Abstract
C4 photosynthesis is a complex trait that boosts productivity in warm environments. Paradoxically, it evolved independently in numerous plant lineages, despite requiring specialised leaf anatomy. The anatomical modifications underlying C4 evolution have previously been evaluated through interspecific comparisons, which capture numerous changes besides those needed for C4 functionality. Here, we quantify the anatomical changes accompanying the transition between non‐C4 and C4 phenotypes by sampling widely across the continuum of leaf anatomical traits in the grass Alloteropsis semialata. Within this species, the only trait that is shared among and specific to C4 individuals is an increase in vein density, driven specifically by minor vein development that yields multiple secondary effects facilitating C4 function. For species with the necessary anatomical preconditions, developmental proliferation of veins can therefore be sufficient to produce a functional C4 leaf anatomy, creating an evolutionary entry point to complex C4 syndromes that can become more specialised.
Keywords: Alloteropsis, bundle sheath, C3‐C4 intermediate, C4 photosynthesis, evolution, grass, leaf anatomy, mesophyll, vein density
Introduction
The vast majority of plants use C3 photosynthesis, but some lineages evolved the C4 pathway to overcome environmentally induced limitations on carbon fixation (Ehleringer et al. 1991; Sage et al. 2011). Net carbon fixation by C3 photosynthesis is decreased in warm, high light, arid and saline environments that lower CO2 concentrations within the leaf and increase photorespiration, the process initiated when O2 instead of CO2 is fixed by the enzyme Rubisco (Chollet & Ogren 1975). To circumvent the losses of carbon and energy caused by photorespiration, the C4 pathway spatially separates the initial fixation of carbon and its assimilation by Rubisco across two leaf compartments, thereby concentrating CO2 at the enzyme's active site to promote CO2 rather than O2 fixation (Downton & Tregunna 1968; Hatch 1976). A number of anatomical and biochemical functions must work in concert to sustain the high fluxes of the C4 cycle, and comparisons of average C4 and C3 plants suggest that the evolution of the C4 phenotype required a large number and scale of changes (Hattersley 1984). Despite this apparent complexity, the C4 trait evolved many times independently (Sage et al. 2011). Resolving this paradox requires the quantitative distinction of changes that were involved in the evolutionary transition from C3 to C4, from those that preceded or followed it.
In most C4 plants, carbon fixation within leaf mesophyll tissue (M) is used to concentrate CO2 and boost Rubisco activity within bundle sheath tissue (BS), whereas Rubisco in C3 plants operates within the M where it depends on atmospheric CO2 diffusion (Fig. 1; Brown 1975; Hattersley et al. 1977; Hatch 1987). Efficient C4 leaves require large BS volumes to accommodate the necessary photosynthetic organelles, including chloroplasts containing abundant Rubisco, and a small distance between M and BS compartments to allow the rapid transfer of metabolites (Fig. 1; Hattersley & Watson 1975; Lundgren et al. 2014). These traits vary among C3 plant lineages, and in grasses, C4 photosynthesis evolved only within those groups with large fractions of BS (Christin et al. 2013; Lundgren et al. 2014). Comparisons of multiple C4 lineages with their C3 relatives indicate that the evolution of C4 leaf anatomy involved ultrastructural rearrangements and further decreases to the relative volume of M compared to BS tissue (Hattersley 1984; Dengler et al. 1994; McKown & Dengler 2007; Christin et al. 2013). These properties can be achieved via a variety of leaf structural modifications, allowing C4 anatomy to be realised differently each time it evolved, in some cases involving the use of different tissue types for the C4 BS function (Brown 1975; Soros & Dengler 2001; Christin et al. 2013; Freitag & Kadereit 2014; Lundgren et al. 2014). While the differences between a diverse range of C3 and C4 species are well known, the minimum set of leaf anatomical modifications required to carry out C4 photosynthesis remains to be established.
Figure 1.
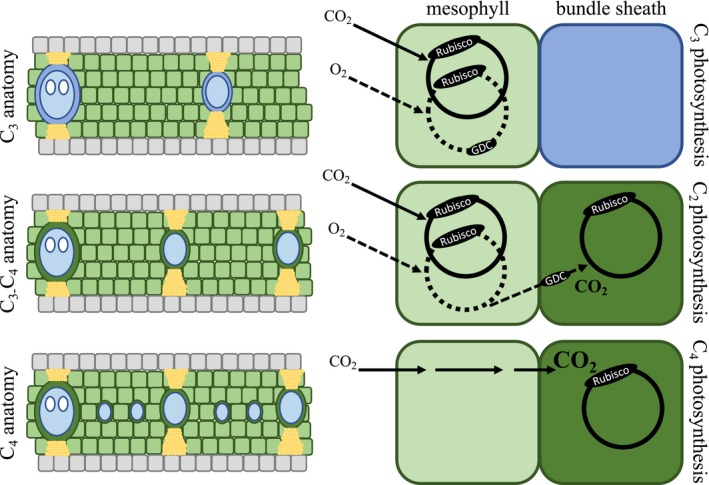
Schematic of leaf anatomy and photosynthetic pathway in C3, C3‐C4 and C4 grasses. In C3 plants, CO 2 assimilation via the Calvin–Benson cycle (solid black circle) and CO 2 release via photorespiration (dashed black circle) both occur in mesophyll cells (light green). C3 leaves consequently have larger areas of mesophyll tissue than bundle sheath tissue, where no photosynthetic activity occurs. C3‐C4 plants use an intermediate physiology called C2 photosynthesis, where the Calvin‐Benson cycle occurs in mesophyll cells, like in C3 plants. However, because glycine decarboxylase (GDC) is specifically localised to bundle sheath cells in these plants, the photorespiratory cycle is split across these two cell types, creating a weak CO 2‐concentrating mechanism, where CO 2 is released in the bundle sheath and can be reassimilated via the Calvin cycle. C2 photosynthesis, therefore, requires large areas of mesophyll for photosynthesis via an initial Calvin‐Benson cycle, but also close contact between mesophyll and bundle sheath cells for the photorespiratory CO 2 pump. C4 plants have a strong CO 2 concentrating mechanism whereby CO 2 is biochemically shuttled from the mesophyll into the bundle sheath. The high CO 2 concentration in the bundle sheath largely avoids oxygenation and thus, photorespiration. Photosynthesis via the C4 cycle therefore requires large areas of bundle sheath tissue, but less mesophyll, which can be achieved via the insertion of minor veins. Dark blue, bundle sheath lacking chloroplasts; dark green, bundle sheaths with chloroplasts; light green, mesophyll cells; yellow, extraxylary fibres/bundle sheath extensions; grey, epidermal cells; light blue, veins; white, metaxylem.
The grass Alloteropsis semialata (R.Br.) Hitchc. provides an outstanding system to capture the early events during C4 evolution because it includes genetically divergent C4 individuals as well as a diversity of non‐C4 plants encompassing C3 and C3‐C4 intermediate phenotypes (Ellis 1974; Lundgren et al. 2016), which emerged in the palaeotropics (Lundgren et al. 2015). The inner sheath (i.e., the mestome sheath), which is present in all C3 grasses, has been co‐opted for the C4 BS function in A. semialata. Previous studies have compared leaf properties among C4 and non‐C4 leaves of a few A. semialata accessions (Ellis 1974; Frean et al. 1983; Ueno & Sentoku 2006; Lundgren et al. 2016; Dunning et al. 2017), but a broader sampling is required to establish which properties are unique to each photosynthetic type.
