Summary
Talimogene laherparepvec (T‐VEC) is a intralesional oncolytic virotherapy, licensed in the European Union for locoregional advanced melanoma of American Joint Committee on Cancer stages IIIB, IIIC and IVM1a. Organ transplant recipients are currently excluded from all clinical trials dealing with immunotherapies due to the risk of transplant rejection. A 58‐year‐old white man with a history of heart and kidney transplantation in 2014 was diagnosed with melanoma (Breslow thickness 1·6 mm, stage pT2a) on the left arm in September 2015. In March 2016 he developed in transit metastases, and local therapy with a combination of topical imiquimod (5%) and cryotherapy of individual lesions was initiated. However, in November 2016 therapy was stopped following local progression of the metastases. An interdisciplinary decision to treat the patient with T‐VEC was taken. After 11 cycles of T‐VEC, the patient showed a complete response. As of June 2018, 11 months after the last treatment cycle of T‐VEC, the patient continues to be tumour free. The patient tolerated the therapy well with only mild adverse events and did not show any sign of graft rejection or loss of function of the transplanted organs. We conclude that T‐VEC can be a potentially effective and safe treatment in patients with a history of organ transplantation. Nevertheless, due to this special situation, the risks and benefits should always be discussed with an interdisciplinary tumour board.
Short abstract
What's already known about this topic?
Talimogene laherparepvec (T‐VEC) is a intralesional oncolytic virotherapy, licensed in the European Union for locoregional advanced melanoma of American Joint Committee on Cancer stages IIIB, IIIC and IVM1a.
Organ transplant recipients have so far been excluded from all clinical trials dealing with immunotherapies due to the risk of transplant rejection.
What does this study add?
We conclude that T‐VEC can be a potentially effective and safe treatment in patients with a history of organ transplantation.
Nevertheless, due to this special situation, the risks and benefits should always be discussed with an interdisciplinary tumour board.
Linked Comment: https://doi.org/10.1111/bjd.18103.
Talimogene laherparepvec (T‐VEC) is an intralesional oncolytic virotherapy. It has been licensed in the European Union since December 2015 for locoregional advanced melanoma of American Joint Committee on Cancer (AJCC) stages IIIB, IIIC and IV M1a. T‐VEC is a modified double‐stranded DNA herpes simplex virus type 1, and its ability to mediate tumour regression is dependent on two different mechanisms.1, 2, 3, 4 Firstly, viral replication leads to preferential lysis of tumour cells, due to disrupted protein kinase R activity and disrupted type I interferon signalling.4 Secondly, the release of new viral particles induces continuous inflammation, which is amplified by expression of human granulocyte colony‐stimulating factor from the altered viral genome.
Besides T‐VEC, other approved drugs like checkpoint inhibitors and targeted therapies can also be used to treat locoregional metastatic disease.5, 6 However, none of these treatments has been formally tested in organ transplant recipients (OTRs), as these patients have been excluded from all pivotal clinical studies. To date only one previous case of administration of T‐VEC in a heart transplant patient with inoperable recurrent melanoma has been described,7 but no cases of patients with double organ transplantation have been reported so far.
Case report
A 58‐year‐old white man had a history of heart and kidney transplantation in 2014, due to dilated cardiomyopathy and consecutive renal failure. He was diagnosed with melanoma (Breslow thickness 1·6 mm, stage pT2a) on the left arm in September 2015. Following a positive sentinel lymph node biopsy, a left axillary lymph node dissection was performed in October 2015 (26 negative nodes). In March 2016 the patient progressed to AJCC stage IIIC with multiple small epidermotropic metastases along his left arm, confirmed by five punch biopsies. Local therapy with a combination of topical imiquimod (5%) and cryotherapy of individual lesions was initiated. However, in November 2016 the therapy was stopped following local progression of the metastases (Fig. 1).
Figure 1.
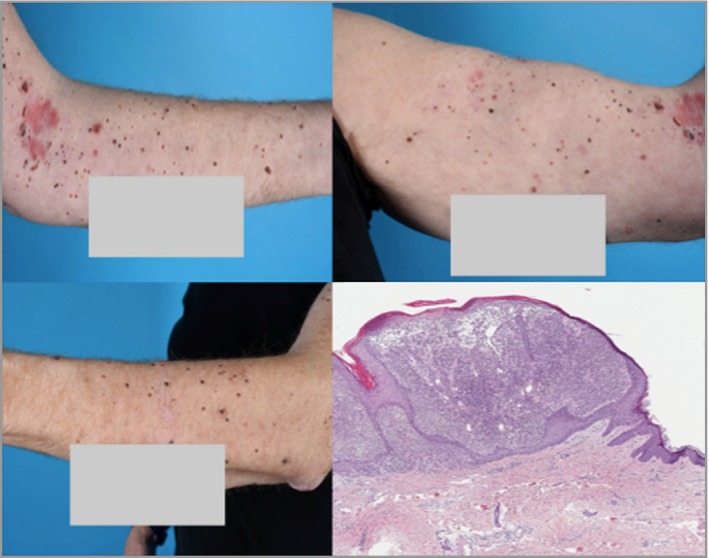
In November 2016, before the first therapy with talimogene laherparepvec, there were multiple epidermotropic cutaneous metastases confirmed by histopathology. The findings showed atypical melanoma cells limited to the epidermis.
