Summary
Background
Previous clinical trials with birch pollen subcutaneous immunotherapy have been conducted over a 1‐ to 2‐year treatment period and involved mostly a single geographic location.
Objective
This study (EudraCT‐Number: 2005‐000025‐35) intended to evaluate the effect of subcutaneous immunotherapy with high‐dose hypoallergenic birch pollen allergoid in patients with confirmed moderate to severe seasonal allergic rhinitis/rhinoconjunctivitis over a 3‐year course in 19 European centres.
Methods
Adults with confirmed birch pollen allergy (n = 253) were randomized to preseasonal placebo (n = 129) or active treatment (n = 124). Primary endpoint was change in Symptom Medication Score after 2 years treatment (2007).
Results
The change in Symptom Medication Score of active‐ vs placebo‐treated patients for the Full Analysis Set (n = 227, 15.2% reduction, P = 0.0710) and Per‐Protocol Set (n = 216, 16.7% reduction, P = 0.0523) showed a positive trend, although significance was not achieved. The primary endpoint, assessed in 2007, coincided with the lowest pollination during the study period. In a subgroup analysis of patients in the north‐eastern region (n = 102), where birch is the major tree and consequently patients’ exposure is higher, changes in Symptom Medication Score (32.7% reduction, P = 0.0034) and median number of well days (P = 0.0232) were highly significant in favour of the active group. During the open‐label third year of treatment, the mean Symptom Medication Score of active‐treated patients was further reduced despite an increased pollen count. Subcutaneous immunotherapy was well tolerated and consistent with the known safety profile.
Conclusions and clinical relevance
Although the primary endpoint was not reached for the Full Analysis Set, a significant and clinically relevant effect on Symptom Medication Score was clearly demonstrated for the subgroup of patients in the north‐eastern region of Europe, where birch is the predominant tree species. Proving efficacy of birch allergen subcutaneous immunotherapy is challenging due to the numerous factors influencing birch pollen allergen exposure in field studies.
Keywords: allergens and epitopes, immunotherapy and tolerance induction, rhinitis
1. INTRODUCTION
Birch is the most allergenic tree pollen in north, central and eastern Europe and the major pollen allergen‐producing tree in northern Europe.1 The abundance of birch trees is highest in northern and eastern Europe, decreasing towards southern and western Europe.2, 3 This correlates with the prevalence of sensitization, with low prevalence in southern Europe and high prevalence in northern Europe4; approximately 15% of the population in northern Europe are sensitized to birch pollen allergens.5 Allergen immunotherapy is, to date, the only causal treatment option for allergic rhinoconjunctivitis with the potential to modify the natural course of allergic disease.6, 7 Very few double‐blind placebo‐controlled (DBPC) studies have demonstrated efficacy for birch pollen immunotherapy, either sublingually 4 or subcutaneously.8, 9, 10 Proving efficacy of birch pollen immunotherapy in field studies is challenging due to the peculiarities of birch pollination and the nature of quantifying pollen exposure. In addition, symptoms depend more on birch allergen content than on the amount of pollen, as well as on patient sensitivity to specific birch allergens.11, 12, 13 Previous studies looking at efficacy of birch pollen subcutaneous immunotherapy (SCIT) have been conducted over a 1‐ to 2‐year treatment period and involved mostly a single geographic location for pollen collection.8, 10, 14 This trial evaluates the safety and efficacy of SCIT using a birch pollen allergoid preparation (Allergovit® Birch, Allergopharma GmbH & Co. KG) in patients with confirmed moderate to severe seasonal allergic rhinitis/rhinoconjunctivitis, with or without asthma, over a 3‐year treatment period (2‐year DBPC phase followed by a 1‐year open‐label phase [OLP]) as recommended by guidelines.6, 7, 15
2. METHODS
2.1. Trial design
The trial was designed as a multi‐national, multi‐centre, randomized, DBPC, phase 3 (for marketing authorization in Sweden and Finland)/phase 4 (post‐marketing in Germany and Poland) study with two parallel treatment groups (EudraCT‐Number: 2005‐000025‐35). The study flow for the trial is described in Figure 1. After 2 years of preseasonal treatment with active therapy or placebo, patients on active treatment continued for 1 year in the OLP.
Figure 1.
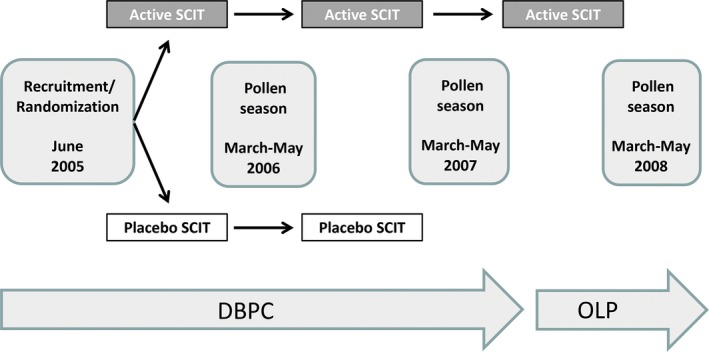
Study flowchart. Preseasonal treatment with active therapy or placebo commenced in October and continued to the start of the birch pollen season the following year. DBPC, double‐blind placebo‐controlled phase (2006‐2007); OLP, open‐label phase (2008); SCIT, subcutaneous immunotherapy
Eligible patients were randomized (1:1) to treatment in 19 trial centres in Sweden, Finland, Poland and Germany and stratified by asthma diagnosis to ensure balanced allocation of asthmatic and non‐asthmatic patients. Randomization was performed according to standard operating procedures (see Appendix S1). The study protocol was approved by the Central Ethics Committees in Germany and respective Independent Ethics Committees of the participating countries (see Appendix S1) and conducted in accordance with the 2002 Declaration of Helsinki, the International Council for Harmonisation guideline for Good Clinical Practice (ICH E6[R1], 1996, Directive 2001/20/EC, 2001), and relevant local laws and regulations.
2.2. Patients
The study included patients aged 18‐60 years with IgE‐mediated moderate to severe seasonal allergic rhinitis/rhinoconjunctivitis16 with or without allergic bronchial asthma to birch pollen allergens (Global Initiative for Asthma [GINA] grade I or II).17 Birch pollen allergy was documented as described in the Appendix S1.
