Summary
Animals are embedded in dynamically changing networks of relationships with conspecifics. These dynamic networks are fundamental aspects of their environment, creating selection on behaviours and other traits. However, most social network‐based approaches in ecology are constrained to considering networks as static, despite several calls for such analyses to become more dynamic.
There are a number of statistical analyses developed in the social sciences that are increasingly being applied to animal networks, of which stochastic actor‐oriented models (SAOMs) are a principal example. SAOMs are a class of individual‐based models designed to model transitions in networks between discrete time points, as influenced by network structure and covariates. It is not clear, however, how useful such techniques are to ecologists, and whether they are suited to animal social networks.
We review the recent applications of SAOMs to animal networks, outlining findings and assessing the strengths and weaknesses of SAOMs when applied to animal rather than human networks. We go on to highlight the types of ecological and evolutionary processes that SAOMs can be used to study.
SAOMs can include effects and covariates for individuals, dyads and populations, which can be constant or variable. This allows for the examination of a wide range of questions of interest to ecologists. However, high‐resolution data are required, meaning SAOMs will not be useable in all study systems. It remains unclear how robust SAOMs are to missing data and uncertainty around social relationships.
Ultimately, we encourage the careful application of SAOMs in appropriate systems, with dynamic network analyses likely to prove highly informative. Researchers can then extend the basic method to tackle a range of existing questions in ecology and explore novel lines of questioning.
Keywords: animal communities, dynamics, individual‐based models, network‐based diffusion analysis, social networks, transmission
As a highly social species, we are fascinated by our changing social relationships and those of animals. The dynamic analysis of social networks is regularly called for but infrequently delivered. The authors review a dynamic network analysis technique that can be used to relate social relationships to ecological and evolutionary processes.
Introduction
Social networks in ecology
Animals compete, cooperate and reproduce with conspecifics, and so are engaged in a network of social interactions. These networks represent the social environment of individuals, which influences various evolutionary and ecological processes (Proulx, Promislow & Phillips 2005; Bascompte 2007; Kurvers et al. 2014). By simultaneously considering both the traits of the individuals in these networks and their patterns of interactions, networks have been used to study diverse subject areas, such as disease epidemiology and individuality (Weber et al. 2013), and the dynamics of group formation (Wilson et al. 2014). The importance of links between individual variation and group‐level processes is increasingly appreciated (Farine, Montiglio & Spiegel 2015), and networks are especially useful as a tool to quantify the social environment to which animals are presumed to be adapted. For instance, by quantifying an individual's social network we gain insights into the social information available to it (Aplin et al. 2012; Atton et al. 2012; Farine et al. 2015), the diseases it is exposed to (Hamede et al. 2009; Bull, Godfrey & Gordon 2012), the intensity of local competition it experiences (Oh & Badyaev 2010; Formica et al. 2011; Fisher, Rodríguez‐Muñoz & Tregenza 2016a) and the strength of its cooperative relationships (Voelkl & Kasper 2009; Apicella et al. 2012).
Typically, these networks of relationships are analysed as being static, i.e. a network is built that summarises a period of time, and this network is related to the processes of interest. However, this ignores the fact that individuals may change their interaction patterns over time (Blonder & Dornhaus 2011; Blonder et al. 2012). If a relationship between two flexible traits exists (e.g. social connectedness and individual dominance) change in one could drive change in the other, but it is difficult to tease apart which trait drives this relationship when only observing the product. This is true of many processes; for instance, if infected individuals show different levels of behaviour, are they infected because of their behaviour or did the infection change their behaviour or that of those around them? Without an experiment, inference of causality is difficult, but strong evidence can be provided where a process or behaviour is observed to consistently happen before, and lead to a change in, another process or behaviour. This is beyond the reach of static network analyses as it requires time‐ordering to be incorporated into the analyses (Blonder et al. 2012; Pinter‐Wollman et al. 2013). By modelling change in a network over time, it is possible to identify not only how social and non‐social processes drive each other (Burk, Steglich & Snijders 2007) but also what processes govern the development of network structure (Kossinets & Watts 2006). Furthermore, transmission dynamics, such as the spread of information or disease across a population, can be examined, allowing us to identify factors important for the contraction and transmission of information or disease (Weber et al. 2013; Van der Waal et al. 2014; Adelman et al. 2015; Aplin et al. 2015a).
Despite the evident potential in the dynamic network analysis approach, applications in ecology remain relatively limited (but see Blonder & Dornhaus 2011; Jeanson 2012; Wilson et al. 2014; Ilany, Booms & Holekamp 2015; Aplin et al. 2015a; Borgeaud et al. 2016; Pasquaretta et al. 2016). Recent calls for the implementation of dynamic network analyses (e.g. Pinter‐Wollman et al. 2013; Croft, Darden & Wey 2016) provided theoretical impetus for the use of dynamic networks, but little discussion of appropriate analytical techniques. Furthermore, contemporary introductions to social network analysis for ecologists state that ‘temporal dynamics represent a significant analytical challenge’ and that tools developed by computer scientists ‘are not realistic for many animal social networks’ (Farine & Whitehead 2015). This indicates that we require more accessible methods. Here, we review recent applications of a method for the dynamic analysis of networks: stochastic actor‐oriented models (SAOMs). In the Supporting Information, we provide a practical guide outlining the data requirements, the process of model fitting and how to interpret the results. We also provide a full worked‐through example, complete with an annotated R script and a data set, to allow readers to implement a SAOM.
The stochastic actor‐oriented model
SAOMs are a class of individual‐based models characterising the behaviour of each actor (individual) in the system, rather than calculating an average effect over a population. The latter approach can be problematic if even small nonlinear dynamics occur (Lehmann 2009). Additionally, linear‐modelling based approaches are often inappropriate for network‐based analyses, as the assumption of independence of residuals is clearly violated when individuals are embedded in an entire network of connections (Croft, James & Krause 2008; Whitehead 2008; Croft et al. 2011; Snijders 2011). It is therefore preferable to use a statistical tool specifically designed to model relationships between individuals, and the non‐independence this implies. For a more detailed mathematical description of SAOMs, we refer readers to the RSiena user manual (Ripley et al. 2015). Alongside our guide in the Supporting Information, further information on data requirements, model fitting and statistical inference is also available in previous papers that have used SAOMs in animals (e.g. Ilany, Booms & Holekamp 2015; Borgeaud et al. 2016; Pasquaretta et al. 2016). We will mainly discuss the application of SAOMs as implemented through the program SIENA (Steglich, Snijders & West 2006; Snijders, van de Bunt & Steglich 2010) and the R package RSiena (Ripley et al. 2015) as this program allows the full breadth of effects we describe to be implemented and comes with a large body of examples, R code and user guides (see: http://www.stats.ox.ac.uk/~snijders/siena/ for these and http://r-forge.r-project.org/R/?group_id=461 for the package itself).
SAOMs model gradual change in the network and traits of the individuals across discrete time points using hidden Markov models. Individuals may possess consistent positions in their social networks (Blumstein, Petelle & Wey 2013; Brent et al. 2013; Jacoby et al. 2014; Aplin et al. 2015b; Formica et al. 2016), and the networks themselves have been shown to be quite consistent across contexts (Dey et al. 2015; Firth & Sheldon 2015, 2016), time (Dey et al. 2013; Shizuka et al. 2014; Ilany, Booms & Holekamp 2015) and even generations (Fisher, Rodríguez‐Muñoz & Tregenza 2016b), so gradual change may be reasonably expected. Individuals are recorded as associating or not at each time point, i.e. the networks are binary. The duration of each time period will be determined by the study system, the questions and processes being investigated and the resolution of the data available, and/or through pilot‐analyses to determine the most appropriate resolution (e.g. Pasquaretta et al. 2016). Some studies of human associations have used up to yearly censuses (e.g. Steglich, Snijders & West 2006), although shorter time frames are more likely to be used for animal social networks (for instance we used 8 days for the example in our Supporting Information).
