Abstract
Background and Aims
One of the most used treatments for achalasia is pneumatic dilation of the lower esophageal sphincter to improve esophageal emptying. Multiple treatment protocols have been described with a varying balloon size, number of dilations, inflation pressure, and duration. We aimed to identify the most efficient and safe treatment protocol.
Methods
We performed a systematic review and meta‐analysis of studies on pneumatic dilation in patients with primary achalasia. Clinical remission was defined as an Eckardt score ≤3 or adequate symptom reduction measured with a similar validated questionnaire. We compared the clinical remission rates and occurrence of complications between different treatment protocols.
Results
We included 10 studies with 643 patients. After 6 months, dilation with a 30‐mm or 35‐mm balloon gave comparable mean success rates (81% and 79%, respectively), whereas a series of dilations up to 40 mm had a higher success rate of 90%. Elective additional dilation in patients with insufficient symptom resolution was somewhat more effective than performing a predefined series of dilations: 86% versus 75% after 12 months. Perforations occurred most often during initial dilations, and significantly more often using a 35‐mm balloon than a 30‐mm balloon (3.2 vs 1.0%); P = 0.027. A subsequent 35‐mm dilation was safer than an initial dilation with 35 mm (0.97% vs 9.3% perforations), P = 0.0017.
Conclusions
The most efficient and safe method of dilating achalasia patients is a graded approach starting with a 30‐mm dilation, followed by an elective 35‐mm dilation and 40 mm when there is insufficient symptom relief.
Keywords: achalasia, balloon dilation, efficacy, safety
The optimal pneumatic dilation therapy for untreated achalasia patients is a 30‐mm dilation, followed by an elective dilation to 35 and 40 mm in patients with persisting or recurrent symptoms. Dilation to 35 and 40 mm is relatively safe when it is preceded by a 30‐mm dilation.

Abbreviations
- LES
lower esophageal sphincter
- POEM
per‐oral endoscopic myotomy
- psi
pound‐force per square inch
Key Points
Multiple treatment protocols for pneumatic dilation in achalasia have been described with a varying balloon size, number of dilations, inflation pressure, and duration. It is unknown which treatment protocol is most efficient and safe.
Dilation with a 40‐mm balloon gave higher success rates than dilation with a 30‐mm or 35‐mm balloon. The risk of perforation increases when using a 35‐mm balloon in the first dilation session. However, when preceded by a 30‐mm dilation, a dilation to 35 and 40 mm is relatively safe.
An initial 30‐mm balloon dilation followed by an elective 35‐mm and 40‐mm balloon dilation in patients with persisting or recurrent symptoms results in the optimal therapeutic efficacy with acceptable perforation risks.
1. INTRODUCTION
Achalasia is a primary motor disease of the esophagus, manometrically characterized by loss of peristalsis and a non‐relaxing lower esophageal sphincter (LES).1 The classic presentation is progressive dysphagia to both solids and liquids, often accompanied by regurgitation of undigested food and chest pain.2, 3 On radiography, poor esophageal emptying and a very narrow LES are seen, and histopathology shows loss of neural cells in the myenteric plexus of the esophagus.4
Unfortunately, there is no curative treatment that can target the neurodegenerative process. Therefore, all treatments are symptomatic, aiming to improve esophageal emptying by means of LES tone reduction.1 Currently, the most common and effective interventions are surgical Heller myotomy, per‐oral endoscopic myotomy (POEM), and pneumatic dilation.1, 3 Many different techniques and treatment protocols have been described for pneumatic dilation.5 In general, a non‐compliant polyethylene balloon (Rigiflex, Boston Scientific, Natick, MA, USA) is positioned across the LES under fluoroscopic guidance, aided by radiopaque markers on the balloon catheter, and the balloon is inflated with a handheld manometer.1 Various balloon sizes, number of dilation sessions, inflation pressures, and inflation durations can be used.5 Consequently, the reported series are heterogeneous with respect to the treatment protocol. Reported treatment success rates vary from 52% to 99%.6, 7 The current American College of Gastroenterology (ACG) guideline for pneumatic dilation in achalasia patients recommends a graded approach using a 30‐mm balloon, followed by a 35‐mm balloon, and thereafter a 40‐mm balloon in non‐responding patients.3
Due to the chronic and progressive character of the disease, many achalasia patients have to undergo several treatments during their life.8 Therefore, it is important to identify the most efficient and safe way of performing pneumatic dilations. In this systematic review, we compare the clinical remission rates and occurrence of complications associated with different dilation protocols in untreated patients with primary achalasia. We describe the effect of different balloon diameters and the effect of a predefined series of dilations versus elective additional dilation sessions based on insufficient symptom resolution. Additionally, we examine which treatment protocol has the lowest risk of complications, specifically perforation, postprocedural retrosternal pain, and reflux symptoms.