2.3. Allergen immunotherapy
The investigational product (Allergovit® Birch, Allergopharma GmbH & Co. KG) is an aluminium hydroxide‐adsorbed allergoid preparation of Betula verrucosa pollen, injected subcutaneously into the upper arm. It was provided in two strengths: Strength A (1000 standardized therapeutic units [TU]/mL) and Strength B (10000 TU/mL). Strength B contained 23 μgeq (microgram equivalent) Bet v 1 (the major birch pollen allergen) in the 0.6 mL maintenance dose.18 Treatment consisted of an injection at intervals of 7 days, according to the standard dosing scheme: initial injections of 0.1, 0.2, 0.4 and 0.8 mL of Strength A followed by 0.15, 0.3 and 0.6 mL of Strength B. In exceptional cases, patients (n = 3) with a high sensitization started on a 1:10 dilution of Strength A (Strength 0 [100 TU/mL]: 0.1, 0.2, 0.4 and 0.8 mL), followed by Strengths A and B. Placebo contained 0.00125 mg/mL (Strength 0), 0.0125 mg/mL (Strength A) or 0.125 mg/mL (Strength B) histamine dihydrochloride in an aluminium hydroxide suspension. After individual maximum dose was reached, maintenance treatment followed. The intervals between injections were gradually extended to 2 weeks and then 4 weeks. Treatment was started each year in October and continued until approximately 2 weeks before the start of the birch pollen season in the following year.
A patient diary was used to document allergy symptoms and use of rescue medication. Prior to start of the birch pollen season, patients were issued permitted rescue medication (see Appendix S1).
2.4. Pollen counts
Each study centre was linked to one or two regional pollen count stations. Information on atmospheric pollen load was supplied by the European Aeroallergen Network (EAN). The respective birch pollen loads were measured for each region for each of the 3 study years, and onset of each birch pollen season was determined by the sponsor, based on EAN data.
2.5. Assessment of efficacy
Patients recorded their daily symptoms and medication intake for 8 weeks during the birch pollen season, and a Symptom Medication Score (SMS) was calculated as previously described.19 Evaluation of the SMS comprised the 7 days before and 13 days after the day of peak birch pollen count. The primary endpoint was the LS‐mean area under the curve (AUC) of the daily sum of the validated SMS during the 21‐day evaluation period in the birch pollen season of 2007, after 2 years DBPC treatment.
Secondary endpoints included the AUC of the SMS after 3 years treatment, number of “well days” (Symptom Score ≤ 4 and a Medication Score = 0), and immunologic changes (allergen‐specific IgE, IgG1 and IgG4, evaluated as described by Corrigan et al20). Samples were taken before the first injection, after the final dose of the preseasonal treatment at least 1 week before the start of the birch pollen season, and after each birch pollen season.
A post hoc exploratory subgroup analysis evaluated differences in the SMS and number of “well days” in the Full Analysis Set (FAS) of the north‐east region (FAS‐NE [Finland, Sweden and Poland, without the most south‐western centre in Poland] vs the FAS [the FAS‐NE plus Germany and the most south‐western centre in Poland]).
2.6. Safety measures
Safety parameters collected included adverse events (AEs), clinical chemistry and haematology parameters. Adverse events were assessed for their relationship to treatment and coded using the Medical Dictionary for Regulatory Activities.
2.7. Statistics
The study was planned for a sample size of 91 evaluable patients per group assuming a standard deviation of 130 per group (1:1 randomization), a mean difference of 60 (a reduction of the AUC by about 30%) and a significance level of 2.5% (level required by the Scandinavian authorities to grant a marketing authorization based on a single phase 3 study) achieving at least 80% statistical power (analysis of covariance [ANCOVA] model adjusted for asthma status at randomization and centre). It was planned to screen 420 patients and to randomize and treat 252 patients. Exploratory subgroup analyses of the primary variable AUC of SMS (ie, differences according to geographic region) and analyses of secondary parameters were tested using the Wilcoxon two‐sample test at a 5% level. At least 75% of the SMS values in the corresponding evaluation period had to be evaluable.
3. RESULTS
3.1. Patients
In total, 403 patients were screened and 253 patients were randomized into the study. The disposition of patients is shown in Figure 2.
Figure 2.
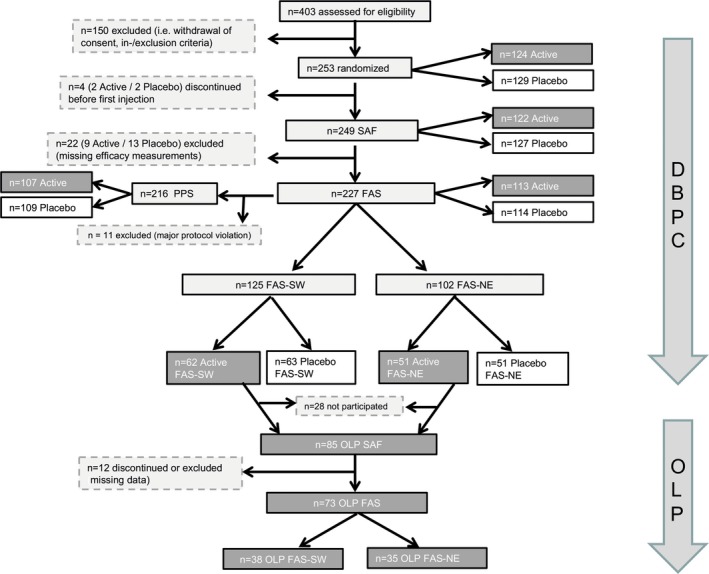
Patient disposition. DBPC, double‐blind placebo‐controlled phase (2006‐2007); FAS, Full Analysis Set; FAS‐NE, FAS north‐east subgroup (patients of centres in Sweden, Finland and Poland, excluding the most south‐western centre in Poland); FAS‐SW, FAS south‐west subgroup (patients of German centres and the most south‐western centre in Poland); n, number of patients; OLP, open‐label phase (2008); PPS, Per Protocol Set; SAF, Safety Analysis Set
In the Safety Analysis Set (SAF), demographics and screening characteristics showed no remarkable differences between the two treatment groups, including comparable frequency of birch allergy symptoms (eg, allergic asthma: active 27.0%, placebo 23.0%), sex (female: active 56.6%, placebo 47.2%) and age (mean [SD], years: active 37.6 [12.2], placebo 35.5 [11.2]; Table 1).
