Abstract
Microplastics have been detected in freshwaters all over the world in almost all samples, and ecotoxicological studies have shown adverse effects of microplastics on organisms. However, no risk assessment of microplastics has been performed specifically in freshwater so far. The aim of the present study was therefore to review all exposure and ecotoxicity data available for microplastics in freshwaters and to perform a preliminary probabilistic risk assessment. The exposure probability distribution was based on 391 concentrations measured in Asia, Europe, and North America. Because exposure data are mainly available in particle number–based metrics but results from hazard studies are mostly mass‐based, the hazard results were converted into particle number concentrations. A statistical analysis of the hazard data showed that there was no significant influence of particle shape or type of polymer on the no‐observed‐effect concentration. The predicted‐no‐effect concentration (PNEC) was calculated as the fifth percentile of the probabilistic species sensitivity distribution, based on 53 values from 14 freshwater species, to have a mode of 7.4 × 105 particles · m−3 (25th and 75th quantiles of 6.1 × 105 and 1.3 × 106 particles · m−3, respectively). The exposure probability distribution was divided by the PNEC probability distribution to calculate risk characterization ratios (RCRs), with modes of 1.3 × 10−6 in North America, 3.3 × 10−6 in Europe, and 4.6 × 10−3 in Asia. Probability distributions associated with the RCRs showed that ecological risks cannot be entirely excluded in Asia, where 0.4% of the RCR values were above 1. Environ Toxicol Chem 2019;38:436–447. © 2018 SETAC
Keywords: Microplastics, Freshwater, Probabilistic species sensitivity distribution, Probabilistic risk assessment
The environmental risk of microplastics was assessed using a probabilistic approach in the freshwaters of Asia, Europe, and North America. Results show that such risks cannot be excluded in Asia.

INTRODUCTION
Microplastic has emerged as a new pollutant in the last years for both the oceans and freshwaters. These particles are defined as having the largest dimension below 5 mm, as opposed to macroplastics (Faure et al. 2015; Duis and Coors 2016). Microplastics are called “primary” when they were intentionally produced at the microscale. They are used in consumer products such as personal care products and in industrial scrubbers (GESAMP Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection 2015; Ivleva et al. 2017) and are mostly spherical in shape (beads). Secondary microplastics are produced by the weathering of larger plastic items, such as plastic films, fishing nets, or household items (Cole et al. 2011; Ivar do Sul and Costa 2014; GESAMP 2015), and most often occur in irregular shapes. Fragments of textile fibers are also mostly considered secondary microplastics (Hidalgo‐Ruz et al. 2012; Stolte et al. 2015; GESAMP 2015; Eerkes‐Medrano et al. 2015).
Research is carried out on both sides of microplastic environmental risk assessment, which encompasses both environmental exposure and ecotoxicity. Most exposure studies have focused on marine environments (Wagner et al. 2014; Horton et al. 2017; Li et al. 2018), but microplastics have also been found in freshwaters in significant concentrations (up to several million pieces per cubic meter; see, e.g., Zhang et al. 2015). Because of their small size, microplastics can be ingested at all levels of the trophic chain (Cole et al. 2013; Imhof et al. 2013). They may then have adverse effects on organisms by affecting the gastrointestinal tract, causing wounds, blockage, starvation, and death (Laist 1987; Derraik 2002; Gregory 2009; Wright et al. 2013; Gall and Thompson 2015). In addition, toxic substances such as persistent organic pollutants or metals can be carried by microplastics (Teuten et al. 2009; Gregory 2009; Hidalgo‐Ruz et al. 2012) or leach from the ingested particles (Ivleva et al. 2017).
The presence of microplastics in the environment and their effects observed in ecotoxicological studies have raised public concern. Regulations are being implemented with the aim of reducing the levels of exposure to primary and secondary microplastics (Xanthos and Walker 2017; European Parliament 2018). However, because plastic production has increased (Plastics Europe 2018) and most microplastics released in the environment are secondary microplastics with low biodegradability (Hidalgo‐Ruz et al. 2012), their environmental concentration may still increase in the near‐ to medium‐term future (Eerkes‐Medrano et al. 2015; Syberg et al. 2015; Koelmans et al. 2017). It is thus of utmost importance to put efforts toward an ecotoxicological risk assessment of microplastics, to obtain a clear idea of the threat they might pose to organisms.
To the best of our knowledge, only 2 risks assessments of microplastics in water have been published in the peer‐reviewed literature. Everaert et al. (2018) analyzed these risks in the marine environment. Based on the procedure outlined by the European Chemicals Agency (2016), the authors calculated a risk characterization ratio (RCR), dividing a predicted environmental concentration by a predicted‐no‐effect concentration (PNEC). Environmental concentrations were modeled based on global plastic production and environmental parameters, whereas ecotoxicity data were collected from the literature. On this global scale, the authors found no immediate risk in marine waters until 2100. Burns and Boxall (2018) published a risk evaluation of microplastics in both freshwaters and marine waters. Their critical review highlighted that organisms are usually exposed in the laboratory to smaller particles and in higher concentrations than those found in the environment. Comparing measured environmental concentrations with ecotoxicity values reported in the literature, the authors concluded that microplastics did not seem to pose a risk to aquatic organisms. They did, however, combine data related to both freshwater and marine environments in the same assessment. An environmental risk assessment of microplastics specifically performed for freshwaters is therefore still missing.
Because ecotoxicity data on microplastics in freshwater are scarce and environmental concentrations are highly variable (see, e.g., the review by Eerkes‐Medrano et al. 2015), we argue that it is preferable to assess the variability and uncertainty by using a probabilistic risk assessment. This approach consists in comparing a probability distribution for environmental concentration with a probability distribution derived from toxicity values (Solomon et al. 2000). The overall principle of this method is to determine, on the one hand, a PNEC distribution based on ecotoxicity data and, on the other hand, a probability distribution of environmental concentration based on measured concentrations or modeling results. Both distributions are then compared, and a risk is assumed to occur if they overlap. A probabilistic RCR can also be calculated, dividing the probability distribution of the exposure concentration by that of the PNEC. Such a method was developed by Gottschalk and Nowack (2012) and applied to engineered nanomaterials, where also both exposure and hazard data are highly uncertain (Gottschalk et al. 2013; Wang et al. 2016; Coll et al. 2016).
The aim of the present study was to perform a probabilistic risk assessment of microplastics in freshwater, following Gottschalk and Nowack (2012). Exposure and ecotoxicity data were analyzed, selected, and compiled. A statistical analysis of the effect of the shape and polymer composition of microplastics on their ecotoxicity was performed. Their potential ecotoxicological risks were then assessed on global and continental scales. This served as a basis for highlighting the most abundant types of studies, data gaps, and discrepancies, as well as research priorities for as accurate an ecotoxicological risk assessment as possible.