The primary focus of this study is to compare leaf anatomy in accessions spanning the diversity of each photosynthetic type to distinguish the structural diversifications that occurred before, during and after C4 emergence in this species. We hypothesise that the properties that predate C4 evolution will be shared by at least some of the non‐C4 individuals, while those that happened after C4 evolution in a phase of subsequent adaptation will be restricted to a subset of the C4 populations. Properties unique to, and common among all, C4 accessions represent those that were involved in the initial transition to a C4 physiology. We conducted a large scan of the diversity within the species using traits linked to the number and size of different cell types and used controlled growth experiments to verify that anatomical differences are not environmentally induced. This evaluation of the gross leaf morphology was accompanied by a focused study in some individuals to identify ultrastructural changes that may also differ between C4 and non‐C4 accessions. Overall, our work shows that a complex trait of large ecological significance can evolve via a few key developmental changes.
Materials and methods
Characterising photosynthetic types
Photosynthetic type was determined by a combination of stable isotope and CO2 compensation point (CCP) data (Table S1; Data set S1), as previously described (Lundgren et al. 2016). The carbon isotope composition of plant tissues (δ13C) distinguishes photosynthetic types (von Caemmerer et al. 2014), such that plants with δ13C values higher than −17‰ were considered to have a fully functioning C4 system, while those with values lower than this threshold were considered either C3 or C3‐C4. CCPs were used to distinguish C3‐C4 from C3 plants and to support the δ13C results. The CCP indicates the CO2 concentration within the leaf at which CO2 assimilation via photosynthesis equals CO2 loss via photorespiration and respiration. As less CO2 is ultimately lost to photorespiration in C3‐C4 plants, they have very low CCPs compared to C3 plants. Thus, non‐C4 plants with CCPs greater than or equal to 35 μmol mol−1 were classified as C3, while those < 35 μmol mol−1 were classified as C3‐C4. CCPs were calculated on 27 living accessions (6 C3, 4 C3‐C4 and 17 C4), following published protocols (Bellasio et al. 2016a,b; Lundgren et al. 2016). Non‐C4 accessions for which live material was unavailable were assumed to have the same photosynthetic type as their closest relatives, as identified by phylogenetic relationships (Table S1).
Leaf samples
Fifty Alloteropsis semialata (R.Br.) Hitchc. accessions distributed across the species’ geographic range, including 17 C3, 6 C3‐C4 and 27 C4, were used to assess intraspecific anatomical variation. Leaf samples from 44 of the 50 accessions were collected from their original field site and preserved until embedding was possible. For the remaining six accessions, leaf samples were taken from plants grown under controlled environment conditions as in Lundgren et al. (2016). For all samples, leaf pieces 3–5 mm in length were embedded in methacrylate embedding resin (Technovit 7100, Heraeus Kulzer GmbH, Wehrhein, Germany), sectioned 6–8 μm thick on a manual rotary microtome (Leica Biosystems, Newcastle, UK) and stained with Toluidine Blue O (Sigma‐Aldrich, St. Louis, MO, USA). Stained leaf sections were imaged using microscopy imaging software with a camera mounted on a microscope (Cell A, Olympus DP71 and Olympus BX51, respectively; Olympus, Hamburg, Germany) and the images were stitched together using DoubleTake (v2.2.9, Echo One, Frederikssund, Denmark).
Leaf anatomy measurements
Anatomical traits were measured using ImageJ (Fig. S1; Schneider et al. 2012) from the cross section of a single leaf segment from the centre of the leaf blade, avoiding segments immediately adjacent to the midrib and lateral edges of the cross section. Vein orders were distinguished following Renvoize (1987). A single segment was defined as the leaf area falling between two secondary veins, which are large veins with metaxylem. Tertiary and minor veins (e.g. quaternary and quinary orders) lack metaxylem. In this species, the extraxylary fibres that flank both the adaxial and abaxial edges of tertiary veins distinguish them from higher order minor veins, which can be flanked by fibres on one side only (Fig. S1).
The cross‐sectional area of the whole segment, combining M, BS, epidermis and bulliform cells, extraxylary fibres and BS extensions as well as any transverse veins or tear spaces was measured. For all accessions, the total BS (i.e. the inner sheath; the compartment used for the Calvin cycle in C4 A. semialata), outer sheath and vein areas were measured separately for secondary, tertiary and any minor veins. The area of M tissue was calculated as the total area remaining after accounting for all other tissue types. In addition, the cross‐sectional area of individual M and BS cells (hereafter ‘size’) was measured (Fig. S1). Although the depth of individual cells can vary, it is their cross‐sectional areas, and not their three‐dimensional volumes, that primarily influence the proportion of each tissue in the leaf.
Linear discriminant analysis
We used a linear discriminant analysis (LDA) to explain the variation between photosynthetic types (i.e. the test maximises between‐group variance while minimising within‐group variance). We performed the LDA on the 50 accessions with leave‐one‐out cross‐validation and then bootstrapping over 100 runs, using the MASS package in R (Venables & Ripley 2002). Prior probabilities were based on the relative sample size of the categorical variable (i.e. 0.34, 0.12 and 0.54 for C3, C3‐C4 and C4 groups respectively). We chose predictor variables that were likely to influence the M : BS ratio, including the number of M cells between major veins, average size of individual M cells, number of minor veins per segment, leaf thickness and the average size of BS cells on tertiary veins.
To test the generality of our findings from A. semialata, we carried out an equivalent LDA for a larger sample of 157 grasses including one C3 and one C4 A. semialata and representing 17 independent C4 lineages. Predictor variables in this analysis were based on the anatomical measurements of Christin et al. (2013) and chosen to best match the variables used in the A. semialata LDA described above, including the number of mesophyll cells between veins, mesophyll cell width, proportion of veins that are minor, leaf thickness, inner BS cell width and outer BS cell width. The species were grouped as C3, C4 species using the inner BS and C4 species using the outer BS.
Vein order analysis
To determine whether the pattern of vein density observed in the main data set was maintained across a larger sample of A. semialata, we counted the total number of veins per segment and the presence or absence of minor veins in 91 additional accessions consisting of herbarium specimens that had been rehydrated in distilled water overnight at 4°C prior to embedding, sectioning, staining and imaging as described above. Together with the 50 previous samples, this larger data set included a total of 72 C4 (i.e. δ13C > −17‰) and 69 non‐C4 (i.e., δ13C < −17‰) accessions distributed across the species’ geographic range.
Ultrastructure and immunohistochemistry
To investigate whether ultrastructural changes might differ between photosynthetic types within this species, we analysed the spatial distributions of organelles and enzymes in one population representing each of the C3, C3‐C4 and C4 types. Recently expanded mature leaf tissue was prepared for transmission electron microscopy and processed for immunodetection of the large subunit of Rubisco (RBCL) and glycine decarboxylase H subunit (GLDH) as previously described (see Supporting Information Materials 1; Khoshravesh et al. 2017).
Leaf anatomy in a common environment
To determine the degree to which the various leaf anatomical phenotypes arose from plastic development responses to their differing native growth environments, we compared field phenotypes to those obtained from live tillers of 17 A. semialata accessions (5 C3, 4 C3‐C4 and 8 C4) after growing for a minimum of 3 months in a common growth chamber, with conditions as described in Lundgren et al. 2016. The environmental conditions (Fick & Hijmans 2017) at the field collection sites are detailed in Table S2. On controlled environment samples, the number and order of veins, minimum number of M cells separating veins, area of inner BS cells, segment length and thickness, and IVD were determined on one segment per leaf.
