Abstract
PURPOSE
We aimed to assess the temporal change in radiation doses from coronary computed tomography angiography (CCTA) during a 6-year period. High CCTA radiation doses have been reduced by multiple technologies that, if used appropriately, can decrease exposures significantly.
METHODS
A total of 1277 examinations performed from 2005 to 2010 were included. Univariate and multivariable regression analysis of patient- and scan-related variables was performed with estimated radiation dose as the main outcome measure.
RESULTS
Median doses decreased by 74.8% (P < .001), from 13.1 millisieverts (mSv) (interquartile range 9.3–14.7) in period 1 to 3.3 mSv (1.8–6.7) in period 4. Factors associated with greatest dose reductions (P < .001) were all most frequently applied in period 4: axial-sequential acquisition (univariate: −8.0 mSv [−9.7 to −7.9]), high-pitch helical acquisition (univariate: −8.8 mSv [ − 9.3 to −7.9]), reduced tube voltage (100 vs 120 kV) (univariate: −6.4 mSv [ − 7.4 to −5.4]), and use of automatic exposure control (univariate: −5.3 mSv [ − 6.2 to −4.4]).
CONCLUSIONS
CCTA radiation doses were reduced 74.8% through increasing use of dose-saving measures and evolving scanner technology.
Keywords: Cardiac computed tomography, Coronary artery disease, Dose-savings methods, Radiation dose, Scan protocols
Coronary computed tomography angiography (CCTA) has considerable but variable adoption in clinical practice.1–5 Because of radiation dose concerns, numerous innovations have been introduced (Figure 1).6–14 Prior studies have shown the potential of protocols tailored for specific parameters, chiefly heart rate and body mass index (BMI).15–17 Substantial dose reductions have been demonstrated in selected populations,9,11,13,18–21 but few studies have documented the combined impact of these measures in a large population.6,22,23 We assessed CCTA radiation dose during a period spanning 3 scanners, several new dose-saving technologies, and standardization of protocols.
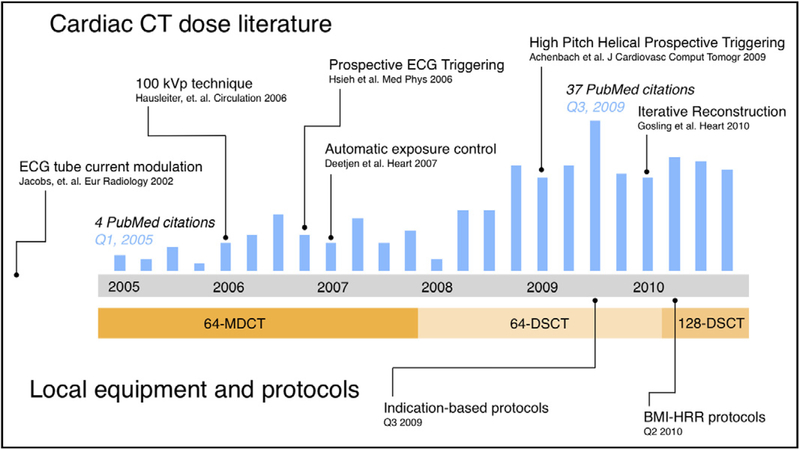
Figure 1.
Study period and context: The timeline plots total quarterly PubMed citations (blue bars) resulting from the search “cardiac CT dose reduction” and notes key developments in the cardiac CT literature during the study period. The locally available equipment during the study period is listed beneath. CT = computed tomography; DSCT = dual-source computed tomography; MDCT = multidetector computed tomography.
MATERIAL AND METHODS
Study Population
The study was approved by the human research committee of Partners Healthcare Institutional Review Board and is compliant with the Health Insurance Portability and Accountability Act. The requirement for informed consent was waived. We analyzed all patients who underwent clinical gated CCTA of the native coronaries at an academic center. Electrocardiogram (ECG)-gated computed tomography (CT) for indications other than native coronary artery disease (ie, pulmonary vein, coronary artery bypass graft, valve, cardiac masses, congenital heart disease, or research examinations) were excluded. A total of 5694 examinations were performed between January 1, 2005, and December 31, 2010. We surveyed all consecutive examinations performed in a given month at 3-month intervals. This yielded 24 sampled months and a total cohort of 1277 examinations. Examinations per month varied from 28 t0 77
Cardiac Computed Tomography Examination
Three scanners were used, depending on available resources (Figure 1). Between January 2005 and December 2007, scans were performed using a 32-detector row, single-source multidetector computed tomography (MDCT) scanner with a flying focal spot (z-Sharp) with an effective 64 overlapping 0.6-mm slices acquired per rotation (64-slice MDCT) (SO-MATOM Sensation 64, Siemens AG, Healthcare Sector, Forchheim, Germany). Beginning in January 2008, scans were performed on a first-generation dual-source computed tomography (DSCT) scanner (SOMATOM Definition 64, Siemens AG) with 2 × 64 × 0.6-mm collimation and gantry rotation time of 330 ms (64-slice DSCT). Beginning in April 2010, scans were performed using a second-generation 128-slice DSCT (SOMATOM Definition Flash, Siemens AG), and volume datasets were acquired with 2 × 128 × 0.6 collimation and a gantry rotation time of 280 ms.
