thus, tissue contrast between damaged and healthy AC.68
Clinical diagnostic and prognostic value
Currently, the application of gagCEST techniques for AC assessment is uncommon in clinical practice due to the technical requisites described above. Nevertheless, several studies have evaluated the potential applications of this approach for AC assessment.69 In these studies, higher MTRasym values have been detected in healthy volunteers than in patients with OA reflecting, in a very specific manner, a loss of GAGs from cartilage even in early stages.70 Some authors have even compared the accuracy of dGEMRIC and gagCEST, both of which are able to assess loss of GAGs with similar results. For cartilage repair assessment or even the introduction of new therapies in the firsts stages of OA, the gagCEST approach has shown promising results in patient monitoring; showing normalization of MTRasym, which suggests recovery of GAGs concentration within AC.71
Sodium imaging
Physical principle
Most MRI systems are designed for the detection and characterization of 1H protons. However, there are other compounds within the tissues, such as sodium which can also be evaluated. +23Na is an ion characterized by a quadrupolar moment able to interact with surrounding protons, which condition a biexponential decay of relaxation times.72 +23Na (positively charged) and PGs (negatively charged) establish an electromagnetic equilibrium inside the AC with a direct correlation between both compounds. Sulfate and carboxylate PGs groups are fixed to +23Na, so a loss of PGs involves a decrease of +23Na concentration within cartilage.8 However, SI of AC has two major drawbacks: its very low concentration and its very short transverse relaxation time. These disadvantages result in poor quality images due to low SNR, poor resolution and partial volume effects from synovial fluid and subchondral bone.73 To try and overcome these limitations, and increase the resolution and SNR of sodium acquisitions, the use of dedicated coils with long scan times and high magnetic field systems is mandatory (Supplementary Video 7).74,75
Ex vivo/in vivo validation
In vivo and ex vivo studies have demonstrated the existence of a fixed charge density (FCD) within AC due to a balance between +23Na and GAGs. That FCD enables the calculation of the GAGs concentration in the AC. Animal studies with bovine cartilage and PGs damage induced by trypsin demonstrate changes in sodium concentration with an almost linearly relationship.76 Sodium MRI has also been applied for OA evaluation in experimental animal models after cytokine injection; detecting a decrease of sodium concentration as well as FCD. The results are consistent with loss of PGs and these studies have shown histological correlation.29
Clinical diagnostic and prognostic value
The use of sodium MRI in common clinical practice is quite limited due to the drawbacks listed above. However, some studies have tested the feasibility of sodium MRI for cartilage evaluation in healthy volunteers and patients with early signs of OA.77 Lower signal in the sodium image, consistent with loss of fixed charge density, has been identified in patients with knee OA compared with healthy controls, which suggests a decrease in PGs concentration within AC.75 Nevertheless, no large series have yet been published evaluating the clinical impact of this approach for assessment of AC damage. Further studies are needed to establish the potential role of this promising tool as well as the sensitivity and specificity with regard the rest of functional imaging modalities for cartilage assessment.
Conclusions
Currently, there is a wide range of functional MRI techniques that are allowing radiologist to evaluate AC in a more accurate manner than with morphological MRI sequences alone. These techniques predominantly evaluate the integrity of each one of AC components, mainly collagen and GAGs/PGs. T2-mapping can be considered the most robust and ready for practical use in current clinical radiology practice due to its relative short acquisition time and its almost worldwide implementation. Other approaches, such as dGEMRIC, also used in clinical practice, have the major drawback of the need for GBCAs administration. To this point, non-contrast techniques like DWI or DTI may become viable alternatives. T1ρ, gagCEST and Sodium imaging have also show high specificity for GAGs/PGs assessment but they are used uncommonly in clinical practice due to the longer acquisition times and higher magnetic fields strength requirement. Nevertheless, the knowledge of the physical basis of how each technique allows one to evaluate ECM in a specific manner is important for radiologists. Being familiar with these techniques will allow physicists and radiologists to optimize the acquisition and interpretation of these studies, not only for investigational purposes but also for their application in current and future clinical radiologic practice.
