Abstract
Background
Gastroenteritis has been associated with complications such as irritable bowel syndrome (IBS) and chronic fatigue (CF). Little is known about the implications for quality of life (QoL) in this setting. The aims of this study were to evaluate the association between exposure to Giardia infection and QoL ten years after the infection, and how this related to IBS and CF.
Methods
We followed 1252 patients with laboratory‐verified Giardia lamblia infection and a matched control group for 10 years after an epidemic in Bergen, Norway, in 2004. The main outcome was QoL after ten years as defined by the Short‐form 12 version 2 with a physical component summary (PCS) and a mental component summary (MCS), both with range 0‐100 (T‐score). Regression analyses were performed using mixed modeling.
Key Results
Mean PCS T‐score in the exposed group (51.4; 95% CI: 50.6‐52.1) was 2.8 T‐score points (95% CI: −3.8 to −1.9, P < 0.001) lower than that in the control group (54.2; 95% CI: 53.7‐54.8). The mean MCS T‐score was also 2.8 T‐score points (95% CI: −3.8 to −1.9, P < 0.001) lower among the exposed (48.9; 95% CI: 48.2‐49.6) than the controls (51.7; 95% CI: 51.1‐52.4). Further analyses found that the effect of Giardia exposure on QoL was mediated by IBS and CF.
Conclusions & Inferences
Exposure to Giardia infection was associated with a lower QoL ten years later as compared to a control group, an effect that was mediated by IBS and CF.
Keywords: epidemiology, infectious disease, Irritable bowel syndrome, quality of life
We assessed quality of life ten years following a Giardia lamblia infection and found that exposure to Giardia was associated with a reduced quality of life as compared to a control group. The reduced quality of life was mainly due to the coexistence of irritable bowel syndrome and chronic fatigue.

Abbreviations
- CF
chronic fatigue
- CFS
chronic fatigue syndrome
- CI
confidence interval
- IBS
irritable bowel syndrome
- MCS
mental component summary
- OR
odds ratio
- PCS
physical component summary
- QoL
Quality of life
- SD
Standard deviation
- SF‐12v2
Short‐form 12 version 2
Key Points.
Irritable bowel syndrome (IBS) and fatigue are known complications following gastroenteritis. This paper assessed the quality of life ten years after a Giardia lamblia gastroenteritis and how this related to IBS and fatigue.
Quality of life was lower among patients who suffered from gastroenteritis, mainly due to the development of IBS and fatigue.
Clinicians should be aware that gastroenteritis can have a lasting impact on quality of life in patients, especially in those who have long‐term complications.
1. INTRODUCTION
Gastrointestinal infections can lead to long‐term complications after the microbial agent has been eradicated. Post‐infectious irritable bowel syndrome (PI‐IBS) has been recognized for decades1 and is clinically similar to sporadic irritable bowel syndrome (IBS). Chronic fatigue syndrome (CFS) is another known condition following some infections,2 including giardiasis.3, 4 Chronic fatigue (CF) is a useful and validated concept in epidemiologic studies where clinical examination is not feasible.5 CF has also been found to be a long‐lasting complication after giardiasis.6, 7, 8 Few studies have investigated quality of life (QoL) after gastrointestinal infections. One study found that QoL was impaired six months after Shiga toxin‐producing Escherichia coli gastroenteritis, and that the physical QoL normalized after one year, whereas the mental QoL remained impaired.9 CFS and IBS have been shown to affect QoL.10, 11, 12, 13, 14
In 2004, one of the main drinking‐water reservoirs of the city of Bergen, Norway, was contaminated by Giardia lamblia cysts. IBS and CF were associated with exposure to Giardia infection as long as ten years after the outbreak,6 but how this may affect QoL is not well known. The main aim of this study was to evaluate the association between exposure to Giardia infection and QoL ten years after the Bergen outbreak, as compared to a control group. The secondary aim was to assess how QoL related to IBS and CF in the exposed and the control group.
2. MATERIALS AND METHODS
2.1. Design and participants
This was a prospective cohort study following 1252 infected patients with laboratory‐verified Giardia lamblia (the exposed group) and a control group three, six, and ten years after an epidemic of giardiasis in Bergen, Norway, 2004. The group of 2504 controls was matched 2:1 by sex and age to the exposed group and was recruited from the Bergen area by Statistics Norway, on our request. We only included participants who were 18 years or older in 2014 for this study.