Table 1.
Demographic and other patient characteristics (SAF)
| Active (n = 122) | Placebo (n = 127) | |
|---|---|---|
| Sex, n (%) | ||
| Male | 53 (43.4) | 67 (52.8) |
| Female | 69 (56.6) | 60 (47.2) |
| Age (y) | ||
| Mean (SD) | 37.6 (12.2) | 35.5 (11.2) |
| Range | 18‐59 | 18‐59 |
| Patients with birch allergy symptoms, n (%) | ||
| Allergic asthma | 33 (27.0) | 29 (23.0) |
| Allergic conjunctivitis | 122 (100.0) | 126 (100.0) |
| Allergic rhinitis | 122 (100.0) | 126 (100.0) |
| Cough/sibilant rhonchi | 34 (27.9) | 41 (32.5) |
| Neurodermatitis | 8 (6.6) | 10 (7.9) |
| Birch allergy diagnostics, mean (SD) | ||
| SPT weal area (mm) | 10.5 (3.1) | 10.9 (3.2) |
| EAST/CAP value (kUA/L) | 25.9 (27.6) | 25.5 (28.7) |
EAST/CAP, Enzyme Allergo Sorbent Test/CAPACITY (Pharmacia ThermoFisher Scientific); kUA/L, kilo units of allergen‐specific IgE per litre; n, number of patients; SAF, Safety Analysis Set; SD, standard deviation; SPT, skin prick test.
3.2. Pollen count
For the FAS and FAS‐NE, the lowest pollen load of all 3 treatment years was observed in 2007 (year of primary endpoint assessment). The average 2007 peak pollen counts in the investigated countries (for all centres) were similar: 1364 (pollen/m3) in Germany, 1141 (pollen/m3) in Poland, 1119 (pollen/m3) in Finland and 1030 (pollen/m3) in Sweden. Figure 3 illustrates the course of the pollen count for the FAS and FAS‐NE, over the three pollen seasons.
Figure 3.
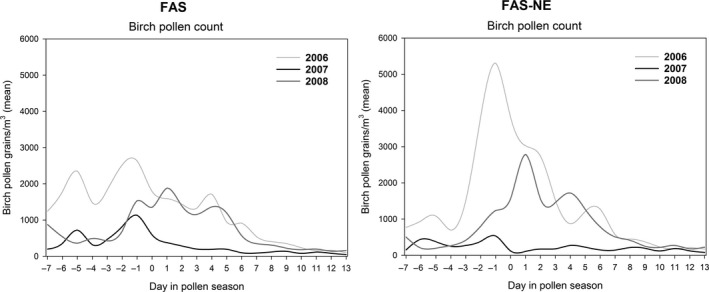
Course of birch pollen count during the 21‐day evaluation period from 2006 to 2008. FAS, Full Analysis Set; FAS‐NE, FAS north‐east subgroup (patients of centres in Sweden, Finland and Poland, excluding the most south‐western centre in Poland)
3.3. Efficacy
Patients in the DBPC phase who received at least one dose of study medication and for whom the efficacy assessment of the primary variable was available for at least 1 year were included in the FAS (n = 227). After 2 years of treatment (primary endpoint), a positive trend was observed: active‐treated patients in the FAS had a reduction in SMS of 15.2% (LS‐mean) compared with placebo‐treated patients, although the significance level of 5% was not achieved (P = 0.0710; Figure 4 and Table 2). For the Per‐Protocol Set (PPS, n = 216, post hoc analysis) patients with major protocol violations were excluded (including possible allergy to a range of other allergens causing symptoms in and around the birch pollen season—alder, hazel, poplar, elm, willow‐tree, beech, oak, ash, rape, Dermatophagoides pteronyssinus, Dermatophagoides farinae, dog, cat, Aspergillus, Penicillium). The comparison of active vs placebo in the PPS after 2 years of treatment consolidated the positive trend observed in the FAS (16.7% reduction, LS‐mean), but again a significance level of 5% level was not achieved (P = 0.0523). However, the SMS of the active‐treated patients of the FAS‐NE (n = 102) was significantly lower compared to the placebo group after 2 years of treatment (P = 0.0034; Figure 4 and Table 2). These results were associated with a clinically relevant difference in SMS between active and placebo of 32.7% (LS‐mean). After 3 years of treatment, during the OLP of the study, the mean SMS of the active‐treated patients of the FAS, PPS and FAS‐NE had decreased further from the second year (Table 2), despite an increasing birch pollen load (Figure 3). The analysis of the first year data (secondary endpoint) did not reveal a significant difference in SMS of active‐ vs placebo‐treated patients for any analysis sets (FAS, PPS and FAS‐NE; Table 2). Figure S1 shows the mean SMS of the active‐ and placebo‐treated patients in the context of the birch pollen counts during the pollen season for the FAS and FAS‐NE during the 3 years of treatment.
Figure 4.
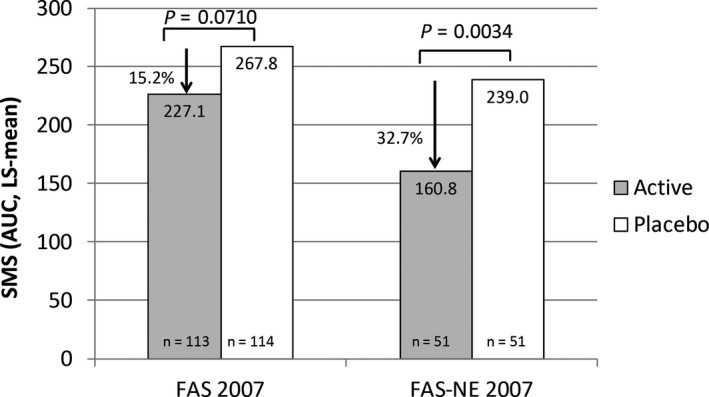
Reduction in LS‐mean AUC of the SMS in the second year of therapy (2007): FAS and FAS‐NE. The figure shows the reduction in SMS of the active‐ vs placebo‐treated patients of the FAS and FAS‐NE subgroup in 2007 (primary endpoint). AUC, area under the curve; FAS, Full Analysis Set; FAS‐NE, FAS north‐east subgroup (patients of centres in Sweden, Finland and Poland, excluding the most south‐western centre in Poland); LS, least‐squares; n, number of patients; SMS, Symptom Medication Score
Table 2.