Plasticity for leaf anatomy in response to low CO2
To further test whether C4‐compatible phenotypes could emerge from plastic responses to the environment, as previously suggested (Li et al. 2014), we carried out a CO2 manipulation experiment designed to promote photorespiration. One C4 (MDG, South Africa) and one C3 (GMT, South Africa) plant were initially grown from seed in a controlled environment chamber set as described in Lundgren et al. 2016, but with 400 μmol mol−1 CO2 concentration. Both plants were split into five replicate cuttings and kept in the same growth chamber conditions to re‐establish for four months. From each replicated clone, one fully expanded, mature leaf was sampled and fixed in 4 : 1 ethanol : acetic acid solution. The growth chamber was then set to 180 μmol mol−1 CO2 concentration for the next four months to promote photorespiration, while maintaining the other environmental conditions, and one new fully expanded leaf was again sampled and fixed. All leaf samples from the 400 (i.e. ambient) and 180 (i.e. low) CO2 treatments were embedded, sectioned and imaged as described above. To determine whether the plants used different photosynthetic pathways in the two CO2 growth environments, we determined CCP and carboxylation efficiency, as described in Lundgren et al. (2016).
Results
Alloteropsis semialata presents a continuum of leaf anatomy
The ratio of M to BS tissue, a trait known to differ among C3 and C4 species (Hattersley 1984), forms a continuum within Alloteropsis semialata, along which photosynthetic types are sorted (Fig. 2). Indeed, the smallest values are restricted to C4 accessions and the largest are found in C3 individuals. When considering the area in cross section between two secondary veins (i.e., a leaf segment from here onwards; Fig. S1), the M area is over 10 times larger than the BS area in C3 accessions, but less than five times larger in C4 accessions (Data set S1). As expected, C3‐C4 accessions are intermediate in their overall leaf anatomy, with 5–10 times more M than BS. These M : BS ranges are consistent with those measured in other C3 and C4 grasses (Christin et al. 2013).
Figure 2.
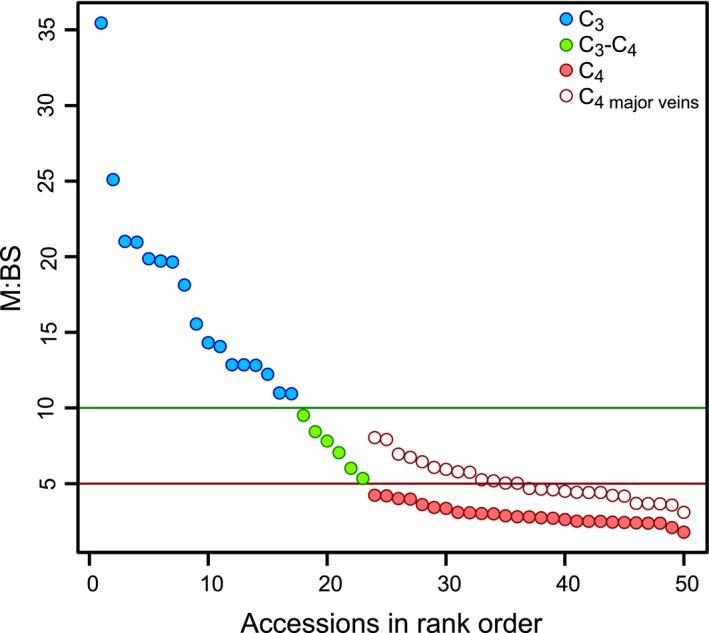
Continuous variation in Alloteropsis semialata leaf anatomy, but distinct division among C3, C3‐C4 and C4 types. Ratios of mesophyll (M) to bundle sheath (BS) area of individual accessions of C3 (blue circles), C3‐C4 (green circles) and C4 (solid red circles) plants, ranked by M:BS value. n = 50. Lines delineating M:BS ratios that distinguish C3 from C3‐C4 (green) and C3‐C4 from C4 (red) are shown. For C4 individuals, M:BS ratios are also calculated in the absence of minor veins (open red circles).
C3, C3‐C4 and C4 Alloteropsis semialata have distinct leaf anatomy
Variation in M : BS ratios can arise via changes to several underlying traits (Lundgren et al. 2014). Our modelling shows that M area is the product of leaf thickness and interveinal distance (IVD; Table 1). The latter is predicted by the number and size of M cells between veins (Table 1). BS area is explained by the number of BS units (i.e. the number of veins per segment) and the size of BS cells (Table 1). When these potential explanatory traits are incorporated within an LDA, all variance between the three photosynthetic types is captured (Fig. 3a). In a bootstrapped sample, the mean overall predictive accuracy is 0.986, which is statistically indistinguishable from 1.0 (95% CI = 0.966–1.000). The mean predictive accuracy for C4 (0.999, 95% CI = 0.995–1.000), C3 (0.976, 95% CI = 0.918–1.000) and C3‐C4 (0.926, 95% CI = 0.805–1.000) accessions is also statistically indistinguishable from 1.0. The analysis, therefore, confirms that leaf anatomy varies among photosynthetic types in a statistically predictable manner.
Table 1.
Results of linear regression analyses on leaf components underlying M : BS ratios in Alloteropsis semialata
| F | df | Adj R 2 | P | t | P | |
|---|---|---|---|---|---|---|
| Total M area/segment (μm2) | 59.35 | 2, 47 | 0.704 | 1.38 × 10−13 | ||
| Leaf thickness (μm) | 3.98 | 0.00024 | ||||
| Interveinal distance* (μm) | 10.58 | 5.07 × 10−14 | ||||
| Interveinal distance (μm) | 343.4 | 2, 47 | 0.933 | < 2.2 × 10−16 | ||
| Number M cells between veins† | 25.48 | < 2 × 10−16 | ||||
| M cell size (μm2) | 6.97 | 9.22 × 10−9 | ||||
| Total BS area/segment (μm2) | 124.8 | 2, 47 | 0.835 | < 2.2 × 10−16 | ||
| BS cell size‡ (μm2) | 9.52 | 1.54 × 10−12 | ||||
| Vein density (veins/segment) | 4.23 | 0.00011 | ||||
*Average distance between the center points of all veins.
†Number of mesophyll (M) cells between all veins.
‡Cross‐sectional area of inner bundle sheath (BS) cells on tertiary order veins.
Figure 3.