REFERENCES
- 1.Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med 2010; 26: 355–69. doi: 10.1016/j.cger.2010.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eckstein F, Collins JE, Nevitt MC, Lynch JA, Kraus VB, Katz JN, et al. Brief report: cartilage thickness change as an imaging biomarker of knee osteoarthritis progression: data from the foundation for the National Institutes of health osteoarthritis biomarkers Consortium. Arthritis & Rheumatology 2015; 67: 3184–9. doi: 10.1002/art.39324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Podlipská J, Guermazi A, Lehenkari P, Niinimäki J, Roemer FW, Arokoski JP, et al. Comparison of diagnostic performance of semi-quantitative knee ultrasound and knee radiography with MRI: Oulu knee osteoarthritis study. Sci Rep 2016; 6: 22365. doi: 10.1038/srep22365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi W, Tian D, Liu D, Yin J, Huang Y. The comparison of measurement between ultrasound and computed tomography for abnormal degenerative facet joints: a STROBE-compliant article. Medicine 2017; 96: e7680. doi: 10.1097/MD.0000000000007680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duc SR, Pfirrmann CWA, Schmid MR, Zanetti M, Koch PP, Kalberer F, et al. Articular cartilage defects detected with 3D water-excitation true FISP: prospective comparison with sequences commonly used for knee imaging. Radiology 2007; 245: 216–23. doi: 10.1148/radiol.2451060990 [DOI] [PubMed] [Google Scholar]
- 6.Crema MD, Roemer FW, Marra MD, Burstein D, Gold GE, Eckstein F, et al. Articular cartilage in the knee: current MR imaging techniques and applications in clinical practice and research.. Radiographics 2011; 31: 37–61. doi: 10.1148/rg.311105084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alizai H, Virayavanich W, Joseph GB, Nardo L, Liu F, Liebl H, et al. Cartilage lesion score: comparison of a quantitative assessment score with established semiquantitative Mr scoring systems. Radiology 2014; 271: 479–87. doi: 10.1148/radiol.13122056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Binks DA, Hodgson RJ, Ries ME, Foster RJ, Smye SW, McGonagle D, et al. Quantitative parametric MRI of articular cartilage: a review of progress and open challenges. Br J Radiol 2013; 86: 20120163. doi: 10.1259/bjr.20120163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nebelung S, Post M, Knobe M, Tingart M, Emans P, Thüring J, et al. Detection of early-stage degeneration in human articular cartilage by multiparametric MR imaging mapping of tissue functionality. Sci Rep 2019; 9: 5895. doi: 10.1038/s41598-019-42543-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guermazi A, Alizai H, Crema MD, Trattnig S, Regatte RR, Roemer FW. Compositional MRI techniques for evaluation of cartilage degeneration in osteoarthritis. Osteoarthritis Cartilage 2015; 23: 1639–53. doi: 10.1016/j.joca.2015.05.026 [DOI] [PubMed] [Google Scholar]
- 11.Raya JG. Techniques and applications of in vivo diffusion imaging of articular cartilage. J Magn Reson Imaging 2015; 41: 1487–504. doi: 10.1002/jmri.24767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rehnitz C, Kupfer J, Streich NA, Burkholder I, Schmitt B, Lauer L, et al. Comparison of biochemical cartilage imaging techniques at 3 T MRI. Osteoarthritis Cartilage 2014; 22: 1732–42. doi: 10.1016/j.joca.2014.04.020 [DOI] [PubMed] [Google Scholar]