2. METHODS
2.1. Literature search and screening
To identify studies describing the efficacy of pneumatic dilation in achalasia patients, we searched PubMed, Embase, and Cochrane. We performed our search on December 8, 2016, using the following terms (including synonyms): “esophageal achalasia,” “pneumatic dilation” and “size” or “effect.” The exact search is displayed in Appendix S1, and Figure 1 shows a summary of our literature search, screening, and selection. During title and abstract screening, we used the following inclusion criteria: adult patients with primary achalasia; treatment with pneumatic dilation and article type: no reviews, commentaries, meta‐analyses, or case reports. Next, two authors (FH and LP) separately screened the full text of the remaining articles more accurately using stricter inclusion criteria: no previous treatments; use of Rigiflex balloon and full description of the procedure; use of Eckardt score or a similar validated questionnaire and full‐text availability. Publications that did not meet the abovementioned criteria were excluded from further analysis.
Figure 1.
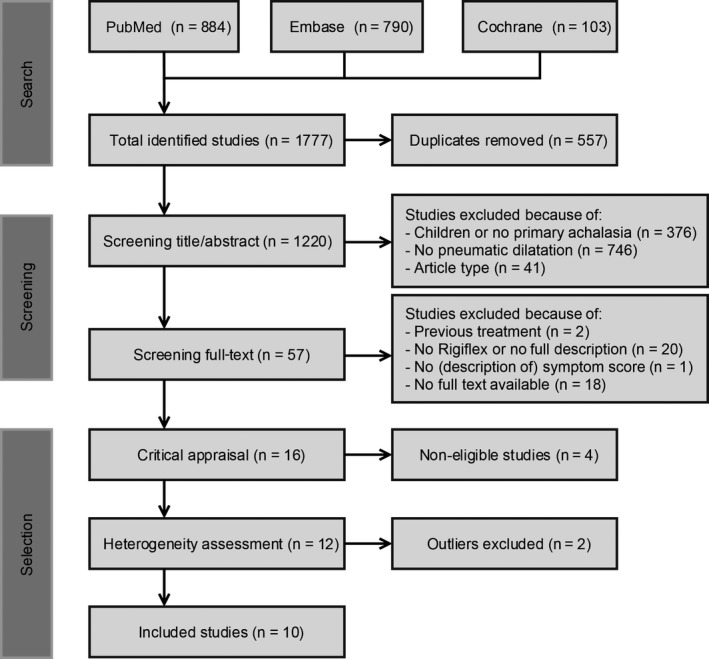
Flowchart of literature search, screening, and selection
2.2. Critical appraisal and article selection
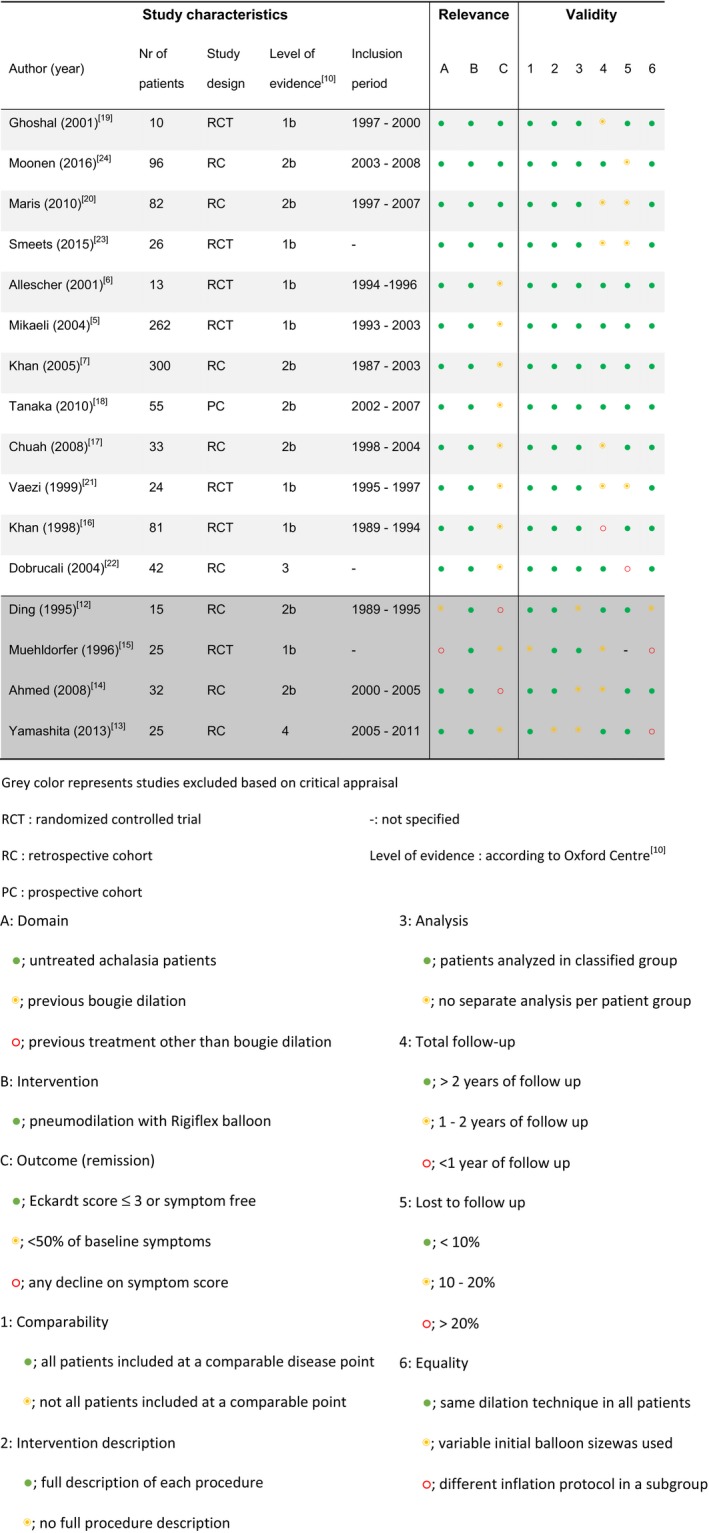
Of the remaining articles, two authors (FH and LP) independently determined the relevance and validity during critical appraisal (Table 1), using the Newcastle Ottawa Quality assessment scale as a guideline.9 We noted the number of included patients, the study design, the level of evidence,10 and the inclusion period of the patients. The relevance of the articles was assessed by critically appraising the patient group, intervention, and outcome. The validity of the articles was assessed using the following criteria: (a) comparability: All patients were included at a comparable point in the course of their disease; (b) intervention description: A full description of each procedure is included; (c) analysis: All patients were analyzed in the group to which they were classified; (d) total follow‐up duration; (e) percentage of patients lost to follow‐up; and (f) equality: All patients were treated with the same dilation technique.