MATERIALS AND METHODS
Data collection, extraction, and harmonization
A data search was conducted on the Web of Science and Google Scholar with the keyword “microplastic” until the end of June 2018. All references cited in each selected paper were also searched. Only peer‐reviewed experimental studies conducted in freshwater were included in the present study.
Exposure data
Data on microplastic concentrations measured in freshwaters of different regions of the world were collected, including Europe, North America, and Asia (Supplemental Data, Table S1). Concentrations were reported in the studies either as numbers of particles per unit of volume or as numbers of particles per surface unit. To homogenize the data, all concentrations expressed per unit of surface were converted to concentrations per unit of volume using the given height of trawl or neuston nets used for sampling. The use of the total height of the nets might lead to approximate calculations of concentrations, for 2 main reasons. First, microplastic particles might concentrate in a layer of water that is thinner than the sampled surface layer. With the averaging of this surface concentration over the whole height of the nets, this surface concentration is therefore underestimated. However, as organisms move up and down the whole water column, within a higher height than those of the nets, the overall concentration to which they are exposed is actually overestimated. The proper concentration to which the organisms are exposed would depend on their movement (or absence of movement) within the water column, but we can consider that the approach followed in the present study is rather conservative because most organisms would move in lower layers of water and therefore be exposed to a lower concentration than what was calculated. Second, the amount of water passing through the net depends on many factors, such as clogging. This might lead to actual volumes sampled being smaller than expected, this time resulting in an underestimation of the exposure. Although we acknowledge potential over‐ or underestimations of the risk, such considerations are very difficult to include in a quantitative way and were left out of our calculations. Concentrations measured in estuaries were excluded from our assessment because brackish waters host different species than freshwaters.
Ecotoxicity data
Ecotoxicity data for pelagic and benthic freshwater organisms were collected. Preferred ecotoxicological endpoints were survival, growth, reproduction, and changes in significant metabolic processes such as photosynthesis, which are most significant on the ecosystem level (European Chemicals Agency 2008). Other endpoints were excluded. When both chronic and acute data were reported in the same study, the former were preferred. Results obtained for different sizes, shapes, and materials in the same study were considered separate input values.
In effect studies, exposure concentrations are normally reported in mass‐based units, whereas measurements in the environment are normally reported in particle numbers. One evaluation of the effect data was therefore made based on mass as a metric and a second one on particle numbers. The conversion of mass concentrations into particle number concentrations was based on the reported particle shape, mean size, and material density. Because of the lack of material characterization given in some papers, 3 data points from 2 species included in the mass‐based ecotoxicity assessment could not be included in the assessment performed in particle number concentration. This represented 6% of all data points and 14% of the species. Spheres, fragments, and fibers were distinguished; but because sizes of fragments were most often reported as mean diameters, they were treated as spheres in the present calculations. The values used for the calculations are reported for each study in Supplemental Data, Table S2.
Different studies had various purposes and experimental setups, reporting various dose descriptors. Concentrations causing 50% growth inhibition, lethality, or any other effect (IC50, LC50, and EC50, respectively) were the most common descriptors. To maximize the homogeneity of the dose descriptors, the lowest‐observed‐effect concentration, which is the lowest tested concentration leading to an effect significantly different from the control, was extracted only if no LC50, EC50, or IC50 values were reported. The highest observed‐no‐effect concentration (HONEC) was used only if no other endpoints were reported. Moreover, because the lowest part of the species sensitivity curve is the most sensitive to derive a PNEC (see below, Probabilistic risk assessment), HONEC values were included in the assessment only if they were higher than 1 mg · L−1. This ensured that the lowest part of the species sensitivity curve would not be skewed by such highly uncertain data.
According to the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) guidance (European Chemicals Agency 2008), the chronic no‐observed‐effect concentration (NOEC) is the preferred endpoint to derive a PNEC. The different dose descriptors were therefore converted into chronic NOECs based on 2 assessment factors (AFs; European Chemicals Agency 2008): AFtime was used to derive the long‐term (or chronic) effect based on the short‐term (or acute) effect, whereas AFdescriptor allowed the extrapolation of any dose descriptor into a NOEC. The values that were used are available in Supplemental Data, Tables S3 and S4.
Testing the influence of microplastic shape or composition on their ecotoxicity
Correlation tests were performed on the ecotoxicity data set to test any statistical difference between the values obtained with different shapes and polymers. Because the ecotoxicity of a substance depends highly on the species exposed, it was necessary to select one single species at a time to run such tests. Daphnia magna was the species for which the highest number of data points was available and the only one for which the number of data points was high enough to perform a relevant statistical analysis. Mann‐Whitney tests were thus performed on this restricted data set, which can be applied to samples that do not follow a normal distribution. The null hypothesis is the equality of 2 sample means; it was rejected if the p value obtained was <0.05.
Three pairs of subsamples were tested, which were those for which sufficient data points were available: “spheres” versus “not spheres,” “polyethylene” versus “not polyethylene,” and “polystyrene” versus “not polystyrene.” The means of these samples were analyzed using the software SPSS Statistics 25 (IBM, 2017).
Probabilistic risk assessment
The rationale of probabilistic risk assessment is to compare the probability distribution of exposure with that of hazard (here, ecotoxicity). These probability distributions were built on the described data, as detailed in this section. The exposure assessment was based on building a cumulative exposure probability curve of the measured environmental concentrations (MEC). The first step of building the exposure probability distribution was to define a distribution for each sampling point. As detailed in Supplemental Data, Table S1, a triangular distribution was built when the minimum, mean, and maximum concentrations measured at each location were available (Supplemental Data, Figure S1A); and a normal distribution was built when means and standard deviations were reported in the study (Supplemental Data, Figure S1B). When only one value was available for a given location, no variability could be associated, which corresponded to a Dirac function (Supplemental Data, Figure S1C). Multiple cumulative functions were then sampled from these distributions, representing the range of uncertainty associated with available exposure concentrations.
The hazard assessment was performed based on species sensitivity distributions (SSDs). To build the probability distribution associated with the ecotoxicity data, all available data relevant to one species were grouped into a single probability distribution, according to the method based on the probabilistic SSD (PSSD) developed by Gottschalk and Nowack (2012) and modified by Wigger et al. (Empa, St. Gallen, Switzerland, unpublished manuscript). These probability distributions accounted for interlaboratory variation and uncertainty associated with AFtime and AFdescriptor. Multiple PSSDs were then sampled from these species‐specific distributions. Finally, as recommended by the European Chemicals Agency (2008), the probability distribution of the PNEC was extracted as the fifth percentiles of these PSSDs.