Acquisition parameters were adjusted to individually optimize scans, based on the available technology: range of pitch 0.2 to 3.4, range of tube current 63 to 961 milliampere-seconds (mAs) per rotation (when available after April 2008, scout-based automatic reference tube current selection, or automatic exposure control was used [CAREDose 4D, Siemens AG, Healthcare Sector]), range of tube potential 80 to 140 kilovolt-potential (kVp). Axial images were reconstructed using conventional filtered back-projection. Contrast agent administration was timed by means of a 20-mL test bolus acquisition. Typically, 60 to 100 mL of contrast agent (iopamidol 370 g/cm3 Isovue 370, Bracco Diagnostics, Princeton, NJ), adjusted in accordance with scan time, was administered as a bolus at 4 to 8 mL/sec (depending on patient size) followed by a 40-mL saline flush, during a single breath-hold at end inspiration. ECG synchronization was achieved with retrospective ECG-gated helical scanning, prospective ECG-triggered axial scanning, or prospective ECG-triggered high-pitch helical scanning; selections were made from the scanner’s available options, based on site protocols when applicable, and the supervising physician’s decision, after beta-blocker and nitroglycerine administration at the time of the scan.
Protocol Changes
A team of at least 1 imaging fellow (board-eligible radiologist or cardiologist), supervised by an attending radiologist or cardiologist, decided on specific imaging parameters and premedication regimen.
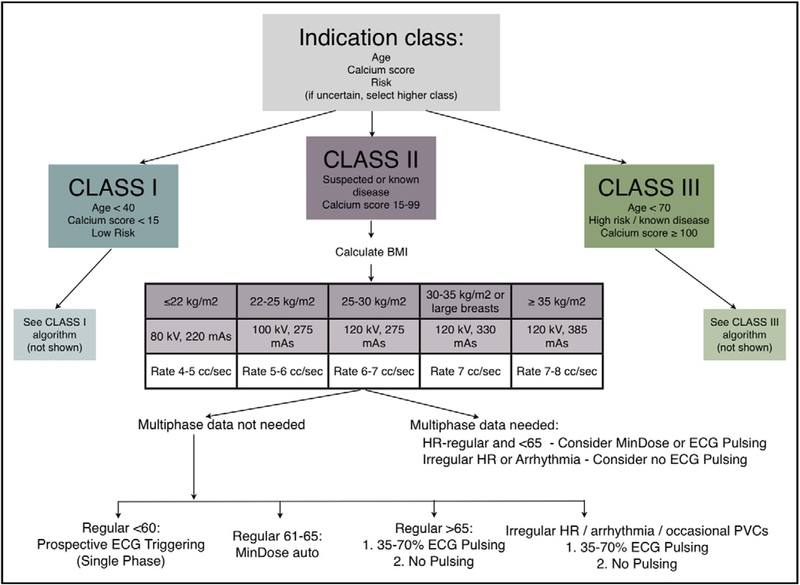
After an initial period without protocol recommendations, guidelines were developed (May 2009; Figure 2). These detailed recommended protocols were based primarily on pretest probability and are documented as “indication-based protocols,” and were publicized, although adherence was not enforced. Compliance remained at the discretion of the supervising team.
Figure 2.
Indication-based protocols: The primary decision point revolved around the indication class, which was intended to reflect the pretest probability. Detailed, specific charts were tailored within each class. ECG = electrocardiogram; HR = heart rate; PVC = premature ventricular contraction.
Subsequently, we developed simplified recommended settings based on body mass index (BMI), heart rate, and heart rhythm-based nomograms (“BMI/HRR-based protocols”), and these were saved on the CT console (Table 1). This intervention coincided with the acquisition of 128-slice DSCT. Initial stratification was selected by the technologist, who was responsible for determining the BMI. The BMI/HRR protocols involved preset default kVp and reference mAs settings (Table 1). The imaging team then decided on the method of ECG synchronization (ie, prospective triggering or retrospective gating). Again, the protocols were not enforced; the physicians had the option to override any or all settings.
Table 1.
Body Mass Index, Heart Rate, and Heart Rhythm–Based Protocols
| BMI | HR | kVp | Reference mAs | Gating/Triggering |
|---|---|---|---|---|
| <20 | Low, stable | 80 | 430 | Prospective high-pitched helical |
| Irregular heart rates (if padding applied) | Axial-sequential (prospective) | |||
| Irregular/high heart rate; or if functional information needed | Retrospective gating, with ECG-based mAs pulsing to 4% of the reference tube current (interval increasing with HR) | |||
| 20–25 | Low, stable | 100 | 320 | Prospective high-pitched helical |
| Irregular heart rates (if padding applied) | Axial-sequential (prospective) | |||
| Irregular/high heart rate; or if functional information needed | Retrospective gating, with ECG-based mAs pulsing to 4% of the reference tube current (interval increasing with HR) | |||
| >25–31 | Low, stable | 120 | 320 | Prospective high-pitched helical |
| Irregular heart rates (if padding applied) | Axial-sequential (prospective) | |||
| Irregular/high heart rate; or if functional information needed | Retrospective gating, with ECG-based mAs pulsing to 4% of the reference tube current (interval increasing with HR) | |||
| >31 | Low, stable | 120 | 430 | Prospective high-pitched helical |
| Irregular heart rates (if padding applied) | Axial-sequential (prospective) | |||
| Irregular/high heart rate; or if functional information needed | Retrospective gating, with ECG-based mAs pulsing to 4% of the reference tube current (interval increasing with HR); pulse width maximized to allow low temporal resolution/high mAs images* |
BMI = body mass index; HR = heart rate; ECG = electrocardiogram; kVp = kilovolt-potential; mAs = milliampere-seconds.