Patients consented to participate upon answering the questionnaire. The Regional Committee for Ethics in Medical Research approved the study (ref.no. 2014/1372).
2.2. Variables
Health‐related QoL was the main outcome of the study, as measured by the Short‐form 12 version 2 (SF‐12v2). The SF‐12v2 consists of a physical component summary (PCS) and a mental component summary (MCS), measuring physical and mental QoL, respectively. The PCS and MCS range from 0‐100 and are based on a 2009 US norm for QoL with a mean of 50 and a standard deviation of 10. The points on the scale are referred to as T‐score points. The two scores are based on the score of eight sub‐scales, that is, physical functioning, role‐physical (how the physical QoL affects daily functioning), bodily pain, general health, vitality, social functioning, role‐emotional (how the mental QoL affects daily functioning), and mental health. Although all eight sub‐scales contribute to the scoring of both PCS and MCS, the former three have the strongest correlation with PCS, and the latter three have the strongest correlation with MCS. Also, the use of an orthogonal scoring algorithm applies negative weights to sub‐scales most strongly correlated with MCS when scoring the PCS (and vice versa), ensuring validity in discriminating between physical and mental health outcomes. In addition, PCS and MCS scores were both dichotomized based on a score of 45 and above, or lower than 45. Scoring of the QoL variables was done using the QualityMetric Health Outcomes™ Scoring Software 5.0, as recommended by the developers of the SF‐12.15 The software's option to estimate missing scores was used. We used PCS and MCS means and standard deviations from Gandek et al16 to compare our results with a Norwegian norm. The SF‐12 was translated to Norwegian and validated for use on a Norwegian population as part of that study. The developers of the SF‐12 suggest that when comparing QoL between groups, a difference in three or more T‐score points is considered clinically important.15
IBS was defined according to the Rome‐III criteria.17 Respondents were defined as having IBS if reporting recurrent abdominal pain or discomfort for at least three days per month in the last three months, associated with at least two or more of the additional IBS‐criteria related to defecation or stool changes, if onset of symptoms was at least 6 months prior to completing the questionnaire.
CF was defined using the Fatigue Questionnaire.5 This validated questionnaire consists of 13 questions, where 11 of these measure different aspects of fatigue on a four‐item Likert scale: “less than usual” (0), “as usual” (1), “more than usual” (2), and “much more than usual” (3). The sum of these scores constitutes the total fatigue score with a range of 0‐33. The Likert scale scores are also dichotomized (0 and 1 into 0, 2 and 3 into 1), and CF is defined as a dichotomized score of four or more and a duration of six months or more. Cases with 4 or less missing answers on the 11 fatigue‐related questions were included, and the missing responses for the questions were estimated based on the average for non‐missing responses to that particular question.
IBS and CF were assessed at follow‐ups of 3, 6, and 10 years after the outbreak, whereas QoL was assessed only at the 10‐year follow‐up.
To better assess and illustrate the relationships between exposure status, IBS and CF (all dichotomous), and the outcomes PCS and MCS, the three former variables were combined into one eight‐category variable with the categories “Neither condition among controls” (reference category in regression analyses), “Neither condition among exposed”, “IBS‐only among controls”, “IBS‐only among exposed”, “CF‐only among controls”, “CF‐only among exposed”, “IBS and CF among controls”, and “IBS and CF among exposed”.
Demographic variables recorded were sex (dichotomous), age (continuous and categorized according to the SF12v2 user's manual15), marital status (four categories), level of education (three categories), and source of income (four categories).
2.3. Analyses and statistical methods
We calculated descriptive statistics as percentage, mean, standard deviation (SD), and 95% confidence intervals (CI). We used Fisher's exact 2‐sided mid‐p test in 2 × 2 tables for binary outcomes18 and Pearson's chi‐square exact 2‐sided test for multilevel outcomes.
To account for dependence between matched subjects, we used mixed modeling with unstructured covariance when performing regression analyses.
All means presented are the observed means, to give the reader the unadjusted values of PCS and MCS for the different study groups. All differences between means presented are estimated, as they are the results of regression analyses, and hence, they do not necessarily equal the crude differences between the observed means.
Confounding was evaluated with regression analyses. Level of education, source of income, and marital status were considered potential confounders. Sex and age were matched for and hence were not considered potential confounders. Possible interactions from IBS, CF, sex, or age on the effect of exposure on PCS and MCS were evaluated in the regression models and with the Breslow‐Day test for homogeneity of the OR in stratified cross‐tabulations.