Table 1.
Critical appraisal
2.3. Data extraction
The primary outcome was the percentage of patients in clinical remission, as defined by an Eckardt score ≤3. The only other remission criterion that was considered valid was being completely symptom‐free or having>50% symptom reduction on a similar validated questionnaire comparable to the Eckardt score. For each study, we noted the number of patients, the description of the symptom score, the definition of clinical remission, and the clinical remission rates after 6 and 12 months. Furthermore, we noted the treatment characteristics: balloon size, number of dilation sessions, inflation pressure, inflation duration, and usage of predefined series of dilations versus elective additional dilations only in patients with recurrent symptoms. Secondary outcomes were the number and type of complications: perforation, postprocedural pain, and reflux symptoms.
2.4. Statistical analyses
After extracting all data from all articles, the total number of treated patients, the number of patients in remission after 6 and 12 months, and the 95% confidence interval were imported in Comprehensive Meta‐Analysis software (version 3, Biostat, Englewood, NJ). The inconsistency between studies was assessed and quantified by calculating the heterogeneity (I 2) and the between‐study variance (τ 2).11 Studies yielding extreme effects that appeared to be outlying were tested and excluded when significantly influencing the heterogeneity. Next, a random‐effects analysis was performed creating a Forest plot to compare the remission rates between dilation up to 30, 35, and 40 mm. The same analysis was performed to compare the remission rate between articles using a predefined treatment protocol versus additional dilation only in patients with recurrent symptoms. The number of perforations was compared between different groups using a two‐tailed Fisher's exact test in GraphPad Prism software (version 7, San Diego, CA). A P‐value <0.05 was regarded significant.
3. RESULTS
3.1. Literature search, screening, and selection
The search yielded 1777 records: PubMed 884, Embase 790, and Cochrane 103. After removing 557 duplicates, 1220 unique studies were identified. Of these, 1163 studies were excluded during title and abstract screening and another 41 articles during full‐text screening (Figure 1). The remaining 16 articles were critically appraised. Four studies were excluded during critical appraisal, and two other studies were excluded because the reported success rates were outliers that caused significant heterogeneity. Finally, 10 articles were found eligible and included in our systematic review.
3.2. Critical appraisal and heterogeneity assessment
The four excluded studies during critical appraisal are visible in Table 1. Two of the four excluded studies were excluded because of an inconsistent treatment protocol: Both Ding12 and Yamashita et al13 dilated some of their patients initially with a 30 mm and others with a 35‐mm balloon without distinguishing between these patients in the results. Furthermore, Ding et al did not describe their final success rate after follow‐up or their outcome measurement. A third study, by Ahmed et al,14 was excluded because no definition of clinical remission was specified. The fourth study to be discarded was that of Muehldorfer et al15 because it included a heterogeneous group of treated and untreated patients without stating the previous treatment or making a distinction in treatment results between these patients.
The two excluded outlier studies during heterogeneity assessment were Khan et al.7, 16 These studies yielded extreme results that appeared to be outlying. The percentage of total inconsistency across studies due to true heterogeneity (I 2) was 79% including these studies and dropped to 64% without these studies. Moreover, the between‐study variance (τ 2) dropped from 0.64 to 0.25 when excluding these studies. Based on this, both studies were considered outliers significantly influencing the true heterogeneity between studies and therefore excluded from further analyses.
3.3. Comparing efficacy between different balloon sizes
A total of 10 studies with 643 patients were included and subdivided into three groups: dilation up to 30, 35, and 40 mm. Dilation with 30 mm and 35 mm showed comparable mean remission rates after 6 months (81% and 79%) whereas dilation up to 40 mm had a higher remission rate of 90%. After 12 months, the success rates decreased in all groups to 77%, 70%, and 87%, respectively. The results are shown in Table 2 and Figure 2.