Being modelled as a Markov chain means that information about the past is not included by default and is assumed to not bring any additional predictive power (Burk, Steglich & Snijders 2007; Snijders, van de Bunt & Steglich 2010). While this may initially seem an oversight, it should be noted that SAOMs model states, e.g. ‘X and Y are currently connected’, rather than events, e.g. ‘X interacted with Y’ (Snijders, van de Bunt & Steglich 2010), so historical information on long‐term social associates is included in present information. However, even if researchers have recorded events rather than states, these data can be used in a SAOM. Events can be aggregated to infer states (Snijders, van de Bunt & Steglich 2010), e.g. ‘X and Y were grooming each other 4 days out of 7 this week, suggesting they are socially affiliated’. Between each time point, it is assumed that individuals optimise their position in the network according to a utility function, with this function determined by their links with others in the network and the links between these others, short‐term preferences and unknown tendencies (modelled as residual/random deviance; Burk, Steglich & Snijders 2007). The process of change between each time‐step consists of the objective function and the rate function. The objective function determines the manner of the change occurring, e.g. which individuals form social relationships, while the rate function models the speed of change of relationships, e.g. if individuals slowly form bonds that last a long time, or rapidly form short‐term relationships. Each of these functions can be influenced by covariates related to individuals or the environment. Two further assumptions worth highlighting in our description of the modelling framework are that (i) it is assumed that each individual controls it outgoing interactions, and (ii) that each individual has complete information about the network. However, neither of these assumptions is overly restrictive. For the former, in directed networks, outgoing interactions are typically defined as those the individual initiates, while for undirected networks, SAOMs allow multiple definitions of interaction that can correspond to the studied system, for example a relationship formed either by the actions of a single individual or the mutual agreement of both individuals in a dyad (Ripley et al. 2015). Regarding complete knowledge of the network, individuals typically only need limited information to act as they do, as the local network is typically the main driver of network change (Snijders, van de Bunt & Steglich 2010). Therefore, findings are generally robust to minor violations of this assumption.
SAOMs model network change in a series of network snapshots, including each member of the population and their observed social associations at each time point. Change in the network can be modelled in response to connections in the network and in response to a range of covariate types. Furthermore, SAOMs can include traits of individuals as response variables, which may be modelled to vary due to covariates or covary with social relationships. We describe each of these below. Figure 1 provides a pictorial representation of a SAOM, indicating the breadth of effects that can be specified. Descriptions of some of the network and trait processes that SAOMs can model of interest to ecologists are provided in Table 1, with some implemented in our worked example in the Supporting Information. A more complete list is available in the RSiena manual (Ripley et al. 2015).
Figure 1.
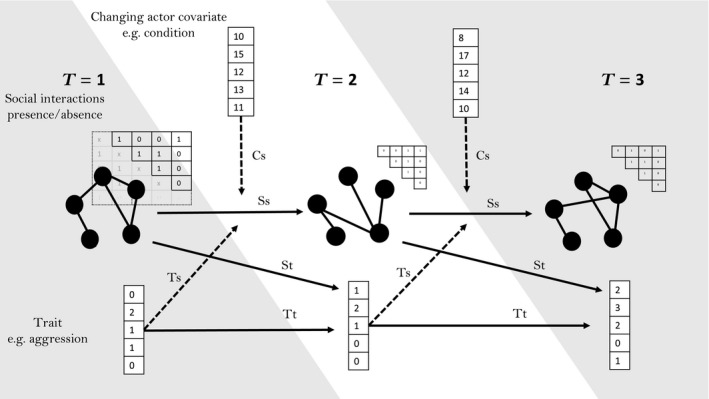
Pictorial representation of a SAOM, to illustrate the kind of effects that can be modelled. Note that our recommendations on network size still apply (see Supporting Information). Here there are three time periods, where five individuals change (or not) their social associations over time. Simultaneously, there is another dependant variable (a trait value, e.g. aggression) changing across each of the three time periods. Processes depicted model effects of: the social structure at one time point depending on the social structure at previous time points (lines labelled ‘Ss’); social structure influencing the value of traits at the next time point (lines labelled ‘St’); the trait at one time point influencing the trait at the next time point (lines labelled ‘Tt’); the trait influencing how the social structure changes from one time point to the next (lines labelled ‘Ts’) and some changing actor variable (e.g. condition) influencing the social structure change from one time point to the next (lines labelled ‘Cs’). Here the network is undirected/symmetrical, so only the above‐diagonal of the association matrices are shown at time points two and three, but full association matrices would be entered as data for all.
Table 1.
A list of possible effects of particular interest to ecologists that can be modelled with SAOMs in the SIENA software. In general, a positive value for the effect indicates the process outlined is occurring, but if otherwise this will be described. Effect type indicates whether the effect is a structural term, a covariate influencing the network, or if it involves the relationship between tie formation and the change in a trait, and whether the effect is relevant for undirected and/or directed networks. ‘Ego’ refers to the individual who is initiating the interaction, ‘alter’ to the receiver of the interaction
| Effect name | Effect type | Description of effect | Behavioural process |
|---|---|---|---|
| Ego/alter effects on tie formation | Covariate, directed and undirected | Traits of the individual on the ties it sends/receives | Traits of individuals, e.g. their sex or age influencing the likelihood to form ties |
| Ego/alter effects on rate | Covariate, directed and undirected | Traits of an individual on rate of change of relationships | Individuals of different sex, age or personality forming or dissolving ties at different rates |
| Ego‐alter trait interactions | Covariate, directed and undirected | Properties of both individuals on the chance of tie formation between them |
Positive: ties form within classes/homophily, e.g. intra‐sex aggression Negative: ties form between classes, e.g. producer‐scrounger |
| Outdegree | Structural term, directed | Number of existing associations of an individual on its tendency to form new associations |
Positive: Social behavioural types, e.g. consistently social or non‐social individuals Negative: optimising group size |
| Popularity/indegree | Structural term, directed and undirected | Tendency for individual to associate with others who already have a large number of associates | Attractive/susceptible phenotypes for affiliative/aggressive interactions |
| Triadic closure | Structural term, directed and undirected | Tendency of individuals to associate with ‘friends of friends’ | Coalition/clique formation |
| Reciprocity | Structural term, directed | Individuals repeat interactions with those that interact with them | Preferred associations, tit‐for‐tat cooperation |
| Balance | Structural term, directed and undirected | Tendency to have/lack the same ties as another associate | Partner choice copying, community formation |
| Three cycles | Structural term, directed | Directed social interactions, e.g. grooming or aggression, from X to Y, Y to Z and Z to X |
Positive: generalised reciprocity Negative: linear dominance hierarchies |
| Influence | Network‐behaviour co‐dynamic, directed and undirected | Changes in individuals’ traits due to the behaviour of their associates | Social learning and information or disease transmission |
| Selection | Network‐behaviour co‐dynamic, directed and undirected | Forming ties due to the behaviour of the other individuals |
Positive: partner choice based on phenotype Negative: avoidance of aggressive or diseased individuals |
| Dyadic covariates | Covariate, directed and undirected | Properties of a relationship between two individuals, e.g. distance | Accounting for separation in space, time or degree of genetic relatedness between individuals |
| Degree on behaviour | Network‐behaviour co‐dynamic, directed and undirected | Influence of number of relationships on behaviour | Social behaviour carry‐overs to non‐social contexts, e.g. Winner‐loser effects |
| Behaviour on degree | Network‐behaviour co‐dynamic, directed and undirected | Influence of behaviour level on formation of new ties | Behavioural carry‐overs to social contexts, e.g. boldness covaries with frequency of aggressive interactions |
The SAOM framework can estimate the importance of a variety of structural network processes (e.g. the tendency of individuals to form associations with individuals with whom they already share a mutual associate: ‘triadic closure’) on network change. Modelling these kinds of structural processes allows the researcher to determine how particular aspects of individuals’ social environments, such as the presence of a mutual associate, influence their choice of association partners. Such effects also enable researchers to control for structure in the data or biases generated by the method of data collection (see below).
