Abstract
Objectives
To test whether high-dose statin treatment would result in a reduction in periodontal inflammation as assessed by fluorodeoxyglucose-positron emission tomography/computed tomography, FDG-PET/CT.
Background
Periodontal disease (PD) is an independent risk factor for atherosclerosis.
Methods
Eighty-three adults with risk factors or with established atherosclerosis, who were not taking high-dose statins, were randomized to atorvastatin 80 mg vs. 10mg in a multicenter, double-blind trial to evaluate the impact of atorvastatin on arterial inflammation. Subjects were evaluated using FDG-PET/CT at baseline, 4 and 12 weeks. Arterial and periodontal tracer activity was assessed while blinded to treatment allocation, clinical characteristics, and temporal sequence. Periodontal bone loss (an index of PD severity) was evaluated using contrast-enhanced CT images while blinded to clinical and imaging data.
Results
Seventy-one subjects completed the study and 59 provided periodontal images for analysis. At baseline, areas of severe PD had higher target-to-background ratio (TBR) compared to areas without severe PD (mean TBR [95% CI] = 3.83 [3.36, 4.30] vs. 3.18 [2.91, 3.44], p=0.004). After 12 weeks, there was a significant reduction in periodontal inflammation in patients randomized to atorvastatin 80 vs. 10 mg (ΔTBR mean [95 CI], 80mg vs. 10mg group = −0.43 [−0.83, −.02], p=0.04). Between-group differences were greater in patients with higher periodontal inflammation at baseline (−0.74 [−1.29, −0.19], p=0.01) and in patients with severe bone loss at baseline (−0.61 [−1.16, −0.054], p=0.03). Furthermore, the changes in periodontal inflammation correlated with changes in carotid inflammation (R=0.61, p<0.001).
Conclusion
High-dose atorvastatin reduces periodontal inflammation, suggesting a newly recognized effect of statins. Given the concomitant changes observed in periodontal and arterial inflammation, these data raise the possibility that a portion of that beneficial impact of statins on atherosclerosis relate to reductions in extra-arterial inflammation, e.g. periodontitis.
Keywords: Statins, Atherosclerosis, Imaging, Nuclear Medicine, Inflammation
Introduction
Periodontal disease (PD) affects over 47% of adults in the US (1) and the combined cost for periodontal and preventive dental services amount to over $ 14 billion in the US alone.(2) Moreover, PD is a common, independent risk factor for atherosclerotic disease.(3,4) Multiple pathogenic mechanisms linking PD and cardiovascular disease have been proposed. Most prominently, local periodontal inflammation, through pro-inflammatory cytokine release, leads to increased systemic inflammation as measured by CRP, TNF-α, IL-6 and other biomarkers.(5–7) Augmented circulating inflammatory mediators, in turn, promote inflammatory activity within atherosclerotic plaque.(8,9) Interestingly, local treatment of PD has also been shown to reduce systemic inflammation in patients with a history of cardiovascular events.(10) To date, however, no definitive evidence exist that treatment of periodontal disease decreases cardiovascular disease progression or cardiovascular events.
18F-Flurodeoxyglucose positron emission tomography (FDG-PET) imaging provides a non-invasive measure of inflammation including inflammatory activity within atherosclerotic plaques. Several studies have demonstrated a strong correlation between carotid FDG uptake with histopathological measures of macrophage infiltration and inflammatory gene expression.(11–15) The arterial FDG signal is reproducible,(16) correlates with atherosclerotic inflammatory burden, and is modifiable by anti-atherosclerotic therapies.(17–20) We have previously demonstrated that periodontal inflammation correlates with carotid artery inflammation,(21) and, most recently, others have shown that periodontal FDG uptake correlates with periodontal disease severity as measured by alveolar bone loss.(22)
HMG-CoA reductase inhibitors, so called statins, have clear benefits in atherosclerotic diseases.(23) These drugs effectively decrease low-density lipoprotein cholesterol (LDL-C) levels and have other beneficial pleiotropic effects beyond lipid lowering, especially with respect to reducing systemic inflammation and also inflammatory activity within atherosclerotic plaques.(17,24) Multiple retrospective epidemiologic studies have demonstrated that statin therapy is also associated with reduced severity of periodontitis.(25–28) Most recently, a small prospective study suggested an additional benefit of combined statin and standard local periodontal treatment compared to standard local therapy alone.(29) Nevertheless, the direct anti-inflammatory actions of statins in periodontal tissue have not been previously demonstrated. Accordingly, we used FDG-PET/CT imaging to evaluate a potentially novel pleiotropic effect of statin treatment on periodontal tissue. We specifically tested the hypothesis that atorvastatin treatment would lower periodontal disease activity, mirroring its action on atherosclerotic plaque activity,(20) thereby providing a link between both disease states.
Methods
Study design
This double-blind, randomized, active-comparator study (www.clinicaltrials.gov, ) was conducted at 10 U.S. centers in order to study the impact of high-dose statin therapy on arterial inflammation. The protocol was reviewed and approved by each center’s institutional review board and all participants provided written informed consent prior to any study procedures. In the current study, we performed a separate, blinded analysis of FDG uptake in the periodontium to assess the impact of statin treatment on periodontal tissue inflammation. Permission was received from the Partners Healthcare IRB to evaluate periodontal disease indices on the anonymized imaging data.
Patients
163 subjects were initially screened and 83 subjects (n= 83, median age 59 yrs, range 37–78 yrs, 78% men), were randomized in this study. Men and women 30 to 80 years old were included if they had documentation or history of any one of the following: 1) carotid artery disease, 2) coronary artery disease, 3) cerebrovascular disease, 4) peripheral arterial disease (ankle-brachial index ≥0.5 and ≤0.9), 5) type 2 diabetes mellitus, or 6) BMI 30 to 40 kg/m2 (inclusive) and waist circumference >102 cm in men, and >88 cm in women. Patients were excluded if they had a history of: 1) type 1 diabetes mellitus; 2) significant cardiovascular event or intervention within 12 weeks of screening; 3) significant heart failure (e.g., NYHA Class III or IV, defibrillators); 4) active or chronic hepatobiliary disease; or 5) a chronic systemic inflammatory condition (such as rheumatoid arthritis or psoriasis), or chronic infection. Additionally, eligible subjects were required to have LDL-C ≥60 mg/dL and triglyceride level <350 mg/dL and were required to be statin naïve or taking no more than low-dose statins (defined as: atorvastatin ≤10 mg, simvastatin ≤20 mg, rosuvastatin ≤5 mg, pravastatin ≤40 mg, or fluvastatin ≤40 mg.)