Table 2.
Summary of dilation protocols and efficacy
| Author (y) | Number of patients per dilation session | Treatment protocola | Time in‐between (wk) | Remission rates | Total FU (mo) | Prespecified protocol | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 6 mo (%) | 12 mo (%) | End of studyb (%) | |||||
| Chuah (2008)17 | 33 | ‐ | ‐ | 30 | ‐ | 91 | 81 | ‐ | 12 | Yes |
| Tanaka (2010)18 | 55 | ‐ | ‐ | 30 | ‐ | 75 | 73 | 73 | 74 | Yes |
| Ghoshal (2001)19 | 10 | 4 | ‐ | 30 − 30 | NS | 80 | 80 | ‐ | 12 | No |
| Maris (2010)20 | 82 | 14 | 2 | 30 − 30 − 30 | 4 − 80 | 83 | 78 | ‐ | 12 | No |
| Dobrucali (2004)22 | 42 | 18 | 4 | 30 − 35 − 35 | 6 − 8 | 88 | 67 | 54 | 60 | No |
| Smeets (2015)23 | 26 | 26 | ‐ | 30 + 35 | 1 | 73 | 73 | ‐ | 12 | Yes |
| Vaezi (1999)21 | 24 | 7 | ‐ | 30 − 35 | 14 | 75 | 70 | ‐ | 12 | No |
| Allescher (2001)6 | 13 | 9 | ‐ | 35 − 40 | 4 − 200 | 69 | 62 | 45 | 48 | No |
| Mikaeli (2004)5, c |
A: 62 B: 200 |
A: 18 B: 56 |
A: 3 B: 8 |
A: 35 − 40 − 40 B: 30 − 35 − 40 |
4 − 192 |
A: 92 B: 95 |
A: 88 B: 90 |
A: 70 B: 89 |
60 | No |
| Moonen (2016)24 | 96 | 96 | 24 | 30 + 35 − 40 | 4 | 90 | 90 | 82 | 120 | No |
‐, Not applicable; FU, follow‐up; mo, months; NS, not specified; wk, weeks.
+ = predefined scheme of two dilations; − = a next dilation only in case of symptom recurrence.
End of study = remission rate at total follow‐up duration in months.
Two different dilation protocols were used, which are separately represented.
Figure 2.
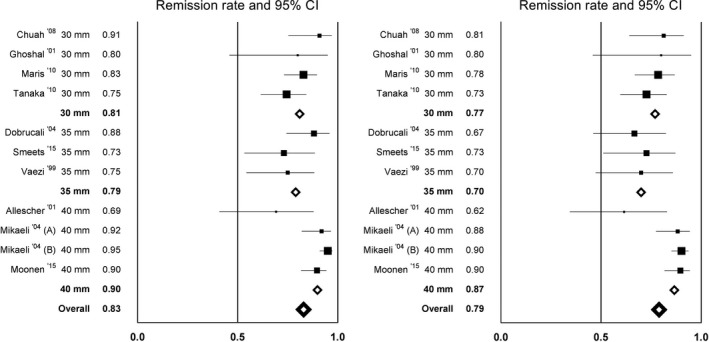
Remission rates per article after 6 mo (left) and 12 mo (right) of follow‐up, comparing dilation up to 30, 35, and 40 mm
Dilation up to 30 mm led to a mean clinical remission rate of 81% after 6 months and 77% after 12 months (Figure 2). Four articles were included in this subgroup, with a mean follow‐up time of 28 months and a total of 180 patients at 12 months follow‐up. Chuah et al17 and Tanaka et al18 treated the patients with one 30‐mm dilation. These two studies show very different success rates, ranging from 75% to 91% after 6 months and from 73% to 81% after 12 months. In the study by Tanaka et al,18 the lowest efficacy rates were found and also the lowest inflation pressure was used (Table 3). Ghoshal et al19 and Maris et al20 both initially dilated with 30 mm and repeated dilation with 30 mm when symptoms recurred. Their success rates were 80%‐83% after 6 months and 80%‐78% after 12 months, respectively. Maris et al20 offered their patients up to three dilations with a 30‐mm balloon, based on symptom recurrence. All of these studies used an inflation time of 60 seconds, with inflation pressures varying between 3 and 15 psi.
Table 3.