The inclusion of covariates (at both individual and dyadic levels) enables the assessment of the role of individual traits and other relationships between individuals in influencing network structure. Individual traits can be included as constant actor covariates over the time period (e.g. sex) and, equivalently, fixed dyadic traits (e.g. relatedness) can be included as constant dyadic covariates. Additionally, dynamic covariates can also be included, either for changing individual traits (e.g. body condition), changing environmental conditions (e.g. rainfall) or changing dyadic covariates (e.g. spatial proximity). Interactions between network effects and covariates can also be specified. For instance, in some social systems it might be hypothesised that males are more likely to form coalitions than females. One would then specify an interaction between sex and triadic closure, and evaluate its importance.
Finally, behaviours or traits, as long as they change in a similar time frame to social relationships, can be considered as response variables alongside network change, allowing their change to be directly modelled alongside the change in social relationships. If the trait does not vary at a similar temporal scale as the variation in the social network, then their relationship cannot be assessed, as SAOMs model network dynamics and trait changes on the same temporal scale. By modelling how an individual's trait changes over time, affected by the trait values of those it interacts with, one can also model the spread of information, a cultural trait or disease across a population (see Example research area 1 below).
Previous applications of SAOMs
SAOMs were initially developed in the social sciences, and have been used extensively to study human behaviour. Example research questions include how music preferences and drug taking habits develop within and among friendship groups (Steglich, Snijders & West 2006) and how unethical behaviour can spread within organisations (Zuber 2014), see Wölfer, Faber & Hewstone (2015) for a review. Such questions have clear analogies for non‐human animal behaviour (such as the spread of a novel foraging technique through a group; Boogert et al. 2008; Allen et al. 2013; Aplin et al. 2015a). To date however, there have been only limited applications of SAOMs by those investigating animal interactions, although this has started to change in the last few years.
Jones (2011) investigated patterns of interactions in farmed salmon Salmo salar, and found that fish were either consistent givers or receivers of aggression, suggesting social personality types (Krause, James & Croft 2010; Wilson et al. 2012; Aplin et al. 2015b). More recently, Ilany, Booms & Holekamp (2015) investigated the long‐term dynamics of spotted hyena Crocuta crocuta social networks. Some of their key findings were that structural constraints, individual's traits and environmental conditions all shape network dynamics, and that female hyenas are more flexible in their social bonding tendencies, possibly reflecting their dominance in hyena groups (Ilany, Booms & Holekamp 2015). SAOMs have also been used to investigate social information transmission in Drosophila melanogaster (Pasquaretta et al. 2016), showing that uninformed flies tend to change social contacts faster. Boucherie et al. (2016) used SAOMs to explore changes in relationships in captive rooks Corvus frugilegus. Rooks preferentially interacted with paired congeners and were more likely to develop a relationship with connections of a social partner. They also found that sex had no significant effect on social dynamics. Finally, Borgeaud et al. (2016) investigated the dynamics of multiple social groups of vervet monkeys Chlorocebus pygerythrus, and found that some processes (e.g. triadic closure) were key to all groups, while others varied in their importance.
These studies provide fundamental insights into how and why animal groups from a range of taxa possess their observed structure, in particular highlighting the varying importance sex plays in different social systems and the importance of accounting for topological network effects on social relationships. Each of these studies had access to a large enough population of animals that were reliably individually recognisable, where social relationships could be clearly defined and when (relatively) complete covariate data were available. The same will need to be true for other studies that attempt to utilise SAOMs. In fact, the data requirements may be even more severe to fully exploit SAOMs. Specifically, as we have highlighted in the preceding section, SAOMs can model multiple traits changing over time, not just social relationships, and the individual or environmental covariates that affect them. Furthermore, it is possible to model the effect of greater network structure (beyond immediate connections) on the formation of new ties. These elements will require high‐resolution data on the changing social relationships and traits of a whole population of individuals at multiple time points, which will prove a challenge for the application of SAOMs to typical data sets in ecology.
In summary, this approach allows researchers to model the combination of the change in a trait over time, social relationships, group behaviour and transmission dynamics. Having outlined the opportunities that exist when using SAOMs (above and Table 1), we now describe some of the challenges that still need to be addressed. The data requirements for a SAOM will be the major challenge for ecologists wishing to use this method (see Supporting Information for technical detail). We then provide examples of research areas SAOMs seem particularly suited to tackle and discuss the modelling and simulation work needed to establish the performance of SAOMs with data sets more typical of those in ecology.
Challenges when using SAOMs to study animal networks
Above we highlighted the diversity of effects that SAOMs can model, and the recent applications in a range of organisms. This should make clear the opportunities to be exploited when using SAOMs to investigate dynamic network processes. However, as touched upon above, there are significant challenges when applying SAOMs to animal network data. Five principal concerns are that: (i) SAOMs only model relationships as existing or not, i.e. they cannot accept relationships of different strengths, (ii) they are not designed to deal with situations where there is uncertainty surrounding network edges, (iii) missing individuals (i.e. those not identifiable in the population) or interactions can lead to problems with estimation, (iv) methods of data collection may bias the network and (v) adequate controls for spatial proximity for non‐social reasons are required.
Binary networks and the inclusion of interaction strength
SAOMs are designed to study change in the presence and absence of social relationships, i.e. binary networks. Reducing weighted networks to binary descriptions can have major implications in animal social network analysis, for instance if weak ties are important for processes such as information transmission (Granovetter 1973) and may result in incorrect network metrics (Franks, Ruxton & James 2009; Farine 2014). As SAOMs split the data collected into distinct time periods, some information on repeated associations is retained in the form of relationships being present in multiple time periods rather than a single interaction with a weight. Furthermore, through the use of ‘ordered’ networks, RSiena can model relationships from a small range of different strengths. Essentially, a binary network of ‘strong’ associations among a population is entered alongside another binary network of ‘weak or stronger’ associations (theoretically three or more levels of association strength could be used, although the tractability of the subsequent model may limit such extensions). The SAOM then estimates what influences weak association formation and dissolution, and what predicts the transitions between weak and strong associations. This avoids some of the problems associated with ‘filtering’, where ties below a certain threshold are removed, as ties can be represented as belonging to a small set of different strengths. Determining these association strengths still requires a degree of thresholding however, so the problems mentioned above are still present to some degree. Therefore, SAOMs are likely to be most appropriate when the key biological implications of the interaction depends on whether it happened or not (e.g. sharing a nest or roost, creating the opportunity for direct transmission of a parasite), with limited additional information provided by assigning a range of weights to relationships. Methods to analyse networks with edge weights drawn from a greater range through (the related) exponential random graph models are being developed (Krivitsky 2012), so in the future, SAOMs may be able to include such information.