- 13.Bruno F, Arrigoni F, Palumbo P, Natella R, Maggialetti N, Reginelli A, et al. New advances in MRI diagnosis of degenerative osteoarthropathy of the peripheral joints. Radiol Med 2019; 260. doi: 10.1007/s11547-019-01003-1 [DOI] [PubMed] [Google Scholar]
- 14.Novakofski KD, Pownder SL, Koff MF, Williams RM, Potter HG, Fortier LA. High-Resolution Methods for Diagnosing Cartilage Damage In Vivo. Cartilage 2016; 751. doi: 10.1177/1947603515602307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baum T, Joseph GB, Karampinos DC, Jungmann PM, Link TM, Bauer JS. Cartilage and meniscal T2 relaxation time as non-invasive biomarker for knee osteoarthritis and cartilage repair procedures. Osteoarthritis Cartilage 2013; 21: 1474–84. doi: 10.1016/j.joca.2013.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kijowski R, Blankenbaker DG, Munoz Del Rio A, Baer GS, Graf BK. Evaluation of the articular cartilage of the knee joint: value of adding a T2 mapping sequence to a routine MR imaging protocol. Radiology 2013; 267: 503–13. doi: 10.1148/radiol.12121413 [DOI] [PubMed] [Google Scholar]
- 17.Li X, Benjamin Ma C, Link TM, Castillo D-D, Blumenkrantz G, Lozano J, et al. In vivo T(1rho) and T(2) mapping of articular cartilage in osteoarthritis of the knee using 3T MRI. Osteoarthritis Cartilage 2007; 15: 789–97. doi: 10.1016/j.joca.2007.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith HE, Mosher TJ, Dardzinski BJ, Collins BG, Collins CM, Yang QX, et al. Spatial variation in cartilage T2 of the knee. J Magn Reson Imaging 2001; 14: 50–5. doi: 10.1002/jmri.1150 [DOI] [PubMed] [Google Scholar]
- 19.Mosher TJ, Dardzinski BJ. Cartilage MRI T2 relaxation time mapping: overview and applications. Semin Musculoskelet Radiol 2004; 8: 355–68. doi: 10.1055/s-2004-861764 [DOI] [PubMed] [Google Scholar]
- 20.Bae WC, Du J, Bydder GM, Chung CB, Conventional CCB. Conventional and ultrashort Time-to-Echo magnetic resonance imaging of articular cartilage, meniscus, and intervertebral disk. Topics in Magnetic Resonance Imaging 2010; 21: 275–89. doi: 10.1097/RMR.0b013e31823ccebc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bae WC, Dwek JR, Znamirowski R, Statum SM, Hermida JC, D’Lima DD, et al. Ultrashort echo time MR imaging of osteochondral junction of the knee at 3 T: identification of anatomic structures contributing to signal intensity. Radiology 2010; 254: 837–45. doi: 10.1148/radiol.09081743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watanabe A, Boesch C, Siebenrock K, Obata T, Anderson SE. T2 mapping of hip articular cartilage in healthy volunteers at 3T: a study of topographic variation. J Magn Reson Imaging 2007; 26: 165–71. doi: 10.1002/jmri.21014 [DOI] [PubMed] [Google Scholar]
- 23.Guermazi A, Roemer FW, Alizai H, Winalski CS, Welsch G, Brittberg M, et al. State of the art: MR imaging after knee cartilage repair surgery. Radiology 2015; 277: 23–43. doi: 10.1148/radiol.2015141146 [DOI] [PubMed] [Google Scholar]
- 24.Welsch GH, Trattnig S, Scheffler K, Szomonanyi P, Quirbach S, Marlovits S, et al. Magnetization transfer contrast and T2 mapping in the evaluation of cartilage repair tissue with 3T MRI. J Magn Reson Imaging 2008; 28: 979–86. doi: 10.1002/jmri.21516 [DOI] [PubMed] [Google Scholar]