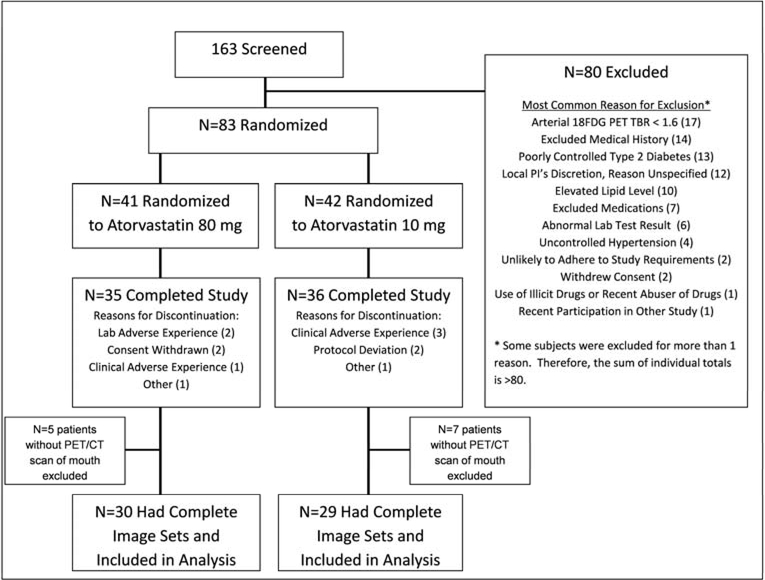
After the initial clinical screening, patients underwent baseline imaging using 18F-fluorodeoxyglucose-positron emission tomography/computed tomography (FDG-PET/CT). Since the parent study was designed to evaluate the effect of statin treatment on arterial inflammation, subjects without evidence of any arterial inflammation at baseline were excluded from randomization (i.e. TBR ≥1.6 present in either aorta, right or left carotid), which resulted in the exclusion of approximately 10% of the initially screened population. Eligible patients were randomized (after prior statin therapy was discontinued), in a double-blinded manner to 10 mg atorvastatin tablet (Lipitor, Pfizer, New York, NY) plus 80 mg atorvastatin matching placebo daily or 80 mg atorvastatin tablet plus 10 mg atorvastatin matching placebo daily for 12 weeks. Following randomization, FDG-PET/CT images were obtained again after 4 and 12 weeks. Seventy-one subjects completed the 12 week drug treatment period. In 12 subjects, coverage of the mouth was not included within the PET/CT field of view hence the final analysis set for the current study included 59 subjects (Figure 1).
Figure 1: Trial consort diagram.
* Some subjects were excluded for more than 1 reason. Therefore, the sum of individual totals is >80.
FDG-PET-CT imaging
FDG-PET-CT imaging was performed using previously validated methods.(11,12) Patients were asked to adhere to a low-carbohydrate diet for 24 hours before the test and to fast overnight prior to imaging. Imaging was performed 2 h after administration of 10 mCi of 18F-FDG, and images were acquired in 3-dimensional mode over 15 minutes. The image data were attenuation-corrected and reconstructed using ordered-subsets expectation-maximization (OSEM) algorithm. Blood sugar concentration was <200 mg/dl at the time of imaging for all subjects.
Measurement of periodontal tissue FDG uptake with PET
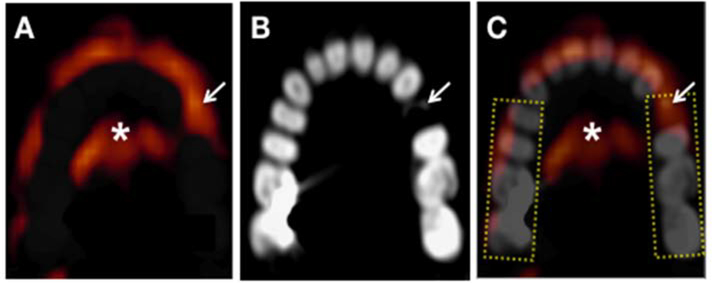
PET and CT images were analyzed using previously detailed methods.(21) Investigators were blinded to the clinical history, randomization details, and temporal sequence of imaging time points. Subsequently, the datasets (week 0, 4, and 12 images) were batch analyzed after manual co-registration of PET and CT images (Leonardo TrueD, Siemens Forchheim, Germany) as previously reported.(21) Co-registration was performed using anatomical landmarks such as teeth and periodontal tissue, brain, spinal cord, spine, and jaw. After co-registration, the periodontal tissues were identified and the maximum standardized uptake value (SUV) of FDG was measured by drawing a rectangular region of interest (ROI) around the teeth. ROIs were drawn from mesial surface of first premolar to distal surface of second molar teeth in each of 4 quadrants (Figure 2). Care was taken to avoid spill-over activity from the tongue, as well as from pharyngeal and buccal structures. Periodontal FDG uptake around anterior teeth (incisor and canine) and third molars (if present) were not assessed. Target-to-background ratios (TBR) were derived from the ratio of the SUV of the periodontal tissue to background blood activity from the internal jugular vein. The inter- and intra-observer correlation of periodontal TBR measurement (based on blinded analysis of 24 subjects) was 0.986 and 0.996, respectively. The inter- and intra-reader variability was 4.58% and 2.36%, respectively.
Figure 2: Measurement of periodontal FDG uptake on an axial mandibular section.
Rectangular regions of interest (ROIs), shown as dotted yellow rectangles, drawn around the teeth from the premolar to the second molar in each quadrant. The maximum SUV value is then recorded for each ROI. Arrow indicates an area of a broken tooth. A) PET, B) CT and C) composite PET/CT image. Brighter red to yellow colors represent higher FDG PET activity.
*=tongue FDG activity
Measurement of FDG uptake in carotid tissue
Carotid FDG uptake was measured using previously described methods.(12) ROIs were placed at each axial plane along the length of the carotid to measure the maximum SUV. TBR was calculated as the ratio between the carotid arterial to the venous blood SUV measured in the internal jugular vein. Thereafter, FDG uptake (TBR) was calculated using an average of the maximum TBR activity for all of the axial segments that compose the vessel.