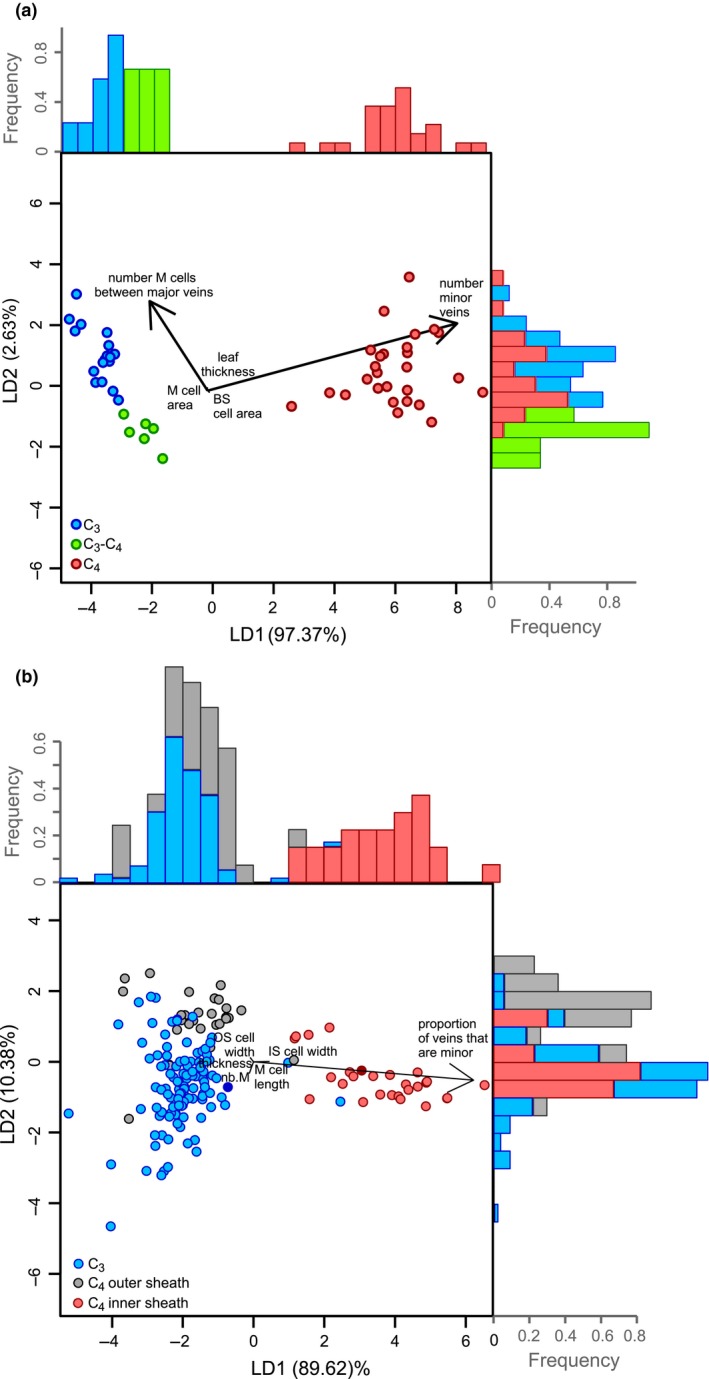
Linear discriminant analysis of leaf anatomical traits. The first (LD1) and second (LD2) dimensions of the LDA are plotted against each other with histograms of each dimension shown on the opposing axis for (a) the LDA on C3, C3‐C4 and C4 Alloteropsis semialata accessions and (b) the LDA on 157 C3, C4 inner sheath and C4 outer sheath grass species. In addition, one C3 and one C4 A. semialata accession were included in this larger LDA and are denoted by solid blue and red circles respectively. Loading plots are overlaid via black arrows. M, mesophyll; IS, inner sheath; OS, outer sheath; nb.M, number of mesophyll cells between veins.
The first axis of the LDA explains 97.37% of the variance between photosynthetic types and clearly distinguishes C4 from non‐C4 accessions (Fig. 3a). This axis is most strongly associated with the number of minor veins per segment, which were absent from all non‐C4 accessions in this analysis (Table 2). The second axis explains 2.63% of the variance between groups, clearly distinguishes C3 from C3‐C4 plants, and is most strongly associated with the number of M cells between major veins (i.e. secondary and tertiary order veins) and the number of minor veins (Fig. 3a; Table 2). Since minor veins are restricted to C4 individuals, their contribution to LD2 is linked to diversity within the C4 group. These results indicate that most of the variance in the data set stems from the contrast between C4 and non‐C4 individuals and is driven entirely by a single underlying trait, the presence of minor veins. The phenotypic distance between C3 and C3‐C4 individuals is very small, being explained by the number of M cells between major veins. Conversely, leaf thickness and the cross‐sectional areas of individual BS and M cells poorly distinguish photosynthetic types in this species.
Table 2.
Coefficients of linear discriminants in a linear discriminant analysis on (top) five leaf anatomical traits expected to drive overall mesophyll to bundle sheath area ratios in Alloteropsis semialata and on (bottom) six leaf anatomical traits in 157 grass species, grouped as C3 species, C4 species using the inner sheath and C4 species using the outer sheath
| LDA on Alloteropsis semialata accessions | ||
|---|---|---|
| Trait | LD1 | LD2 |
| Number of minor veins per segment | 1.3591 | 0.3663 |
| Number of mesophyll cells between major veins | −0.315 | 0.4881 |
| Average area inner bundle sheath cell on tertiary veins (μm2) | 0.0123 | −0.0104 |
| Average area mesophyll cell (μm2) | −0.0012 | −7.82E‐05 |
| Leaf thickness (μm) | −5.52E‐04 | 0.0189 |
| LDA on 157 grass species + 1 C3 and 1 C4 Alloteropsis semialata accession | ||
|---|---|---|
| Trait | LD1 | LD2 |
| Proportion of veins that are minor | 4.145779 | −0.34605 |
| Outer bundle sheath (BS) cell width (μm) | −0.06433 | 0.080823 |
| Inner BS cell width (μm) | 0.301257 | 0.004749 |
| Leaf thickness (μm) | −0.0083 | −0.00729 |
| Mesophyll cell width (μm) | 0.01989 | −0.01048 |
| Number of mesophyll cells between veins | −0.08007 | −0.22237 |
The first two axes of an LDA of anatomical traits on the larger species data set explain 89.62% and 10.38% of the variation respectively. The first axis clearly distinguishes C4 species that use the inner bundle sheath from C4 species using the outer sheath and the C3 species (Fig. 3b) and is mostly associated with the proportion of minor veins, while the remaining anatomical traits are weakly correlated with both axes (Table 2).
Differences between C4 and non‐C4 phenotypes arise from the development of minor veins
In A. semialata, the presence of minor veins is the only variable consistently distinguishing C4 and non‐C4 accessions. When the M : BS ratio is calculated in the absence of minor veins, the clear distinction between C3‐C4 and C4 accessions disappears, with nearly half the C4 accessions overlapping with C3‐C4 plants (Fig. 2). This shows that the development of minor veins in C4 accessions reduces the M : BS ratio by increasing BS area and displacing M area. To confirm the restriction of minor veins to C4 individuals, we screened vein architecture in a larger data set of A. semialata (Fig. 4a,b; Data set S2). Minor veins were present in all C4 accessions and absent in all but five non‐C4 accessions. Four of these had only occasional and irregularly spaced minor veins, while the final accession is an individual originating from a natural cross between C3‐C4 and C4 individuals (Olofsson et al. 2016). Our data therefore show that the presence of frequent and regularly spaced minor veins is universally and uniquely associated with the C4 genomic background, captures nearly all of the anatomical variation between C4 and non‐C4 phenotypes and explains overall differences in relative M and BS areas.
Figure 4.