Technologists calculated the BMI, and physicians decided on the type of ECG synchronization. Physicians always had the option of overriding any settings at their discretion.
Preferred protocol for very large patients.
Thus, the cohort was divided into 4 periods: period 1, 64-slice MDCT without default protocols; period 2, 64-slice DSCT without default protocols; period 3, 64-slice DSCT with indication-based protocols; and period 4, 128-slice DSCT with BMI/HRR-based protocols.
Estimation of Radiation Dose
The effective radiation dose was calculated by multiplying dose-length-product by the European Working Group for Guidelines on Quality Criteria in Computed Tomography conversion coefficient (k = 0.014 millisievert [mSv] × [mGy × cm]−1).24
Data Collection
The dose-length-product, tube potential (kVp), tube current (mAs), summed volume-weighted CT dose index, and volume CT dose index for the CCTA series were extracted manually from the dose exposure record. The extracted study data contained the total radiation dose including the summed dose-length-product from all parts of the examination, including topogram, test bolus, CCTA, and calcium scoring scan (if performed). Scan length was derived by using the formula (dose-length-product ÷ volume-weighted CT dose index for the CCTA acquisition).
Certain patient demographics (age and gender) and scan parameters (scanner type, presence of tube current modulation and window, prospective triggering, and automatic exposure control) were extracted from the image metadata.
Further patient characteristics (beta-blockade, BMI, presence of sinus rhythm, mean heart rate, range of heart rate) and the incidence and source(s) of nonevaluable segments were identified through review of all clinical reports. Height and weight were surveyed or measured and recorded by the technologist. The BMI was available for only 532 patients. For all patients, chest area was measured on an axial full field-of-view image at the z-axis level of the mid left atrium as a surrogate for patient size as described previously.25
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation or median with interquartile range (25th and 75th percentiles); nominal variables were expressed as percentage or frequencies, as appropriate. Differences in continuous variables were assessed using the Kruskal-Wallis test. Differences with respect to nondiagnostic segments were calculated using analysis of variance. Nominal variables were compared among the 3 groups using chi-square and Fisher exact tests, when appropriate. A 2-tailed P value less than .05 was considered statistically significant. In the subset of patients for whom all variables were available, univariate and multivariable analyses were performed to identify the effect of patient and imaging characteristics on the radiation dose. All analyses were performed using SAS (v 9.2, SAS Institute Inc, Cary, NC).
Finally, to adjust for the effects of the different scanner technologies, we applied the effect estimates derived from the multivariable model to adjust the effective radiation dose assuming that the first-generation DSCT was used in the entire cohort.
RESULTS
Patient characteristics are shown in Table 2. Scan parameters and radiation dose parameters are summarized in Table 3.
Table 2.
Patient Demographics
| Total (n = 1277) | 64-Slice MDCT (n = 713) | 64-Slice DSCT (n = 220) | 64-Slice DSCT: Indication-based Protocols (n = 90) | 128-Slice DSCT: BMI-based Protocols (n = 254) | P Value | |
|---|---|---|---|---|---|---|
| Patient Demographics | ||||||
| Age, y | 58.6 ± 13.8 | 61.7 ± 12.9 | 56.3 ± 13.3 | 55.5 ± 14.4 | 53.2 ± 13.9 | <.001 |
| Male, n (%) | 725 (56.8) | 396 (56.8) | 124 (56.4) | 46 (51.1) | 159 (62.6) | .158 |
| BB, n (%) | 813 (67.6) | 435 (67.7) | 107 (49.3) | 76 (85.4) | 195 (77.1) | <.001 |
| Heart rate, beats/min | 61.9 ± 9.8 | 61.9 ± 9.4 | 65.1 ± 10.9 | 61.5 ± 10.6 | 59.1 ± 8.3 | <.001 |
| Range, beats/min | 57.1–68.9 | 54.3–74.7 | 60.4–71.5 | 56.9–68.4 | 54.5–66.5 | |
| SR, n (%) | 1244 (97.4) | 697 (97.8) | 216 (98.2) | 90 (100.0) | 241 (98.0) | .570 |
| BMI, kg/m2 | 27.9 ± 5.0 | 27.6 ± 4.3 | 28.3 ± 4.9 | 27.6 ± 4.1 | 28.6 ± 6.8 | .024 |
| Chest area, cm2 | 808.2 ± 180.0 | 805.4 ± 172.4 | 832.7 ± 197.1 | 805.1 ± 165.4 | 796.5 ± 189.7 | .191 |
MDCT = multidetector computed tomography; DSCT = dual-source computed tomography; BB = beta-blockade; SR = sinus rhythm; BMI = body mass index.
Table 3.