The PNEC probability distribution was then compared to that of the MECs, and RCRs were obtained. The RCR is defined by the European Chemicals Agency (2016) as the exposure concentration divided by the PNEC. The probabilistic RCRs were obtained by dividing each point of the PNEC distribution by each point of the MEC distributions, as outlined by Coll et al. (2016). We calculated RCRs for each region and for the whole data set. If exposure and ecotoxicity probability distributions overlap (or RCR ≥ 1), a risk can be expected to occur toward the organisms living in the relevant compartment; no overlap (RCR < 1) indicates no immediate concern for risk.
RESULTS AND DISCUSSION
Probabilistic exposure assessment
In total, 391 measurements of microplastic concentrations in freshwaters were included in our assessment, of which 56% were reported from North American locations, 28% from Asian locations, and 16% from Europe. Within Europe, samples were collected in France (34%), Switzerland, Italy (25% each), Germany (10%), Austria, and the Netherlands (3% each). Asian samples came from China (86%), Mongolia (8%), and Vietnam (6%). Of the North American samples, 81% were collected in the United States and 19% were collected in Canada. Even though data were available on the most plastic‐polluted river (Lebreton et al. 2017), the Yangtze River, data were missing for other highly polluted rivers, for example, the Ganges in India and the Amazon in South America. In a more general perspective, it can be argued that the scientific focus should put priority on sampling rivers in those countries where the waste‐management system is often not performant enough to handle properly the amounts produced, leading to plastic waste occurring in close proximity to water bodies (Henry et al. 2006; Parrot et al. 2009). It is then highly probable that this mishandled plastic waste enters freshwater, where it can degrade to secondary microplastics (Blettler et al. 2018; Khan et al. 2018).
When studies reported variability, it was often among replicates taken at the same location and at the same time. Temporal variability over 1 yr was assessed only in the United States’ tributaries to the Great Lakes (Baldwin et al. 2016). Spatial variability was assessed across the River Seine (Dris et al. 2015), along the River Danube (Lechner et al. 2014), and on Swiss lakes (Faure et al. 2015). The variability of measurement at a certain point of time within sampling stations was assessed in the Great Lakes (United States and Canada; Cable et al. 2017). Although these measured variabilities could sometimes cover several orders of magnitude (Lechner et al. 2014; Cable et al. 2017), 59% of the data points included in the present study were not associated with any measurement of variability and could consequently not be attributed any probability distribution.
All available data were compiled in a cumulative way in Figure 1, whether they were probability distributions associated with measurements or single data points to which no uncertainty or variability could be associated. We built 10 000 cumulative curves from 10 000 runs. In each run, one value was reported for each measurement, which was sampled from the associated probability distribution. If no distribution could be defined, the concentration measured was reported as such. Then, all points taken from a single run were ordered in ascending order and plotted on the figure as one cumulative curve. This procedure was repeated 10 000 times and resulted in the ranges of microplastic concentrations associated with each probability value that can be observed in Figure 1.
Figure 1.
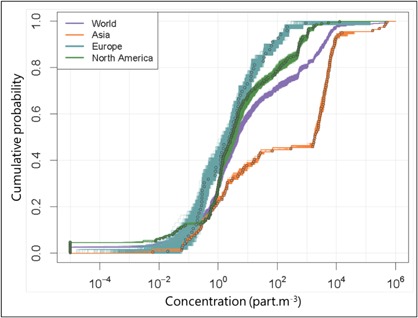
Cumulative probability curves of concentrations of microplastics measured in freshwater. Samples with no detectable microplastics have been converted to very low concentrations (10−5 particles · m−3) to show them on the logarithmic scale of the graph. Points represent means or medians (means preferred, according to availability) of concentrations measured in replicates. The areas visible on the figure are the result of the combination of 10 000 cumulative curves.
Most microplastic concentrations measured were between 10−2 and 104 particles · m−3. The highest concentrations were found in Asia (up to 5.2 × 105 particles · m−3; Supplemental Data, Table S5). North America presented the lowest concentrations, with many “nondetects,” but also the widest span, with concentrations up to 1.3 × 104 particles · m−3. In Asia, approximately 45% of the data were found to be in the high concentration range between 103 and 104 particles · m−3, which resulted in a clear step in the cumulative distribution (Figure 1). Europe and North America presented more homogeneous frequencies of concentrations. In North America, almost 40% of the measured concentrations were <1 particle · m−3. In Europe, more than half of the concentrations were measured between 0.1 and 10 particles · m−3.
Microplastic sampling in freshwater was most often performed with neuston or trawling nets (76% of exposure data points). Bottles and pumps were used in a few cases for bulk sampling, but in all cases microplastics were recovered by sieving or filtration. Yet, different cutoff sizes were used, ranging from 0.45 to 500 µm. More than half of the samples were collected with cutoff sizes >100 μm, but more than a quarter were filtered through pores ≤50 μm (Figure 2A). Filtration removes the lowest size fraction of the particulate material, so exposure concentrations might then be underestimated; but the different cutoff sizes used lead to different levels of underestimation of the total microplastics amount (Hidalgo‐Ruz et al. 2012; Dris et al. 2015). There is therefore an urgent need for sampling protocol harmonization to make the collected data as comparable as possible. This is also important because the smallest particles are potentially those that are most easily ingested (Dris et al. 2015). Moreover, because fibers have one very small dimension, although some of their dimensions are above the filtration threshold, they might pass through the filters and, again, result in biased measured concentrations (Horton et al. 2017).
Figure 2.
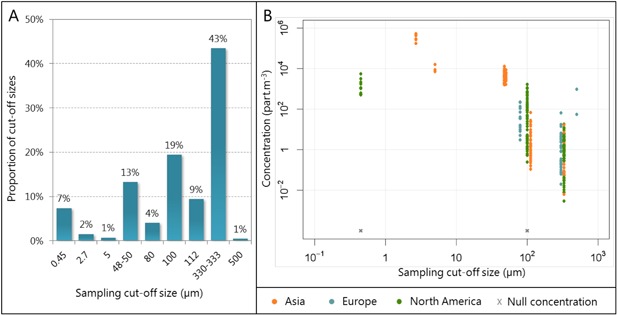
(A) Proportions of cutoff sizes used among studies to sample microplastics from freshwater. (B) Concentrations of microplastics measured in freshwater with different sampling cutoff sizes.