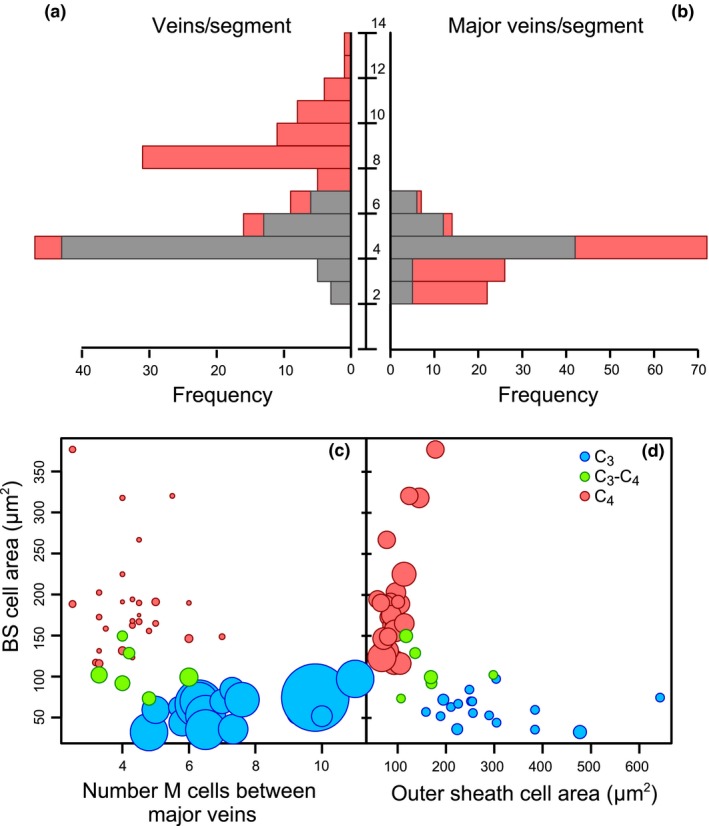
Diversity of intraspecific anatomical components. Histograms of (a) vein density (i.e., the total number of veins per segment) and (b) the number of major veins per segment in C4 (red; n = 72) and non‐C4 (grey, n = 69) accessions. Scatter plots show (c) the average number of mesophyll (M) cells between major veins vs. the average area of individual bundle sheath (BS) cells, with dot size proportional to the M:BS ratio, and (d) BS cell area vs. outer sheath cell area, with dot size proportional to the number of veins per segment. Colours indicate photosynthetic type with C3 (blue; n = 17), C3‐C4 (green; n = 6) and C4 (red; n = 27).
The proliferation of minor veins explains a number of patterns associated with C4 anatomy. As expected, the number of M cells between consecutive veins differs among photosynthetic types, being the smallest in C4 accessions (1–3), compared to C3‐C4 (3–6) and C3 (5–11) plants (Fig. S2). However, the number of M cells between major veins overlaps between the C4 and non‐C4 groups (Fig. 4c), which indicates that the reduced distance between any pair of M and BS cells in C4 accessions is caused by the differentiation of ground meristem cells into minor veins rather than a reduced proliferation of M cells. The high vein density of C4 plants following the development of minor veins is accompanied by more than a twofold increase in extraxylary fibres (i.e. tissue area per segment length) than is found in non‐C4 accessions (Fig. S3). As the area of extraxylary fibres per vein does not differ between the photosynthetic types (Fig. S3), the increased fibre area in C4 plants derives entirely from their greater vein density.
Other anatomical changes happened before or after the transition from C3‐C4 to C4 physiology
The development of minor veins explains the overall anatomical difference between C4 and non‐C4 accessions and is therefore linked to the emergence of a fully functioning C4 physiology from a C3‐C4 intermediate state. Evolutionary changes that happened once this C4 physiology was in place would be restricted to some, but not all, C4 individuals. In our data set, such changes include further reductions to the M : BS ratio, potentially achieved via contractions to M airspace (Byott 1976) or increases in BS cell size. Indeed, although BS cell sizes of different photosynthetic types overlap, large increases to BS cell size characterise some African C4 accessions (Figs S4 and S5). The BS cell enlargement was therefore involved in the adaptation of C4 physiology after it had emerged, possibly to accommodate more or larger organelles for a more efficient C4 cycle, rather than being involved in its origin. Occasional hybridisation between C4 and non‐C4 individuals could affect the distribution of trait values; however, non‐C4 A. semialata individuals are restricted to Africa, so that hybridisation outside of Africa is unlikely. Yet, Asia and Australian accessions exhibit some of the smallest BS cells among C4 accessions (Fig. S5).
Some characters observed in C4 accessions are also present in C3‐C4 individuals, but not C3 ones, indicating that they are not associated with the transition to fully functional C4 physiology, but might have facilitated it. These include a small increase in BS cell sizes in C3‐C4 compared with C3 plants and a decrease in outer sheath cell size, with C3‐C4 accessions bridging the anatomical gap between C3 and C4 outer sheath cell sizes (Fig. 4c,d). This reduced outer sheath in C3‐C4 and C4 A. semialata likely facilitates metabolite exchanges between M and BS cells.
Differences between C4 and non‐C4 leaves are not environmentally induced
Alloteropsis semialata plants grow naturally in diverse environments, depending on their photosynthetic background and evolutionary history (Lundgren et al. 2015). To verify that the differences we observe among photosynthetic types are not induced by environmental variations, we compared the leaves of field‐collected plants after transplanting and growing them in a common controlled environment growth chamber for at least 3 months (Data set S3; Fig. S6). Compared to field conditions, C3 accessions produced more M cells between veins (P = 0.044) in the common environment. Moreover, C3‐C4 plants produced thicker leaves (P = 0.040), such that leaf thickness of the three photosynthetic types converged in the common environment, which is likely a result of the non‐limiting light, nutrients and water available in these conditions. However, the other traits were not influenced by growth conditions and leaf anatomy of the three photosynthetic types remained distinct when grown in the common environment.
We further verified that historical changes in atmospheric composition did not influence the leaf phenotype by comparing C3 and C4 A. semialata under current ambient (400 ppm) and the Pleistocene minimum (180 ppm) CO2 concentrations. Plants grown under the low CO2 concentration experience elevated photorespiration rates, which might have induced a more C4‐like anatomy. However, we found that plants did not shift photosynthetic state under the differing CO2 conditions (i.e. mean CCPs in ambient/low CO2 for C3 = 49.8/53.1 and C4 = 4.6/8.1 μmol mol−¹; Data set S4). Both C3 and C4 plants produced thinner leaves in the low CO2 environment (P = 0.0049 C3/0.0065 C4), and C4 plants developed smaller BS cells (P = 0.011; Fig. S6), probably because the lower carbon supply restricted development (Ripley et al. 2013). Importantly, the C3 plants did not produce more veins (or any minor veins), larger BS cells or fewer M cells between veins when grown under this high photorespiration condition. These results show that, even when photorespiration is high, a C4‐like phenotype is not plastically induced in C3 A. semialata.
Discussion
Photosynthetic types form a continuum, along which multiple biochemical, anatomical and ultrastructural alterations increase the proportion of CO2 fixed via the C4 cycle. The emerging model of C4 evolution involves gradual and overlapping phenotypic changes (Heckmann et al. 2013; Sage et al. 2014; Bräutigam & Gowik 2016; Schlüter & Weber 2016; Dunning et al. 2017), with traits acquired in differing orders among C4 lineages (Williams et al. 2013). Different traits may be involved in the initial transition to a C4 phenotype and the subsequent adaptation and diversification of that phenotype (Christin & Osborne 2014; Watcharamongkol et al. 2018). Within the grass Alloteropsis semialata, we have shown that the only gross leaf property distinguishing all C4 from all non‐C4 phenotypes is the development of frequent minor veins. The presence of these minor veins has multiple consequences, including an overall increase in vein density, enlargement of the total volumes of BS tissue and a displacement of M tissue. These anatomical changes combine to facilitate C4 cycle activity, as demonstrated by a strong correlation between leaf vein frequency and carbon isotope composition observed for this species (Lundgren et al. 2016). Our analyses of leaf ultrastructure indicate that the evolution of C4 photosynthesis in A. semialata may have involved additional changes in organelle distribution among cell types (Supporting Information Materials 1; Figs S7–S9), although the small sample of populations prevents us from differentiating ultrastructural changes linked to the transition to C4 from those that happened later.