Assessment of periodontal bone loss
Contrast enhanced CT imaging was performed once (at baseline or week 4) to provide additional anatomical information to ensure that the same locations within the arterial segments are measured across time. In 59 patients, the CT images included the oral cavity in the field of view, thus allowing for post-hoc evaluation of alveolar bones. Imaging parameters included rotation time of 420ms or less, tube current of ~750 mAs and voltage of 120 kVp. Image acquisition characteristics were section thicknesses of 0.75 mm and pitch of 0.2–0.4. Iopamidol 300mg/ml or similar was used as an intravenous contrast agent and infused at 5–6 mls/second. While the multi-section CT image acquisition was optimized for arterial imaging, the current and voltage used for arterial imaging is substantially higher than that typically used for dental imaging.(30) Evaluation of the periodontal CT images was performed by an experienced periodontist (TV), blinded to all PET data, clinical information and treatment assignment. Alveolar bone resorption was semi-quantitatively assessed in each of the four dental quadrants: 0, 1, 2, or 3 for none, mild (limited to coronal 1/3 of the root), moderate (including the middle 1/3 of the root) or severe (to the apical 1/3 of the root) bone loss, respectively. Periodontium of anterior teeth (incisors and canines) and third molars were not evaluated for bone resorption scoring.
Assessment of blood biomarkers
High-sensitivity CRP, LDL-C, and high-density lipoprotein (HDL) concentrations were assessed in plasma at 0, 4 and 12 weeks. All serum biomarker analyses were performed in batches.
Statistical analysis
“Generalized Estimating Equations” test was used to compare FDG uptake in dental quadrants with vs. without severe periodontal disease (based on CT evidence of alveolar bone loss). The same test was employed to adjust for the covariates in that analysis. Student’s T-test was used to assess the impact of atorvastatin treatment (80 mg compared with 10 mg) on periodontal inflammation. Univariate associations were tested using Pearson’s correlation coefficient. Linear regression was used to evaluate the independence of periodontal TBR as a predictor of periodontal bone loss, with periodontal disease risk factors as co-variants. A forward enter approach was employed to evaluate the robustness of these relationships, wherein the significant-or near-significant (p<0.10) variables identified in univariate analysis were entered. Between group differences were based on observed data values, without adjusting for missing data. Unstandardized regression coefficients are reported as β and 95% confidence interval. Statistical analysis was performed using SPSS version 20 (IBM, Armonk, NY). All reported P-values are two-tailed; statistical significance was set at p<0.05. Multiplicity adjustments were not applied for secondary comparisons of interest; as such, nominal p-values are reported for all comparisons.
RESULTS
Subject characteristics
A total of 163 patients were screened and 83 patients were randomized. Twelve patients discontinued early due to: adverse experiences (n=6), withdrawal of consent (n=2), protocol deviation, or other reason (n=4). Of the 83 patients randomized to the study, 71 subjects completed the study and among those, periodontal tissue images were available for 59 subjects. The demographic and clinical characteristics of the patients at the baseline are shown in Table 1.
Table 1.
Baseline Patient Characteristics
| Variables | Total N=59 | Atorvastatin 10 mg N=29 | Atorvastatin 80 mg N=30 | P-value |
|---|---|---|---|---|
| Age | 59.78±9.73 | 60.31±10.52 | 59.27±9.04 | 0.684 |
| Male gender | 44 (74.57) | 21 (72.41) | 23 (76.66) | 0.882 |
| Caucasian race | 45 (76.27) | 21 (72.41) | 24 (80) | 0.648 |
| Body Mass Index (BMI) | 31.68±8.24 | 31.62±10.56 | 31.73±5.32 | 0.975 |
| Diabetes mellitus | 18 (30.5) | 11 (37.93) | 7 (23.33) | 0.223 |
| Current smoker | 17 (28.81) | 9 (31.03) | 8 (26.66) | 0.711 |
| Ever smoked | 30 (50.84) | 16 (55.17) | 14 (46.66) | 0.514 |
| Coronary artery disease | 26 (44.06) | 13 (44.82) | 13 (43.33) | 0.908 |
| Pre-study statin use | 33 (55.93) | 18 (62.06) | 15 (50) | 0.351 |
| LDL-C | 104.66±26.51 | 103.36±24.05 | 105.87±28.98 | 0.722 |
| hs-CRP | 2.61±2.56 | 2.4±2.41 | 2.8±2.72 | 0.551 |
| HDL Cholesterol | 46.98±15.23 | 50.64±14.92 | 43.57±14.96 | 0.077 |
| Gingival TBR | 3.78±1.22 | 3.6±1.01 | 3.95±1.38 | 0.284 |
Values are presented as Mean±SD or N (%).
Relationship between periodontal disease and periodontal FDG uptake at baseline
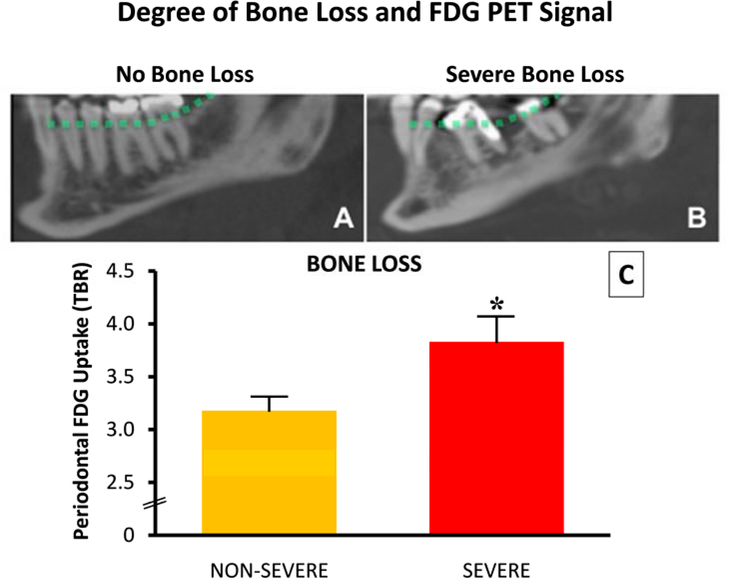
Periodontal FDG uptake at baseline was compared to CT measures of alveolar bone resorption. Mean baseline TBR was significantly higher in quadrants with vs. without severe alveolar bone loss (dental quadrant TBR: 3.83 [3.36, 4.30] vs. 3.18 [2.91, 3.44], p=0.004; Figure 3). The association between periodontal TBR and alveolar bone loss remained significant after adjusting for age and gender (β=0.64, p=0.005) and major risk factors of periodontal disease: diabetes mellitus and smoking (31,32) (β=0.65, P=0.004).