Perforation rates per study
| Author (y) | Treatment protocola |
Perforation n (%) |
Initial/subsequent | Inflation time (s) | Inflation pressure (psi) |
|---|---|---|---|---|---|
| Perforation | |||||
| Chuah (2008)17 | 30 | 1/33 (3%) | Initial | 60 + 30 | 12 + 12 |
| Dobrucali (2004)22 | 30 − 35 − 35 | 1/42 (2.3%) | Initial | 60 | 15 |
| Vaezi (1999)21 | 30 − 35 | 1/24 (4.2%) | Initial | 60 | 9 − 15 |
| Moonen (2016)24, b | 30 + 35 − 40 | 3/96 (3.1%) 2/96 (2%) | Initial subsequent | 60 + 60 | 5 + 8 |
| Moonen (2016)24, b | 35 | 4/13 (32%) | Initial | 60 + 60 | 5 + 8 |
| Mikaeli (2004)5, b | 35 – 40 − 40 | 3/62 (5%) | Initial | 10 | 10 |
| No perforation | |||||
| Tanaka (2010)18 | 30 | 0/55 | ‐ | 60 + 60 + 60 | 3 − 4 + 4 − 5 + 5 − 7 |
| Ghoshal (2001)19 | 30 − 30 | 0/10 | ‐ | 60 | 10 − 15 |
| Maris (2010)20 | 30 − 30 − 30 | 0/82 | ‐ | 60 − 180 | 9 |
| Smeets (2015)23 | 30 + 35 | 0/26 | ‐ | 180 | 5 |
| Mikaeli (2004)5, b | 30 − 35 − 40 | 0/200 | ‐ | 10 | 10 |
| Not specified | |||||
| Allescher (2001)6 | 35 − 40 | ‐ | ‐ | 120 | 8 − 10 |
Bold numbers represent the dilation where a perforation occurred.
−, Not specified; n, number; psi, pound‐force per square inch; s, seconds.
+ = predefined scheme of two dilations; − = a next dilation only in case of symptom recurrence.
Two different dilation protocols are used, which are presented separately.
Dilation protocols up to 35 mm resulted in a comparable mean clinical remission rate: 79% after 6 months and 70% after 12 months (Figure 2). Three studies are included in this group. The total number of included patients at 12 months is 92 patients, with a mean follow‐up of 28 months. Hence, not all patients were dilated with a 35‐mm balloon, depending on the treatment protocol. Overall, 55% of patients received a 35‐mm dilation. Vaezi et al21 and Dobrucali et al22 used a comparable dilation protocol, namely 30 mm followed by 35 mm within a few weeks in patients with insufficient symptom relief. When necessary, Dobrucali et al22 repeated the 35‐mm dilation one extra time. Vaezi et al had a success rate of 75% after 6 months. Dobrucali et al had a considerably higher success rate of 88% on the 6‐month interval, although this decreased to only 54% after 5 years of follow‐up. In the last study, by Smeets et al,23 a success rate of 73% after 6 months was attained, comparable to Vaezi et al, even though all patients underwent two dilations (30 mm followed by 35 mm within a few weeks) and the duration of balloon inflation was longer (180 s). However, they used a low inflation pressure of 5 psi, whereas the other studies used a pressure of 10‐15 psi (Table 3).
The average success rate of dilation up to 40 mm was considerably higher than dilation to 30 or 35 mm: 90% after 6 months and 87% after 12 months (Figure 2). The total number of included patients at 6 and 12 months was 371 and 348, respectively, with a mean follow‐up of 76 months. Three studies are included in this group. Again, not all patients but only the minority with insufficient effect of 35‐mm dilation (16%) received a 40‐mm dilation. Mikaeli et al5 used two different treatment protocols: 35 − 40 − 40 mm dilation (group A) and 30 − 35 − 40 mm dilation (group B), and will therefore be described as two different studies. Moonen et al24 used the same balloon sizes as in group B of Mikaeli. These two studies showed the highest success rates attained with dilation up to 40 mm, both after 6 months (95% and 90%) and after 12 months (both 90%). Allescher et al6 described the lowest success rates at the 6‐month (69%) and 12‐month interval (62%), using 35‐40 mm as gradation protocol. All studies using dilation up to 40 mm used inflation pressures of 8‐10 psi for 60 seconds (Table 3). Using a higher inflation pressure does not result in an increased success rate after 12 months (P = 0.22).
3.4. Comparing efficacy between different dilation protocols
We also compared studies that followed a predefined series of dilations (three studies) with studies that performed elective additional dilations in patients that had persisting or recurrent symptoms (seven studies). The second group had a higher remission rate after 6 months (88%) and 12 months of follow‐up (86%), when compared to the group that underwent dilations according to a predefined protocol (78% and 75%). The additional dilation group, however, had a wider range of final success rates (Figure 3). Regarding the studies performing a predefined series of dilations, 2 out of 3 only dilated up to 30 mm: Chuah et al17 and Tanaka et al,18 and one study dilated up to 35 mm: Smeets et al.23 This partially explains the lower remission rates in the predefined treatment group when compared to the elective additional dilation group.
Figure 3.