Scan Parameters and Dose Estimates
| Total (n = 1277) | 64-Slice MDCT (n = 713) | 64-Slice DSCT (n = 220) | 64-Slice DSCT: Indication-based Protocols (n = 90) | 128-Slice DSCT: BMI-based Protocols (n = 254) | P Value | |
|---|---|---|---|---|---|---|
| Scan Parameters | ||||||
| Tube voltage, kVp | ||||||
| 80 kVp, n (%) | 18 (1.4) | 1 (0.14) | 3 (1.4) | 0 (0) | 14 (5.51) | <.001 |
| 100 kVp, n (%) | 150 (11.7) | 7 (0.98) | 25 (11.4) | 6 (6.7) | 112 (44.1) | <.001 |
| 120 kVp, n (%) | 1104 (86.5) | 705 (98.9) | 188 (85.5) | 84 (93.3) | 127 (50.0) | <.001 |
| 140 kVp, n (%) | 5 (0.4) | 0 (0) | 4 (1.8) | 0 (0) | 1 (0.39) | .008 |
| RGT, n (%) | 1092 (85.5) | 713 (100) | 193 (87.7) | 66 (73.3) | 120 (47.2) | <.001 |
| ECTCM, n (%) | 344 (26.9) | 0 (0) | 178 (80.9) | 58 (64.4) | 108 (42.5) | <.001 |
| ECG-PW, % | 20.1 ± 14.2 | 0 (0) | 22.9 ± 12.7 | 21.8 ± 12.8 | 15.3 ± 15.5 | <.001 |
| PGT, n (%) | 225 (17.6) | 0 (0) | 26 (11.8) | 24 (26.7) | 175 (68.9) | <.001 |
| Axial, n (%) | 75 (5.9) | 0 (0) | 26 (11.8) | 24 (26.7) | 25 (9.84) | <.001 |
| High-pitch helical, n (%) | 150 (11.7) | 0 (0) | 0 (0) | 0 (0) | 150 (59.1) | <.001 |
| AEC, n (%) | 442 (34.6) | 0 (0) | 135 (61.4) | 86 (95.6) | 221 (87.0) | <.001 |
| Scan length, cm | 16.3 ± 2.1 | 16.3 ± 1.7 | 16.1 ± 2.6 | 15.9 ± 2.6 | 16.8 ± 2.5 | <.001 |
| Dose Estimates | ||||||
| CTDIvol, mGy | 58.1 (46.4–77.0) | 70.5 (53.4–79.1) | 56.3 (42.1–70.9) | 46.2 (29.9–61.2) | 25.9 (16.9–46.4) | <.001 |
| DLP, mGy × cm | 724.0 (552.0–1010.0) | 934.0 (664.0–1051.0) | 729.0 (543.8–988.8) | 584.0 (341.0–785.25) | 239.0 (125.3–481.8) | <.001 |
| ED, mSv | 10.4 (7.7–14.1) | 13.1 (9.3–4.7) | 10.2 (7.6–13.8) | 8.2 (4.8–11.0) | 3.3 (1.8–6.7) | <.001 |
kVp = kilovolt-potential; RGT = retrospective gating; ECTCM = electrocardiogram-based tube current modulation; ECG-PW = electrocardiogram-based pulsing window; PGT = prospective triggering; AEC = automatic exposure control;CTDIvol = volume-weighted computed tomography dose index; mGy = milligray; DLP = dose-length-product; ED = effective dose estimate; mSv = millisievert.
Patient Characteristics
A total of 1277 subjects were surveyed; 713 (55.8%) were imaged with 64-slice MDCT (period 1) and 310 (24.3%) were imaged with 64-slice DSCT (ie, first-generation DSCT) (220 [17.2%] scanned without protocols (period 2) and 90 [7.0%] with indication-based protocols [period 3]). The 128-slice DSCT (ie, second-generation DSCT) with BMI/HRR-based protocols was used in the remaining 254 patients (19.9%) (period 4); all periods differed significantly in the total number of patients (P < .001).
There were significant differences between all periods with regard to age, use of beta-blockers, heart rate, and BMI. In all 4 periods, patients did not differ significantly regarding gender, presence of sinus rhythm, and chest area at the level of the mid left atrium.
Scan Parameters
Significant differences across the 4 periods were found for the majority of scan parameters.
The frequency of prospectively ECG-triggered axial-sequential scans, prospective ECG-triggered high-pitch helical (“flash-mode”) scans, and retrospective ECG-gated helical scans (with ECG-based tube current modulation) differed significantly (P < .001). Significant differences also were found in the frequency of various tube potential, with 100 kVp most frequently used for 128-slice DSCT (44.1%) and 120 kVp most frequently used with 64-slice MDCT (98.9%). Likewise, all periods significantly differed in the frequency of automatic exposure control use.
Radiation Dose Estimates
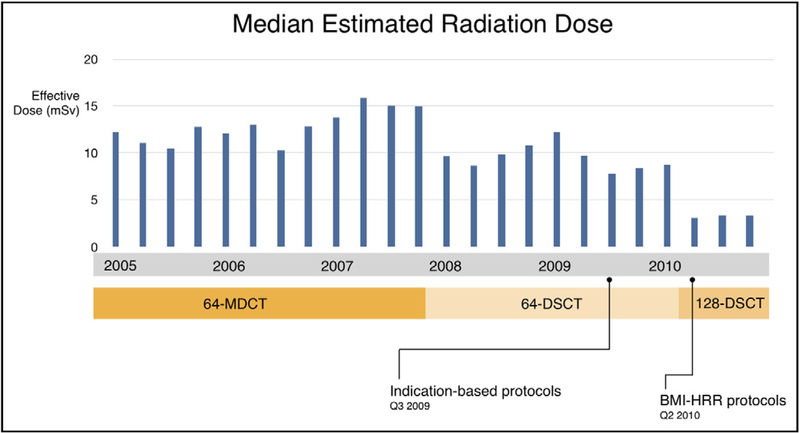
The overall median effective dose was 10.4 mSv (7.7–14.1). Median radiation dose for periods 1 to 4 was 13.1 mSv (9.3–14.7), 10.2 (7.6–13.8), 8.2 mSv (4.8–11.0), and 3.3 mSv (1.8–6.7), respectively. Differences between the periods were statistically significant. When compared with the initial doses in period 1, radiation doses were reduced by 74.8% in period 4 (Figure 3).