Figure 3: Lateral view of the mandible from an angulated sagittal plane for patients without (A) and with severe bone loss (B).
Dotted line indicates the estimated alveolar bone margin without bone loss. Bone loss on CT is dichotomized into severe and non-severe categories, and plotted against target to background ratio (TBR) (C). *= p <0.05, error bars represent SEM.
Statins and periodontal tissue inflammation
We observed a normal distribution of periodontal FDG uptake values across the subjects in both treatment groups at baseline, week 4 and 12. In the entire cohort (before subset selection based on the presence of periodontitis at baseline), a significant reduction in periodontal FDG uptake was seen after 12 weeks of treatment with high vs. low dose atorvastatin (mean change TBR ± SD= − 0.29 ± 0.85 vs. 0.13 ± 0.68, atorva 80 vs. 10 mg, p=0.04, Table 2). The impact of high-dose atorvastatin on PD activity remained significant after adjusting for (1) age and gender (β=−0.45, P=0.034), (2) Diabetes mellitus and smoking (β=−0.43, P=0.04), and (3) prior CAD, baseline HDL, LDL and CRP levels (β=−0.45, P=0.034).
Table 2.
Summary of Changes of target to background ratio (TBR) at 4 and 12 weeks
| TBR Endpoints | Atorvastatin 10 | Atorvastatin 80 | 80 vs. 10 mg atorvastatin § | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 4 | 12 | Δ4 weeks | Δ12 weeks | 0 | 4 | 12 | Δ4 weeks | Δ12 weeks | Δ4 weeks | Δ12 weeks | ||
| All Patients* | 3.60 [3.22,3.99] | 3.71 [3.31,4.06] | 3.79 [3.30,4.27] | 0.014 [−0.20,0.23] P=0.89 | 0.13 [−0.13,0.39] P=0.31 | 3.95 [3.43,4.46] | 3.58 [3.17,3.98] | 3.65 [3.19,4.11] | −0.395 [−0.76, −0.03] P=0.035 | −0.29 [−0.61, 0.02] P=0.067 | −0.381 [−0.763,0.00] P=0.05 | −0.43 [−0.83, −0.020] P=0.04 | |
| Subgroup of Patients With Moderate to Severe Periodontal Disease at Baseline | PD (by PET)† | 4.07 [3.70,4.43] | 4.10 [3.67,4.52] | 4.29 [3.80,4.78] | 0.028 [−0.26,0.32] p=0.844 | 0.22 [−0.14,0.59] P=0.226 | 4.64 [4.14,5.14] | 3.97 [3.55,4.39] | 4.12 [3.61,4.62] | −0.74 [−119, −0.28] P=0.003 | −0.52 [−0.96, −0.08] P=0.022 | −0.522 [−1.03,−0.12] P=0.045 | −0.743 [−1.29, −0.187] P=0.01 |
| PD (by CT) ‡ | 3.72 [3.10,4.34] | 3.88 [3.05,4.72] | 4.14 [3.21,5.06] | 0.16 [−0.28,0.61] P=0.443 | 0.41 [−0.04,0.88] P=0.074 | 3.54 [2.65,4.44] | 3.51 [2.69,4.33] | 3.35 [2.60,4.10] | −0.03 [−0.49,0.43] P=0.883 | −0.19 [−0.56,0.17] P=0.278 | −0.229 [−0.85,0.40] P=0.458 | − 0.609 [−116, −0.054] P=0.033 | |
Data is presented as Mean [95% CI]
N = 30 for 80 mg atorvastatin group and N = 29 for 10 mg atorvastatin group
Patients with lower tertile baseline TBR excluded. N = 20 for 80 mg atorvastatin group and N = 20 for 10 mg atorvastatin group
Patients with CT evidence of moderate to severe periodontal bone resorption. N = 13 for 80 mg atorvastatin group and N = 12 for 10 mg atorvastatin group
Group comparisons are adjusted for baseline gingival TBR
No adjustment is made for multiple comparisons.
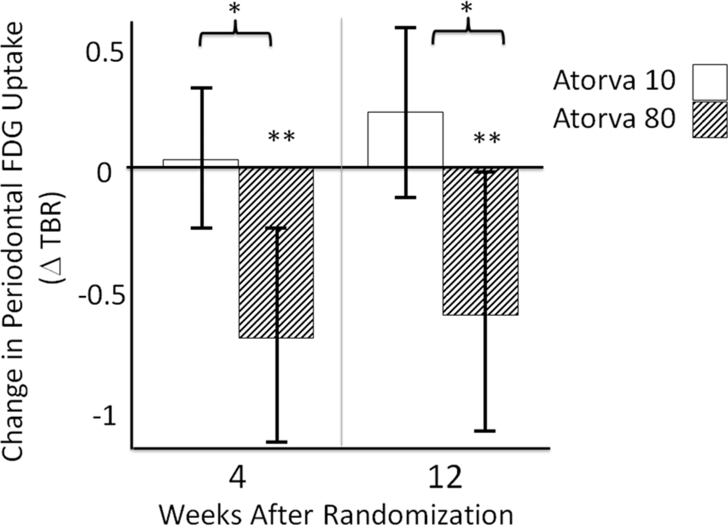
Moreover, the effect of high-dose statin was more notable in subjects with imaging evidence of periodontal disease at baseline. In the first PD-based subset analysis, individuals with PD were identified based on the presence of PET evidence of active periodontitis (subjects with highest two tertiles of baseline periodontal TBR). Between-group differences became more substantial within individuals with active periodontitis (by PET) at baseline, (change TBR= −0.52 ± 0.94 vs. 0.22 ± 0.79, ATV 80 vs. 10 mg, P=0.01, Figure 4, Table 2). Furthermore, the difference in changes in PD activity between treatment groups was significant starting at 4 weeks (Figure 4, Table 2). Similarly, in the second sub-group analysis, individuals with PD were identified based on CT evidence of alveolar bone loss to indicate a history of PD. In that analysis, subjects without moderate or severe alveolar bone loss in at least one tooth were excluded. In this second PD based analysis, between-group differences remained significant in subgroup of patients with moderate to severe periodontal bone loss at baseline as determined by CT (change in TBR= −0.19 ± 0.61 vs. 0.41 ± 0.73, ATV 80 vs. 10mg, P=0.033, Table 2).
Figure 4: Change in periodontal FDG uptake (TBR) at 4 and 12 weeks from the baseline in patients with active periodontitis (by PET).