Comparison of the different concentrations reported with different cutoff sizes does not show any clear correlation between cutoff sizes <100 μm and measured concentration, although >5 μm there seems to be a lower chance of measuring high concentrations of microplastics with higher cutoff sizes (Figure 2B). In particular, European waters were only sampled with the highest size thresholds. The exposure concentrations on this continent might therefore be underestimated compared to Asia or North America, where several studies used much lower cutoff sizes. This confirms the need for standardized protocols, preferably using small cutoff sizes.
Probabilistic hazard assessment
In total, 53 ecotoxicity values were included in our assessment, covering 14 species (Supplemental Data, Table S2). Most species for which the sensitivity to microplastics was tested were invertebrates (6 arthropods, 1 cnidarian, and 1 mollusk). Two algae, 1 higher plant, and 3 fish species were also tested, although the data related to 2 fish species could not be converted in number concentrations and were consequently not included in the risk assessment. Daphnia magna was by far the most represented species (29 data points, or 52% of the data points collected). Nevertheless, this data set complied well with REACH criteria (European Chemicals Agency 2008) because more than 10 species were represented on the PSSD. Two of these criteria were not met (Supplemental Data, Table S6): the occurrence of a second fish species (for the PSSD built on number concentrations) and that of an insect species.
The compositions and shapes of the microplastics tested differ among ecotoxicological studies (Supplemental Data, Figure S2). Most particles were spherical (66%), approximately a quarter were of irregular shape (“fragments,” 24%), and the remainder were fibers or of unknown shape. The polymers most frequently tested were polyethylene (38%), polystyrene (23%), and polyethylene terephthalate (9%). All pairs of shape and polymer subsamples tested with the Mann‐Whitney test presented p values >0.2 (Table 1). This confirmed that, with the available data set, no difference could be made between the toxicities of 1) spherical and nonspherical microplastics, 2) polyethylene and nonpolyethylene microplastics, and 3) polystyrene and nonpolystyrene microplastics. It is therefore reasonable to assess the overall ecotoxicity of microplastics, combining data on all shapes and polymer types together. However, because this data set is very limited, the conclusion driven by statistics might not reflect the actual situation, and differences in toxicities among different types of materials could be captured in the future when more data become available. Avio et al. (2015) also showed, albeit experimentally, that polyethylene and polystyrene gave similar effects on marine organisms.
Table 1.
p values obtained with Mann‐Whitney tests comparing the no‐observed‐effect concentrations of subsamples of different shapes and compositions
| Subsamples | p value |
|---|---|
| Sphere vs other | 0.465 |
| Polyethylene vs other | 0.829 |
| Polystyrene vs other | 0.213 |
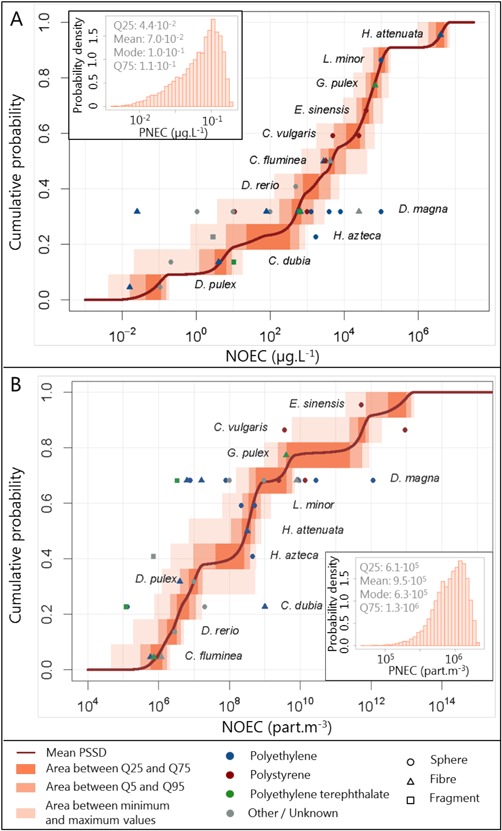
All ecotoxicity data were thus used to build the PSSD expressed in mass concentration (Figure 3A). If these data could be converted to number concentrations, they were used to build the PSSD based on number concentrations (Figure 3B), which was most useful for risk assessment because it allowed a comparison of hazard and exposure data. Exposure number concentrations were not converted to mass concentrations because the characterization of the particles collected in freshwaters was most often less accurate than in ecotoxicity tests. On each of these graphs, 10 000 PSSDs were reported. In the same way as for the exposure assessment, in each of the 10 000 runs, one value was sampled from each species‐specific probability distribution. These values were then ordered in an ascending order and plotted as one cumulative curve. The NOECs themselves are also reported in the figures. They were aggregated by species, which were ordered on the graph according to the geometric means of their NOECs.
Figure 3.
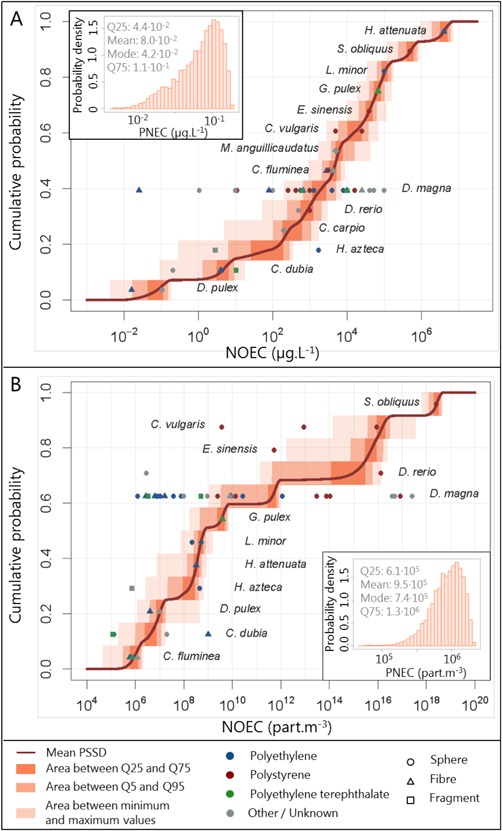
Probabilistic species sensitivity distributions and probability distribution of the predicted‐no‐effect concentration of microplastics in freshwater. The upper panel (A) shows mass‐based concentrations; the lower panel (B) shows particle number concentrations. NOEC = no‐observed‐effect concentration; PNEC = predicted‐no‐effect concentration; PSSD = probability species sensitivity distribution; Q = quantile (e.g., Q25 = 25th quantile).