Edge uncertainty
Animal networks typically contain greater uncertainty than human networks, as we must infer unobservable social states from observable behaviours. Ideally, we would use SAOMs when limited inference of unobserved social relationships is required (e.g. when two primates are observed to groom one another). However, if association‐based methods are used (i.e. social relationships are inferred from repeated spatio‐temporal co‐occurrence, e.g. Sundaresan et al. 2007; Shorrocks & Croft 2009; Aplin et al. 2012; Allen et al. 2013; Ilany, Booms & Holekamp 2015) during network construction then a high level of confidence that these data represent true states of association is required. For some study systems and some methods, this may require a large number of observations (Lusseau, Whitehead & Gero 2008; Franks, Ruxton & James 2009; Farine & Strandburg‐Peshkin 2015). This however does not preclude their use (e.g. Ilany, Booms & Holekamp 2015) as long as the inference of associations is a confident one. To increase confidence in the results, one can use multiple thresholds to construct binary networks, and then to model network dynamics using each data set to evaluate the sensitivity of the results to a given threshold. Assuming any major biases are accounted for, transitions from one network to the next should still approximate real changes in the animals’ social environments. RSiena parameter estimates are provided with standard errors, which will be large if the studied effects are weak or highly variable. As mentioned above, if you cannot reliably infer states of association from your data, we do not recommend the use of SAOMs.
The impact of missing individuals and interactions
Animal networks typically contain both missing individuals (e.g. when an individual was not observed for the entirety of the study period) and missing edges (e.g. an association between two individuals was missed). Individuals appearing or disappearing from the network during a study can be accounted for in the modelling process. In RSiena, the absence of an association due to missing data can be entered so that the absence of the relationship will not inform parameter estimates (see the Supporting Information and Ripley et al. 2015), which we feel is an acceptable solution. However, individuals or edges that are never detected throughout the entire study period represent uncertainty that cannot be addressed in this way. In general, this is a problem that applies across statistical approaches to network analysis rather than to just SAOMs (Kossinets 2006), the consequences of which depend on the proportion of individuals missing and whether they were missing at random or not (Smith & Moody 2013; Silk et al. 2015; Smith, Moody & Morgan 2017). Missingness can affect parameter estimates through a reduction in network size, which has knock‐on effects on network parameters, and non‐random missingness related to variables of interest that may bias results (Huisman & Steglich 2008). Hipp et al. (2015) found that some parameters in a SAOM are robust no matter how missing data are dealt with, but other parameters are dependent on how missingness is handled. Methods of imputation of this missing data are viable, provided that the method is mindful of the study design (e.g. the ‘Held‐Out Predictive Evaluation’ of Wang et al. 2016). Methods that generate uncertainty around observations of animal networks (e.g. Farine & Strandburg‐Peshkin 2015) and simulation studies that explore the effect of missing information on the outcome of analytical approaches (e.g. Silk et al. 2015; Smith, Moody & Morgan 2017) will be essential for exploring how robust SAOMs are to the kinds of missing data common in ecological rather than sociological data sets.
Biases introduced by the method of data collection
The method of data collection may influence the network structure, creating spurious patterns. For example, individuals will be assigned many mutual associations if all individuals in a group are linked when they are observed together (the ‘gambit of the group’; Whitehead & Dufault 1999). This gives strong importance to the effect of triadic closure, as individuals will be connected to all their groupmates as well as to each other (Franks, Ruxton & James 2009). Performing enough censuses or surveys can ameliorate this problem (Franks, Ruxton & James 2009). Furthermore, there are additional features of SAOMs that allow the control of various factors that may bias results if they are known: particular structural network terms, or covariates, can be used to model aspects of the social network that may stem from the method of data collection. For example, when modelling networks constructed using a group‐based approach, the estimate for triadic closure could be considered to be (at least in part) controlling for this effect rather than being a parameter of interest. Additionally, dummy variables that interact with certain effects can be used to control for the confounding effect of a methodological bias, e.g. higher detection rate and so a higher frequency of interactions in certain study areas. This then allows conclusions about hypotheses of interest to be made having accounted for the confounding factors. The type of controlling factor specified will depend on the likely biases a particular method of data collection introduces, provided this is known. If the biases a method introduces are not known, then simulations may need to be performed to determine what the null expectations in the system are, to compare to the observed outcome.
The effect of spatial structure in networks
Spatial factors can influence the likelihood of two individuals interacting in a wide range of animal networks (Frère et al. 2010; Carter et al. 2013; Best et al. 2014). This raises the possibility that we are studying co‐choice of spatial location rather than choice of social partners, as certain patterns of resource exploitation can create the impression of complex social behaviour (Ramos‐Fernández, Boyer & Gómez 2006). Therefore, we need to account for animals’ space use when assessing their choice of interaction partners. Within a SAOM, this issue can be tackled through the use of dyadic covariates in the model. Shared group membership or a spatial relationship such as the distance between locations at the start of each time point can be entered as a dyadic covariate which accounts for the fact that individuals in the same group or near each other are more likely to interact. This effectively incorporates an appropriate null model into the analysis, as the significance of other parameters in the model is calculated alongside the influence of these spatial control terms. Therefore, preference for associating with (for example) individuals of the opposite sex can be estimated given the degree to which the sexes use the same space. A similar approach is advocated by Whitehead & James (2015), who suggest calculating ‘generalised affiliation indices’ (GAIs) that represent relationships that occur beyond what is expected based on factors such as spatio‐temporal overlap to enter into further network analyses. Such GAIs could be entered into a SAOM, but we recommend using the original association data and the factors that need controlling for within the SAOM. Spiegel et al. (2016) have recently developed a modelling strategy to separate spatial proximity from social associations, using randomisations of movement patterns within individuals with particular time periods. The resulting ‘expected’ patterns of social association could then be entered as changing dyadic covariates into a SAOM, as described above. An outstanding problem for these approaches is the degree to which the spatial position of individuals itself represents social behaviour, so should not be ‘accounted for’ and discarded from inferences on social behaviour. Assessing how different controls for spatial location influence our inferences about social behaviour in different systems should inform us on how problematic this issue is.
Example research area 1: SAOMs and transmission dynamics
A primary interest for those studying epidemiology is how individual behaviour relates to infection at the individual and the population level (Tompkins et al. 2011). If a disease is transmitted directly, its spread depends on the social relationships of the entire population, making it a network‐based problem. With a SAOM, being infected or not can be modelled as a dynamically changing trait with multiple levels (e.g. uninfected, infected but dormant, infective). This can then be influenced by (i) individual characteristics (e.g. sex, condition), (ii) network position (e.g. connectedness) and (iii) the characteristics of associates, including their own disease state. This allows the tendency to be infected to be influenced by the infection status of social partners, allowing the spread of a disease across the dynamically changing network to be modelled. The researcher can then explore whether the infection status alters the rate or choice of interactions, or the tendency to be targeted with interactions. As well as modelling disease status as more complicated than infected/uninfected, differences between classes (e.g. sex) in infection or transmission rates can be examined (McDonald et al. 2014). Furthermore, the change in infection status could be constrained to becoming infected, with returns to an uninfected state being impossible (Greenan 2015; Ripley et al. 2015). A similar framework can be applied to information transmission, modelling the spread of information across a population (Greenan 2015). This has previously been investigated in animals using network‐based diffusion analysis (NBDA; Aplin et al. 2012, 2015a; Atton et al. 2012; Allen et al. 2013; Boogert et al. 2014; Farine et al. 2015) with Hobaiter et al. (2014) extending traditional NBDA to account for the build‐up of relationships over time. However, SAOMs explicitly model both the change in the network and in the trait over time as mutually connected dependant variables. This allows the extent and direction of any causal relationship(s) and the effect of external variables on the change in both networks and the trait to be modelled. This gives different information to NBDA, which may instead be used to estimate the transmission speed across different parts of and the whole of the network.