Error bars represent 95% Confidence Interval, † p=0.003, ‡ p=0.022, * p= 0.045, § p= 0.01
Relationship between atherosclerotic inflammation and periodontal inflammation
Baseline FDG uptake (TBR) in periodontal tissue correlated with baseline carotid plaque TBR (Figure 5). Additionally, after 12 weeks of statin therapy, changes in periodontal inflammation correlated with changes in carotid inflammation (Figure 6). Moreover, subjects with greater than median reduction of periodontal TBR demonstrated a significantly greater decrease of arterial inflammation after adjusting for changes of LDL-C and CRP (β= −0.29, p=0.001).
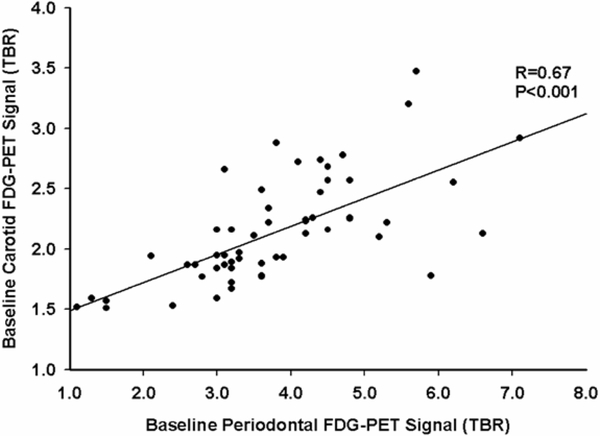
Figure 5. Prior to Randomization, Periodontal FDG uptake Correlated with Carotid Inflammation.
FDG uptake within periodontal tissues was compared with carotid FDG uptake (a measure of carotid inflammation) at baseline. A significant relationship was observed (r= 0.67, p<0.001). FDG=18F-fluorodeoxyglucose; TBR= target-to-background ratio
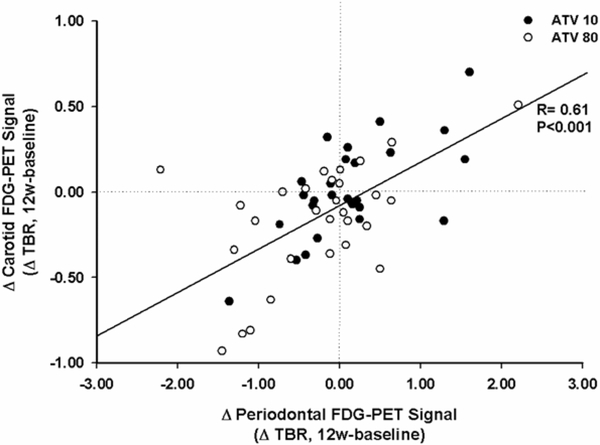
Figure 6. In the Setting of Statin Therapy, Changes in Periodontal Activity Correlate with Changes in Carotid Inflammation.
After 12-weeks of statin treatment, a significant relationship was observed between changes in periodontal activity (FDG uptake measured as ΔTBR) and changes in carotid arterial inflammation (measured as ΔTBR). FDG=18F-fluorodeoxyglucose; TBR= target-to-background ratio
Periodontal Disease Inflammation vs. Lipids and CRP
Baseline PD activity (periodontal TBR) correlated inversely with baseline HDL concentration (R=−0.35, P=0.007) but did not correlate with baseline CRP or baseline LDL. The presence of severe alveolar bone loss (by CT) was associated with higher CRP concentrations (β=1.97, p=0.022), after correcting for age, gender, smoking, and diabetes. Changes in PD activity did not correlate with changes in CRP, LDL or HDL.
Discussion
This report demonstrates that 12 weeks of high-dose atorvastatin therapy, significantly reduces periodontal inflammation. Between group differences were more pronounced in individuals with PET imaging evidence of periodontal disease. The reduction in the PD signal was observed as early as 4 weeks after randomization and correlated with changes in FDG uptake within the wall of the carotid artery (a marker of arterial inflammation).
Periodontal FDG uptake as a marker of periodontitis
While evaluation of malignant and infectious oral lesions using FDG-PET is established in clinical practice, the assessment of periodontal disease using FDG-PET imaging is relatively novel. We have previously reported the correlation between periodontal FDG uptake and arterial plaque inflammation,(21) and hypothesized that periodontal FDG uptake reflects dynamic periodontal inflammation and thus the severity of PD. Recently, Kito et al.(22) provided supportive evidence for this contention, demonstrating that periodontal FDG uptake is indeed correlated with PD severity as measured by the magnitude of bone resorption on radiographs, a destructive end result of inflammation.(33) In the current study, we provide further confirmation of these findings and, importantly, provide additional evidence that periodontal FDG uptake reflects periodontal inflammation. However, in contrast to bone loss that identifies the longer-term consequences of severe PD, the FDG PET signal likely reflects the current inflammatory burden within the target tissue and indicates on-going active PD.
Relationship between periodontitis and atherosclerosis
While an epidemiological association between PD and atherosclerosis is well known, the degree of influence of PD on atherosclerosis is not fully understood. Nevertheless, the consensus of two independent reviews was that PD is an independent risk factor for atherosclerosis.(34,35) Imaging studies have also established a direct association between PD and carotid atherosclerosis by correlating increased intima-media thickness with extent of PD.(36) Importantly, the ICARAS (Inflammation and Carotid Artery- Risk from Atherosclerosis Study) revealed that PD was associated with subsequent progression of atherosclerosis.(37) In this study, we provide additional evidence of a link between PD and atherosclerosis, by showing a significant correlation between PD and carotid imaging parameters at baseline (thus confirming prior studies), and additionally demonstrating a strong association between changes of the two imaging parameters over a 12-week period upon atorvastatin treatment. The close association that we observed between the two tissues supports the hypothesis that periodontal and atherosclerotic inflammations (as assessed by FDG-PET arterial wall imaging) are inter-related, though the nature of that association is undefined.