- 25.Jungmann PM, Baum T, Bauer JS, Karampinos DC, Erdle B, Link TM, et al. Cartilage repair surgery: outcome evaluation by using noninvasive cartilage biomarkers based on quantitative MRI techniques? Biomed Res Int 2014; 201417. doi: 10.1155/2014/840170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wáng Y-XJ, Zhang Q, Li X, Chen W, Ahuja A. Yuan J. T1ρ magnetic resonance: basic physics principles and applications in knee and intervertebral disc imaging. Quant Imaging Med Surg 2015; 5: 858–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hatcher CC, Collins AT, Kim SY, Michel LC, Mostertz WC, Ziemian SN, et al. Relationship between T1rho magnetic resonance imaging, synovial fluid biomarkers, and the biochemical and biomechanical properties of cartilage. J Biomech 2017; 55: 18–26. doi: 10.1016/j.jbiomech.2017.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Souza RB, Feeley BT, Zarins ZA, Link TM, Li X, Majumdar S. T1Rho MRI relaxation in knee OA subjects with varying sizes of cartilage lesions. Knee 2013; 20: 113–9. doi: 10.1016/j.knee.2012.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wheaton AJ, Dodge GR, Borthakur A, Kneeland JB, Schumacher HR, Reddy R. Detection of changes in articular cartilage proteoglycan byT1ρ magnetic resonance imaging. J Orthop Res 2005; 23: 102–8. doi: 10.1016/j.orthres.2004.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atkinson HF, Birmingham TB, Moyer RF, Yacoub D, Kanko LE, Bryant DM, et al. MRI T2 and T1ρ relaxation in patients at risk for knee osteoarthritis: a systematic review and meta-analysis. BMC Musculoskelet Disord 2019; 20: 182. doi: 10.1186/s12891-019-2547-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lozano J, Li X, Link TM, Safran M, Majumdar S, CB M. Detection of posttraumatic cartilage injury using quantitative T1rho magnetic resonance imaging: a report of two cases with arthroscopic findings. J Bone Jt Surg - Ser A 2006; 88: 1349–52. [DOI] [PubMed] [Google Scholar]
- 32.Hulvershorn J, Borthakur A, Bloy L, Gualtieri EE, Reddy R, Leigh JS, et al. T1ρ contrast in functional magnetic resonance imaging. Magn. Reson Med 2005; 54: 1155–62. doi: 10.1002/mrm.20698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duvvuri U, Reddy R, Patel SD, Kaufman JH, Kneeland JB, Leigh JS. T1ρ-relaxation in articular cartilage: effects of enzymatic degradation. Magn Reson Med 1997; 38: 863–7. doi: 10.1002/mrm.1910380602 [DOI] [PubMed] [Google Scholar]
- 34.Akella SVS, Regatte RR, Borthakur A, Kneeland JB, Leigh JS, Reddy R. T1ρ MR imaging of the human wrist in vivo. Acad Radiol 2003; 10: 614–9. doi: 10.1016/S1076-6332(03)80079-X [DOI] [PubMed] [Google Scholar]
- 35.Wang L, Regatte RR. Quantitative mapping of human cartilage at 3.0T: parallel changes in T2, T1ρ, and dGEMRIC. Acad Radiol 2014; 21: 463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Le J, Peng Q, Sperling K. Biochemical magnetic resonance imaging of knee articular cartilage: T1rho and T2 mapping as cartilage degeneration biomarkers. Ann N Y Acad Sci 2016; 1383: 34–42. doi: 10.1111/nyas.13189 [DOI] [PubMed] [Google Scholar]