Figure 3.
Unadjusted median estimated radiation dose (mSv) versus scanner and protocol type. Progressive decreases in radiation doses were documented with successive scanners and protocols. BMI = body mass index; HRR = heart rate and rhythm; DSCT = dual-source computed tomography; MDCT = multidetector computed tomography.
Predictors of Cardiac Computed Tomography Angiography Radiation Dose
The results of regression analysis are listed in Table 4. A total of 532 patients had complete data that could be included in the regression analyses. Univariate regression analysis demonstrated a significant association among 3 patient-related variables (BMI, chest area, and heart rate) and 7 scan-related variables (scan length, kVp, prospective modes—all types, and scanner model). In the multivariable analysis, the same variables were significantly associated, although the strength of associations changed. Of note, when adjusted for the other variables, the effect of the scanner type on dose was smaller relative to the other variables. Increasing body size and high and unstable heart rates were related to a higher effective dose. Likewise, an increasing scan length was significantly associated with a higher radiation dose. The use of automatic exposure control resulted in a decrease of −1.0 mSv (−2.1 to 0.1), although a higher dose reduction was achieved with other radiation dose-saving methods, such as reduced tube potential (100 kVp) (−1.7 mSv [−2.7 to −0.7]).
Table 4.
Predictors of Coronary Computed Tomography Angiography Radiation Dose
| Univariate Linear Regression Analysis |
Multivariable Linear Regression Analysis |
|||
|---|---|---|---|---|
| Predictors | Estimate (mSv) | P Value | Estimate (mSv) | P Value |
| BMI normal (20–25 kg/m2 vs <20 kg/m2) | 0.7 (−1.8 to 3.2) | .567 | 2.4 (0.6–4.1) | .010 |
| BMI overweight (>25–31 kg/m2 vs <20 kg/m2) | 2.0 (−0.4 to 4.4) | .107 | 2.4 (0.6–4.2) | .010 |
| BMI obese (>31 kg/m2 vs <20 kg/m2) | 5.1 (2.6–7.7) | <.001 | 3.5 (1.3–5.8) | .002 |
| Chest area (cm2) | 0.1 (0.1–0.1) | <.001 | 0.0 (0.0–0.1) | .015 |
| Heart rate (60–70 vs <60 beats/min) | 2.5 (1.5–3.4) | <.001 | 0.5 (−0.2 to 1.2) | .187 |
| Heart rate (>70 vs <60 beats/min) | 3.9 (2.2–5.6) | <.001 | 1.3 (0.1–2.1) | .042 |
| Rhythm (sinus vs nonsinus) | −2.1 (−5.7 to 1.4) | .240 | −2.4 (−4.8 to −0.0) | .046 |
| Use of beta-blockade | −0.6 (−1.7 to 0.4) | .252 | 0.2 (−0.6 to 0.9) | .627 |
| Scan length (cm) | −0.3 (−0.5 to 0.1) | .004 | 0.2 (0.0–0.4) | .028 |
| AEC | −5.3 (−6.2 to −4.4) | <.001 | −1.0 (−2.1 to 0.1) | .076 |
| Low kVp (100 vs 120 kVp) | −6.4 (−7.4 to −5.4) | <.001 | −1.7 (−2.7 to −0.7) | <.001 |
| Prospective ECG-triggered axial scan mode | −8.0 (−9.7 to v7.9) | <.001 | −5.7 (−7.2 to −4.3) | <.001 |
| High-pitch helical scan mode | −8.8 (−9.3 to −7.9) | <.001 | −5.5 (−6.6 to −4.4) | <.001 |
| First-generation DSCT (64-slice DSCT) | −1.1 (−2.1 to 0.0) | .05 | −0.1 (−1.4 to 1.3) | .937 |
| Second-generation DSCT (128-slice DSCT) | −7.0 (− 8.0 to −6.0) | <.001 | −2.7 (−4.2 to −1.1) | <.001 |
BMI = body mass index; mSv = millisievert; AEC = automatic exposure control; kVp = kilovolt-potential; ECG = electrocardiogram; DSCT = dual-source computed tomography.
Nonevaluable Coronary Segments
The frequency of nondiagnostic examinations was 2.7% (580/21,709) on a per coronary segment basis. The rate of nondiagnostic segments was 3.3% (396/12,121), 1.7% (65/3,740), 0.8% (12/1,530), and 2.5% (107/4,318) in periods 1 to 4, respectively.