AUC of the SMS after 1, 2 and 3 years of preseasonal treatment
| Parameter | AUC of SMS | |||||
|---|---|---|---|---|---|---|
| FAS | PPS | FAS‐NE | ||||
| Active | Placebo | Active | Placebo | Active | Placebo | |
| DBPC (1 y of treatment) | ||||||
| n | 108 | 113 | 103 | 108 | 50 | 51 |
| Mean (SD) | 293.5 (168.1) | 298.3 (174.2) | 292.8 (166.0) | 301.3 (173.0) | 266.7 (146.5) | 313.8 (160.4) |
| Median | 291.5 | 261.0 | 286.0 | 263.0 | 261.3 | 271.0 |
| Min‐Max | 0.0‐651.0 | 11.0‐698.0 | 0.0‐651.0 | 11.0‐698.0 | 32.0‐540.0 | 57.0‐698.0 |
| Adjusteda LS‐mean (SE) | 311.5 (18.1) | 324.2 (17.6) | 309.3 (18.4) | 325.5 (17.8) | 286.5 (23.4) | 338.5 (22.1) |
| P‐value | 0.5755 | 0.4780 | 0.0733 | |||
| Active vs Placebo (% reduction) | 3.9 | 5.0 | 15.4 | |||
| DBPC (2 y of treatment) | ||||||
| n | 113 | 114 | 107 | 109 | 51 | 51 |
| Mean (SD) | 207.6 (173.8) | 238.9 (182.4) | 206.5 (172.9) | 239.3 (183.7) | 136.1 (115.6) | 205.7 (163.3) |
| Median | 155.0 | 213.0 | 155.0 | 216.0 | 104.0 | 174.0 |
| Min‐Max | 0.0‐633.0 | 0.0‐888.0 | 0.0‐633.0 | 0.0‐888.0 | 0.0‐485.0 | 0.0‐642.0 |
| Adjusteda LS‐mean (SE) | 227.1 (17.8) | 267.8 (17.4) | 222.4 (18.1) | 266.9 (17.8) | 160.8 (21.0) | 239.0 (20.0) |
| P‐value | 0.0710 | 0.0523 | 0.0034 | |||
| Active vs Placebo (% reduction) | 15.2 | 16.7 | 32.7 | |||
| DBPC + OLP (3 y of treatment) | ||||||
| n | 73 | n.d. | 69 | n.d. | 35 | n.d. |
| Mean (SD) | 128.5 (129.6) | n.d. | 130.5 (132.3) | n.d. | 106.0 (108.8) | n.d. |
| Median | 76.0 | n.d. | 76.0 | n.d. | 49.0 | n.d. |
| Min‐Max | 0.0‐492.0 | n.d. | 0.0‐492.0 | n.d. | 0.0‐382.0 | n.d. |
ANCOVA, analysis of covariance; AUC, area under the curve; DBPC, double‐blind placebo‐controlled; FAS, Full Analysis Set; FAS‐NE, FAS north‐east subgroup (patients of centres in Sweden, Finland, and Poland, excluding the most south‐western centre in Poland); LS, least‐squares; n, number of patients; n.d., no data; OLP, open‐label phase; PPS, Per Protocol Set; SD, standard deviation; SE, standard error; SMS, Symptom Medication Score.
ANCOVA model adjusted for asthma status at randomization and centre.
The analysis of the number of “well days” and the immunologic changes in the patients were other secondary objectives of this study. In the FAS, the median number of “well days” during the 21‐day evaluation period of birch pollen flight in 2007 was higher in the active treatment group (42.9%, n = 100) compared to the placebo group (19.0%, n = 91) but not statistically significant (P = 0.1062). However, in the FAS‐NE, this increase was significant (active 61.9%, n = 47; placebo 28.6%, n = 42; P = 0.0232; Figure 5). After 3 years of active treatment (OLP in 2008), the median number of “well days” improved to 61.9% in the FAS (n = 73) and 71.4% FAS‐NE (n = 35).
Figure 5.
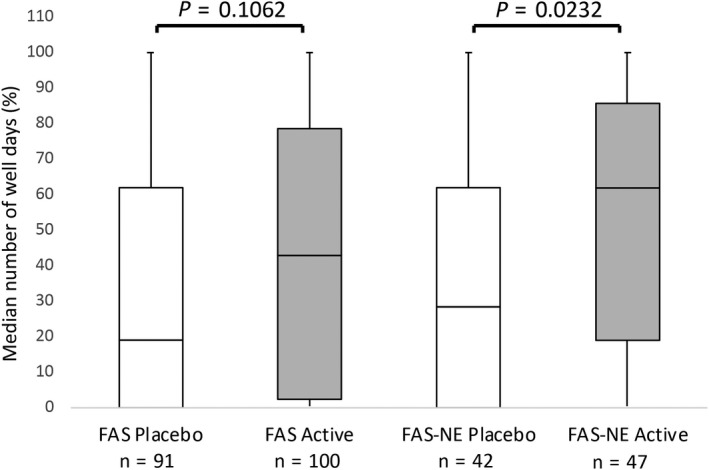
Median number of “well days” in the second year of treatment (2007, DBPC). This figure shows the percentage of median number of well days during the 21‐day evaluation period of birch pollination for the FAS and the FAS‐NE subgroup (% median well days = median number of well days/21‐day evaluation period). FAS, Full Analysis Set; FAS‐NE, FAS north‐east subgroup (patients of centres in Sweden, Finland and Poland, excluding the most south‐western centre in Poland); n, number of patients
In the FAS and FAS‐NE, median IgG4 and IgG1 levels increased significantly (P < 0.001) in favour of the active treatment group after the first and second preseasonal treatment courses and continued to rise in the third year of treatment (Figure S2). Median IgG4 and IgG1 levels of the placebo group remained largely unchanged during the entire study period.
3.4. Tolerability and safety
All patients who received at least one dose of the study medication (n = 249) were included in the SAF. For all 3 years of treatment, patients received an average of 3 maintenance doses after reaching the maximum dose. Active‐treated patients received an average cumulative dose of 25600 TU (98.1 μgeq Bet v 1) in 2006, 30000 TU (115.0 μgeq Bet v 1) in 2007 and 24000 TU (92.0 μgeq Bet v 1) in 2008. The total average cumulative dose after 3 years of therapy was 79600 TU (305 μgeq Bet v 1).