All tests were two‐sided with the level of statistical significance set to 0.05. The analyses were done using IBM SPSS Statistics for Windows, version 25.0 (IBM Corp, Armonk, NY, USA).
3. RESULTS
Response rate, demographic data, prevalence of IBS and CF, and non‐responder analyses of this cohort ten years after the Giardia outbreak have been published previously,6 and some of these data are summarized in Table 1. For PCS, there were 0.3% missing values among exposed (2/590) and 0.9% missing among controls (6/696). For MCS, there were no missing values. For IBS, there were 14 missing out of 590 (2.4%) among exposed and 11 of 696 (1.6%) among controls. For CF, the corresponding numbers were 3/590 (0.5%) and 4/696 (0.6%), respectively.
Table 1.
Response rate, demographics, and prevalence of irritable bowel syndrome and chronic fatigue of the cohorts ten years after a Giardia lamblia outbreak in Bergen, Norway, in 2004
| Characteristics | Respondents who answered at ten‐year follow‐up, N = 1286 | ||||
|---|---|---|---|---|---|
| Exposed (n = 590) | Controls (n = 696) | P‐valueb | |||
| n | %a | n | %a | ||
| Response rate | 592/1176 | 50.3 | 708/2330 | 30.4 | |
| Female sex | 395 | 66.9 | 455 | 65.4 | NAc |
| Age in years | |||||
| Mean/range | 42.9d | 18‐88d | 43.6d | 18‐89d | NAc |
| 18‐24 | 12 | 2.0 | 9 | 1.3 | NAc |
| 25‐34 | 174 | 29.5 | 184 | 26.4 | |
| 35‐44 | 189 | 32.0 | 258 | 37.1 | |
| 45‐54 | 103 | 17.5 | 98 | 14.1 | |
| 55‐64 | 72 | 12.2 | 88 | 12.6 | |
| 65‐74 | 32 | 5.4 | 44 | 6.3 | |
| 75‐89 | 8 | 1.4 | 15 | 2.2 | |
| Marital status | |||||
| Single | 124 | 21.1 | 113 | 16.3 | 0.04 |
| Married | 423 | 71.9 | 536 | 77.1 | |
| Divorced | 35 | 6.0 | 32 | 4.6 | |
| Widowed | 6 | 1.0 | 14 | 2.0 | |
| Education | |||||
| Primary school | 23 | 3.9 | 31 | 4.5 | 0.31 |
| Secondary school | 128 | 21.9 | 172 | 25.1 | |
| University | 434 | 74.2 | 481 | 70.3 | |
| Main occupation | |||||
| Worker | 478 | 81.2 | 580 | 83.6 | 0.30 |
| Student | 16 | 2.7 | 16 | 2.3 | |
| Unemployed/retired | 78 | 13.2 | 88 | 12.7 | |
| Other | 17 | 2.9 | 10 | 1.4 | |
| IBS prevalence | 248 | 43.1 | 94 | 13.7 | <0.001 |
| CF prevalence | 153 | 26.1 | 73 | 10.5 | <0.001 |
CF, chronic fatigue; IBS, irritable bowel syndrome.
Percentages may not total to 100 because of rounding.
Pearson's chi‐squared exact 2‐sided, except for IBS and CF (Fisher's exact 2‐sided mid‐p).
NA = not applicable; respondents were matched on sex and age, and hence, we did not perform significance testing for these variables.
Mean age, age range.
Mean QoL T‐score for the entire cohort regardless of group was 52.9 (SD: 8.7) for PCS and 50.4 (SD: 9.1) for MCS.
Mean PCS T‐score in the exposed group (51.4; 95% CI: 50.6‐52.1) was 2.8 T‐score points (95% CI: −3.8 to −1.9; P < 0.001) lower than for the control group (54.2; 95% CI: 53.7‐54.8). The mean MCS T‐score was also 2.8 T‐score points (95% CI: −3.8 to −1.9, P < 0.001) lower among the exposed (48.9; 95% CI: 48.2‐49.6) than the controls (51.7; 95% CI: 51.1‐52.4; Figure 1).
Figure 1.