- 37.Bolbos RI, Link TM, Benjamin Ma C, Majumdar S, Li X. T1ρ relaxation time of the meniscus and its relationship with T1ρ of adjacent cartilage in knees with acute ACL injuries at 3T. Osteoarthr Cartil 2009; 17: 12–18. doi: 10.1016/j.joca.2008.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li X, Kuo D, Theologis A, Carballido-Gamio J, Stehling C, Link TM, et al. Cartilage in Anterior Cruciate Ligament–Reconstructed Knees: MR Imaging T1rho and T2—Initial Experience with 1-year Follow-up. Radiology 2011; 258: 505–14. doi: 10.1148/radiol.10101006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li W, Scheidegger R, Wu Y, Edelman RR, Farley M, Krishnan N, et al. Delayed contrast-enhanced MRI of cartilage: comparison of nonionic and ionic contrast agents. Magn Reson Med 2010; 64: 1267–73. doi: 10.1002/mrm.22555 [DOI] [PubMed] [Google Scholar]
- 40.Burstein D, Velyvis J, Scott KT, Stock KW, Kim YJ, Jaramillo D, et al. Protocol issues for delayed Gd(DTPA)2--enhanced MRI (dGEMRIC) for clinical evaluation of articular cartilage. Magn Reson Med 2001; 45: 36–41. doi: [DOI] [PubMed] [Google Scholar]
- 41.Bashir A, Gray ML, Boutin RD, Burstein D. Glycosaminoglycan in articular cartilage: in vivo assessment with delayed Gd(DTPA)(2-)-enhanced MR imaging. Radiology 1997; 205: 551–8. doi: 10.1148/radiology.205.2.9356644 [DOI] [PubMed] [Google Scholar]
- 42.Bashir A, Gray ML, Hartke J, Burstein D. Nondestructive imaging of human cartilage glycosaminoglycan concentration by MRI. Magn. Reson. Med. 1999; 41: 857–65. doi: [DOI] [PubMed] [Google Scholar]
- 43.Peterson P, Tiderius CJ, Olsson E, Lundin B, Olsson LE, Svensson J. Knee dGEMRIC at 7 T: comparison against 1.5 T and evaluation of T1-mapping methods. BMC Musculoskelet Disord 2018; 19: 149. doi: 10.1186/s12891-018-2071-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim YJ, Jaramillo D, Millis MB, Gray ML, Burstein D. Assessment of early osteoarthritis in hip dysplasia with delayed gadolinium-enhanced magnetic resonance imaging of cartilage. J Bone Jt Surg - Ser A 2003; 85: 1987–92. doi: 10.2106/00004623-200310000-00019 [DOI] [PubMed] [Google Scholar]
- 45.Basser P, Pierpaoli C. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med 1996; 36: 893–906. [DOI] [PubMed] [Google Scholar]
- 46.Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology 2014; 201: 637–48. doi: 10.1148/radiology.201.3.8939209 [DOI] [PubMed] [Google Scholar]
- 47.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B 1996; 111: 209–19. doi: 10.1006/jmrb.1996.0086 [DOI] [PubMed] [Google Scholar]
- 48.Burstein D, Gray ML, Hartman AL, Gipe R, Foy BD. Diffusion of small solutes in cartilage as measured by nuclear magnetic resonance (NMR) spectroscopy and imaging. J Orthop Res 1993; 11: 465–78. doi: 10.1002/jor.1100110402 [DOI] [PubMed] [Google Scholar]
- 49.Raya JG. Techniques and applications of in vivo diffusion imaging of articular cartilage. J Magn Reson Imaging 2015; 1504: 1487–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferizi U, Ruiz A, Rossi I, Bencardino J, Raya JG. A robust diffusion tensor model for clinical applications of MRI to cartilage. Magn Reson Med 2018; 79: 1157–64. doi: 10.1002/mrm.26702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Toffanin R, Mlynárik V, Russo S, Szomolányi P, Piras A, Vittur F. Proteoglycan depletion and magnetic resonance parameters of articular cartilage. Arch Biochem Biophys 2001; 390: 235–42. doi: 10.1006/abbi.2001.2338 [DOI] [PubMed] [Google Scholar]
- 52.Berg A, Singer T, Moser E. High-resolution diffusivity imaging at 3.0 T for the detection of degenerative changes: a trypsin-based arthritis model. Invest Radiol 2003; 38: 460–6. [DOI] [PubMed] [Google Scholar]