During the DBPC phase, 61 of 122 patients (50.0%) in the active treatment group and 37 of 127 patients (29.1%) in the placebo treatment group experienced at least 1 AE with a suspected relationship to study medication. During the DBPC phase, the most common AEs (occurring in >4% of patients in either treatment group) with a suspected relationship to study medication were injection site reaction, injection site pruritus, injection site swelling, injection site pain, rhinitis, injection site erythema and conjunctivitis (Table 3). No serious AEs considered related to study treatment occurred. During the DBPC phase, systemic adverse reactions occurred with similar frequency in both treatment groups (active 13.9% vs placebo 16.5%). Local reactions were more frequent in the active treatment group (42.6% vs placebo 23.6%), and all local reactions were related to injection site conditions. Adverse events for the OLP SAF (ie, patients who received 2 years DBPC active treatment and 1‐year open‐label active treatment) were similar to the active treatment group of the DBPC phase (Table 3). There were no clinically relevant changes in vital signs or laboratory values in the DBPC or OLP phases. Overall, the SCIT was well tolerated and AEs were as expected.
Table 3.
Adverse events with a suspected relationship to study medication occurring in >4% of patients (SAF)
| n (%) | DBPC | OLPa | |
|---|---|---|---|
| Active (n = 122) | Placebo (n = 127) | Active (n = 85) | |
| Patients with at least 1 AE | 61 (50.0) | 37 (29.1) | 45 (52.9) |
| Injection site reaction | 52 (42.6) | 29 (22.8) | 38 (44.7) |
| Injection site pruritus | 42 (34.4) | 16 (12.6) | 31 (36.5) |
| Injection site swelling | 32 (26.2) | 10 (7.9) | 25 (29.4) |
| Injection site pain | 14 (11.5) | 8 (6.3) | 12 (14.1) |
| Rhinitis | 6 (4.9) | 8 (6.3) | 4 (4.7) |
| Injection site erythema | 6 (4.9) | 3 (2.4) | 8 (9.4) |
| Conjunctivitis | 5 (4.1) | 3 (2.4) | 4 (4.7) |
| Injection site warmth | 1 (0.8) | 3 (2.4) | 4 (4.7) |
AE, adverse event; DBPC, double‐blind placebo‐controlled; n, number of patients; OLP, open‐label phase; SAF, Safety Analysis Set.
Patients who received 3 years (y) of active treatment (2‐y DBPC and 1‐y open‐label treatment).
4. DISCUSSION
Allergoids are chemically modified natural allergens with reduced allergenicity and retained immunogenicity, thus permitting the administration of high allergen doses in shorter dose escalation schemes without an increased risk of AEs.18 This study tested a high‐dose hypoallergenic birch pollen allergoid preparation and showed a positive change in SMS in active‐ vs placebo‐treated patients in the FAS and PPS after 2 years treatment, although a significance level of 5% was not achieved (15.2% lower LS‐mean SMS in active‐treated patients, P = 0.0710). A significant reduction in SMS (32.7%, P = 0.0034, LS‐mean) was, however, clearly demonstrated in the FAS‐NE subgroup after 2 years of active treatment. A statistically significant improvement in SMS of >30% was previously considered indicative of clinically relevant efficacy21; nowadays >20% indicates efficacy.22 After 3 years, during the non‐controlled OLP of the study, the mean SMS of the active‐treated patients of the FAS (n = 73), PPS (n = 69), and FAS‐NE (n = 35) had decreased further, despite an increasing birch pollen load, suggesting increased efficacy and continued desensitization towards the allergen. The median number of “well days” after 2 years treatment in the FAS favoured active treatment, although a significance level of 5% was not achieved. Again, a significantly greater percentage of median “well days” (P = 0.0232) was found in the FAS‐NE after 2 years treatment, which, in both the FAS‐NE and FAS, improved further after 3 years treatment. In addition, no serious related AEs occurred during the study and the allergoid SCIT was well tolerated.
While there is no clear relationship between immunoglobulin changes and clinical efficacy,23 increases in immunological parameters, notably IgG1 and IgG4, suggest immunological change and desensitization for the active‐treated patients in the FAS and FAS‐NE. The significant clinical benefit seen in the FAS‐NE patients in an apparently low birch pollen season may be explained by a greater birch pollen exposure. Birch trees are much more abundant in northern and eastern European regions than in southern and western regions and, because most birch pollen sediments near the pollen‐producing tree, this points towards a higher birch pollen exposure for patients from northern and eastern Europe.2, 3, 5, 12, 24
There are multiple confounding variables when studying the efficacy of SCIT with field‐based birch pollen studies, which may explain the difficulty in demonstrating significant benefit in the FAS and PPS patients. The PPS excluded 11 patients with major protocol violations, 6 of whom were due to possible allergies to other allergens that may cause symptoms around the birch pollen season. If these patients are not included, the influence of interfering allergens is reduced. The northern parts of Europe usually show less interference from other pollen species during the birch pollen season because the allergologically most relevant pollen types have virtually no overlapping seasons, and therefore interfering allergens are less of an issue.1
In the southern and western regions, pollen seasons are not that well separated. Despite similar average peak birch pollen counts in all four countries in 2007, the SMS values for active‐ and placebo‐treated patients in the FAS and PPS were higher than those for patients in the corresponding treatment groups of the FAS‐NE. This indicates that there may be an influence of other allergens in the SMS of patients in the FAS and PPS due to overlapping pollen seasons in southern and western regions, which may have distorted the primary endpoint evaluation. The potential overlap of exposure to different kinds of pollen was exemplary analysed for 2007, the year of the primary endpoint. In Germany, the flowering seasons of oak, plane tree, ash tree, beech and hornbeam overlapped with the birch pollen season whereas in Poland, Sweden and Finland the pollen seasons were better separated (just oak and hornbeam pollination overlapped with the birch pollen season in Sweden and Poland, no interfering allergens were recorded in Finland). The highest impact of interfering allergens was seen in Germany, thus supporting the rationale for the subgroup analysis (Figure S3). Therefore, depending on location, interfering pollens may have increased the symptom load in the population during the birch pollen season and influenced the outcome of this study.