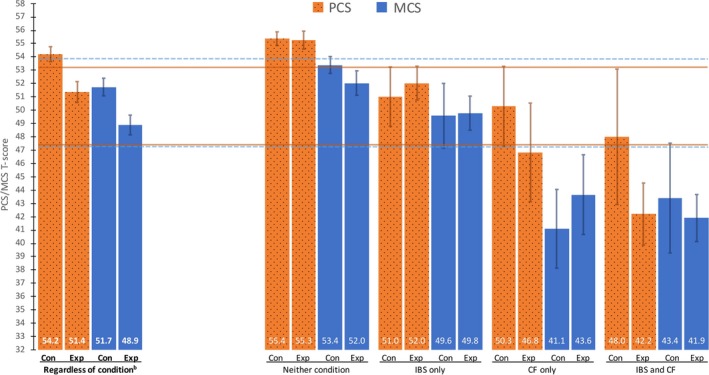
Observed mean PCS and MCS with 95% confidence intervals 10 years after a Giardia lamblia outbreak in Bergen, Norway, in 2004, as compared to a Norwegian Norma. PCS, physical component summary; MCS, mental component summary; IBS, irritable bowel syndrome; CF, chronic fatigue; Con, controls; Exp, exposed to Giardia. aSF‐12 scores for a Norwegian sample population from Gandek et al.16 The horizontal lines on the figure are one‐third of a standard deviation T‐score points under/over the mean T‐score from that population. Dotted for MCS, solid for PCS. bThe first four columns (with bold labels) depict PCS and MCS according to exposure status. The next sixteen columns depict PCS and MCS according to the eight‐category variable described in the methods section
“Neither condition among controls” (after ten years) was the reference category in regression analyses and was the subgroup with the highest QoL for both PCS and MCS (Tables 2 and 3), with mean T‐scores of 55.4 (95% CI: 54.9‐55.9) and 53.4 (95% CI: 52.8‐54.0), respectively. The exposed with neither condition after ten years had the same PCS (mean T‐score difference: −0.1; 95% CI: −1.2 to 1.0; P‐value: 0.84), but a lower MCS (mean T‐score difference: −1.4; 95% CI: −2.5 to −0.2; P‐value: 0.023) than the reference category. All other categories in the eight‐category variable described in the methods section had a lower QoL than the reference category, both for PCS and for MCS. This eight‐category variable was also analyzed with PCS and MCS as a dichotomized outcome with a T‐score below 45 points or not (Tables 2 and 3). Over 50% of the respondents in the category “IBS and CF among exposed” had a PCS lower than 45 points. For MCS, all categories comprising respondents with CF‐only or CF and IBS had over 50% of respondents with an MCS score below 45.
Table 2.
Quality of life, physical component summary analyzed by simple regression analyses, as continuous and dichotomized variable according to exposure group 10 years after a Giardia lamblia outbreak in Bergen, Norway, in 2004 (N = 1247)
| Exposure group | N | Mean/difference | 95% CI for mean/difference | P‐value | PCS score below 45 | |||
|---|---|---|---|---|---|---|---|---|
| n | % | OR | 95% CI | |||||
| Neither condition among controls | 532 | 55.4a | 54.9 to 55.9 | ref | 35 | 6.6 | ref | ref |
| Neither condition among exposed | 280 | −0.1 | −1.2 to 1.0 | 0.84 | 18 | 6.4 | 1.0 | 0.6‐1.8 |
| IBS‐only among controls | 75 | −4.4 | −6.2 to −2.5 | <0.001 | 17 | 22.7 | 4.4 | 2.3‐8.3 |
| IBS‐only among exposed | 146 | −3.3 | −4.7 to −1.9 | <0.001 | 20 | 13.7 | 2.4 | 1.3‐4.3 |
| CF‐only among controls | 50 | −5.1 | −7.3 to −2.9 | <0.001 | 16 | 32.0 | 6.1 | 3.0‐12.2 |
| CF‐only among exposed | 45 | −8.6 | −10.9 to −6.2 | <0.001 | 22 | 48.9 | 14.0 | 7.1‐27.6 |
| IBS and CF among controls | 18 | −7.5 | −11.0 to −3.9 | <0.001 | 6 | 33.3 | 6.9 | 2.4‐20.2 |
| IBS and CF among exposed | 101 | −13.1 | −14.8 to −11.5 | <0.001 | 55 | 54.5 | 17.4 | 10.3‐29.5 |
CF, chronic fatigue; IBS, irritable bowel syndrome; PCS, physical component summary; Ref, reference group.
Mean in reference group of eight‐category exposure variable.
Table 3.