Exploratory Analyses to Assess the Effect of Scanner Technology, Protocols, and Compliance on Radiation Dose
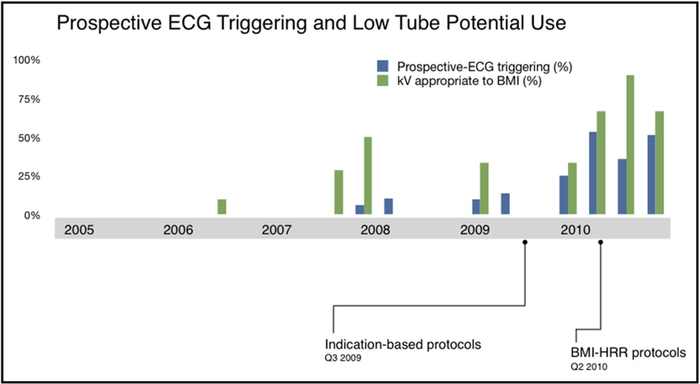
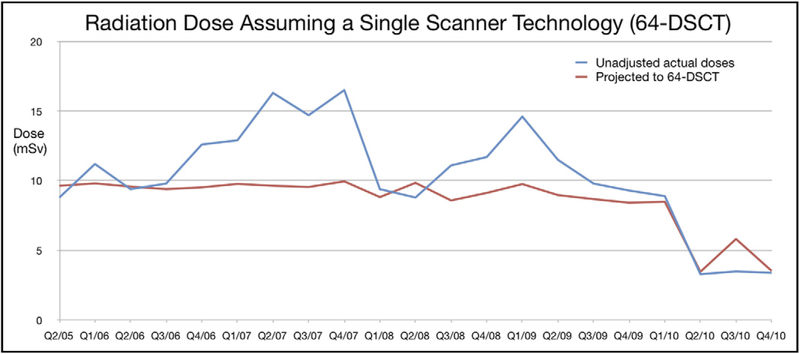
The proportions of patients who received appropriate prospective ECG-triggered modes (axial-sequential or high-pitch helical prospective modes) and kVp settings that were appropriately tailored to BMI per departmental protocols are shown in Figure 4. Figure 5 demonstrates a potential dose reduction to all patients if the same patient characteristics were applied using the multivariate model adjusted for 64-slice DSCT characteristics.
Figure 4.
The proportion of patients who received prospective ECG triggering (including high-pitch helical modes) when actual heart rate and rhythm were compatible according to protocol, (blue bars) and tube potential (kVp) was appropriate to actual patient BMI per department protocol (green bars). The lowest radiation doses coincided with the increased appropriate adjustments. BMI = body mass index; ECG = electrocardiogram; HRR = heart rate and rhythm.
Figure 5.
Estimated radiation dose (red line) demonstrates the potential changes in dose to all patients if the same patient characteristics were applied using the multivariate model, adjusted for 64-slice DSCT technology and ideal compliance. DSCT = dual-source computed tomography.
DISCUSSION
Our single-center study surveyed CCTA radiation doses in the 6 years after the early adoption of 64-slice MDCT, an era including the introduction of numerous dose-protection technologies and the eventual standardization of our site’s protocols. The approximately 75% dose reduction was achieved through a combination of factors, and at first glance our data might suggest that the reductions resulted from new CT purchases. However, our analyses reveal that although newer scanners contributed to dose reductions, a significant amount was due to increasing physician selection use of dose-protection methods (Figure 4). We speculate that these effects were facilitated by general advances in knowledge and by our site protocols, because during the entire study period, highly trained subspecialist physicians supervised each scan.
While doses were trending downward, we observed a slight overall decrease in the rate of nondiagnostic segments. A slight increase in the nondiagnostic segment rate was noted with 128-slice DSCT (ie, second-generation DSCT), which we speculate is due to a “learning curve” effect. Specifically, a new mode—prospective ECG-triggered high-pitch helical scanning—was more frequently chosen, which was associated with a slightly higher rate of nondiagnostic segments of 2.5%.
The 128-slice DSCT, acquired at the same time as we implemented our BMI, heart rate, and rhythm-based (BMI-HRR-based) protocols, does allow further dose reductions beyond earlier scanner generations and offers slightly higher temporal resolution than 64-slice DSCT (ie, first-generation DSCT). The prospective ECG-triggered high-pitch helical scan mode allows a small incremental dose reduction beyond the “step and shoot” or prospective ECG-triggered axial mode by completely eliminating z-axis overlap.26 Yet the radiation doses of axial-sequential modes on 64- and 128-slice DSCT were both significantly below our overall median doses. On the basis of our exploratory analysis, we suggest that similar dose reductions would have been possible even if all patients were instead scanned using earlier 64-slice DSCT technology, without the incremental benefits of 128-slice MDCT (Figure 5, red line).
Significant dose savings can be accomplished by almost all of the currently installed worldwide base of cardiac-capable 64-slice MDCT and higher scanners, using lower kVp settings and prospective ECG-triggered axial scans, regardless of vendor, assuming that careful heart rate control is performed (using beta-blockers). In addition, before our protocol implementations, our CCTA doses were already trending downward. This could be expected because of increasing physician awareness of dose protection in this evolving field.27 Indeed, after our study period, CCTA dose guidelines have been recently published, based on the extensive prior work in this field.14
Our study also demonstrates that despite significant progress, we still have considerable room to reduce doses via increased compliance (Figure 4). More than 10 physician decisions determine the dose-related parameters chosen for each scan, from premedication to advanced ECG synchronization mode selections. On simplifying the decision process (via default BMI-HRR-based protocols), we reduced the minimum number of necessary radiation-related decision steps to 2 (premedication and choosing the ECG synchronization), yet demonstrated improvement in the dose-lowering methods used.