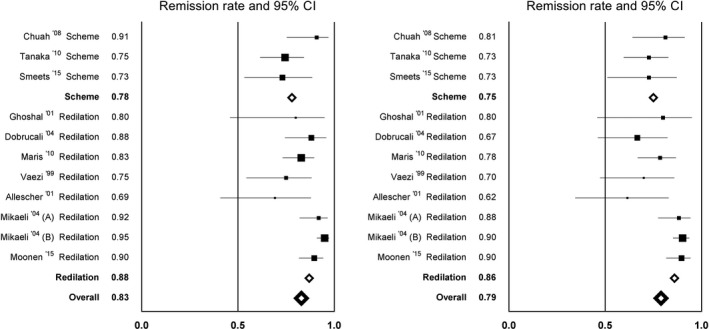
Remission rates per article after 6 mo (left) and 12 mo (right) of follow‐up, comparing studies that used a predefined dilation protocol (“Scheme”) with studies in which additional dilations were performed when symptom recurrence occurred (“Redilation”)
3.5. Complications
All studies except one reported the occurrence of complications such as perforations (Table 3). In 7 of 10 studies, a perforation occurred in one or more patients. The risk of perforation using a 30‐mm balloon was very low (6/588, 1.0%) and, interestingly, all of these perforations occurred during the initial dilation. When only initial dilations were considered, the chance of perforation with a 35‐mm balloon was significantly higher (7/75, 9.3%) than with a 30‐mm balloon (6/568, 1.1%); P < 0.001. In one study,24 a high perforation rate during initial balloon dilation of 35 mm was encountered (4/13 patients, 32%), prompting a change of protocol into starting with a 30‐mm balloon.
When all dilations (initial and subsequent dilations together) were considered, the chance of perforation using a 35‐mm balloon was significantly higher (9/282 patients, 3.2%) than using a 30‐mm balloon (6/588 patients, 1.0%); P < 0.001. But, when only looking at 35‐mm dilations, a subsequent dilation was evidently safer than an initial dilation (0.97% vs 9.3% perforations), P = 0.008. Surprisingly, none of the 62 dilations with 40‐mm balloons caused a perforation.
There are little data available on other side effects. After dilation up to 30 mm, two studies described chest pain after the procedure in 2 of 10 (20%) and 7 of 81 (8.6%) patients. This pain occurred directly after dilation and reduced within 48 hours. Symptoms suggestive of gastroesophageal reflux were reported by 6.9% of patients that received a dilation up to 35 mm. After 40‐mm dilation, no side effects were specified.
3.6. Inflation time and inflation pressure
Inflation time and pressure did not seem to influence treatment efficacy and perforation risk. The inflation time did not correlate with the success rate after 12 months (r −0.554; P = 0.097), or with the perforation rate (r −0.512; P = 0.130). Also, there was no correlation between inflation pressure and treatment efficacy after 12 months (r 0.361; P = 0.305), or between inflation pressure and risk of perforation (r 0.378; P = 0.281).
4. DISCUSSION
In this large systematic review and meta‐analysis, we compared different pneumatic dilation treatment protocols in patients with primary achalasia. Regarding efficacy, we found that elective additional dilation with 40 mm increases the success rate after an initial 30‐mm and 35‐mm dilation and that, in general, elective additional dilation is slightly more successful than following a predefined dilation protocol. Regarding safety, we found that perforations occurred significantly more often when the first dilation was performed using a 35‐mm balloon than when the first dilation was performed with a 30‐mm balloon. A subsequent 35‐mm dilation was significantly safer than initial dilation with a 35‐mm balloon. No perforations were described in patients undergoing a 40‐mm dilation.
We conclude that the safest and most efficient dilation method for patients with primary achalasia is to start with a 30‐mm balloon, followed by an elective 35‐mm and an elective 40‐mm balloon dilation in patients with insufficient symptom relief. It is surprising that we could not find a higher efficacy of 35 mm after 30 mm. Numerous previous studies did report additional benefit of a 35‐mm dilation after a 30‐mm dilation in a subgroup of patients.5, 21, 22 Our relatively low efficacy of dilation up to 35 mm could be caused by the smaller sample size in this group, as compared to the number of patients dilated with a 30‐mm or 40‐mm balloon.
Although we did not find additional benefit from a 35‐mm balloon dilation after initial 30‐mm dilation, dilation up to 40 mm gave higher remission rates than 30 and 35 mm (90% vs 77%‐81% after 6 months). It is hard to distinguish whether the additional benefit of a 40‐mm dilation is caused by the larger balloon size or by the higher number of dilations, because it was always performed in a series of two or three dilations. It is most likely a combination of the two. A previous large study calculated that a series of three dilations was significantly more successful higher than one or two dilations.25 Furthermore, a cumulatively rising efficacy per larger balloon diameter has been reported, with the highest efficacy of a graded series dilations up to 40 mm.3 This is in line with our findings and also with the recommendation of the current ACG guideline for achalasia: a graded series of balloon dilations, starting with 30 mm and using a larger diameter in patients who continue to be symptomatic, up to 40 mm.3 On the other hand, in patients after previous Heller myotomy, it has been described that patients with insufficient symptom relief after a 35‐mm dilation will not experience any improvement from a 40‐mm dilation.26
Clearly, the use of a larger balloon can only be justified when the benefits outweigh the risks. For 35 and 40 mm, there seems to be a certain benefit. Unfortunately, there also seems to be an undeniable increase in perforation risk. As expected, perforations occurred significantly more often during 35‐mm dilations than during 30‐mm dilations, even when not only looking at initial dilations (3.8% vs 0.6%, P < 0.001). The dilation protocol used by Moonen et al24 starting with a 35‐mm balloon and inflating it twice for 60 seconds resulted in a high perforation rate, despite the fact that low inflation pressures (5 and 8 psi) were used. This suggests that the size of the balloon plays a role.24 The majority of the perforations in our review occurred during the initial dilation, and more often with 35 mm than 30 mm. This again stresses the importance of a graded approach. Surprisingly, no perforation occurred with the usage of 40‐mm balloon, although the number of studies using this balloon size was small. It must be considered that all studies used a graded approach with 40 mm as the final step, which is also in line with the hypothesis of a graded approach being safer.