Example research area 2: Behavioural types and networks
Social network analyses determine how specifics of individuals’ social environments, as measured by network traits such as degree or betweenness, are related to other aspects of their ecology. This indicates that individual‐level behavioural traits are important for ecological and evolutionary processes. This conclusion has become an established orthodoxy in behavioural ecology (Koolhaas et al. 1999; Dall, Houston & McNamara 2004; Réale et al. 2007), with within‐population, among‐individual differences in behaviour observed to be widespread (Bell, Hankison & Laskowski 2009), linked to fitness (Smith & Blumstein 2008) and various ecological and evolutionary dynamics (Wolf & Weissing 2012). By modelling both as responses, SAOMs allow the integration of these two branches of individual specific behaviours from social and non‐social domains, studying their codependent change over time. For instance, one could model how the level of risk‐taking behaviour relates to the number of social associates. This would allow ecologists to determine whether there are social ‘personality’ types (Krause, James & Croft 2010; Wilson et al. 2012), and whether they are associated with a suite of non‐social behavioural traits, i.e. as part of a ‘behavioural syndrome’ (Sih et al. 2004; Sih, Chang & Wey 2014) or associated with ‘social carry‐over effects’ (Niemelä & Santostefano 2015). Furthermore, it has been observed that animal social groups show assortativity, where individuals of similar behavioural types are more likely to associate (Aplin et al. 2013; Wilson et al. 2014; Carter et al. 2015). This could result from ‘selection’ where individuals choose associates of a similar behavioural type, or ‘influence’ where individuals change their behavioural type to match that of their associates (Steglich, Snijders & West 2006; Burk, Steglich & Snijders 2007; Steglich, Snijders & Pearson 2010). Identifying exactly which processes are more influential will indicate the cognitive process occurring and therefore the selection pressures at work, yet cannot happen unless the change in traits is ordered in a dynamic analysis. Furthermore, assortativity can arise as a byproduct of triadic closure (Ilany & Akcay 2016), suggesting that proper control for this effect is necessary. Finally, the social niche hypothesis suggests that repeated social interactions lead to an increase in within‐individual consistency (Bergmüller & Taborsky 2010; Montiglio, Ferrari & Reale 2013). Evidence is mixed however (Laskowski & Bell 2014; Laskowski & Pruitt 2014; Modlmeier et al. 2014). Investigating this question further with SAOMs will allow broader trends to be identified.
Future work
It is clear that SAOMs have great potential to inform the study of animal networks, but have potentially problematic assumptions and significant limitations. For many of these potential drawbacks, there is currently a lack of precise understanding of the impact they might have. Therefore, a fundamental next step is to test the susceptibility of SAOMs to type I and II errors in networks with a range of structures and constructed with a range of different interaction definitions, for a variety of missing levels of information. This work could use simulation‐modelling to determine the ability of SAOMs to detect a signal in simulated network data sets with different degrees of missing data (e.g. Huisman & Steglich 2008; Hipp et al. 2015).
It is also apparent that some of the current problems for using SAOMs with animal networks could be addressed by continued development of the modelling framework, or by making use of major advantages of SAOMs (e.g. the individual‐based approach) to develop or adapt alternative approaches specifically designed for animal networks. Many of the statistical processes used within a SAOM, such as Markov chains, are becoming increasingly familiar to ecologists and there is no reason why extensions, such as the ability to incorporate edge weights into the models, could not be developed specifically for animal networks. We hope that by highlighting this methodological tool we can stimulate further developments to enhance its utility.
Summary
SAOMs are a potentially useful tool for studying animal social networks, and their use in ecology is increasing rapidly. By providing a review of their uses, and a practical guide in the Supporting Information, we hope to aid those interested in applying it to their own data. Appreciating the range of effects that can and have been implemented in SAOMs in other fields should enable ecologists to ask new questions of existing data sets or formulate new questions surrounding social and non‐social behaviour. However, there are still a number of key challenges that must be addressed. First, how well the key assumptions of SAOMs are satisfied in different animal study systems, and second, how SAOMs can be modified to improve their applicability to the types of data sets generated in ecological research. Satisfying these concerns, while exploring a range of network‐based questions in ecology promises to provide new insights into the relationships between social systems and broader evolutionary and ecological processes.
Authors’ contributions
D.N.F. and M.J.S. conceived the idea for the manuscript, with D.N.F. leading the writing. All authors contributed to the writing of successive drafts. D.N.F. and T.T. contributed to collecting the data for the Supporting Information as members of the WildCrickets research group. D.N.F. analysed these data with contributions from A.I. and M.J.S. All authors gave final approval for publication.
Data accessibility
This article used no data. For the data used as an example in the practical guide, see the online Supporting Information.
Supporting information
Figure S1. SAOMs Practical guide. Flow chart.
Figure S2–S4. SAOMs Practical guide. RSiena bad GOF plots.
Figure S5–S7. SAOMs Practical guide. RSiena good GOF plots.
Figure S8. SAOMs Practical guide. Cricket social network.
Data S1. SAOMs Practical guide text.
Data S2. SAOMs R Code.
Data S3. SAOMs behaviour data.
Data S4. SAOMs network data 1.
Data S5. SAOMs network data 2.
Data S6. SAOMs network data 3.
Data S7. SAOMs sex data.
Acknowledgements
We thank Bea Downing, Lucy Steward, Mike Kings and Jonathan Jarrett for useful discussions surrounding network analysis and for testing the R code. We also thank Tom Snijders for providing useful comments that improved the manuscript. Alecia Carter, Damien Farine and an anonymous reviewer provided extensive reviews of the manuscript that substantially improved it. Finally, we thank Rolando Rodríguez‐Muñoz and Luke Meadows who helped collect the data for the Supporting Information. We have no competing interests. Funding for this research was provided by NERC (studentship no.: NE/H02249X/1; grant no.: NE/H02364X/1 [Correction added after online publication on 3 April 2017: Funding information added].