Quality of life, mental component summary analyzed by simple regression analyses, as continuous and dichotomized variable according to exposure group ten years after a Giardia lamblia outbreak in Bergen, Norway, in 2004, (N = 1255)
| Exposure group | N | Mean/difference | 95% CI for mean/difference | P‐value | MCS score below 45 | |||
|---|---|---|---|---|---|---|---|---|
| n | % | OR | 95% CI | |||||
| Neither condition among controls | 536 | 53.4a | 52.8 to 54.0 | ref | 67 | 12.5 | ref | ref |
| Neither condition among exposed | 282 | −1.4 | −2.5 to −0.2 | 0.023 | 50 | 17.7 | 1.6 | 1.0‐2.3 |
| IBS‐only among controls | 75 | −3.8 | −5.8 to −1.8 | <0.001 | 15 | 20.0 | 1.7 | 0.9‐3.3 |
| IBS‐only among exposed | 146 | −3.6 | −5.1 to −2.1 | <0.001 | 33 | 22.6 | 2.1 | 1.3‐3.3 |
| CF‐only among controls | 51 | −12.3 | −14.7 to −10.0 | <0.001 | 30 | 58.8 | 9.6 | 5.2‐17.8 |
| CF‐only among exposed | 45 | −9.8 | −12.2 to −7.3 | <0.001 | 23 | 51.1 | 7.6 | 4.0‐14.4 |
| IBS and CF among controls | 19 | −10.1 | −13.8 to −6.3 | <0.001 | 13 | 68.4 | 15.9 | 5.9‐43.1 |
| IBS and CF among exposed | 101 | −11.5 | −13.3 to −9.8 | <0.001 | 62 | 61.4 | 11.4 | 7.1‐18.4 |
CF, chronic fatigue; IBS, irritable bowel syndrome; MCS, mental component summary; Ref, reference group.
Mean in reference group of eight‐category exposure variable.
In a regression model with exposure status, IBS and CF after ten years as independent variables, and PCS as the outcome, there was no longer a significant effect of exposure status on the outcome with a mean PCS that was 0.50 T‐score points lower among the exposed (95% CI: −1.4 to 0.40; P‐value: 0.28). The same was found for mean MCS, which was 0.75 T‐score points lower among the exposed (95% CI: −1.7 to 0.22; P‐value: 0.13).
We found a significant interaction between CF and exposure status on both measures of QoL (Table 4). The mean PCS among those with CF in the exposed group was −9.2 points lower than for respondents without CF, whereas for controls, the difference was −4.7 (P < 0.001). For MCS, the relationship was inverse: The mean MCS among those with CF in the control group was −10.9 T‐score points lower than for respondents without CF, whereas for exposed, the difference was −8.0 (P‐value: 0.027). We found no significant interaction between IBS and exposure on QoL (P‐value: 0.78 for PCS; P‐value: 0.34 for MCS).
Table 4.
Physical component summary and mental component summary according to chronic fatigue status at ten years follow‐up, in exposed and control cohorts of the Giardia lamblia outbreak in Bergen, Norway, in 2004a
| Outcome | Cohort | CF | n | Mean | 95% CI for mean or difference | P‐value for interactionb | Difference between differencesc | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| PCS | Exposed | Yes | 146 | 43.7 | 41.7 | 45.6 | <0.001 | −4.5 |
| No | 426 | 54.2 | 53.5 | 54.8 | ||||
| Differenced | −9.2 | −10.7 | −7.7 | |||||
| Control | Yes | 68 | 49.7 | 47.1 | 52.2 | |||
| No | 607 | 54.8 | 54.3 | 55.4 | ||||
| Differenced | −4.7 | −6.6 | −2.7 | |||||
| MCS | Exposed | Yes | 146 | 42.4 | 40.9 | 43.9 | 0.027 | 2.9 |
| No | 428 | 51.3 | 50.5 | 52.0 | ||||
| Differenced | −8.0 | −9.6 | −6.4 | |||||
| Control | Yes | 70 | 41.7 | 39.3 | 44.1 | |||
| No | 611 | 52.9 | 52.3 | 53.6 | ||||
| Differenced | −10.9 | −12.9 | −8.9 | |||||
CF, chronic fatigue; CI, confidence interval; MCS, mental component summary (0‐100); PCS, physical component summary (0‐100).
In a linear regression model including the factors cohort (exposed/control), irritable bowel syndrome (yes/no), CF (yes/no), and the interaction term cohort ×CF.
Interaction between cohort and CF on the outcomes PCS and MCS.
The difference in quality of life between CF and no CF among exposed, minus that among controls.
Means are observed, with CI. Differences are estimated in a mixed linear model and hence does not necessarily equal observed mean value for CF minus mean value for no CF.