Our data suggest that inflation pressure does not influence the success rates of pneumatic dilation. Clinical experience tells us that even low pressures of 5‐8 psi are enough to completely open the balloon and entirely eliminate shouldering. Furthermore, we have no indication that a high inflation pressure is more likely to cause perforations. Theoretically, once the balloon is completely filled with air and the waist of the tight LES has gone, it should not make a difference whether the pressure in the balloon is high or low as the diameter will be the same. The recommendation of the current ACG guideline is 8‐15 psi for 15‐60 seconds.3 The characteristics of the patients, however, need to be taken into account. For example, one study described perforation in a short Taiwanese patient with a very low body mass.17 In children, often the same balloon sizes are used as in adults and perforations occur more often with larger balloons.27
In almost all studies, the remission rates eventually decrease with time, which suggests that pneumatic dilation is for many patients a temporary solution for their achalasia symptoms. The higher efficacy of additional dilation in patients with symptom recurrence, rather than following a predefined treatment protocol, also suggests a temporary effect.7 The mechanism of effect of pneumatic dilation is not completely understood.28 A previous study showed only stretching of the LES and no muscular disruption on endoscopic ultrasound after pneumatic dilation.28 Hypothetically, regeneration of the muscle fiber cells could cause the temporary effect. Another reason could be the progressive neurodegenerative character of achalasia that can cause symptom increase. Unfortunately, there are no data to support these hypotheses.
In conclusion, in untreated achalasia patients, an initial 30‐mm balloon dilation followed by an elective 35‐mm and 40‐mm balloon dilation in patients with persisting or recurrent symptoms results in the optimal therapeutic efficacy with acceptable perforation risks. Although using a 35‐mm balloon in the first dilation session increases the risk of perforation, dilation to 35 and 40 mm is relatively safe when it is preceded by a 30‐mm dilation.
CONFLICT OF INTEREST
AB received research funding from Endostim, Medical Measurement Systems, Danone and given and received speaker and/or consulting fees from MMS, Astellas, AstraZeneca and Almirall. LP, FH, and AS have no conflict of interest to declare.
AUTHOR CONTRIBUTIONS
FH involved in study concept and design, study selection, data extraction, quality assessment, interpretation of results, drafting of the manuscript, and approval of final submitted draft; LP contributed to the literature search, study selection, data extraction, quality assessment, and approval of final submitted draft; AS and AB involved in study concept and design, interpretation of results, reviewing the manuscript for important intellectual content, and approval of final submitted draft.
Supporting information
van Hoeij FB, Prins LI, Smout AJPM, Bredenoord AJ. Efficacy and safety of pneumatic dilation in achalasia: A systematic review and meta‐analysis. Neurogastroenterol Motil. 2019;31:e13548 10.1111/nmo.13548
REFERENCES
- 1. Pandolfino JE, Gawron AJ. Achalasia: a systematic review. JAMA. 2015;313(18):1841‐1852. [DOI] [PubMed] [Google Scholar]
- 2. Cheng P, Shi H, Zhang Y, et al. Clinical effect of endoscopic pneumatic dilation for achalasia. Medicine (Baltimore). 2015;94(28):e1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vaezi MF, Pandolfino JE, Vela MF. ACG clinical guideline: diagnosis and management of achalasia. Am J Gastroenterol. 2013;108(8):1238‐1249. [DOI] [PubMed] [Google Scholar]
- 4. Hirano I. Pathophysiology of achalasia. Curr Gastroenterol Rep. 1999;1(3):198‐202. [DOI] [PubMed] [Google Scholar]
- 5. Mikaeli J, Bishehsari F, Montazeri G, Yaghoobi M, Malekzadeh R. Pneumatic balloon dilatation in achalasia: a prospective comparison of safety and efficacy with different balloon diameters. Aliment Pharmacol Ther. 2004;20(4):431‐436. [DOI] [PubMed] [Google Scholar]
- 6. Allescher HD, Storr M, Seige M, et al. Treatment of achalasia: botulinum toxin injection vs. pneumatic balloon dilation. A prospective study with long‐term follow‐up. Endoscopy. 2001;33(12):1007‐1017. [DOI] [PubMed] [Google Scholar]
- 7. Khan AA, Shah SW, Alam A, Butt AK, Shafqat F. Sixteen years follow up of achalasia: a prospective study of graded dilatation using Rigiflex balloon. Dis Esophagus. 2005;18(1):41‐45. [DOI] [PubMed] [Google Scholar]
- 8. Vaezi MF, Richter JE. Current therapies for achalasia: comparison and efficacy. J Clin Gastroenterol. 1998;27(1):21‐35. [DOI] [PubMed] [Google Scholar]
- 9. Stang A. Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol. 2010;25(9):603‐605. [DOI] [PubMed] [Google Scholar]
- 10. OCEBM . Levels of Evidence Working Group. The Oxford Levels of Evidence 2. Oxford Centre for Evidence‐Based Medicine. http://wwwcebmnet/indexaspx?o=5653. Accessed December 20, 2017.