References
- Adelman, J.S. , Moyers, S.C. , Farine, D.R. & Hawley, D.M. (2015) Feeder use predicts both acquisition and transmission of a contagious pathogen in a North American songbird. Proceedings of the Royal Society B: Biological Sciences, 282, 20151429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, J. , Weinrich, M. , Hoppitt, W. & Rendell, L. (2013) Network‐based diffusion analysis reveals cultural transmission of lobtail feeding in humpback whales. Science, 340, 485–488. [DOI] [PubMed] [Google Scholar]
- Apicella, C.L. , Marlowe, F.W. , Fowler, J.H. & Christakis, N.A. (2012) Social networks and cooperation in hunter‐gatherers. Nature, 481, 497–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aplin, L.M. , Farine, D.R. , Morand‐Ferron, J. & Sheldon, B.C. (2012) Social networks predict patch discovery in a wild population of songbirds. Proceedings of the Royal Society B: Biological Sciences, 279, 4199–4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aplin, L.M. , Farine, D.R. , Morand‐Ferron, J. , Cole, E.F. , Cockburn, A. & Sheldon, B.C. (2013) Individual personalities predict social behaviour in wild networks of great tits (Parus major). Ecology Letters, 16, 1365–1372. [DOI] [PubMed] [Google Scholar]
- Aplin, L.M. , Farine, D.R. , Morand‐Ferron, J. , Cockburn, A. , Thornton, A. & Sheldon, B.C. (2015a) Experimentally induced innovations lead to persistent culture via conformity in wild birds. Nature, 518, 538–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aplin, L.M. , Firth, J.A. , Farine, D.R. et al (2015b) Consistent individual differences in the social phenotypes of wild great tits, Parus major . Animal Behaviour, 108, 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atton, N. , Hoppitt, W. , Webster, M.M. , Galef, B.G. & Laland, K.N. (2012) Information flow through threespine stickleback networks without social transmission. Proceedings of the Royal Society B: Biological Sciences, 279, 4272–4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bascompte, J. (2007) Networks in ecology. Basic and Applied Ecology, 8, 485–490. [Google Scholar]
- Bell, A.M. , Hankison, S.J. & Laskowski, K.L. (2009) The repeatability of behaviour: a meta‐analysis. Animal Behaviour, 77, 771–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmüller, R. & Taborsky, M. (2010) Animal personality due to social niche specialisation. Trends in Ecology & Evolution, 25, 504–511. [DOI] [PubMed] [Google Scholar]
- Best, E.C. , Dwyer, R.G. , Seddon, J.M. & Goldizen, A.W. (2014) Associations are more strongly correlated with space use than kinship in female eastern grey kangaroos. Animal Behaviour, 89, 1–10. [Google Scholar]
- Blonder, B. & Dornhaus, A. (2011) Time‐ordered networks reveal limitations to information flow in ant colonies. PLoS ONE, 6, e20298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blonder, B. , Wey, T.W. , Dornhaus, A. , James, R. & Sih, A. (2012) Temporal dynamics and network analysis. Methods in Ecology and Evolution, 3, 958–972. [Google Scholar]
- Blumstein, D.T. , Petelle, M.B. & Wey, T.W. (2013) Defensive and social aggression: repeatable but independent. Behavioral Ecology, 24, 457–461. [Google Scholar]
- Boogert, N.J. , Reader, S.M. , Hoppitt, W. & Laland, K.N. (2008) The origin and spread of innovations in starlings. Animal Behaviour, 75, 1509–1518. [Google Scholar]
- Boogert, N.J. , Nightingale, G.F. , Hoppitt, W. & Laland, K.N. (2014) Perching but not foraging networks predict the spread of novel foraging skills in starlings. Behavioural Processes, 109, 135–144. [DOI] [PubMed] [Google Scholar]
- Borgeaud, C. , Sosa, S. , Sueur, C. , Bshary, R. & van de Waal, E. (2016) Intergroup variation of social relationships in wild vervet monkeys: a dynamic network approach. Frontiers in Psychology, 7, 915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucherie, P.H. , Sosa, S. , Pasquaretta, C. & Dufour, V. (2016) A longitudinal network analysis of social dynamics in rooks Corvus frugilegus: repeated group modifications do not affect social network in captive rooks. Current Zoology, zow083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent, L.J.N. , Heilbronner, S.R. , Horvath, J.E. , Gonzalez‐Martinez, J. , Ruiz‐Lambides, A. , Robinson, A.G. , Skene, J.H.P. & Platt, M.L. (2013) Genetic origins of social networks in rhesus macaques. Scientific Reports, 3, 1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull, C.M. , Godfrey, S.S. & Gordon, D.M. (2012) Social networks and the spread of Salmonella in a sleepy lizard population. Molecular Ecology, 21, 4386–4392. [DOI] [PubMed] [Google Scholar]
- Burk, W.J. , Steglich, C.E.G. & Snijders, T.A.B. (2007) Beyond dyadic interdependence: actor‐oriented models for co‐evolving social networks and individual behaviors. International Journal of Behavioral Development, 31, 397–404. [Google Scholar]
- Carter, K.D. , Seddon, J.M. , Frère, C.H. , Carter, J.K. & Goldizen, A.W. (2013) Fission–fusion dynamics in wild giraffes may be driven by kinship, spatial overlap and individual social preferences. Animal Behaviour, 85, 385–394. [Google Scholar]
- Carter, A.J. , Lee, A.E.G. , Marshall, H.H. , Tico, M.T. & Cowlishaw, G. (2015) Phenotypic assortment in wild primate networks: implications for the dissemination of information. Royal Society Open Science, 2, 140444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft, D.P. , Darden, S.K. & Wey, T.W. (2016) Current directions in animal social networks. Current Opinion in Behavioral Sciences, 12, 52–58. [Google Scholar]
- Croft, D.P. , James, R. & Krause, J. (2008) Exploring Animal Social Networks. Princeton University Press, Oxford, UK. [Google Scholar]
- Croft, D.P. , Madden, J.R. , Franks, D.W. & James, R. (2011) Hypothesis testing in animal social networks. Trends in Ecology & Evolution, 26, 502–507. [DOI] [PubMed] [Google Scholar]
- Dall, S.R.X. , Houston, A.I. & McNamara, J.M. (2004) The behavioural ecology of personality: consistent individual differences from an adaptive perspective. Ecology Letters, 7, 734–739. [Google Scholar]
- Dey, C.J. , Reddon, A.R. , O'Connor, C.M. & Balshine, S. (2013) Network structure is related to social conflict in a cooperatively breeding fish. Animal Behaviour, 85, 395–402. [Google Scholar]
- Dey, C.J. , Tan, Q.Y.J. , O'Connor, C.M. , Reddon, A.R. , Caldwell, J.R. & Balshine, S. (2015) Dominance network structure across reproductive contexts in the cooperatively breeding cichlid fish Neolamprologus pulcher . Current Zoology, 61, 45–54. [Google Scholar]
- Farine, D.R. (2014) Measuring phenotypic assortment in animal social networks: weighted associations are more robust than binary edges. Animal Behaviour, 89, 141–153. [Google Scholar]