Daphnia magna was the species for which the widest range of NOECs was calculated, with a minimum of 2.5 × 10−2 μg · L−1 and a maximum of 105 μg · L−1 (Supplemental Data, Table S2). The assessment of the species sensitivity performed in mass concentrations and their ranking based on the geometric means of their NOECs showed that Hydra attenuata was the least sensitive species (NOEC = 4 × 106 μg · L−1) and Daphnia pulex the most sensitive species (NOEC = 6 × 10−2 μg · L−1). Because the particles used in the tests were of different sizes, the order of NOECs was different when they were expressed in mass concentration or in number concentration: for a same mass‐based NOEC value, the number‐based NOEC would be lower for large particles than for small particles. Therefore, when using number concentrations, a different order of species sensitivity was found. Scenedesmus obliquus was then the least sensitive species, presenting a NOEC of approximately 2.7 × 1018 particles · m−3, whereas Corbicula fluminea appeared to be the most sensitive species, showing a mean NOEC of 8.8 × 105 particles · m−3. However, Ceriodaphnia dubia, the second most sensitive species based on geometric means of NOECs, presented the lowest NOEC of all (1.2 × 105 particles · m−3).
The microplastic PNEC distribution was taken as the fifth percentiles of the PSSDs (insets in Figure 3A and B). When expressed in mass concentration, the mode of the PNEC was 4.2 × 10−2 μg · L−1, with 25th and 75th quantiles of 4.4 × 10−2 and 1.1 × 10−1 μg · L−1, respectively. When using number‐based metrics, the PNEC took a similar shape, also unimodal, but with lower probabilities associated with the lowest values than when expressed in mass‐based metrics. Its mode was 7.4 × 105 particles · m−3, its 25th quantile was 6.1 × 105 particles · m−3, and its 75th quantile was 1.3 × 106 particles · m−3.
The approach used to derive PSSDs in the present study presents several limitations, which could be overcome when more appropriate data are available. First, the number concentrations were calculated based on the mean diameter of the particles tested: because the size distributions of the particles were not always reported, the polydispersity could not be taken into account. Consequently, the actual concentration of particles tested could be different from those that were calculated. Second, the assessment factors describing the relationship between chronic and acute toxicities and the extrapolation from various dose descriptors to NOECs were not specific to microplastics. This uncertainty was represented by the coefficients of variation attributed to the values we could find in the literature, but those coefficients of variation could be assessed more accurately based on studies specific to microplastics, which would work on describing better the relationships between acute and chronic toxicity and between dose descriptors. Finally, even if the data set complies with most of the REACH criteria (European Chemicals Agency 2008), it is still limited in terms of number of studies, especially regarding the number of data points available for some species: 7 species could not be attributed to more than one NOEC. Although those are not among the most sensitive and do not affect significantly the PNEC distribution, the overall hazard assessment could be more accurate if it included more data.
Probabilistic risk assessment
To assess the risk that microplastics might pose in the world's freshwaters, the probability distribution of the MECs and that of the PNEC were plotted on the same graph (Figure 4). The probability distribution of the global exposure concentration overlapped to a small extent with the PNEC probability distribution: the range of overlapping values (from the minimum of the PNEC distribution to the maximum of the MEC distribution) represented approximately 23% of the total range of MEC and PNEC values combined (from the minimum of the MEC distribution to the maximum of the PNEC distribution). Overlapping values ranged from 3.8 × 104 (minimum of the PNEC) to 5.2 × 105 particles · m−3 (maximum of the MEC). Therefore, it cannot be excluded that microplastics represent an ecological risk in freshwater.
Figure 4.
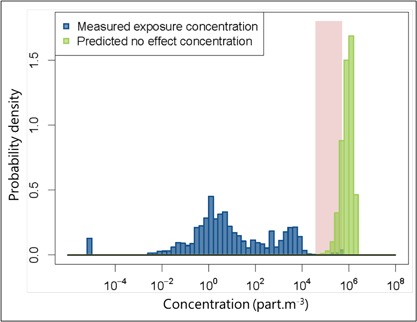
Probability distributions of the measured environmental concentration of microplastics and their predicted‐no‐effect concentration in freshwater. The overlap of the 2 distributions is enhanced by the shaded area.
A probability distribution can be obtained for the global RCR by dividing the MEC distribution by the PNEC distribution. This calculation was also performed with continent‐specific exposure data, resulting in RCR distributions specific to Asia, Europe, and North America (Figure 5).
Figure 5.
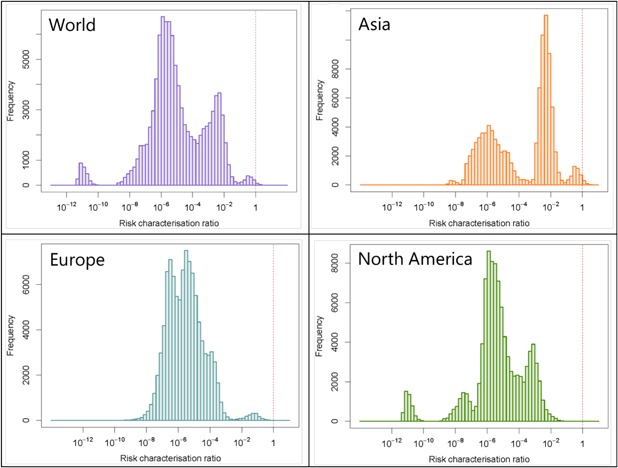
Probability distributions of risk characterization ratios in the world, Asia, Europe, and North America.
A very small percentage (0.12%) of the probability distribution calculated for the global RCR was >1. The ranges of the probability distributions were <1 in Europe and North America, meaning that no risk should currently be expected there. The modes of the European and North American distributions were 3.3 × 10−6 and 1.3 × 10−6, respectively. However, 0.4% of the RCR probability distribution in Asia was >1, which is why the global RCR also ranges up to values >1. Therefore, even if the highest modal value, that is the most probable value, was well below 1 (4.6 × 10−3), an ecotoxicological risk cannot be completely excluded on this continent.
The main advantage of a probabilistic risk assessment as it was performed in the present study is that it allows inclusion of all data available at one point in time and gives an overall picture of the situation as it is known. In this way, the risk assessment is sounder than if it was performed using single values or fitting mathematical models to multiple data. It also helps in identifying data gaps and research priorities. It should be kept in mind that this probabilistic risk assessment highly depends on the available data. For such new fields as microplastic environmental measurements and microplastic ecotoxicity, future data might indicate higher or lower risk than those reported in the present study. Our goal was to follow the method of a probabilistic risk assessment to underline potential data gaps and research priorities rather than to perform a final risk assessment.
The polymers most often used in ecotoxicity tests were polyethylene and polypropylene. Although these are the most used polymers (Plastics Europe 2018), they were most often tested as primary microplastics, in a spherical shape. However, secondary microplastics such as fibers and fragments were found more often than primary microplastics in freshwaters (see, e.g., Cable et al. 2017; Wang et al. 2017; Vermaire et al. 2017). The comparability of the ecotoxicity data set with the exposure data set would therefore be improved if more studies focused on testing the ecotoxicity of secondary microplastics.