The simplification of workflow by default protocols is a compelling narrative and has been successful in other facets of medicine.28 However, we do not believe that CCTA acquisition can be fully automated; physicians are intimately involved in CCTA, making high-level decisions from the time of examination request through post-processing and reporting. However, our results suggest that simplified protocols can result in significantly decreased radiation burden and high diagnostic image quality. This finding has implications for all practice settings from academic centers to community practices without the steady presence of a physician during the scan.
Further work to reduce doses at our institution is necessary. Our protocols did not use iterative reconstruction algorithms.29,30 We used conservative thresholds for BMI used to lower the tube potential (kVp).6 Finally, images could be more systematically analyzed to ensure minimization of z-axis scan length and centering within the gantry.
We acknowledge the limitations inherent to this retrospective, observational cohort study. We are unable to isolate the contribution of recommended protocols from the contribution of the various techniques and scanners. Although prospective randomized trials could be performed, the evolving, numerous dose reduction technologies may yield this impractical.
Another limitation is that the determination of nonevaluable segments was based on clinical reporting rather than research interpretation. This more practical method was chosen to identify only quality issues that would have clinical impact. In addition, BMI was not available for the entire cohort, particularly early in our study period. To mitigate this limitation, we used anthropometric measures (chest area) for the entire cohort. Finally, we used the dose-length-product method, which might underestimate or overestimate true radiation exposure.
CONCLUSIONS
We observed a significant reduction in CCTA radiation doses of 74.8% by using a combination of improved dose-saving technology and standardized protocols.
CLINICAL SIGNIFICANCE.
Technical and practical innovations to coronary computed tomography angiography (CCTA) techniques have reduced radiation exposure.
New scanner technology coupled with simple protocol recommendations resulted in a 74.8% decrease in radiation doses to all patients undergoing CCTA at an experienced tertiary referral center. This resulted in a median dose of 3.3 mSv.
Similar dose reductions are possible when these protocols are carefully applied and used with older-generation scanners.
Acknowledgments
Funding: Drs Ghoshhajra, Blankstein, and Goehler received funding from National Institutes of Health 1T32 HL076136. Dr Ghoshhajra received funding from Radiological Society of North America RF0908. Partners Healthcare Institutional Review Board specifically approved the waiver of consent for this study.
Footnotes
Conflict of Interest: None.
Authorship: All authors had access to the data and played a role in writing this manuscript.
References
- 1.Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–2284. [DOI] [PubMed] [Google Scholar]
- 2.Min JK, Shaw LJ, Berman DS. The present state of coronary computed tomography angiography a process in evolution. J Am Coll Cardiol. 2010;55:957–965. [DOI] [PubMed] [Google Scholar]
- 3.Tsai IC, Choi BW, Chan C, et al. ASCI 2010 appropriateness criteria for cardiac computed tomography: a report of the Asian Society of Cardiovascular Imaging Cardiac Computed Tomography and Cardiac Magnetic Resonance Imaging Guideline Working Group. Int J Cardiovasc Imaging. 2010;26(Suppl 1):1–15. [DOI] [PubMed] [Google Scholar]
- 4.Carbonaro S, Villines TC, Hausleiter J, Devine PJ, Gerber TC, Taylor AJ. International, multidisciplinary update of the 2006 Appropriateness Criteria for cardiac computed tomography. J Cardiovasc Comput Tomogr. 2009;3:224–232. [DOI] [PubMed] [Google Scholar]
- 5.Taylor AJ, Cerqueira M, Hodgson JM, et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol. 2010;56: 1864–1894. [DOI] [PubMed] [Google Scholar]
- 6.Hausleiter J, Meyer T, Hermann F, et al. Estimated radiation dose associated with cardiac CT angiography. JAMA. 2009;301:500–507. [DOI] [PubMed] [Google Scholar]
- 7.Hermann F, Martinoff S, Meyer T, et al. Reduction of radiation dose estimates in cardiac 64-slice CT angiography in patients after coronary artery bypass graft surgery. Invest Radiol. 2008;43:253–260. [DOI] [PubMed] [Google Scholar]
- 8.Deetjen A, Mollmann S, Conradi G, et al. Use of automatic exposure control in multislice computed tomography of the coronaries: comparison of 16-slice and 64-slice scanner data with conventional coronary angiography. Heart. 2007;93:1040–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheffel H, Alkadhi H, Leschka S, et al. Low-dose CT coronary angiography in the step-and-shoot mode: diagnostic performance. Heart. 2008;94:1132–1137. [DOI] [PubMed] [Google Scholar]