In the first treatment year (2006), the difference in SMS (LS‐mean) of active‐ vs placebo‐treated patients was not statistically significant, although the birch pollen exposure was higher than in the second year (primary endpoint). In 2006, the peak SMS (FAS) occurred 1 week after the peak birch pollen count, when the pollen concentration had already been reduced substantially. During all 3 treatment years, after the peak pollen count, the course of SMS (FAS and FAS‐NE) and the course of birch pollen counts developed independently from each other: the SMS remained high or increased despite the pollen counts having dropped to low levels (Figure S1). This effect was also seen in another birch pollen study 14 but has not been observed in grass pollen studies.20, 25 As discussed above for the second year of treatment, interfering allergens may be responsible.
Strong variability of the annual birch pollen count, as seen in this study, is a well‐known phenomenon 24, 26, 27 reported previously.8, 9, 14 In seasons with a low pollen load, that is, 2007 when the primary endpoint was estimated, placebo‐treated patients may have no symptoms or need of rescue medication (as seen in minimum SMS values of 0 in Table 2). This makes it difficult to show a significant difference in the SMS between active‐ and placebo‐treated patients. In a study stratifying study centres according to the disease severity of the placebo group, active‐treated patients from centres with low symptom severity in the placebo group showed no or only limited treatment benefit.28 In this same study, for active‐treated patients from centres with placebo groups with medium or severe symptom levels, a clinically relevant difference between active‐ and placebo‐treated patients could be demonstrated, indicating that treatment efficacy may be underestimated if a study includes too many centres with low pollen exposure and low disease severity.28
Previous field studies have failed to show a difference in SMS between active‐ and placebo‐treated patients in seasons with low birch pollen loads,9, 14 yet demonstrated significant efficacy in seasons with higher birch pollen.4, 9, 14 In this study, the SMS and birch pollen counts courses showed differences, which are normally not observed in grass pollen studies.20, 25 Importantly, the average counting error of Hirst‐type pollen traps used in Europe is ≥25% 29 and, because they are usually mounted at rooftop level, may not accurately reflect the pollen exposure of a moving person at street level.30, 31 It is assumed that data from a pollen sampler typically represent the pollen load within a certain area with similar vegetation and climate.29 However, significant discrepancies in pollen counts (mainly due to differences in local vegetation) have been known among pollen traps located within a 30‐kilometre radius.32 In another study, deviations in pollen concentration from 283% to 1962% were measured among pollen samplers located a few kilometres from each other.30 In Germany, some study centres and pollen‐monitoring stations were located up to 94 km apart and patients may live even more distant. The distribution of birch trees in central Europe is patchy and presents local differences, whereas in northern Europe birch trees are the most abundant and cover the area continuously.2, 30 All pollen monitoring stations in Sweden, Finland and Poland were located in the same town or within 18 km of the study centre and therefore may better reflect patients’ actual pollen exposure. Collection of local or patient individualized data on pollen concentrations may result in more reliable data on pollen exposure in clinical trials.30, 32 Nevertheless, personalized methods are still under development and local pollen samplers cannot capture differences in pollen exposure due to patients’ lifestyle or spatial movements.30, 32
Even if birch pollen counts are representative of a patient's local environment, they serve only as a proxy for actual allergen exposure.33, 34, 35 A comparable pollen load provokes different levels of symptom severity in different regions and years.36 Up to 10‐fold daily,33, 34, 35 >fivefold yearly, and threefold regional differences in the release of the major allergen Bet v 1 from birch pollen have been reported, and numerous local factors influence its release.11 Measurement of the pollens’ allergen content is not feasible in a large clinical trial, and no data were obtained in this study. The use of environmental challenge chambers is an option to overcome the unpredictability of birch pollen exposure and the effect of low pollen exposure on treatment effect in field pollen studies,37, 38 but the comparability of chamber studies to field studies needs still to be confirmed.
This trial was performed without a baseline phase, which has implications for treatment allocation and documentation of clinical efficacy.21 Patients’ recall of their medical condition in the previous season may be subject to memory bias. Therefore, patients who do not experience the appropriate minimum of symptoms could have been included, which risks biasing the treatment groups.21, 39 In fact, the range of median SMS (minimum‐maximum) of placebo‐treated patients indicates that patients with no or very little symptoms were included in the study (2006‐2007, Table 2). Furthermore, non‐responders to treatment may have been among the evaluated study population because there is no option to detect them without a baseline phase.21
In conclusion, although the primary endpoint was not reached (FAS), SCIT with a high‐dose hypoallergenic birch pollen allergoid preparation was found to have a significant and clinically relevant effect on SMS in the subgroup of patients living where birch is the major tree species (FAS‐NE). After 3 years, the mean SMS for all active‐treated patients was further decreased, despite an increasing birch pollen load. This, together with the significant immunological response seen with SCIT in the FAS and FAS‐NE, indicates its efficacy. With highly fluctuating pollen such as birch, future allergy field studies should consider use of more personalized pollen data and/or adaptive study designs permitting the postponement of the primary endpoint evaluation to the next or second next study year in case of unusually low pollination. In addition, studies in environmental challenge chambers may be an option for allergens with unpredictable and strongly fluctuating pollination and varying allergen content. Indeed, the clinical efficacy of Allergovit® Birch in allergic rhinitis has been confirmed using an environmental challenge chamber,40 suggesting that the non‐attainment of a 5% significance level in the FAS and PPS in this study may be due to environmental factors.
CONFLICT OF INTEREST
MW reports honoraria from Allergopharma GmbH & Co. KG for consulting and speaker activities outside the submitted work. BK and MR report personal fees from Allergopharma GmbH & Co. KG outside the submitted work. The remaining authors declare that they have no relevant conflicts of interest.
AUTHOR CONTRIBUTIONS
MW, SR, BS, JA, A‐SH, AL and EV recruited patients and obtained data. MR managed the clinical trial, and BK reviewed the literature and drafted the manuscript. All the authors critically reviewed the manuscript, approved the final version for submission and accept overall accountability for accuracy and integrity of the manuscript. All authors had full access to the data and had final responsibility for the decision to submit for publication.