- 11. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21(11):1539‐1558. [DOI] [PubMed] [Google Scholar]
- 12. Ding PH. Endoscopic pneumatic balloon dilatation for achalasia of the cardia. Med J Malaysia. 1995;50(4):339‐345. [PubMed] [Google Scholar]
- 13. Yamashita H, Ashida K, Fukuchi T, et al. Predictive factors associated with the success of pneumatic dilatation in Japanese patients with primary achalasia: a study using high‐resolution manometry. Digestion. 2013;87(1):23‐28. [DOI] [PubMed] [Google Scholar]
- 14. Ahmed WU, Qureshi H, Maher M, Arif A. Achalasia in a gastroenterology unit of Karachi. J Pak Med Assoc. 2008;58(12):661‐664. [PubMed] [Google Scholar]
- 15. Muehldorfer SM, Hahn EG, Ell C. High‐ and low‐compliance balloon dilators in patients with achalasia: a randomized prospective comparative trial. Gastrointest Endosc. 1996;44(4):398‐403. [DOI] [PubMed] [Google Scholar]
- 16. Khan AA, Shah SW, Alam A, Butt AK, Shafqat F, Castell DO. Pneumatic balloon dilation in achalasia: a prospective comparison of balloon distention time. Am J Gastroenterol. 1998;93(7):1064‐1067. [DOI] [PubMed] [Google Scholar]
- 17. Chuah SK, Hu TH, Wu KL, et al. Endoscope‐guided pneumatic dilatation of esophageal achalasia without fluoroscopy is another safe and effective treatment option: a report of Taiwan. Surg Laparosc Endosc Percutan Tech. 2008;18(1):8‐12. [DOI] [PubMed] [Google Scholar]
- 18. Tanaka Y, Iwakiri K, Kawami N, et al. Predictors of a better outcome of pneumatic dilatation in patients with primary achalasia. J Gastroenterol. 2010;45(2):153‐158. [DOI] [PubMed] [Google Scholar]
- 19. Ghoshal UC, Chaudhuri S, Pal BB, Dhar K, Ray G, Banerjee PK. Randomized controlled trial of intrasphincteric botulinum toxin A injection versus balloon dilatation in treatment of achalasia cardia. Dis Esophagus. 2001;14(3–4):227‐231. [DOI] [PubMed] [Google Scholar]
- 20. Maris T, Kapetanos D, Ilias A, Augerinos A, Xiarhos P, Kitis G. Mid term results of pneumatic balloon dilatation in patients with achalasia. Ann Gastroenterol. 2010;23(1):61‐63. [Google Scholar]
- 21. Vaezi MF, Richter JE, Wilcox CM, et al. Botulinum toxin versus pneumatic dilatation in the treatment of achalasia: a randomised trial. Gut. 1999;44(2):231‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dobrucali A, Erzin Y, Tuncer M, Dirican A. Long‐term results of graded pneumatic dilatation under endoscopic guidance in patients with primary esophageal achalasia. World J Gastroenterol. 2004;10(22):3322‐3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Smeets F, Masclee A, Keszthelyi D, Tjwa E, Conchillo JM. Esophagogastric junction distensibility in the management of achalasia patients: relation to treatment outcome. Neurogastroenterol Motil. 2015;27(10):1495‐1503. [DOI] [PubMed] [Google Scholar]
- 24. Moonen A, Annese V, Belmans A, et al. Long‐term results of the European achalasia trial: a multicentre randomised controlled trial comparing pneumatic dilation versus laparoscopic Heller myotomy. Gut. 2016;65(5):732‐739. [DOI] [PubMed] [Google Scholar]
- 25. Vela MF, Richter JE, Khandwala F, et al. The long‐term efficacy of pneumatic dilatation and heller myotomy for the treatment of achalasia. Clin Gastroenterol Hepatol. 2006;4(5):580‐587. [DOI] [PubMed] [Google Scholar]
- 26. Saleh CM, Ponds FA, Schijven MP, Smout AJ, Bredenoord AJ. Efficacy of pneumodilation in achalasia after failed Heller myotomy. Neurogastroenterol Motility. 2016;28(11):1741‐1746. [DOI] [PubMed] [Google Scholar]
- 27. Smits M, van Lennep M, Vrijlandt R, et al. Pediatric achalasia in the Netherlands: incidence, clinical course, and quality of life. J Pediatr. 2016;169(110–115):e113. [DOI] [PubMed] [Google Scholar]
- 28. Borhan‐Manesh F, Kaviani MJ, Taghavi AR. The efficacy of balloon dilation in achalasia is the result of stretching of the lower esophageal sphincter, not muscular disruption. Dis Esophagus. 2016;29(3):262‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