- Farine, D.R. , Aplin, L.M. , Sheldon, B.C. & Hoppitt, W. (2015) Interspecific social networks promote information transmission in wild songbirds. Proceedings of the Royal Society B: Biological Sciences, 282, 20142804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farine, D.R. , Montiglio, P.‐O. & Spiegel, O. (2015) From individuals to groups and back: the evolutionary implications of group phenotypic composition. Trends in Ecology & Evolution, 30, 609–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farine, D.R. & Strandburg‐Peshkin, A. (2015) Estimating uncertainty and reliability of social network data using Bayesian inference. Royal Society Open Science, 2, 150367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farine, D.R.D. & Whitehead, H. (2015) Constructing, conducting, and interpreting animal social network analysis. The Journal of Animal Ecology, 84, 1144–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth, J.A. & Sheldon, B.C. (2015) Experimental manipulation of avian social structure reveals segregation is carried over across contexts. Proceedings of the Royal Society B: Biological Sciences, 282, 20142350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth, J. & Sheldon, B. (2016) Social carry‐over effects underpin trans‐seasonally linked structure in a wild bird population. Ecology Letters, 19, 1324–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher, D.N. , Rodríguez‐Muñoz, R. & Tregenza, T. (2016a) Comparing pre‐ and post‐copulatory mate competition using social network analysis in wild crickets. Behavioral Ecology, 27, 912–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher, D.N. , Rodríguez‐Muñoz, R. & Tregenza, T. (2016b) Wild cricket social networks show stability across generations. BMC Evolutionary Biology, 16, 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formica, V.A. , McGlothlin, J.W. , Wood, C.W. , Augat, M.E. , Butterfield, R.E. , Barnard, M.E. & Brodie, E.D. (2011) Phenotypic assortment mediates the effect of social selection in a wild beetle population. Evolution, 65, 2771–2781. [DOI] [PubMed] [Google Scholar]
- Formica, V. , Wood, C. , Cook, P. & Brodie, E. (2016) Consistency of animal social networks after disturbance. Behavioral Ecology, arw128. [Google Scholar]
- Franks, D.W. , Ruxton, G.D. & James, R. (2009) Sampling animal association networks with the gambit of the group. Behavioral Ecology and Sociobiology, 64, 493–503. [Google Scholar]
- Frère, C.H. , Krützen, M. , Mann, J. , Watson‐Capps, J.J. , Tsai, Y.J. , Patterson, E.M. , Connor, R. , Bejder, L. & Sherwin, W.B. (2010) Home range overlap, matrilineal and biparental kinship drive female associations in bottlenose dolphins. Animal Behaviour, 80, 481–486. [Google Scholar]
- Granovetter, M. (1973) The strength of weak ties. American Journal of Sociology, 78, 1360–1380. [Google Scholar]
- Greenan, C.C. (2015) Diffusion of innovations in dynamic networks. Journal of the Royal Statistical Society: Series A (Statistics in Society), 178, 147–166. [Google Scholar]
- Hamede, R.K. , Bashford, J. , McCallum, H. & Jones, M. (2009) Contact networks in a wild Tasmanian devil (Sarcophilus harrisii) population: using social network analysis to reveal seasonal variability in social behaviour and its implications for transmission of devil facial tumour disease. Ecology Letters, 12, 1147–1157. [DOI] [PubMed] [Google Scholar]
- Hipp, J.R. , Wang, C. , Butts, C.T. , Jose, R. & Lakon, C.M. (2015) Research Note: the consequences of different methods for handling missing network data in Stochastic Actor Based Models. Social Networks, 41, 56–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobaiter, C. , Poisot, T. , Zuberbühler, K. , Hoppitt, W. & Gruber, T. (2014) Social network analysis shows direct evidence for social transmission of tool use in wild chimpanzees. PLoS Biology, 12, e1001960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman, M. & Steglich, C. (2008) Treatment of non‐response in longitudinal network studies. Social Networks, 30, 297–308. [Google Scholar]
- Ilany, A. & Akcay, E. (2016) Social inheritance can explain the structure of animal social networks. Nature Communications, 7, 12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilany, A. , Booms, A.S. & Holekamp, K.E. (2015) Topological effects of network structure on long‐term social network dynamics in a wild mammal. Ecology Letters, 18, 687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby, D.M.P. , Fear, L.N. , Sims, D.W. & Croft, D.P. (2014) Shark personalities? Repeatability of social network traits in a widely distributed predatory fish. Behavioral Ecology and Sociobiology, 68, 1995–2003. [Google Scholar]
- Jeanson, R. (2012) Long‐term dynamics in proximity networks in ants. Animal Behaviour, 83, 915–923. [Google Scholar]
- Jones, H.C. (2011) Social Network Analysis of Behavioural Interactions Influencing the Development of Fin Damage in Atlantic Salmon (Salmo salar). University of Cambridge, Cambridge, UK. [Google Scholar]
- Koolhaas, J.M. , Korte, S.M. , De Boer, S.F. , Van Der Vegt, B.J. , Van Reenen, C.G. , Hopster, H. , De Jong, I.C. , Ruis, M.A. & Blokhuis, H.J. (1999) Coping styles in animals: current status in behavior and stress‐physiology. Neuroscience and Biobehavioral Reviews, 23, 925–935. [DOI] [PubMed] [Google Scholar]
- Kossinets, G. (2006) Effects of missing data in social networks. Social Networks, 28, 247–268. [Google Scholar]
- Kossinets, G. & Watts, D.J. (2006) Empirical analysis of an evolving social network. Science, 311, 88–90. [DOI] [PubMed] [Google Scholar]
- Krause, J. , James, R. & Croft, D.P. (2010) Personality in the context of social networks. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences, 365, 4099–4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivitsky, P. (2012) Exponential‐family random graph models for valued networks. Electronic Journal of Statistics, 6, 1100–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurvers, R.H.J.M. , Krause, J. , Croft, D.P. , Wilson, A.D.M. & Wolf, M. (2014) The evolutionary and ecological consequences of animal social networks: emerging issues. Trends in Ecology & Evolution, 29, 326–335. [DOI] [PubMed] [Google Scholar]
- Laskowski, K.L. & Bell, A.M. (2014) Strong personalities, not social niches, drive individual differences in social behaviours in sticklebacks. Animal Behaviour, 90, 287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski, K.L. & Pruitt, J.N. (2014) Evidence of social niche construction: persistent and repeated social interactions generate stronger personalities in a social spider. Proceedings of the Royal Society B: Biological Sciences, 281, 20133166. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lehmann, H. (2009) On the Applicability of Agent Based Modelling in Behavioural Ecology. University of Bath, Bath, UK. [Google Scholar]
- Lusseau, D. , Whitehead, H. & Gero, S. (2008) Incorporating uncertainty into the study of animal social networks. Animal Behaviour, 75, 1809–1815. [Google Scholar]
- McDonald, J.L. , Smith, G.C. , McDonald, R.A. , Delahay, R.J. & Hodgson, D. (2014) Mortality trajectory analysis reveals the drivers of sex‐specific epidemiology in natural wildlife‐disease interactions. Proceedings of the Royal Society B: Biological Sciences, 281, 20140526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modlmeier, A.P. , Laskowski, K.L. , DeMarco, A.E. , Coleman, A. , Zhao, K. , Brittingham, H.A. , McDermott, D.R. & Pruitt, J.N. (2014) Persistent social interactions beget more pronounced personalities in a desert‐dwelling social spider. Biology Letters, 10, 20140419. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Montiglio, P.‐O. , Ferrari, C. & Reale, D. (2013) Social niche specialization under constraints: personality, social interactions and environmental heterogeneity. Philosophical Transactions of the Royal Society B: Biological Sciences, 368, 20120343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemelä, P.T. & Santostefano, F. (2015) Social carry‐over effects on non‐social behavioral variation: mechanisms and consequences. Frontiers in Ecology and Evolution, 3, 49. [Google Scholar]
- Oh, K.P. & Badyaev, A.V. (2010) Structure of social networks in a passerine bird: consequences for sexual selection and the evolution of mating strategies. The American Naturalist, 176, E80–E89. [DOI] [PubMed] [Google Scholar]