A novel pleiotropic effect of statins
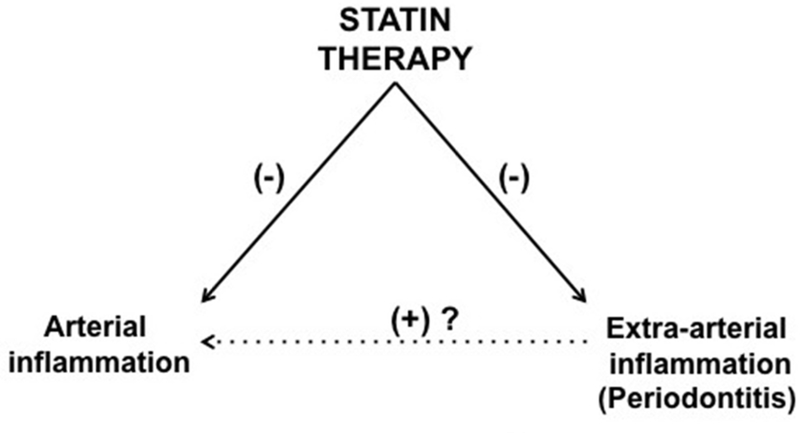
Although it was at first assumed that beneficial impact of statins were mediated via reduction of LDL-C, multiple studies have consistently suggested that not all actions can be accounted for by cholesterol reduction per se. For example, in the CARE (Cholesterol And Recurrent Events) trial, the magnitude of hs-CRP reduction associated with statins over a 5 year observation period was more pronounced than the amount which would be predicted by changes in LDL-C alone.(38) In the PROVE IT-TIMI 22 trial, the rapid event reduction after statin therapy was postulated to be in part due to non-lipid-related properties of statins.(39) These cholesterol-independent or “pleiotropic” effects of statins have been attributed to several mechanisms, including improved endothelial function, decreased smooth muscle cell proliferation, reduced platelet function and attenuated inflammation.(40) Based on the findings of this study, we pose the possibility that statins may exert an additional pleiotropic effect: reduction in non-arterial inflammation, (i.e. within inflamed tissues such as the periodontium). We further postulate that a reduction in local periodontal inflammation (as an example of several clinical models of extra-arterial inflammation) may exert secondary benefits on the systemic arterial milieu, whereby reduction in inflammation within the periodontium leads to a reduction in pro-inflammatory mediators released by periodontal tissue into the systemic circulation, which in-turn may lead to further reductions in atherosclerotic inflammation (Figure 7).
Figure 7. Interrelationships between Arterial and Extra-arterial Inflammatory Processes.
Inflammatory processes in multiple distinct tissues may be inter-related. Within this paradigm, statins can have direct as well as in-direct benefits on arterial inflammation. Statins can impact arterial inflammation through direct actions on the atheromatous milieu. Additionally, statins reduce inflammation in extra-arterial locations (such as periodontal tissues). Theoretically, that reduction in extra-arterial inflammation may decrease chronic cytokine production by those sites hence decreasing pro-inflammatory stimulation of atheroscerlotic plaques.
Indeed, prior studies support the hypothesis that treatment of periodontal disease might yield benefits in atherosclerosis. For instance, PD treatment results in reduced carotid intima-media thickness,(41) and improving oral health decreases both local and systemic markers of inflammation,(42,43) and improves endothelial function.(44,45) Additionally, other extravascular inflammatory diseases have similarly been linked to atherosclerotic disease. The most prominent example is rheumatoid arthritis (RA).(46,47) Atorvastatin therapy has been proved to be beneficial in RA in terms of modulation of clinical and inflammatory markers.(48) Further, treatment of RA and psoriasis with disease modifying anti-inflammatory drugs(49) leads to a reduction in cardiovascular risk thereby supporting the hypothesis that extravascular inflammation is an important and modifiable contributing factor for the promotion of atherosclerosis. However, the exact nature of the interrelationship between statins, arterial inflammation and periodontal inflammation remains elusive by the results of this study and we cannot exclude the possibility that statins may affect both periodontal and arterial inflammation independently without a link between these two tissues.
Future randomized controlled studies will be needed to examine the underlying mechanism of the association between localized inflammation of extra-arterial tissues and atherosclerosis; specifically to test the hypothesis that localized (non-systemic) treatment of extra-arterial inflammation reduces atherosclerotic inflammation and cardiovascular risk. Those studies should also evaluate whether moderate doses of statins also confer anti-inflammatory benefits on the periodontium. Indeed, PD represents an ideal model to test this hypothesis, since treatment of periodontitis can be achieved via local intervention (plaque removal and scaling) without the need for systemic treatment.
If confirmed by larger prospective outcome studies, statins would prove to be a useful adjunctive treatment for periodontal disease, with efficacy seen within one month. Furthermore, the possible interrelationship between periodontitis, atherosclerosis and statins might prove to be of substantial importance, due to the high prevalence of both periodontal and atherosclerotic diseases along with the wide-spread use of statins. Moreover, additional investigations to assess the interrelationships between extra-arterial inflammation and atherosclerosis are warranted, considering the fact that knowledge derived from treatment of extra-arterial inflammatory disorders may lead to useful insights into the inflammatory mechanisms of atherosclerosis in general.(47)
Limitations
While our data suggest that high dose atorvastatin is more effective than low-dose treatment in decreasing inflammation in atherosclerosis and periodontal disease, these findings do not allow to determine the efficacy of low-dose treatment relative to placebo. It is worth noting in this context that the majority of subjects that were randomized to atorvastatin 10mg/day were previously on a low-dose statin prior to study entry, hence might not have experienced a boost in the effective statin dose over the course of the study. This might contribute to the observation that low dose statin treatment was not associated with a reduction in periodontal FDG uptake. Nonetheless, the main observation, of an effect of high-dose statin, remains valid. Further, given that 50% of subjects randomized to atorvastatin 80 were previously on a low-dose statin, the impact of high-dose statin intervention in a statin naïve population might be more profound than seen in the current study. Lastly, the findings of the current study were derived from post-hoc analysis of a population of subjects with PET/CT imaging evidence of atherosclerotic disease. Hence, extrapolation of the findings of this study to the broader population should be done cautiously. Future randomized clinical trials are warranted in order to evaluate effects of statins on periodontal disease regardless of status of atherosclerosis.
Conclusion
We observed that high-dose atorvaststin is associated with a reduction in periodontal inflammation in this multi-center, double-blind trial. The impact of high-dose statins was greatest in individuals with evidence of active periodontitis, and was evident after a 4 week treatment period. Furthermore, we observed a close association between reductions in periodontal and atherosclerotic inflammation. Accordingly, these results identify a potentially novel pleiotropic effect of statins and raise the possibility that an indirect benefit of statins on atherosclerosis may in part relate to a reduction in extra-arterial inflammation.
Acknowledgements
The statistical analysis was conducted with consult from Harvard Catalyst which is supported by National Center for Research Resources and the National Center for Advancing Translational Sciences (NIH Award 8UL1TR000170-05).
Funding Source: Merck & Co., Inc. provided funding for the study.