- 10.Hirai N, Horiguchi J, Fujioka C, et al. Prospective versus retrospective ECG-gated 64-detector coronary CT angiography: assessment of image quality, stenosis, and radiation dose. Radiology. 2008;248:424–430. [DOI] [PubMed] [Google Scholar]
- 11.Hausleiter J, Martinoff S, Hadamitzky M, et al. Image quality and radiation exposure with a low tube voltage protocol for coronary CT angiography results of the PROTECTION II Trial. JACC Cardiovasc Imaging. 2010;3:1113–1123. [DOI] [PubMed] [Google Scholar]
- 12.Leschka S, Stolzmann P, Schmid FT, et al. Low kilovoltage cardiac dual-source CT: attenuation, noise, and radiation dose. Eur Radiol. 2008;18:1809–1817. [DOI] [PubMed] [Google Scholar]
- 13.Achenbach S, Marwan M, Schepis T, et al. High-pitch spiral acquisition: a new scan mode for coronary CT angiography. J Cardiovasc Comput Tomogr. 2009;3:117–121. [DOI] [PubMed] [Google Scholar]
- 14.Halliburton SS, Abbara S, Chen MY, et al. SCCT guidelines on radiation dose and dose-optimization strategies in cardiovascular CT. J Cardiovasc Comput Tomogr. 2011;5:198–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alkadhi H, Stolzmann P, Scheffel H, et al. Radiation dose of cardiac dual-source CT: the effect of tailoring the protocol to patient-specific parameters. Eur J Radiol. 2008;68:385–391. [DOI] [PubMed] [Google Scholar]
- 16.LaBounty TM, Earls JP, Leipsic J, et al. Effect of a standardized quality-improvement protocol on radiation dose in coronary computed tomographic angiography. Am J Cardiol. 2010;106:1663–1667. [DOI] [PubMed] [Google Scholar]
- 17.LaBounty TM, Leipsic J, Mancini GB, et al. Effect of a standardized radiation dose reduction protocol on diagnostic accuracy of coronary computed tomographic angiography. Am J Cardiol. 2010;106:287–292. [DOI] [PubMed] [Google Scholar]
- 18.Achenbach S, Marwan M, Ropers D, et al. Coronary computed tomography angiography with a consistent dose below 1 mSv using prospectively electrocardiogram-triggered high-pitch spiral acquisition. Eur Heart J. 2010;31:340–346. [DOI] [PubMed] [Google Scholar]
- 19.Leschka S, Stolzmann P, Desbiolles L, et al. Diagnostic accuracy of high-pitch dual-source CT for the assessment of coronary stenoses: first experience. Eur Radiol. 2009;19:2896–2903. [DOI] [PubMed] [Google Scholar]
- 20.Maruyama T, Takada M, Hasuike T, Yoshikawa A, Namimatsu E, Yoshizumi T. Radiation dose reduction and coronary assessability of prospective electrocardiogram-gated computed tomography coronary angiography: comparison with retrospective electrocardiogram-gated helical scan. J Am Coll Cardiol. 2008;52:1450–1455. [DOI] [PubMed] [Google Scholar]
- 21.Stolzmann P, Leschka S, Scheffel H, et al. Dual-source CT in step-and-shoot mode: noninvasive coronary angiography with low radiation dose. Radiology. 2008;249:71–80. [DOI] [PubMed] [Google Scholar]
- 22.Hausleiter J, Meyer T, Hadamitzky M, et al. Radiation dose estimates from cardiac multislice computed tomography in daily practice: impact of different scanning protocols on effective dose estimates. Circulation. 2006;113:1305–1310. [DOI] [PubMed] [Google Scholar]
- 23.Raff GL, Chinnaiyan KM, Share DA, et al. Radiation dose from cardiac computed tomography before and after implementation of radiation dose-reduction techniques. JAMA. 2009;301:2340–2348. [DOI] [PubMed] [Google Scholar]
- 24.McCollough C, Cody D, Edyvean S, et al. Rothenberg LAAPM Task Group 23. Diagnostic Imaging Council CT Committee: the measurement, reporting, and management of radiation dose in CT. AAPM Report No. 96. College Park, MD: American Association of Physicists in Medicine; 2008. [Google Scholar]
- 25.Ghoshhajra BB, Engel LC, Major GP, et al. Direct chest area measurement: a potential anthropometric replacement for BMI to inform cardiac CT dose parameters? J Cardiovasc Comput Tomogr. 2011;5: 240–246. [DOI] [PubMed] [Google Scholar]
- 26.Alkadhi H, Stolzmann P, Desbiolles L, et al. Low-dose, 128-slice, dual-source CT coronary angiography: accuracy and radiation dose of the high-pitch and the step-and-shoot mode. Heart. 2010;96:933–938. [DOI] [PubMed] [Google Scholar]
- 27.Abbara S, Arbab-Zadeh A, Callister TQ, et al. SCCT guidelines for performance of coronary computed tomographic angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr. 2009;3:190–204. [DOI] [PubMed] [Google Scholar]
- 28.Bates DW, Kuperman GJ, Wang S, et al. Ten commandments for effective clinical decision support: making the practice of evidence-based medicine a reality. J Am Med Inform Assoc. 2003;10:523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gosling O, Loader R, Venables P, et al. A comparison of radiation doses between state-of-the-art multislice CT coronary angiography with iterative reconstruction, multislice CT coronary angiography with standard filtered back-projection and invasive diagnostic coronary angiography. Heart. 2010;96:922–926. [DOI] [PubMed] [Google Scholar]
- 30.Bittencourt MS, Schmidt B, Seltmann M, et al. Iterative reconstruction in image space (IRIS) in cardiac computed tomography: initial experience. Int J Cardiovasc Imaging. 2011;27:1081–1087. [DOI] [PubMed] [Google Scholar]