- Pasquaretta, C. , Klenschi, E. , Pansanel, J. , Battesti, M. , Mery, F. & Sueur, C. (2016) Understanding dynamics of information transmission in Drosophila melanogaster using a statistical modeling framework for longitudinal network data (the RSiena package). Frontiers in Psychology, 7, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinter‐Wollman, N. , Hobson, E. , Smith, J. et al (2013) The dynamics of animal social networks: analytical, conceptual, and theoretical advances. Behavioral Ecology, 25, 242–255. [Google Scholar]
- Proulx, S.R. , Promislow, D.E.L. & Phillips, P.C. (2005) Network thinking in ecology and evolution. Trends in Ecology & Evolution, 20, 345–353. [DOI] [PubMed] [Google Scholar]
- Ramos‐Fernández, G. , Boyer, D. & Gómez, V.P. (2006) A complex social structure with fission–fusion properties can emerge from a simple foraging model. Behavioral Ecology and Sociobiology, 60, 536–549. [Google Scholar]
- Réale, D. , Reader, S.M. , Sol, D. , McDougall, P.T. & Dingemanse, N.J. (2007) Integrating animal temperament within ecology and evolution. Biological Reviews, 82, 291–318. [DOI] [PubMed] [Google Scholar]
- Ripley, R.M. , Snijders, T.A.B. , Boda, Z. , Voros, A. & Preciado, P. (2015) Manual for SIENA Version 4.0 (Version October 10, 2015). University of Oxford, Oxford, UK. [Google Scholar]
- Shizuka, D. , Chaine, A.S. , Anderson, J. , Johnson, O. , Laursen, I.M. & Lyon, B.E. (2014) Across‐year social stability shapes network structure in wintering migrant sparrows. Ecology Letters, 17, 998–1007. [DOI] [PubMed] [Google Scholar]
- Shorrocks, B. & Croft, D. (2009) Necks and networks: a preliminary study of population structure in the reticulated giraffe (Giraffa camelopardalis reticulata de Winston). African Journal of Ecology, 47, 374–381. [Google Scholar]
- Sih, A. , Chang, A.T. & Wey, T.W. (2014) Effects of behavioural type, social skill and the social environment on male mating success in water striders. Animal Behaviour, 94, 9–17. [Google Scholar]
- Sih, A. , Bell, A.M. , Johnson, J.C. & Ziemba, R.E. (2004) Behavioral syndromes: an intergrative overiew. The Quarterly Review of Biology, 79, 241–277. [DOI] [PubMed] [Google Scholar]
- Silk, M.J. , Jackson, A.L. , Croft, D.P. , Colhoun, K. & Bearhop, S. (2015) The consequences of unidentifiable individuals for the analysis of an animal social network. Animal Behaviour, 104, 1–11. [Google Scholar]
- Smith, B.R. & Blumstein, D.T. (2008) Fitness consequences of personality: a meta‐analysis. Behavioral Ecology, 19, 448–455. [Google Scholar]
- Smith, J.A. & Moody, J. (2013) Structural effects of network sampling coverage I: nodes missing at random. Social Networks, 35, 652–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, J.A. , Moody, J. & Morgan, J.H. (2017) Network sampling coverage II: the effect of non‐random missing data on network measurement. Social Networks, 48, 78–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijders, T.A.B. (2011) Statistical models for social networks. Annual Review of Sociology, 37, 131–153. [Google Scholar]
- Snijders, T.A.B. , van de Bunt, G.G. & Steglich, C.E.G. (2010) Introduction to stochastic actor‐based models for network dynamics. Social Networks, 32, 44–60. [Google Scholar]
- Spiegel, O. , Leu, S.T. , Sih, A. & Bull, C.M. (2016) Socially interacting or indifferent neighbours? Randomization of movement paths to tease apart social preference and spatial constraints (ed T Münkemüller). Methods in Ecology and Evolution, 7, 971–979. [Google Scholar]
- Steglich, C. , Snijders, T.A.B. & Pearson, M. (2010) Dynamic networks and behaviour: separating selection from influence. Sociological Methodology, 40, 329–393. [Google Scholar]
- Steglich, C. , Snijders, T. & West, P. (2006) Applying SIENA: an illustrative analysis of the coevolution of adolescents’ friendship networks, taste in music, and alcohol consumption. Methodology: European Journal of Research Methods for the Behavioral and Social Sciences, 2, 48–56. [Google Scholar]
- Sundaresan, S.R. , Fischhoff, I.R. , Dushoff, J. & Rubenstein, D.I. (2007) Network metrics reveal differences in social organization between two fission‐fusion species, Grevy's zebra and onager. Oecologia, 151, 140–149. [DOI] [PubMed] [Google Scholar]
- Tompkins, D.M. , Dunn, A.M. , Smith, M.J. & Telfer, S. (2011) Wildlife diseases: from individuals to ecosystems. The Journal of Animal Ecology, 80, 19–38. [DOI] [PubMed] [Google Scholar]
- Van der Waal, K.L. , Atwill, E.R. , Isbell, L.A. & McCowan, B. (2014) Linking social and pathogen transmission networks using microbial genetics in giraffe (Giraffa camelopardalis). The Journal of Animal Ecology, 83, 406–414. [DOI] [PubMed] [Google Scholar]
- Voelkl, B. & Kasper, C. (2009) Social structure of primate interaction networks facilitates the emergence of cooperation. Biology Letters, 5, 462–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C. , Butts, C.T. , Hipp, J.R. , Jose, R. & Lakon, C.M. (2016) Multiple imputation for missing edge data: a predictive evaluation method with application to add health. Social Networks, 45, 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, N. , Carter, S.P. , Dall, S.R.X. , Delahay, R.J. , McDonald, J.L. , Bearhop, S. & McDonald, R.A. (2013) Badger social networks correlate with tuberculosis infection. Current Biology, 23, R915–R916. [DOI] [PubMed] [Google Scholar]
- Whitehead, H. (2008) Analysing Animal Societies: Quantitative Methods for Vertebrate Social Analysis. The University Chicago Press, Chicago, IL, USA. [Google Scholar]
- Whitehead, H. & Dufault, S. (1999) Techniques for analyzing vertebrate social structure using identified individuals: review and recommendations Advances in the Study of Behavior, 28th edn (eds Slater P., Rosenblatt J. & Roper T.), pp. 33–73. Academic Press, San Diego, CA, USA. [Google Scholar]
- Whitehead, H. & James, R. (2015) Generalized affiliation indices extract affiliations from social network data. Methods in Ecology and Evolution, 6, 836–844. [Google Scholar]
- Wilson, A.D.M. , Krause, S. , Dingemanse, N.J. & Krause, J. (2012) Network position: a key component in the characterization of social personality types. Behavioral Ecology and Sociobiology, 67, 163–173. [Google Scholar]
- Wilson, A.D.M. , Krause, S. , James, R. , Croft, D.P. , Ramnarine, I.W. , Borner, K.K. , Clement, R.J.G. & Krause, J. (2014) Dynamic social networks in guppies (Poecilia reticulata). Behavioral Ecology and Sociobiology, 68, 915–925. [Google Scholar]
- Wolf, M. & Weissing, F.J. (2012) Animal personalities: consequences for ecology and evolution. Trends in Ecology & Evolution, 27, 452–461. [DOI] [PubMed] [Google Scholar]
- Wölfer, R. , Faber, N.S. & Hewstone, M. (2015) Social network analysis in the science of groups: cross‐sectional and longitudinal applications for studying intra‐ and intergroup behavior. Group Dynamics: Theory, Research, and Practice, 19, 45–61. [Google Scholar]
- Zuber, F. (2014) Spread of unethical behavior in organizations: a dynamic social network perspective. Journal of Business Ethics, 131, 151–172. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. SAOMs Practical guide. Flow chart.
Figure S2–S4. SAOMs Practical guide. RSiena bad GOF plots.
Figure S5–S7. SAOMs Practical guide. RSiena good GOF plots.
Figure S8. SAOMs Practical guide. Cricket social network.
Data S1. SAOMs Practical guide text.
Data S2. SAOMs R Code.
Data S3. SAOMs behaviour data.
Data S4. SAOMs network data 1.
Data S5. SAOMs network data 2.
Data S6. SAOMs network data 3.
Data S7. SAOMs sex data.
Data Availability Statement
This article used no data. For the data used as an example in the practical guide, see the online Supporting Information.