Supporting information
ACKNOWLEDGEMENTS
The authors would like to thank all clinicians involved in the trial, including Randolf Brehler, Rolf Dominicus, Peter Elsner, Susanne Mindt‐Prüfert, Bernhard Homey, Nicolas Hunzelmann, Olga Maus, Anna Bodzenta‐Lukaszyk, Barbara Rogala, Radoslaw Gawlik, Markus Rautiainen, Morgan Andersson, Maria Skedinger, Victoria Strand and Stephan Molitor; and Annemie Narkus as coordinating investigator. The authors would also like to thank Ulrike Lehnigk, Jens Kettner, Miriam Kiene, and Susanne Thum‐Oltmer for helpful discussions and review of the manuscript. We thank Fabian Kreimendahl as statistician, Petra Luebcke for data management and figure preparation, and Sabine Mussler for figure preparation. The trial was sponsored by Allergopharma GmbH & Co. KG. The authors acknowledge the assistance of Helen Brady, Michael Riley, and Laia Pedro‐Roig from Trilogy Writing & Consulting Ltd in the preparation of this manuscript.
Worm M, Rak S, Samoliński B, et al. Efficacy and safety of birch pollen allergoid subcutaneous immunotherapy: A 2‐year double‐blind, placebo‐controlled, randomized trial plus 1‐year open‐label extension. Clin Exp Allergy. 2019;49:516–525. 10.1111/cea.13331
Funding information
This study was funded by Allergopharma GmbH & Co. KG.
REFERENCES
- 1. World Health Organization (WHO) . Phenology and Human Health: Allergic Disorders. 2003; Report No.: EUR/031S036791 ‐ E79129.
- 2. Pauling A, Rotach MW, Gehrig R, Clot B. A method to derive vegetation distribution maps for pollen dispersion models using birch as an example. Int J Biometeorol. 2012;56:949‐958. [DOI] [PubMed] [Google Scholar]
- 3. Siljamo P. Numerical modelling of birch pollen emissions and dispersion on regional and continental scales. Finnish Meteorological Institute Contributions 99 (FMI‐CONT‐99). September 2013.
- 4. Worm M, Rak S, de Blay F, et al. Sustained efficacy and safety of a 300IR daily dose of a sublingual solution of birch pollen allergen extract in adults with allergic rhinoconjunctivitis: results of a double‐blind, placebo‐controlled study. Clin Transl Allergy. 2014;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Siljamo P, Sofiev M, Severova E, et al. Sources, impact and exchange of early‐spring birch pollen in the Moscow region and Finland. Aerobiol. 2008;24:211‐230. [Google Scholar]
- 6. Roberts G, Pfaar O, Akdis CA, et al. EAACI guidelines on allergen immunotherapy: allergic rhinoconjunctivitis. Allergy 2018;73:765‐798. [DOI] [PubMed] [Google Scholar]
- 7. Jutel M, Agache I, Bonini S, et al. International consensus on allergy immunotherapy. J Allergy Clin Immunol. 2015;136:556‐568. [DOI] [PubMed] [Google Scholar]
- 8. Arvidsson MB, Löwhagen O, Rak S. Effect of 2‐year placebo controlled immunotherapy on airway symptoms and medication in patients with birch pollen allergy. Allergy Clin Immunol. 2002;109:777‐783. [DOI] [PubMed] [Google Scholar]
- 9. Höiby AS, Strand V, Robinson DS, Sager A, Rak S. Efficacy, safety, and immunological effects of a 2‐year immunotherapy with Depigoid® birch pollen extract: a randomized, double‐blind, placebo‐controlled study. Clin Exp Allergy. 2010;40:1062‐1070. [DOI] [PubMed] [Google Scholar]
- 10. Bødtger U, Poulsen LK, Jacobi HH, Malling HJ. The safety and efficacy of subcutaneous birch pollen immunotherapy – a one year, randomized, double‐blind, placebo controlled study. Allergy. 2002;57:297‐305. [DOI] [PubMed] [Google Scholar]
- 11. Buters J. Birch pollen allergen release over a 5 year period in Germany: are the differences a consequence of climate change? Allergo J. 2008;17:373‐374. [Google Scholar]
- 12. Buters JTM, Kasche A, Weichenmeier I, et al. Year‐to‐year variation in release of Bet v 1 allergen from birch pollen: evidence for geographical differences between West and South Germany. Int Arch Allergy Immunol. 2008;145:122‐130. [DOI] [PubMed] [Google Scholar]
- 13. Movérare R, Westritschnig K, Svensson M, et al. Different IgE reactivity profiles in birch pollen‐sensitive patients from six European populations revealed by recombinant allergens: an imprint of local sensitization. Int Arch Allergy Immunol. 2002;128:325‐335. [DOI] [PubMed] [Google Scholar]
- 14. Khinchi MS, Poulsen LK, Carat F, André C, Hansen AB, Malling HJ. Clinical efficacy of sublingual and subcutaneous birch pollen allergen‐specific immunotherapy: a randomized, placebo‐controlled, double‐blind, double‐dummy study. Allergy. 2004;59:45‐53. [DOI] [PubMed] [Google Scholar]
- 15. Pfaar O, Bachert C, Bufe A, et al. Guideline on allergen‐specific immunotherapy in IgE‐mediated allergic diseases: S2k Guideline of the German Society for Allergology and Clinical Immunology (DGAKI), the Society for Pediatric Allergy and Environmental Medicine (GPA), the Medical Association of German Allergologists (AeDA), the Austrian Society for Allergy and Immunology (ÖGAI), the Swiss Society for Allergy and Immunology (SGAI), the German Society of Dermatology (DDG), the German Society of Oto‐ Rhino‐Laryngology, Head and Neck Surgery (DGHNO‐KHC), the German Society of Pediatrics and Adolescent Medicine (DGKJ), the Society for Pediatric Pneumology (GPP), the German Respiratory Society (DGP), the German Association of ENT Surgeons (BV‐HNO), the Professional Federation of Paediatricians and Youth Doctors (BVKJ), the Federal Association of Pulmonologists (BDP) and the German Dermatologists Association (BVDD). Allergo J Int. 2014;23:282‐319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bousquet J, Van Cauwenberge P, Khaltaev N. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001;108(5 Suppl):S147‐S334. [DOI] [PubMed] [Google Scholar]
- 17. Global Initiative for Asthma (GINA) . Global Strategy for Asthma Management and Prevention. Pocket guide. Updated 2004.