- 53.Lin PC, Reiter DA, Spencer RG. Classification of degraded cartilage through multiparametric MRI analysis. J Magn Reson 2009; 201: 61–71. doi: 10.1016/j.jmr.2009.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ashinsky BG, Coletta CE, Bouhrara M, Lukas VA, Boyle JM, Reiter DA, et al. Machine learning classification of OARSI-scored human articular cartilage using magnetic resonance imaging. Osteoarthr Cartil 2015; 23: 1704–12. doi: 10.1016/j.joca.2015.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lukas VA, Fishbein KW, Reiter DA, Lin PC, Schneider E, Spencer RG. Sensitivity and specificity of univariate MRI analysis of experimentally degraded cartilage under clinical imaging conditions. J Magn Reson Imaging 2015; 42: 136–44. doi: 10.1002/jmri.24773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Visser SK, Crawford RW, Pope JM. Structural adaptations in compressed articular cartilage measured by diffusion tensor imaging. Osteoarthr Cartil 2008; 16: 83–9. doi: 10.1016/j.joca.2007.05.013 [DOI] [PubMed] [Google Scholar]
- 57.Raya JG, Melkus G, Adam-Neumair S, Dietrich O, Mützel E, Kahr B, et al. Change of diffusion tensor imaging parameters in articular cartilage with progressive proteoglycan extraction. Invest Radiol 2011; 46: 401–9. doi: 10.1097/RLI.0b013e3182145aa8 [DOI] [PubMed] [Google Scholar]
- 58.Juras V, Bittsansky M, Majdisova Z, Szomolanyi P, Sulzbacher I, Gäbler S, et al. In vitro determination of biomechanical properties of human articular cartilage in osteoarthritis using multi-parametric MRI. J Magn Reson 2009; 197: 40–7. doi: 10.1016/j.jmr.2008.11.019 [DOI] [PubMed] [Google Scholar]
- 59.Raya JG, Melkus G, Adam-Neumair S, Dietrich O, Mützel E, Reiser MF, et al. Diffusion-Tensor imaging of human articular cartilage specimens with early signs of cartilage damage. Radiology 2013; 266: 831–41. doi: 10.1148/radiol.12120954 [DOI] [PubMed] [Google Scholar]
- 60.Lukas VA, Fishbein KW, Lin P-C, Schär M, Schneider E, Neu CP, et al. Classification of histologically scored human knee osteochondral plugs by quantitative analysis of magnetic resonance images at 3T. J Orthop Res 2015; 33: 640–50. doi: 10.1002/jor.22810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu SC, Shi DP, Xuan A. Human patellar cartilage: echo planar diffusion-weighted MR imaging findings at 3.0 T. Clin Imaging 2012; 36: 199–202. doi: 10.1016/j.clinimag.2011.08.016 [DOI] [PubMed] [Google Scholar]
- 62.Welsch GH, Trattnig S, Domayer S, Marlovits S, White LM, Mamisch TC. Multimodal approach in the use of clinical scoring, morphological MRI and biochemical T2-mapping and diffusion-weighted imaging in their ability to assess differences between cartilage repair tissue after microfracture therapy and matrix-associated autologous chondrocyte transplantation: a pilot study. Osteoarthr Cartil 2009; 17: 1219–27. doi: 10.1016/j.joca.2009.03.018 [DOI] [PubMed] [Google Scholar]
- 63.Raya JG, Horng A, Dietrich O, Krasnokutsky S, Beltran LS, Storey P, et al. Articular cartilage: in vivo Diffusion-Tensor imaging. Radiology 2012; 262: 550–9. doi: 10.1148/radiol.11110821 [DOI] [PubMed] [Google Scholar]