The cutoff sizes used for microplastic sampling and the sizes of the microplastics tested in ecotoxicology were plotted on cumulative curves for comparison (Figure 6). This showed that >30% of the particles used in ecotoxicological studies were <0.45 μm, which is the smallest cutoff size used to identify microplastics in freshwater in the studies analyzed in the present study. Because the size of a particle could affect its toxicity (Lehtiniemi et al. 2018), this discrepancy could bias the results.
Figure 6.
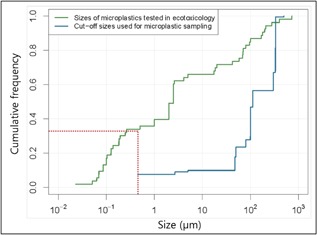
Cumulative curves of cutoff sizes used to recover microplastics from freshwater and mean sizes of microplastics tested for ecotoxicity.
The hazard assessment was therefore performed again, excluding ecotoxicity studies testing particles whose sizes were <0.45 μm. Figure 7 presents the resulting PSSD curves based on mass and particle number. The PNEC distributions extracted from these new PSSDs were very close to those from the complete data set (see Table 2 for 25th quantile, means, modal values, and 75 quantile), indicating that the ecotoxicological experiments with very small particle sizes did not significantly affect the data evaluation. Table 2 also shows that the RCRs calculated based on the restricted data set are practically the same as for the full data set, showing the robustness of the approach and the applied data set.
Figure 7.
Probabilistic species sensitivity distributions and probability distribution of the predicted‐no‐effect concentration of microplastics in freshwater, excluding the studies testing particles smaller than the smallest particle size cutoff used in exposure assessment. The upper panel (A) shows mass‐based concentrations; the lower panel (B) shows particle number concentrations. NOEC = no‐observed‐effect concentration; PNEC = predicted‐no‐effect concentration; PSSD = probabilistic species sensitivity distribution; Q = quantile (e.g., Q25 = 25th quantile).
Table 2.
Statistical analysis of the predicted‐no‐effect concentrations and risk characterization ratios associated with microplastics in freshwater a
| Unit | Q25 | Mean | Mode | Q75 | |
|---|---|---|---|---|---|
| PNEC | |||||
| All data | μg · L−1 | 4.4 × 10−2 | 8.0 × 10−2 | 4.2 × 10−2 | 1.1 × 10−1 |
| particles · m−3 | 6.1 × 105 | 9.5 × 105 | 7.4 × 105 | 1.3 × 106 | |
| Small particles excluded | μg · L−1 | 4.4 × 10−2 | 7.0 × 10−2 | 1.0 × 10−1 | 1.1 × 10−1 |
| particles · m−3 | 6.1 × 105 | 9.5 × 105 | 6.3 × 105 | 1.3 × 106 | |
| RCR | |||||
| Geographical unit | Q25 | Mean | Mode | Q75 | |
| All data | World | 9.2 × 10−7 | 8.3 × 10−3 | 1.8 × 10−6 | 3.6 × 10−4 |
| Asia | 1.7 × 10−6 | 2.7 × 10−2 | 4.6 × 10−3 | 5.7 × 10−3 | |
| Europe | 3.8 × 10−7 | 1.2 × 10−3 | 3.3 × 10−6 | 1.4 × 10−5 | |
| North America | 9.5 × 10−7 | 3.3 × 10−4 | 1.3 × 10−6 | 5.4 × 10−5 | |
| Small particles excluded | World | 9.4 × 10−7 | 7.9 × 10−3 | 1.9 × 10−6 | 3.5 × 10−4 |
| Asia | 1.7 × 10−6 | 2.7 × 10−2 | 4.6 × 10−3 | 5.7 × 10−3 | |
| Europe | 3.7 × 10−7 | 1.1 × 10−3 | 3.6 × 10−6 | 1.4 × 10−5 | |
| North America | 9.5 × 10−7 | 3.5 × 10−4 | 1.4 × 10−6 | 5.9 × 10−5 |
The first assessment was performed with all available data, whereas the second assessment excluded all ecotoxicological values obtained with particles smaller than the smallest cutoff size used to quantify microplastics in freshwater.
PNEC = predicted‐no‐effect concentration; Q25 = 25th quantile; Q75 = 75th quantile; RCR = risk characterization ratio.
This probabilistic risk assessment was made on global and regional scales. But risks will vary locally, especially near point sources such as wastewater‐treatment plants, where exposure can be higher. Moreover, future trends were not assessed. Plastic production is expected to increase (Koelmans et al. 2017), but efforts are being made worldwide to improve waste management. These parameters could be used to assess future exposure and environmental risks. Evaluating different scenarios would help regulators to make decisions on both plastic production and waste management.
CONCLUSIONS
The present study analyzed all available peer‐reviewed data on exposure and ecotoxicity of microplastics in freshwater to perform a preliminary probabilistic environmental risk assessment. Overall, the average risk characterization ratio is several orders of magnitude below 1, indicating no immediate risk to the environment. However, a small risk cannot be excluded, especially in Asia, where there is a certain overlap of the exposure and hazard probability distributions.
Because most microplastics measured in the environment were secondary, these results call for better solid waste and wastewater management to reduce the amounts of fragments and fibers released to freshwater. However, they should not be considered definitive because the model depends highly on the data, whose homogeneity could be improved. For example, European freshwaters were sampled only with cutoff sizes of ≥80 μm. The exposure and therefore the risk that microplastics might pose on this continent could therefore be underestimated. To improve the quality of the model and the accuracy of the results, scientists need to use lower cutoffs when sampling microplastics and to test more secondary microplastics for ecotoxicity. Moreover, the main investigated polymer types in ecotoxicological studies represent only a small fraction of the polymers that flow through our society (Kawecki et al. 2018), and data are missing on some of the most polluted rivers such as the Amazon and Ganges.
Finally, the present results diverge from those of Burns and Boxall (2018), who did not identify any risk. This is partly explained by the fact that the study showing the highest concentrations in Asia, published recently (Lahens et al. 2018), could not be included in their assessment. This illustrates very well that conclusions can change as more data become available. With the improvement of analytical methods to quantify microplastics, better and standardized exposure data will become available, allowing us to exclude in the future published values obtained using nonvalidated methods.
Supplemental Data
The Supplemental Data are available on the Wiley Online Library at DOI: 10.1002/etc.4323. They include exposure and hazard data sets, as well as additional figures and tables.
Data availability
Data, associated metadata, and calculation tools are available from the corresponding author (bernd.nowack@empa.ch).