The change in venation inferred during the evolution of C4 photosynthesis in A. semialata may have a number of physiological and ecological consequences. First, the increase in vein frequency is accompanied by an enhancement of unpigmented extraxylary fibres, which improves light transmission to the BS, and thus ATP production in these cells, facilitating photosynthetic carbon reduction (Bellasio & Lundgren 2016). Enhanced fibre density may also increase leaf toughness, reduce digestibility and consequently deter herbivores (Caswell et al. 1973; Wilson et al. 1983). Secondly, the insertion of additional veins may influence leaf hydraulics. Model simulations for other plant species demonstrate that an increase in minor vein density can lead to greater leaf hydraulic conductance (McKown et al. 2010). However, empirical studies show that this is unlikely to improve drought tolerance, since the decline in hydraulic conductance during drought arises primarily outside veins (Scoffoni & Sack 2017; Scoffoni et al. 2017a), while embolisms arise first in the midrib, not minor veins (Scoffoni et al. 2017b).
Our results complement those from previous comparisons among species, which show that an additional order of minor veins develops during the evolutionary transition from non‐C4 to C4 forms of Flaveria (McKown & Dengler 2009), while BS cell size is large in both C3 and C4 Flaveria species (Kümpers et al. 2017). Our further analysis of leaf gross anatomy across multiple grass species shows that the insertion of additional minor veins is a frequent developmental mechanism for decreasing the M : BS ratio in those C4 grasses that primarily localise Rubisco within the mestome sheath. The insertion of minor veins could occur via relatively few developmental changes, likely underpinned by changes to auxin, brassinosteroids, SHORTROOT/SCARECROW and/or INDETERMINATE DOMAIN transcription factors (Kumar & Kellogg 2018; Sedelnikova et al. 2018). In grasses, vein orders develop sequentially as leaves grow wider, such that minor veins are initiated considerably later than other vein orders, usually once the leaf ceases to widen (Nelson & Langdale 1989; Sedelnikova et al. 2018). Thus, the development of functional minor veins likely arises via the heterochronic regulation of the existing machinery for vein formation, sustaining vein differentiation beyond that of non‐C4 plants (Nelson 2011; Sedelnikova et al. 2018), probably through the prolonged production of auxin during later phases of leaf elongation (Scarpella et al. 2010). Alternatively, minor veins may also result from a heterotopic specialisation of auxin maxima that permits them to form closer together (Kumar & Kellogg 2018).
The possibility that a transition from non‐C4 to C4 states can be caused by a single developmental alteration is a plausible explanation for the recurrent origins of C4 leaf anatomy and helps to resolve the paradox of how this complex trait emerged so many times. We also show that organelle number and size differ among photosynthetic types of A. semialata, but, here too, recent work indicates that one gene can control multiple ultrastructural modifications (Wang et al. 2017). Finally, transcriptome comparisons show that few genes encoding enzymes are upregulated during the transition from non‐C4 to C4 in A. semialata (Dunning et al. 2017). We therefore conclude that the overall transition from a non‐C4 state to the form of C4 photosynthesis observed in A. semialata involved relatively few genetic mutations.
The limited number of changes involved in the emergence of C4 anatomy in A. semialata is partially explained by the presence of relatively enlarged BS in the C3‐C4 accessions, while C3 A. semialata BS size is similar to C3 species from other grass lineages (Lundgren et al. 2014, 2016; Dunning et al. 2017). C3‐C4 A. semialata are also characterised by fewer M cells compared to C3 accessions and higher BS organelle abundance (Figs 3a, 4c and S7–S9). These properties that had been selected for the C3‐C4 physiology eased the subsequent transition to a full C4 state, but it is important to note that the physiology and anatomy of C3‐C4 A. semialata are typical for C3‐C4 plants in general (Lundgren et al. 2016), and their anatomical characteristics can be found among C3 grasses (Hattersley 1984; Christin et al. 2013; Lundgren et al. 2014). The background against which C4 anatomy evolved in A. semialata is therefore not exceptional.
Our conclusion that C4 leaf anatomy can arise from one key developmental modification is apparently incompatible with the great anatomical specialisation of other C4 lineages as well as the large phenotypic gaps separating them from their closest C3 relatives (Dengler et al. 1994; Christin et al. 2013). However, most C3 and C4 sister lineages are separated by long periods of evolution, and comparing these groups therefore captures all of the changes that happened after the origin of C4 photosynthesis to improve the efficiency of C4 physiology and adapt it to various organismal and ecological contexts (Christin & Osborne 2014). Indeed, photosynthetic efficiency may be significantly lower in C4 A. semialata than in species from some older C4 lineages (Lundgren et al. 2016; Bräutigam et al. 2018). This suggests that C4 photosynthesis in A. semialata may represent a rudimentary version of the physiological trait (Ueno & Sentoku 2006). The biochemical characteristics of the C4 cycle in A. semialata may be one reason for this (Bräutigam et al. 2018), and the presence of Rubisco protein in M could be another (Ueno & Sentoku 2006). Anatomical diversity may also explain some of the variation in physiological efficiency among A. semialata populations (Lundgren et al. 2016). Indeed, in A. semialata, enlargements of the BS cells beyond those seen in non‐C4 individuals are restricted to a subset of C4 populations (Fig. 2) and thus happened after the emergence of C4 physiology. Over time, accumulated modifications will move C4 leaf anatomy far beyond that realised via a single developmental change. However, the fact that an initial C4 phenotype and the associated physiology can be accessed via a single modification likely placed multiple groups on a selective highway to highly specialised and successful variants of the C4 syndrome.
Authorship
MRL, PAC and CPO designed the study. MRL produced and analysed the data, with the help of LTD, JJMV and JWB. TS and RK performed the immunolocalisations and TEM imaging. SS assisted with immunolocalisation sample preparation. MS assisted with tissue fixation. MRL, LTD, JKO, BR, MSV, GB, CA, NC, AM, MB, CML, PAC and CPO contributed plant material. MRL, PAC and CPO interpreted the results and wrote the paper, with the help of all the authors.
Supporting information
Acknowledgments
This work was funded by a University of Sheffield Prize Scholarship to MRL, an ERC grant (grant number ERC‐2014‐STG‐638333) and a Royal Society Research Grant (grant number RG130448). LTD and JKO are supported by a NERC grant (grant number NE/M00208X/1) and PAC is supported by a Royal Society University Research Fellowship (grant number URF120119). JWB was supported by 301 and Think Ahead Sheffield Undergraduate Research Experience grants to MRL. The work on ultrastructure was supported by a Natural Sciences and Engineering Research Council of Canada grant (no 2015‐04878) to TLS. The authors thank Peter Westhoff, Stefanie Schulze and Udo Gowik for use of their GLDH antibody, Susanne von Caemmerer for advice about outer bundle sheath cell resistance, Paul Hattersley for leaf samples and 13C isotope data, John Thompson for field assistance and sample collection, Emma Jardine for discussion of linear discriminant analysis, Heather Walker for mass spectrometry assistance, Gareth Fraser for the use of his vibratome and Emanuela Samaritani for histology assistance. Herbarium leaf samples were obtained from the Herbarium at the Royal Botanic Garden Kew, the National Herbarium of South Africa in Pretoria, National Museums of Kenya in Nairobi and the National Botanic Garden of Belgium, Brussels, with the assistance of Martin Xanthos, Lyn Fish, Caroline Mashau and Itambo Malombe.