- 64.Guha A, Wyatt C, Karampinos DC, Nardo L, Link TM, Majumdar S. Spatial variations in magnetic resonance-based diffusion of articular cartilage in knee osteoarthritis. Magn Reson Imaging 2015; 33: 1051–8. doi: 10.1016/j.mri.2015.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Duarte A, Ruiz A, Ferizi U, Bencardino J, Abramson SB, Samuels J, et al. Diffusion tensor imaging of articular cartilage using a navigated radial imaging spin-echo diffusion (RAISED) sequence. Eur Radiol 2019; 29: 2598–607. doi: 10.1007/s00330-018-5780-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Krishnamoorthy G, Nanga RPR, Bagga P, Hariharan H, Reddy R. High quality three-dimensional gagCEST imaging of in vivo human knee cartilage at 7 Tesla. Magn Reson Med 2017; 77: 1866–73. doi: 10.1002/mrm.26265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wei W, Jia G, Flanigan D, Zhou J, Knopp MV. Chemical exchange saturation transfer MR imaging of articular cartilage glycosaminoglycans at 3T: accuracy of B0 field inhomogeneity corrections with gradient echo method. Magn Reson Imaging 2014; 32: 41–7. [Internet]. 10.1016/j.mri.2013.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Singh A, Haris M, Cai K, Kassey VB, Kogan F, Reddy D, et al. Chemical exchange saturation transfer magnetic resonance imaging of human knee cartilage at 3 T and 7 T. Magn Reson Med 2012; 68: 588–94. doi: 10.1002/mrm.23250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schreiner MM, Zbýň S, Schmitt B, Weber M, Domayer S, Windhager R, et al. Reproducibility and regional variations of an improved gagCEST protocol for the in vivo evaluation of knee cartilage at 7 T. Magn Reson mater physics. Biol Med 2016; 29: 513–21. [DOI] [PubMed] [Google Scholar]
- 70.Brinkhof S, Nizak R, Khlebnikov V, Prompers JJ, Klomp DWJ, Saris DBF. Detection of early cartilage damage: feasibility and potential of gagCEST imaging at 7T. Eur Radiol 2018; 28: 2874–81. doi: 10.1007/s00330-017-5277-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Koller U, Apprich S, Schmitt B, Windhager R, Trattnig S. Evaluating the cartilage adjacent to the site of repair surgery with glycosaminoglycan-specific magnetic resonance imaging. Int Orthop 2017; 41: 969–74. doi: 10.1007/s00264-017-3434-1 [DOI] [PubMed] [Google Scholar]
- 72.Zbýň S, Mlynárik V, Juras V, Szomolanyi P, Trattnig S. Evaluation of cartilage repair and osteoarthritis with sodium MRI. NMR Biomed 2016; 29: 206–15. doi: 10.1002/nbm.3280 [DOI] [PubMed] [Google Scholar]
- 73.Borthakur A, Mellon E, Niyogi S, Witschey W, Kneeland JB, Reddy R. Sodium and T1rho MRI for molecular and diagnostic imaging of articular cartilage. NMR Biomed 2006; 19: 781–821. doi: 10.1002/nbm.1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gold GE, Burstein D, Dardzinski B, Lang P, Boada F, Mosher T. MRI of articular cartilage in OA: novel pulse sequences and compositional/functional markers. Osteoarthr Cartil 2006; 14: 76–86. doi: 10.1016/j.joca.2006.03.010 [DOI] [PubMed] [Google Scholar]
- 75.Madelin G, Babb JS, Xia D, Chang G, Jerschow A, Regatte RR. Reproducibility and repeatability of quantitative sodium magnetic resonance imaging in vivo in articular cartilage at 3 T and 7 T. Magn Reson Med 2012; 68: 841–9. doi: 10.1002/mrm.23307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Insko EK, Kaufman JH, Leigh JS, Reddy R. Sodium NMR evaluation of articular cartilage degradation. Magn Reson Med 1999; 41: 30–4. doi: [DOI] [PubMed] [Google Scholar]
- 77.Wheaton AJ, Borthakur A, Shapiro EM, Regatte RR, Akella SVS, Kneeland JB, et al. Proteoglycan loss in human knee cartilage: quantitation with sodium MR Imaging—Feasibility study. Radiology 2004; 231: 900–5. doi: 10.1148/radiol.2313030521 [DOI] [PubMed] [Google Scholar]