Supporting information
This article includes online‐only Supplemental Data.
Supporting Data S1.
Acknowledgment
The authors thank D. Kawecki and H. Wigger for their help in building the method and writing the code to produce probabilistic species sensitivity distributions.
REFERENCES
- Avio CG, Gorbi S, Regoli F. 2015. Experimental development of a new protocol for extraction and characterization of microplastics in fish tissues: First observations in commercial species from Adriatic Sea. Mar Environ Res 111:18–26. [DOI] [PubMed] [Google Scholar]
- Baldwin AK, Corsi SR, Mason SA. 2016. Plastic debris in 29 Great Lakes tributaries: Relations to watershed attributes and hydrology. Environ Sci Technol 50:10377–10385. [DOI] [PubMed] [Google Scholar]
- Blettler MCM, Abrial E, Khan FR, Sivri N, Espinola LA. 2018. Freshwater plastic pollution: Recognizing research biases and identifying knowledge gaps. Water Res 28:416–424. [DOI] [PubMed] [Google Scholar]
- Burns E, Boxall ABA. 2018. Microplastics in the aquatic environment: Evidence for or against adverse impacts and major knowledge gaps. Environ Toxicol Chem 37:2776–2796. [DOI] [PubMed] [Google Scholar]
- Cable RN, Beletsky D, Beletsky R, Wigginton K, Locke BW, Duhaime MB. 2017. Distribution and modeled transport of plastic pollution in the Great Lakes, the world's largest freshwater resource. Front Environ Sci 5 DOI: 10.3389/fenvs.2017.00045. [DOI] [Google Scholar]
- Cole M, Lindeque P, Fileman E, Halsband C, Goodhead R, Moger J, Galloway TS. 2013. Microplastic ingestion by zooplankton. Environ Sci Technol 47:6646–6655. [DOI] [PubMed] [Google Scholar]
- Cole M, Lindeque P, Halsband C, Galloway TS. 2011. Microplastics as contaminants in the marine environment: A review. Mar Pollut Bull 62:2588–2597. [DOI] [PubMed] [Google Scholar]
- Coll C, Notter D, Gottschalk F, Sun TY, Som C, Nowack B. 2016. Probabilistic environmental risk assessment of five nanomaterials (nano‐TiO2, nano‐Ag, nano‐ZnO, CNT, and fullerenes). Nanotoxicology 10:436–444. [DOI] [PubMed] [Google Scholar]
- Derraik JGB. 2002. The pollution of the marine environment by plastic debris: A review. Mar Pollut Bull 44:842–852. [DOI] [PubMed] [Google Scholar]
- Dris R, Imhof H, Sanchez W, Gasperi J, Galgani F, Tassin B, Laforsch C. 2015. Beyond the ocean: Contamination of freshwater ecosystems with (micro‐)plastic particles. Environ Chem 12:539–550. [Google Scholar]
- Duis K, Coors A. 2016. Microplastics in the aquatic and terrestrial environment: Sources (with a specific focus on personal care products), fate and effects. Environ Sci Eur 28:2–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eerkes‐Medrano D, Thompson RC, Aldridge DC. 2015. Microplastics in freshwater systems: A review of the emerging threats, identification of knowledge gaps and prioritisation of research needs. Water Res 75:63–82. [DOI] [PubMed] [Google Scholar]
- European Chemicals Agency. 2008. Characterization of dose [concentration]‐response for environment. In Guidance on Information Requirements and Chemical Safety Assessment European Chemicals Agency, Helsinki, Finland, chap R.10, p 8.
- European Chemicals Agency. 2016. Risk characterisation. In Guidance on Information Requirements and Chemical Safety Assessment European Chemicals Agency, Helsinki, Finland, pt E. ECHA–2016‐G‐04‐EN. [cited 2018 April 13]. Available from: https://echa.europa.eu/documents/10162/13632/information_requirements_part_e_en.pdf.
- European Parliament. 2018. EU strategy to cut plastic waste: More recycling, ban on micro‐plastics.” September 7, 2018. Brussels, Belgium. [cited 2018 August 26]. Available from: http://www.europarl.europa.eu/news/en/headlines/society/20180830STO11347/eu-strategy-to-cut-plastic-waste-more-recycling-ban-on-micro-plastics.
- Everaert G, Van Cauwenberghe L, De Rijcke M, Koelmans AA, Mees J, Vandegehuchte M, Janssen CR. 2018. Risk assessment of microplastics in the ocean: Modelling approach and first conclusions. Environ Pollut 242:1930–1938. [DOI] [PubMed] [Google Scholar]
- Faure F, Demars C, Wieser O, Kunz M, de Alencastro LF. 2015. Plastic pollution in Swiss surface waters: Nature and concentrations, interaction with pollutants. Environ Chem 12:582–591. [Google Scholar]
- Gall SC, Thompson RC. 2015. The impact of debris on marine life. Mar Pollut Bull 92:170–179. [DOI] [PubMed] [Google Scholar]
- GESAMP Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection. 2015. Sources, fate and effects of microplastics in the marine environment: A global assessment. Reports and Studies 90. International Maritime Organization, London, UK.
- Gottschalk F, Kost E, Nowack B. 2013. Engineered nanomaterials in water and soils: A risk quantification based on probabilistic exposure and effect modeling. Environ Toxicol Chem 32:1278–1287. [DOI] [PubMed] [Google Scholar]
- Gottschalk F, Nowack B. 2012. A probabilistic method for species sensitivity distributions taking into account the inherent uncertainty and variability of effects to estimate environmental risk. Integr Environ Assess Manag 9:79–86. [DOI] [PubMed] [Google Scholar]
- Gregory MR. 2009. Environmental implications of plastic debris in marine settings—Entanglement, ingestion, smothering, hangers‐on, hitch‐hiking and alien invasions. Philos Trans R Soc Lond B Biol Sci 364:2013–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry RK, Yongsheng Z, Jun D. 2006. Municipal solid waste management challenges in developing countries—Kenyan case study. Waste Manag 26:92–100. [DOI] [PubMed] [Google Scholar]
- Hidalgo‐Ruz V, Gutow L, Thompson RC, Thiel M. 2012. Microplastics in the marine environment: A review of the methods used for identification and quantification. Environ Sci Technol 46:3060–3075. [DOI] [PubMed] [Google Scholar]
- Horton AA, Walton A, Spurgeon DJ, Lahive E, Svendsen C. 2017. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci Total Environ 586:127–141. [DOI] [PubMed] [Google Scholar]
- IBM. 2017. SPSS Statistics Software v.25.0.