We found no interactions of sex on the association between exposure and PCS (P‐value: 0.16) or MCS (P‐value: 0.39), nor of age on the same associations (P‐values: 0.10 and 0.056, respectively).
We also analyzed how exposure status, IBS and CF after three and six years related to QoL after ten years (Table S1). We found that for the eight‐category variable with “Neither condition among controls” at each follow‐up as reference category, the findings (in terms of direction and statistical significance of the results) were similar to the results above (after ten years) with some exceptions. In addition to the category, “Neither condition among exposed” also the category “IBS‐only among controls” after six years had the same PCS as the reference category. The categories “Neither condition among exposed” and “IBS‐only among controls” after both three and six years had the same MCS after ten years as the reference category. Corresponding analyses on dichotomized PCS and MCS after three and six years are found in Table S2. The effect of exposure on QoL after ten years was absent when controlling for IBS and CF after three and six years in a regression model, with the exception of the analysis after 6 years, where MCS after ten years was significantly reduced when controlling for IBS and CF after 6 years. The interaction between exposure and CF on QoL was not found after three or six years (Table S3).
Scores on the sub‐scales most strongly associated with MCS were assessed descriptively to further elucidate the above‐mentioned interaction between exposure status and CF on QoL (Table S4). The “vitality” subdomain had the lowest score, but this was rather similar between exposed and controls with CF‐only (mean T‐score 38.1 vs 38.4). The subdomain “mental health” was lower among controls with CF‐only (44.9) than among exposed with CF‐only (48.2).
We found no confounding of our results by the demographic variables marital status, level of education, and source of income.
4. DISCUSSION
We found a lower QoL among giardiasis‐exposed persons ten years after the exposure, as compared to a control group. This effect of the exposure to giardiasis on QoL was mediated by IBS and CF. For the association between exposure and QoL, there was an interaction between CF and exposure, as the reduction of physical QoL due to CF was larger among exposed than controls. The opposite was found for mental QoL.
4.1. Interpretation
We found that exposure to Giardia lamblia was associated with a lower QoL ten years later as compared to a control group. However, the difference between the exposed and the controls for both PCS and MCS at 2.8 T‐score points was below the proposed threshold of 3 T‐score points that is considered clinically significant. The mean PCS for the control cohort was higher than the norm for the Norwegian population, whereas the mean PCS among exposed and the mean MCS regardless of exposure group were clinically similar to this norm.16 This may in part be explained by the fact that our study population is relatively young. There is a slight decrease in PCS with age in normal populations, whereas MCS increases somewhat.16
Exposed who had neither condition had a similar PCS to controls with neither condition, whereas the MCS was slightly lower for the exposed. However, both these groups had a QoL higher than or equal to a Norwegian population norm. We found a trend that the presence of IBS alone lowered the QoL in both the exposed and control group, but less than CF alone did. The lowest QoL was found among exposed with both IBS and CF, both for PCS (42.2) and for MCS (41.9), as well as for CF‐only among controls, with an MCS of 41.1. The PCS at 42.2 is comparable to that found in other conditions such as type 2 diabetes and recent myocardial infarction.19 The MCS scores in the two above‐mentioned groups were lower than the cutoff at ≤42 T‐score points used to classify people as being at risk for clinical depression in one study.20 Respondents with IBS‐only had a QoL comparable to a Norwegian norm regardless of exposure status and comparable to a subgroup of IBS patients with low IBS‐symptom severity and no comorbidities in another study on IBS and QoL.10 The PCS of the respondents with IBS‐only in our study is also comparable to that of a group of IBS non‐consulters in a study by Rey et al,21 whereas the MCS is higher in our group. This could be explained by the fact that we have subgrouped our respondents into neither condition, IBS‐only, CF‐only or a combination of the two conditions. The MCS among respondents with IBS dropped markedly when they have comorbid CF, to a level comparable to that of Rey et al's IBS non‐consulters where CF comorbidity is unknown.
The fact that the effect of exposure status disappeared in multiple regression analysis with IBS and CF included in the model supports the choice of these conditions as clinically relevant markers of the consequences of the Giardia epidemic. IBS and CF can be seen as mediators of the effects of Giardia exposure on QoL.