Data Accessibility Statement
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.q7r61k7
References
- Bellasio, C. & Lundgren, M.R. (2016). Anatomical constraints to C4 evolution: light harvesting capacity in the bundle sheath. New Phytol., 212, 485–496. [DOI] [PubMed] [Google Scholar]
- Bellasio, C. , Beerling, D.J. & Griffiths, H. (2016a). An Excel tool for deriving key photosynthetic parameters from combined gas exchange and chlorophyll fluorescence: theory and practice. Plant, Cell Environ., 39, 1180–1197. [DOI] [PubMed] [Google Scholar]
- Bellasio, C. , Beerling, D.J. & Griffiths, H. (2016b). Deriving C4 photosynthetic parameters from combined gas exchange and chlorophyll fluorescence using an Excel tool: theory and practice. Plant, Cell Environ., 39, 1164–1179. [DOI] [PubMed] [Google Scholar]
- Bräutigam, A. & Gowik, U. (2016). Photorespiration connects C3 and C4 photosynthesis. J. Exp. Bot., 67, 2953–2962. [DOI] [PubMed] [Google Scholar]
- Bräutigam, A. , Schluter, U. , Lundgren, M.R. , Flachbart, S. , Ebenhoh, O. , Schonknecht, G. et al (2018). Biochemical mechanisms driving rapid fluxes in C4 photosynthesis. bioRxiv, 387431. [Google Scholar]
- Brown, W.V. (1975). Variations in anatomy, associations, and origins of Kranz tissue. Am. J. Bot., 62, 395–402. [Google Scholar]
- Byott, G.S. (1976). Leaf air space systems in C3 and C4 species. New Phytol., 76, 295–299. [Google Scholar]
- von Caemmerer, S. , Ghannoum, O. , Pengelly, J.J. & Cousins, A.B. (2014). Carbon isotope discrimination as a tool to explore C4 photosynthesis. J. Exp. Bot., 65, 3459–3470. [DOI] [PubMed] [Google Scholar]
- Caswell, H. , Reed, F. , Stephenson, S.N. & Werner, P.A. (1973). Photosynthetic pathways and selective herbivory: a hypothesis. Am. Nat., 107, 465–480. [Google Scholar]
- Chollet, R. & Ogren, W.L. (1975). Regulation of photorespiration in C3 and C4 species. Bot. Rev., 41, 137–179. [Google Scholar]
- Christin, P.A. & Osborne, C.P. (2014). The evolutionary ecology of C4 plants. New Phytol., 204, 765–781. [DOI] [PubMed] [Google Scholar]
- Christin, P.A. , Osborne, C.P. , Chatelet, D.S. , Columbus, J.T. , Besnard, G. , Hodkinson, T.R. et al (2013). Anatomical enablers and the evolution of C4 photosynthesis in grasses. Proc. Natl Acad. Sci., 110, 1381–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dengler, N.G. , Dengler, R.E. , Donnelly, P.M. & Hattersley, P.W. (1994). Quantitative leaf anatomy of C3 and C4 grasses (Poaceae): bundle sheath and mesophyll surface area relationships. Ann. Bot., 73, 241–255. [Google Scholar]
- Downton, W.J.S. & Tregunna, E.B. (1968). Carbon dioxide compensation‐its relation to photosynthetic carboxylation reactions, systematics of the Gramineae, and leaf anatomy. Can. J. Bot., 46, 207–215. [Google Scholar]
- Dunning, L.T. , Lundgren, M.R. , Moreno‐Villena, J.J. , Namaganda, M. , Edwards, E.J. , Nosil, P. et al (2017). Introgression and repeated co‐option facilitated the recurrent emergence of C4 photosynthesis among close relatives. Evolution, 71, 1541–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehleringer, J.R. , Sage, R.F. , Flanagan, L.B. & Pearcy, R.W. (1991). Climate change and the evolution of C4 photosynthesis. Trends Ecol. Evol., 6, 95–99. [DOI] [PubMed] [Google Scholar]
- Ellis, R.P. (1974). The significance of the occurrence of both Kranz and non‐Kranz leaf anatomy in the grass species Alloteropsis semialata . S. Afr. J. Sci., 70, 169–173. [Google Scholar]
- Fick, S.E. & Hijmans, R.J. (2017). Worldclim 2: new 1‐km spatial resolution climate surfaces for global land areas. Int. J. Climatol., 37, 4302–4315. [Google Scholar]
- Frean, M.L. , Ariovich, D. & Cresswell, C.F. (1983). C3 and C4 Photosynthetic and anatomical forms of Alloteropsis semialata (R. Br.) Hitchcock: 2. A comparative investigation of leaf ultrastructure and distribution of chlorenchyma in the two forms. Ann. Bot., 51, 811–821. [Google Scholar]
- Freitag, H. & Kadereit, G. (2014). C3 and C4 leaf anatomy types in Camphorosmeae (Camphorosmoideae, Chenopodiaceae). Plant Syst. Evol., 300, 665–687. [Google Scholar]
- Hatch, M.D. (1976). Photosynthesis: the path of carbon In: Plant Biochemistry (eds Bonner J., Varner J.). Academic Press, New York, USA, pp. 797–844. [Google Scholar]
- Hatch, M.D. (1987). C4 photosynthesis: a unique blend of modified biochemistry, anatomy and ultrastructure. Biochim. Biophys. Acta, 895, 81–106. [Google Scholar]
- Hattersley, P.W. (1984). Characterization of C4 type leaf anatomy in grasses (Poaceae). M:BS area ratios. Ann. Bot., 53, 163–180. [Google Scholar]
- Hattersley, P.W. & Watson, L. (1975). Anatomical parameters for predicting photosynthetic pathways of grass leaves: the ‘maximum lateral cell count’ and the ‘maximum cells distant count’. Phytomorphology, 25, 325–333. [Google Scholar]
- Hattersley, P.W. , Watson, L. & Osmond, C.B. (1977). In situ immunofluorescent labelling of ribulose‐1, 5‐bisphosphate carboxylase in leaves of C3 and C4 plants. Aust. J. Plant Physiol., 4, 523–539. [Google Scholar]
- Heckmann, D. , Schulze, S. , Denton, A. , Gowik, U. , Westhoff, P. , Weber, A.P.M. et al (2013). Predicting C4 photosynthesis evolution: modular, individually adaptive steps on a Mount Fuji fitness landscape. Cell, 153, 1579–1588. [DOI] [PubMed] [Google Scholar]
- Khoshravesh, R. , Lundsgaard‐Nielsen, V. , Sultmanis, S. & Sage, T.L. (2017). Light Microscopy, Transmission Electron Microscopy, and Immunohistochemistry Protocols for Studying Photorespiration. Photorespiration. Humana Press, New York, USA, pp. 243–270. [DOI] [PubMed] [Google Scholar]
- Kumar, D. & Kellogg, E.A. (2018). Getting closer: vein density in C4 leaves. New Phytol. 10.1111/nph.15491. [DOI] [PubMed] [Google Scholar]