- 18. Brehler R, Kahlert H, Thum‐Oltmer S. Hypoallergenic preparations in SCIT. Immunological features and their impact on clinical efficacy and safety, exemplary for the allergoids Allergovit®, Acaroid® and a folding variant of the recombinant birch pollen major allergen Bet v 1. Allergo J 2010;19:477‐484. [Google Scholar]
- 19. Häfner D, Reich K, Matricardi PM, Meyer H, Kettner J, Narkus A. Prospective validation of ‘Allergy‐Control‐SCORETM’: a novel symptom–medication score for clinical trials. Allergy. 2011;66:629‐636. [DOI] [PubMed] [Google Scholar]
- 20. Corrigan CJ, Kettner J, Doemer C, Cromwell O, Narkus A. Efficacy and safety of preseasonal‐specific immunotherapy with an aluminium‐adsorbed six‐grass pollen allergoid. Allergy. 2005;60:801‐807. [DOI] [PubMed] [Google Scholar]
- 21. Malling HJ. Criteria for clinical efficacy–readout and monitoring of clinical studies. Arb Paul Ehrlich Inst Bundesamt Sera Impfstoffe Frankf A M. 2003;94:119‐123. [PubMed] [Google Scholar]
- 22. Canonica GW, Baena‐Cagnani CE, Bousquet J, et al. Recommendations for standardization of clinical trials with allergen specific immunotherapy for respiratory allergy. A statement of a World Allergy Organization (WAO) taskforce. Allergy 2007;62:317‐324. [DOI] [PubMed] [Google Scholar]
- 23. Pfaar O, van Twuijver E, Boot JD, et al. A randomized DBPC trial to determine the optimal effective and safe dose of a SLIT‐birch pollen extract for the treatment of allergic rhinitis: results of a phase II study. Allergy. 2016;71:99‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Spieksma FTM, Corden JM, Detandt M, et al. Quantitative trends in annual totals of five common airborne pollen types (Betula, Quercus, Poaceae, Urtica, and Artemisia), at five pollen‐monitoring stations in western Europe. Aerobiologia. 2003;19:171‐184. [Google Scholar]
- 25. Varney VA, Gaga M, Frew AJ, Aber VR, Kay AB, Durham SR. Usefulness of immunotherapy in patients with severe summer hay fever uncontrolled by antiallergic drugs. BMJ. 1991;302:265‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ranta H, Hokkanen T, Linkosalo T, Laukkanen L, Bondestam K, Oksanen A. Male flowering of birch: spatial synchronization, year‐to‐year variation and relation of catkin numbers and airborne pollen counts. For Ecol Manage. 2008;255:643‐650. [Google Scholar]
- 27. D'Amato G, Spieksma FT, Liccardi G, et al. Pollen‐related allergy in Europe. Allergy. 1998;53:567‐578. [DOI] [PubMed] [Google Scholar]
- 28. Howarth P, Malling HJ, Molimard M, Devillier P. Analysis of allergen immunotherapy studies shows increased clinical efficacy in highly symptomatic patients. Allergy. 2012;67:321‐327. [DOI] [PubMed] [Google Scholar]
- 29. Pfaar O, Bastl K, Berger U, et al. Defining pollen exposure times for clinical trials of allergen immunotherapy for pollen induced rhinoconjunctivitis – an EAACI Position Paper. Allergy. 2017;72:713‐722. [DOI] [PubMed] [Google Scholar]
- 30. Werchan B, Werchan A, Mücke HG, et al. Spatial distribution of allergenic pollen through a large metropolitan area. Environ Monit Assess. 2017;189:169. [DOI] [PubMed] [Google Scholar]
- 31. Spieksma FTM, Noort PV, Nikkels H. Influence of nearby stands of Artemisia on street‐level versus roof‐top‐level ratio's of airborne pollen quantities. Aerobiologia 2000;16:21‐24. [Google Scholar]
- 32. Katelaris CH, Burke TV, Byth K. Spatial variability in the pollen count in Sydney, Australia: can one sampling site accurately reflect the pollen count for a region? Ann Allergy Asthma Immunol. 2004;93:131‐136. [DOI] [PubMed] [Google Scholar]
- 33. Buters JT, Weichenmeier I, Ochs S, et al. The allergen Bet v 1 in fractions of ambient air deviates from birch pollen counts. Allergy. 2010;65:850‐858. [DOI] [PubMed] [Google Scholar]
- 34. Bousquet J, Schunemann HJ, Fonseca J, et al. MACVIA‐ARIA Sentinel Network for allergic rhinitis (MASK‐rhinitis): the new generation guideline implementation. Allergy. 2015;70:1372‐1392. [DOI] [PubMed] [Google Scholar]
- 35. Buters JTM, Thibaudon M, Smith M, et al. Release of Bet v 1 from birch pollen from 5 European countries. Results from the HIALINE study. Atmos Environ 2012;55:496‐505. [Google Scholar]
- 36. Bastl K, Kmenta M, Jäger S, Bergmann K‐C, Berger U. Development of a symptom load index: enabling temporal and regional pollen season comparisons and pointing out the need for personalized pollen information. Aeobiologia 2014;30:269‐280. [Google Scholar]
- 37. Durham SR, Nelson HS, Nolte H, et al. Magnitude of efficacy measurements in grass allergy immunotherapy trials is highly dependent on pollen exposure. Allergy. 2014;69:617‐623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pfaar O, Demoly P, Gerth van Wijk R, et al. Recommendations for the standardization of clinical outcomes used in allergen immunotherapy trials for allergic rhinoconjunctivitis: an EAACI Position Paper. Allergy 2014;69:854‐867. [DOI] [PubMed] [Google Scholar]
- 39. Casale TB, Canonica GW, Bousquet J, et al. Recommendations for appropriate sublingual immunotherapy clinical trials. J Allergy Clin Immunol. 2009;124:665‐670. [DOI] [PubMed] [Google Scholar]
- 40. Wagenmann M, Hohlfeld J, Tribanek M, Homolla V, Karjalainen M, Häfner D. Double‐blind randomized multicenter clinical trial to evaluate the specific and non‐specific effects of AIT with a grass and a birch pollen allergoid. EAACI Congress Vienna. 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