We found an interaction by CF on the effects of exposure on QoL in that the reduction in PCS score among respondents due to CF was significantly larger among the exposed than among controls. For MCS, the relationship was opposite, the reduction was significantly larger among controls than the exposed with CF. A possible explanation for this could be that although both groups probably have a multifactorial cause for their CF, the respondents with CF among the exposed have a more specific organic etiology for their condition (ie, Giardia), whereas among controls mental factors could be more important. This notion is supported by the fact that the subdomain “mental health” was lower among controls with CF‐only, than among exposed with CF‐only (Table S4).
4.2. Strengths and Limitations
One strength of this study was that the use of a control group made the unfortunate event of an outbreak simulate a natural experiment. The number of participants in both groups was high, and all of the exposed had a laboratory‐confirmed diagnosis. The longitudinal aspect of the study variables IBS and CF (measured after three, six, and ten years) makes causal inferences about the relationship between these conditions and QoL after ten years plausible. We used the validated SF‐12 to measure generic QoL. We performed the scoring using the developers recommended algorithm, with the 2009 US norm. The fact that the measure is generic, widely used and has a standardized scoring algorithm, makes direct comparisons of our scores to other studies on various patient groups using the SF‐12 or SF‐36 possible.15
The response rate in the exposed group (50%) is a source of possible bias, but is deemed acceptable and as expected for this kind of survey.22 The control group response rate is lower (30%), and we have made the case against selection bias in a previous study on the same cohort.6 Also, recent research suggests that a declining response rate does not necessarily imply increasing bias in analyses of associations, although simple distributional data may suffer.22
The demographic variables recorded in our study (marital status, level of education, source of income) were measured after the exposure and hence could in part be affected by the exposure, making their role as confounders questionable.
QoL, IBS, and CF were all measured at the same time point, 10 years after the exposure. This makes inferences about the relationships between these outcomes less certain. We therefore included results from analyses of IBS and CF measured at time‐points three and six years after exposure and their effect on QoL after 10 years as well. In terms of direction of the effect and statistical significance, the results from these analyses were generally similar to our main findings, except for the fact that the interaction between CF and exposure on QoL was only found after 10 years. Nevertheless, we believe that the longitudinal analyses support the notion of IBS and CF as causes of reduced QoL and justify discussing the role of IBS and CF as mediators of the effect of exposure on QoL, as well as CF as an interacting variable on the association between exposure status and QoL.
5. CONCLUSION
We found a lower QoL among the exposed 10 years after giardiasis as compared to a control group, and this was mediated by IBS and CF. There was furthermore a significant interaction of CF on the association between the exposure and QoL. The effect of having CF in reducing the physical QoL was larger among the exposed than among controls, whereas for mental QoL, the opposite was found. The findings in this study support the importance of investigating whether patients suffer from PI‐IBS and CF after giardiasis, as these complications explain the reduced QoL in the exposed cohort in our study.
CONFLICTS OF INTEREST
All authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
SL, is the main author, has performed the analyses, and has done the main writing of the article and worked with interpretation of the results. GR, KH, NL, and KAW, contributed to the design, writing, and interpretation of the results. GEE, contributed to the analyses, design, writing, and interpretation of the results. KEE, contributed to the writing and interpretation of the results. All authors have revised the article for intellectual content and approved the final version to be published.
Supporting information
Litleskare S, Rortveit G, Eide GE, et al. Quality of life and its association with irritable bowel syndrome and fatigue ten years after giardiasis. Neurogastroenterol Motil. 2019;31:e13559 10.1111/nmo.13559
Funding information
The first author has a PhD scholarship from the University of Bergen. Bergen Municipality funded parts of the study. All researchers are independent from the sponsors. The sponsors had no role in study design, in collection, analysis, interpretation of data, or in writing or deciding to submit the manuscript.