- Kümpers, M.C. , Burgess, S.J. , Reyna‐Llorens, I. , Smith‐Unna, R. , Boursnell, R. & Hibberd, J.M. (2017). Shared characteristics underpinning C4 leaf maturation derived from analysis of multiple C3 and C4 species of Flaveria . J. Exp. Bot., 68, 177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , Xu, J. , Haq, N.U. , Zhang, H. & Zhu, X.G. (2014). Was low CO2 a driving force of C4 evolution: Arabidopsis responses to long‐term low CO2 stress. J. Exp. Bot., 65, 3657–3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren, M.R. , Osborne, C.P. & Christin, P.A. (2014). Deconstructing Kranz anatomy to understand C4 evolution. J. Exp. Bot., 65, 3357–3369. [DOI] [PubMed] [Google Scholar]
- Lundgren, M.R. , Besnard, G. , Ripley, B.S. , Lehmann, C.E.R. , Chatelet, D.S. , Kynast, R.G. et al (2015). Photosynthetic innovation broadens the niche within a single species. Ecol. Lett., 18, 1021–1029. [DOI] [PubMed] [Google Scholar]
- Lundgren, M.R. , Christin, P.A. , Gonzalez Escobar, E. , Ripley, B.S. , Besnard, G. , Long, C.M. et al (2016). Evolutionary implications of C3‐C4 intermediates in the grass Alloteropsis semialata . Plant, Cell Environ., 39, 1871–1873. [DOI] [PubMed] [Google Scholar]
- McKown, A.D. & Dengler, N.G. (2007). Key innovations in the evolution of Kranz anatomy and C4 vein pattern in Flaveria (Asteraceae). Am. J. Bot., 94, 382–399. [DOI] [PubMed] [Google Scholar]
- McKown, A.D. & Dengler, N.G. (2009). Shifts in leaf vein density through accelerated vein formation in C4 Flaveria (Asteraceae). Ann. Bot., 104, 1085–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKown, A.D. , Cochard, H. & Sack, L. (2010). Decoding leaf hydraulics with a spatially explicit model: principles of venation architecture and implications for its evolution. Am. Nat., 175, 447–460. [DOI] [PubMed] [Google Scholar]
- Nelson, T. (2011). The grass leaf developmental gradient as a platform for a systems understanding of the anatomical specialization of C4 leaves. J. Exp. Bot., 62, 3039–3048. [DOI] [PubMed] [Google Scholar]
- Nelson, T. & Langdale, J.A. (1989). Patterns of leaf development in C4 plants. Plant Cell, 1, 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson, J.K. , Bianconi, M. , Besnard, G. , Dunning, L.T. , Lundgren, M.R. , Holota, H. et al (2016). Genome biogeography reveals the intraspecific spread of adaptive mutations for a complex trait. Mol. Ecol., 25, 6107–6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renvoize, S.A. (1987). A survey of leaf‐blade anatomy in grasses XI. Paniceae. Kew. Bull., 42, 739–768. [Google Scholar]
- Ripley, B.S. , Cunniff, J. & Osborne, C.P. (2013). Photosynthetic acclimation and resource use by the C3 and C4 subspecies of Alloteropsis semialata in low CO2 atmospheres. Glob. Change Biol., 19, 900–910. [DOI] [PubMed] [Google Scholar]
- Sage, R.F. , Christin, P.A. & Edwards, E.J. (2011). The C4 plant lineages of planet Earth. J. Exp. Bot., 62, 3155–3169. [DOI] [PubMed] [Google Scholar]
- Sage, R.F. , Khoshravesh, R. & Sage, T.L. (2014). From proto‐Kranz to C4 Kranz: building the bridge to C4 photosynthesis. J. Exp. Bot., 65, 3341–3356. [DOI] [PubMed] [Google Scholar]
- Scarpella, E. , Barkoulas, M. & Tsiantis, M. (2010). Control of leaf and vein development by auxin. CSH Perspect. Biol., 2, a001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlüter, U. & Weber, A.P. (2016). The road to C4 photosynthesis: evolution of a complex trait via intermediary states. Plant Cell Physiol., 57, 881–889. [DOI] [PubMed] [Google Scholar]
- Schneider, C.A. , Rasband, W.S. & Eliceiri, K.W. (2012). NIH Image to ImageJ: 25 years of image analysis. Nat. Methods, 9, 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoffoni, C. & Sack, L. (2017). The causes and consequences of leaf hydraulic decline with dehydration. J. Exp. Bot., 68, 4479–4496. [DOI] [PubMed] [Google Scholar]
- Scoffoni, C. , Albuquerque, C. , Broderson, C.R. , Townes, S.V. , John, G.P. , Bartlett, M.K. et al (2017a). Outside‐xylem vulnerability, not xylem embolism, controls leaf hydraulic decline during dehydration. Plant Physiol., 173, 1197–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoffoni, C. , Albuquerque, C. , Broderson, C.R. , Townes, S.V. , John, G.P. , Cochard, H. et al (2017b). Leaf vein xylem conduit diameter influences susceptibility to embolism and hydraulic decline. New Phytol., 213, 1076–1092. [DOI] [PubMed] [Google Scholar]
- Sedelnikova, O.V. , Hughes, T.E. & Langdale, J.A. (2018). Understanding the genetic basis of C4 Kranz anatomy with a view to engineering C3 crops. Annu. Rev. Genet., 52, 10.1146/annurev-genet-120417-031217. [DOI] [PubMed] [Google Scholar]
- Soros, C.L. & Dengler, N.G. (2001). Ontogenetic derivation and cell differentiation in photosynthetic tissues of C3 and C4 Cyperaceae. Am. J. Bot., 88, 992–1005. [PubMed] [Google Scholar]
- Ueno, O. & Sentoku, N. (2006). Comparison of leaf structure and photosynthetic characteristics of C3 and C4 Alloteropsis semialata subspecies. Plant, Cell Environ., 29, 257–268. [DOI] [PubMed] [Google Scholar]
- Venables, W.N. & Ripley, B.D. (2002). Modern Applied Statistics with S, 4th edn. Springer, New York, USA. [Google Scholar]
- Wang, P. , Khoshravesh, R. , Karki, S. , Tapia, R. , Balahadia, C.P. , Bandyopadhyay, A. et al (2017). Re‐creation of a key step in the evolutionary switch from C3 to C4 leaf anatomy. Curr. Biol., 27, 3278–3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watcharamongkol, T. , Christin, P.A. & Osborne, C.P. (2018). C4 photosynthesis evolved in warm climates but promoted migration to cooler ones. Ecol. Lett., 21, 376–383. [DOI] [PubMed] [Google Scholar]
- Williams, B.P. , Johnston, I.G. , Covshoff, S. & Hibberd, J.M. (2013). Phenotypic landscape inference reveals multiple evolutionary paths to C4 photosynthesis. eLife, 2, e00961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, J.T.R. , Brown, R.H. & Windham, W.R. (1983). Influence of leaf anatomy on the dry matter digestibility of C3, C4, and C3/C4 intermediate types of Panicum species. Crop Sci., 23, 141–146. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.q7r61k7