Disclosures: A. Alon and S.S. Shankar are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ, USA. A. Alon, owns stock in Merck Sharp & Dohme Corp. A. Tawakol and Z.A. Fayad received consulting fees, and their institutions received grants from Roche and Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ, USA. M.E. Farkouh’s institution received grants from Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ, USA. S. Subramanian, H. Emami, E. Vucic, P. Singh, J. Vijayakumar, K. Fifer and T. Van Dyke have nothing to disclose. JHF Rudd is supported by the NIHR Cambridge Biomedical Research Center.
Abbreviation List
- CAD
coronary artery disease
- CRP
C-reactive protein
- FDG
2–18F-fluoro-2-deoxy-D-glucose
- HDL-C
high-density lipoprotein cholesterol
- LDL-C
low-density lipoprotein cholesterol
- PD
periodontal disease
- PET/CT
positron emission tomography-computed tomography
- ROI
region of interest
- SUV
standardized uptake value
- TBR
target-to-background ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res 2012;91:914–20. [DOI] [PubMed] [Google Scholar]
- 2.Brown LJ, Johns BA, Wall TP. The economics of periodontal diseases. Periodontol 2000 2002;29:223–34. [DOI] [PubMed] [Google Scholar]
- 3.Genco R, Offenbacher S, Beck J. Periodontal disease and cardiovascular disease: epidemiology and possible mechanisms. J Am Dent Assoc 2002;133 Suppl:14S–22S. [DOI] [PubMed] [Google Scholar]
- 4.Beck J, Garcia R, Heiss G, Vokonas PS, Offenbacher S. Periodontal disease and cardiovascular disease. J Periodontol 1996;67:1123–37. [DOI] [PubMed] [Google Scholar]
- 5.Linden GJ, McClean K, Young I, Evans A, Kee F. Persistently raised C-reactive protein levels are associated with advanced periodontal disease. J Clin Periodontol 2008;35:741–7. [DOI] [PubMed] [Google Scholar]
- 6.Noack B, Genco RJ, Trevisan M, Grossi S, Zambon JJ, De Nardin E. Periodontal infections contribute to elevated systemic C-reactive protein level. J Periodontol 2001;72:1221–7. [DOI] [PubMed] [Google Scholar]
- 7.Paraskevas S, Huizinga JD, Loos BG. A systematic review and meta-analyses on C-reactive protein in relation to periodontitis. J Clin Periodontol 2008;35:277–90. [DOI] [PubMed] [Google Scholar]
- 8.Tonetti MS, D’Aiuto F, Nibali L, et al. Treatment of periodontitis and endothelial function. N Engl J Med 2007;356:911–20. [DOI] [PubMed] [Google Scholar]
- 9.Figuero E, Sanchez-Beltran M, Cuesta-Frechoso S, et al. Detection of periodontal bacteria in atheromatous plaque by nested polymerase chain reaction. J Periodontol 2011;82:1469–77. [DOI] [PubMed] [Google Scholar]
- 10.Offenbacher S, Beck JD, Moss K, et al. Results from the Periodontitis and Vascular Events (PAVE) Study: a pilot multicentered, randomized, controlled trial to study effects of periodontal therapy in a secondary prevention model of cardiovascular disease. Journal of periodontology 2009;80:190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rudd JH, Warburton EA, Fryer TD, et al. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation 2002;105:2708–11. [DOI] [PubMed] [Google Scholar]
- 12.Tawakol A, Migrino RQ, Bashian GG, et al. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol 2006;48:1818–24. [DOI] [PubMed] [Google Scholar]
- 13.Tawakol A, Migrino RQ, Hoffmann U, et al. Noninvasive in vivo measurement of vascular inflammation with F-18 fluorodeoxyglucose positron emission tomography. J Nucl Cardiol 2005;12:294–301. [DOI] [PubMed] [Google Scholar]
- 14.Graebe M, Pedersen SF, Borgwardt L, Hojgaard L, Sillesen H, Kjaer A. Molecular pathology in vulnerable carotid plaques: correlation with [18]-fluorodeoxyglucose positron emission tomography (FDG-PET). Eur J Vasc Endovasc Surg 2009;37:714–21. [DOI] [PubMed] [Google Scholar]
- 15.Pedersen SF, Graebe M, Fisker Hag AM, Hojgaard L, Sillesen H, Kjaer A. Gene expression and 18FDG uptake in atherosclerotic carotid plaques. Nucl Med Commun 2010;31:423–9. [DOI] [PubMed] [Google Scholar]
- 16.Rudd JH, Myers KS, Bansilal S, et al. (18)Fluorodeoxyglucose positron emission tomography imaging of atherosclerotic plaque inflammation is highly reproducible: implications for atherosclerosis therapy trials. J Am Coll Cardiol 2007;50:892–6. [DOI] [PubMed] [Google Scholar]
- 17.Tahara N, Kai H, Ishibashi M, et al. Simvastatin attenuates plaque inflammation: evaluation by fluorodeoxyglucose positron emission tomography. J Am Coll Cardiol 2006;48:1825–31. [DOI] [PubMed] [Google Scholar]
- 18.Mizoguchi M, Tahara N, Tahara A, et al. Pioglitazone Attenuates Atherosclerotic Plaque Inflammation in Patients With Impaired Glucose Tolerance or Diabetes A Prospective, Randomized, Comparator-Controlled Study Using Serial FDG PET/CT Imaging Study of Carotid Artery and Ascending Aorta. JACC Cardiovasc Imaging 2011;4:1110–8. [DOI] [PubMed] [Google Scholar]
- 19.Fayad ZA, Mani V, Woodward M, et al. Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-PLAQUE): a randomised clinical trial. Lancet 2011;378:1547–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tawakol A, Fayad ZA, Mogg R, et al. Intensification of Statin Therapy Results in a Rapid Reduction in Atherosclerotic Inflammation: Results of A Multi-Center FDG-PET/CT Feasibility Study. J Am Coll Cardiol 2013. [DOI] [PubMed] [Google Scholar]
- 21.Fifer KM, Qadir S, Subramanian S, et al. Positron emission tomography measurement of periodontal (18)f-fluorodeoxyglucose uptake is associated with histologically determined carotid plaque inflammation. J Am Coll Cardiol 2011;57:971–6. [DOI] [PubMed] [Google Scholar]
- 22.Kito S, Koga H, Kodama M, et al. Reflection of (18)F-FDG accumulation in the evaluation of the extent of periapical or periodontal inflammation. Oral Surg Oral Med Oral Pathol Oral Radiol 2012;114:e62–9. [DOI] [PubMed] [Google Scholar]