- Imhof HK, Ivleva NP, Schmid J, Niessner R, Laforsch C. 2013. Contamination of beach sediments of a subalpine lake with microplastic particles. Curr Biol 23:R867– R868. [DOI] [PubMed] [Google Scholar]
- Ivar do Sul JA, Costa MF. 2014. The present and future of microplastic pollution in the marine environment. Environ Pollut 185:352–364. [DOI] [PubMed] [Google Scholar]
- Ivleva NP, Wieshu AC, Niessner R. 2017. Microplastic in aquatic ecosystems. Angew Chem Int Ed Engl 56:1720–1739. [DOI] [PubMed] [Google Scholar]
- Kawecki D, Scheeder P, Nowack B. 2018. Probabilistic material flow analysis of seven commodity plastics in Europe. Environ Sci Technol 52:9874–9888. [DOI] [PubMed] [Google Scholar]
- Khan FR, Mayoma BS, Biginagwa FJ, Syberg K. 2018. Microplastics in inland African waters: Presence, sources, and fate In Wagner M, Lambert S, eds, The Handbook of Environmental Chemistry, Vol 58 Springer, Cham, Switzerland, pp 101–124. [Google Scholar]
- Koelmans AA, Besseling E, Foekema E, Kooi M, Mintenig S, Ossendorp BC, Redondo‐Hasselerharm PE, Verschoor A, van Wezel AP, Scheffer M. 2017. Risks of plastic debris: Unravelling fact, opinion, perception, and belief. Environ Sci Technol 51:11513–11519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahens L, Strady E, Kieu‐Le T‐C, Dris R, Boukerma K, Rinnert E, Gasperi J, Tassin B. 2018. Macroplastic and microplastic contamination assessment of a tropical river (Saigon River, Vietnam) transversed by a developing megacity. Environ Pollut 236:661–671. [DOI] [PubMed] [Google Scholar]
- Laist DW. 1987. Overview of the biological effects of lost and discarded plastic debris in the marine environment. Mar Pollut Bull 18:319–326. [Google Scholar]
- Lebreton LCM, van der Zwet J, Damsteeg J‐W, Slat B, Andrady A, Reisser J. 2017. River plastic emissions to the world's oceans. Nat Commun 8:15611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner A, Keckeis H, Lumesberger‐Loisl F, Zens B, Krusch R, Tritthart M, Glas M, Schludermann E. 2014. The Danube so colourful: A potpourri of plastic litter outnumbers fish larvae in Europe's second largest river. Environ Pollut 188:177–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtiniemi M, Hartikainen S, Näkki P, Engström‐Öst J, Koistinen A, Setälä O. 2018. Size matters more than shape: Ingestion of primary and secondary microplastics by small predators. Food Webs 17:e00097. [Google Scholar]
- Li J, Liu H, Chen P. 2018. Microplastics in freshwater systems: A review on occurrence, environmental effects, and methods for microplastics detection. Water Res 137:362–374. [DOI] [PubMed] [Google Scholar]
- Parrot L, Sotamenou J, Dia BK. 2009. Municipal solid waste management in Africa: Strategies and livelihoods in Yaoundé, Cameroon. Waste Manag 29:986–995. [DOI] [PubMed] [Google Scholar]
- Plastics Europe. 2018. Plastics—The facts 2017. An analysis of European plastics production, demand and waste data. Brussels, Belgium. [cited 2018 August 19]. Available from: https://www.plasticseurope.org/en/resources/publications/274-plastics-facts-2017.
- Solomon K, Giesy JP, Jones P. 2000. Probabilistic risk assessment of agrochemicals in the environment. Crop Prot 19:649–655. [Google Scholar]
- Stolte A, Forster S, Gerdts G, Schubert H. 2015. Microplastic concentrations in beach sediments along the German Baltic coast. Mar Pollut Bull 99:216–229. [DOI] [PubMed] [Google Scholar]
- Syberg K, Khan FR, Selck H, Palmqvist A, Banta GT, Daley J, Sano L, Duhaime MB. 2015. Microplastics: Addressing ecological risk through lessons learned. Environ Toxicol Chem 34:945–953. [DOI] [PubMed] [Google Scholar]
- Teuten EL, Saquing JM, Knappe DRU, Barlaz MA, Jonsson S, Björn A, Rowland SJ, Thompson RC, Galloway TS, Yamashita R, Ochi D, Watanuki Y, Moore C, Viet PH, Tana TS, Prudente M, Boonyatumanond R, Zakaria MP, Akkhavong K, Ogata Y, Hirai H, Iwasa S, Mizukawa K, Hagino Y, Imamura A, Saha M, Takada H. 2009. Transport and release of chemicals from plastics to the environment and to wildlife. Philos Trans R Soc Lond B Biol Sci 364:2027–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermaire JC, Pomeroy C, Herczegh SM, Haggart O, Murphy M. 2017. Microplastic abundance and distribution in the open water and sediment of the Ottawa River, Canada, and its tributaries. Facets 2:301–314. [Google Scholar]
- Wagner M, Scherer C, Alvarez‐Munoz D, Brennholt N, Bourrain X, Buchinger S, Fries E, Grosbois C, Klasmeier J, Marti T, Rodriguez‐Mozaz S, Urbatzka R, Vethaak AD, Winther‐Nielsen M, Reifferscheid G. 2014. Microplastics in freshwater ecosystems: What we know and what we need to know. Environ Sci Eur 26:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WF, Ndungu AW, Li Z, Wang J. 2017. Microplastics pollution in inland freshwaters of China: A case study in urban surface waters of Wuhan, China. Sci Total Environ 575:1369–1374. [DOI] [PubMed] [Google Scholar]
- Wang Y, Kalinina A, Sun T, Nowack B. 2016. Probabilistic modeling of the flows and environmental risks of nano‐silica. Sci Total Environ 545–546:67–76. [DOI] [PubMed] [Google Scholar]
- Wright S, Thompson RC, Galloway TS. 2013. The physical impacts of microplastics on marine organisms: A review. Environ Pollut 178:483–492. [DOI] [PubMed] [Google Scholar]
- Xanthos D, Walker TR. 2017. International policies to reduce plastic marine pollution from single‐use plastics (plastic bags and microbeads): A review. Mar Pollut Bull 118:17–26. [DOI] [PubMed] [Google Scholar]
- Zhang K, Gong W, Lv J, Xiong X, Wu C. 2015. Accumulation of floating microplastics behind the Three Gorges Dam. Environ Pollut 204:117–123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This article includes online‐only Supplemental Data.
Supporting Data S1.
Data Availability Statement
Data, associated metadata, and calculation tools are available from the corresponding author (bernd.nowack@empa.ch).