REFERENCES
- 1. Grover M, Camilleri M, Smith K, Linden DR, Farrugia G. On the fiftieth anniversary. Postinfectious irritable bowel syndrome: mechanisms related to pathogens. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility. Society. 2014;262:156‐167. [DOI] [PubMed] [Google Scholar]
- 2. Moss‐Morris R, Deary V, Castell B. Chronic fatigue syndrome. Handb Clin Neurol. 2013;110:303‐314. [DOI] [PubMed] [Google Scholar]
- 3. Morch K, Hanevik K, Rivenes AC, et al. Chronic fatigue syndrome 5 years after giardiasis: differential diagnoses, characteristics and natural course. BMC Gastroenterol. 2013;13:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Naess H, Nyland M, Hausken T, Follestad I, Nyland HI. Chronic fatigue syndrome after Giardia enteritis: clinical characteristics, disability and long‐term sickness absence. BMC Gastroenterol. 2012;12:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chalder T, Berelowitz G, Pawlikowska T, et al. Development of a fatigue scale. J Psychosom Res. 1993;372:147‐153. [DOI] [PubMed] [Google Scholar]
- 6. Litleskare S, Rortveit G, Eide GE, Hanevik K, Langeland N, Wensaas KA. Prevalence of irritable bowel syndrome and chronic fatigue 10 years after giardia infection. Clin Gastroenterol Hepatol. 2018;167: 1064–1072.e4. [DOI] [PubMed] [Google Scholar]
- 7. Hanevik K, Wensaas KA, Rortveit G, Eide GE, Morch K, Langeland N. Irritable bowel syndrome and chronic fatigue six years after Giardia infection: a controlled prospective cohort study. Clin Infect Dis. 2014;59:1394‐1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wensaas KA, Langeland N, Hanevik K, Morch K, Eide GE, Rortveit G. Irritable bowel syndrome and chronic fatigue 3 years after acute giardiasis: historic cohort study. Gut. 2012;612:214‐219. [DOI] [PubMed] [Google Scholar]
- 9. Riegel B, Broicher W, Wegscheider K, et al. Quality of life one year post‐Shiga toxin‐producing Escherichia coli O104 infection–a prospective cohort study. Neurogastroenterol Motil. 2015;273:370‐378. [DOI] [PubMed] [Google Scholar]
- 10. Michalsen VL, Vandvik PO, Farup PG. Predictors of health‐related quality of life in patients with irritable bowel syndrome. A cross‐sectional study in Norway. Health Qual Life Outcomes. 2015;13(1):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA. 2015;3139:949‐958. [DOI] [PubMed] [Google Scholar]
- 12. Lowry TJ, Pakenham KI. Health‐related quality of life in chronic fatigue syndrome: predictors of physical functioning and psychological distress. Psychol Health Med. 2008;132:222‐238. [DOI] [PubMed] [Google Scholar]
- 13. Gralnek IM, Hays RD, Kilbourne A, Naliboff B, Mayer EA. The impact of irritable bowel syndrome on health‐related quality of life. Gastroenterology. 2000;1193:654‐660. [DOI] [PubMed] [Google Scholar]
- 14. Sugawara N, Sato K, Takahashi I, et al. Irritable bowel syndrome and quality of life in a community‐dwelling population in Japan. Int J Psychiatry Med. 2018;533:159‐170. [DOI] [PubMed] [Google Scholar]
- 15. Maruish ME, ed. User’s Manual for the SF‐12v2 Health Survey, 3rd edn Lincoln, RI: QualityMetric Incorporated; 2012. [Google Scholar]
- 16. Gandek B, Ware JE, Aaronson NK, et al. Cross‐validation of item selection and scoring for the SF‐12 Health Survey in nine countries: results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol. 1998;5111:1171‐1178. [DOI] [PubMed] [Google Scholar]
- 17. Rome Foundation . Rome III diagnostic questionnaire for the adult functional GI disorders. Available at: https://www.theromefoundation.org/assets/pdf/19_RomeIII_apA_885-898.pdf. Accessed January 15th, 2019.
- 18. Lydersen S, Fagerland MW, Laake P. Recommended tests for association in 2 2 tables. Stat Med. 2009;287:1159‐1175. [DOI] [PubMed] [Google Scholar]
- 19. Ware JE Jr, Kosinski M, Keller S. SF‐12: How to Score the SF‐12 Physical and Mental Health Summary Scales, 2nd edn Boston, MA: The Health Institute, New England Medical Center; 1995. [Google Scholar]
- 20. Vilagut G, Forero CG, Pinto‐Meza A, et al. The mental component of the short‐form 12 health survey (SF‐12) as a measure of depressive disorders in the general population: results with three alternative scoring methods. Value Health. 2013;164:564‐573. [DOI] [PubMed] [Google Scholar]
- 21. Rey E, Garcia‐Alonso MO, Moreno‐Ortega M, Alvarez‐Sanchez A, Diaz‐Rubio M. Determinants of quality of life in irritable bowel syndrome. J Clin Gastroenterol. 2008;429:1003‐1009. [DOI] [PubMed] [Google Scholar]
- 22. Rindfuss RR, Choe MK, Tsuya NO, Bumpass LL, Tamaki E. Do low survey response rates bias results? Evidence from Japan. Demogr Res. 2015;32:797‐828. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