- 23.Ferrieres J Effects on coronary atherosclerosis by targeting low-density lipoprotein cholesterol with statins. Am J Cardiovasc Drugs 2009;9:109–15. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura K, Sasaki T, Cheng XW, Iguchi A, Sato K, Kuzuya M. Statin prevents plaque disruption in apoE-knockout mouse model through pleiotropic effect on acute inflammation. Atherosclerosis 2009;206:355–61. [DOI] [PubMed] [Google Scholar]
- 25.Cunha-Cruz J, Saver B, Maupome G, Hujoel PP. Statin use and tooth loss in chronic periodontitis patients. J Periodontol 2006;77:1061–6. [DOI] [PubMed] [Google Scholar]
- 26.Saver BG, Hujoel PP, Cunha-Cruz J, Maupome G. Are statins associated with decreased tooth loss in chronic periodontitis? J Clin Periodontol 2007;34:214–9. [DOI] [PubMed] [Google Scholar]
- 27.Meisel P, Kohlmann T, Wallaschofski H, Kroemer HK, Kocher T. Cholesterol, C-Reactive Protein, and Periodontitis: HMG-CoA-Reductase Inhibitors (Statins) as Effect Modifiers. ISRN Dent 2011;2011:125168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindy O, Suomalainen K, Makela M, Lindy S. Statin use is associated with fewer periodontal lesions: A retrospective study. BMC Oral Health 2008;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fajardo ME, Rocha ML, Sanchez-Marin FJ, Espinosa-Chavez EJ. Effect of atorvastatin on chronic periodontitis: a randomized pilot study. J Clin Periodontol 2010;37:1016–22. [DOI] [PubMed] [Google Scholar]
- 30.Saavedra-Abril JA, Balhen-Martin C, Zaragoza-Velasco K, Kimura-Hayama ET, Saavedra S, Stoopen ME. Dental multisection CT for the placement of oral implants: technique and applications. Radiographics 2010;30:1975–91. [DOI] [PubMed] [Google Scholar]
- 31.Sima C, Rhourida K, Van Dyke TE, Gyurko R. Type 1 diabetes predisposes to enhanced gingival leukocyte margination and macromolecule extravasation in vivo. J Periodontal Res 2010;45:748–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bergstrom J Tobacco smoking and chronic destructive periodontal disease. Odontology 2004;92:1–8. [DOI] [PubMed] [Google Scholar]
- 33.Graves DT, Li J, Cochran DL. Inflammation and uncoupling as mechanisms of periodontal bone loss. J Dent Res 2011;90:143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Friedewald VE, Kornman KS, Beck JD, et al. The American Journal of Cardiology and Journal of Periodontology editors’ consensus: periodontitis and atherosclerotic cardiovascular disease. J Periodontol 2009;80:1021–32. [DOI] [PubMed] [Google Scholar]
- 35.Lockhart PB, Bolger AF, Papapanou PN, et al. Periodontal disease and atherosclerotic vascular disease: does the evidence support an independent association?: a scientific statement from the American Heart Association. Circulation 2012;125:2520–44. [DOI] [PubMed] [Google Scholar]
- 36.Soder PO, Soder B, Nowak J, Jogestrand T. Early carotid atherosclerosis in subjects with periodontal diseases. Stroke 2005;36:1195–200. [DOI] [PubMed] [Google Scholar]
- 37.Schillinger T, Kluger W, Exner M, et al. Dental and periodontal status and risk for progression of carotid atherosclerosis: the inflammation and carotid artery risk for atherosclerosis study dental substudy. Stroke 2006;37:2271–6. [DOI] [PubMed] [Google Scholar]
- 38.Ridker PM, Rifai N, Pfeffer MA, Sacks F, Braunwald E. Long-term effects of pravastatin on plasma concentration of C-reactive protein. The Cholesterol and Recurrent Events (CARE) Investigators. Circulation 1999;100:230–5. [DOI] [PubMed] [Google Scholar]
- 39.Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004;350:1495–504. [DOI] [PubMed] [Google Scholar]
- 40.Takemoto M, Liao JK. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Arterioscler Thromb Vasc Biol 2001;21:1712–9. [DOI] [PubMed] [Google Scholar]
- 41.Piconi S, Trabattoni D, Luraghi C, et al. Treatment of periodontal disease results in improvements in endothelial dysfunction and reduction of the carotid intima-media thickness. FASEB J 2009;23:1196–204. [DOI] [PubMed] [Google Scholar]
- 42.Al-Katma MK, Bissada NF, Bordeaux JM, Sue J, Askari AD. Control of periodontal infection reduces the severity of active rheumatoid arthritis. J Clin Rheumatol 2007;13:134–7. [DOI] [PubMed] [Google Scholar]
- 43.Montebugnoli L, Servidio D, Miaton RA, et al. Periodontal health improves systemic inflammatory and haemostatic status in subjects with coronary heart disease. J Clin Periodontol 2005;32:188–92. [DOI] [PubMed] [Google Scholar]
- 44.Blum A, Kryuger K, Mashiach Eizenberg M, et al. Periodontal care may improve endothelial function. Eur J Intern Med 2007;18:295–8. [DOI] [PubMed] [Google Scholar]
- 45.Seinost G, Wimmer G, Skerget M, et al. Periodontal treatment improves endothelial dysfunction in patients with severe periodontitis. Am Heart J 2005;149:1050–4. [DOI] [PubMed] [Google Scholar]
- 46.Solomon DH, Karlson EW, Rimm EB, et al. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation 2003;107:1303–7. [DOI] [PubMed] [Google Scholar]
- 47.Ku IA, Imboden JB, Hsue PY, Ganz P. Rheumatoid arthritis: model of systemic inflammation driving atherosclerosis. Circ J 2009;73:977–85. [DOI] [PubMed] [Google Scholar]
- 48.McCarey DW, McInnes IB, Madhok R, et al. Trial of Atorvastatin in Rheumatoid Arthritis (TARA): double-blind, randomised placebo-controlled trial. Lancet 2004;363:2015–21. [DOI] [PubMed] [Google Scholar]
- 49.Prodanovich S, Ma F, Taylor JR, Pezon C, Fasihi T, Kirsner RS. Methotrexate reduces incidence of vascular diseases in veterans with psoriasis or rheumatoid arthritis. J Am Acad Dermatol 2005;52:262–7. [DOI] [PubMed] [Google Scholar]