A positron emission tomography scan performed at this time did not show any sign of metastases in any other part of the body. A multidisciplinary tumour board was held, including members of the patient's heart and kidney transplant team. As the tumour did not carry mutations in BRAF, c‐kit or NRAS, and as the risk of graft rejection was assumed to be higher with treatment with systemic checkpoint inhibitors, it was decided to treat the patient with the intralesional oncolytic virotherapy T‐VEC. The immunosuppressive regimen, which at this time consisted of mycophenolate mofetil 2 g per day, tacrolimus 7·5 mg per day and prednisolone 10 mg per day, was changed to mycophenolate mofetil 2 g per day and sirolimus 1 mg per day before starting therapy with T‐VEC.
In June 2017, after 11 cycles of intralesional infiltration with T‐VEC [first dose of 106 plaque‐forming units (PFU) mL−1, followed 3 weeks later by 10 doses of 108 PFU mL−1 in 2‐week intervals] the patient presented complete regression of all lesions, including those that were not injected, confirmed by representative punch biopsies (Fig. 2). The patient tolerated the therapy well, with mild adverse events. According to the Common Terminology Criteria for Adverse Events these were grade II pyrexia, grade I local erythema and grade I lesional hypopigmentation.
Figure 2.
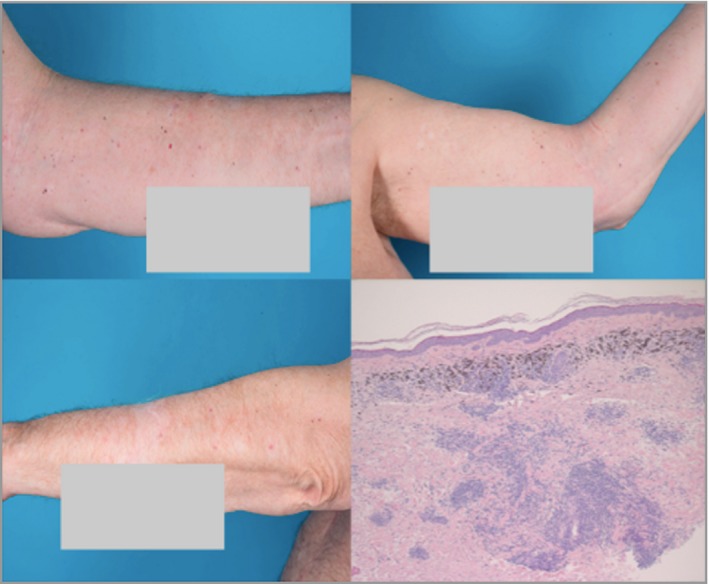
In June 2017, 8 months after 11 cycles of talimogene laherparepvec, there was complete regression of all melanoma metastases on the patient's left arm. Histopathology showed melanophages and plasma cells, with no sign of melanoma residua.
In addition to regular dermato‐oncological follow‐up, the patient was checked by his transplant team every 3 months, including laboratory tests, transthoracic echocardiography (showing stable left ventricular function), electrocardiography, coronary angiography and coronary computer tomography. During the entire treatment period, the patient did not show any sign of graft rejection or changes in the function of the transplanted organs. As of September 2018, 13 months after the last dose of T‐VEC in June 2017, the patient remains well and tumour free.
Discussion
Compared with the general population, OTRs have an estimated 2·4 times higher risk of developing melanoma.8 Additionally, immunosuppressive drugs increase the risk of developing skin cancer.9 Patients treated with calcineurin inhibitors show a higher risk of developing skin cancer after transplantation than those receiving mammalian target of rapamycin inhibitors like sirolimus.10 Therefore, we changed the patient's concomitant immunosuppressive therapy from tacrolimus to sirolimus in order to minimize a possible tumour‐promoting effect.
Only a few cases of OTRs treated with checkpoint inhibitors have been published,9, 11, 12 as immunotherapies are often considered ineffective due to the existing immunosuppressive medication,13 and they could at the same time pose a risk of transplant rejection.9 BRAF–mitogen‐activated protein kinase kinase (MEK) inhibitors can also be considered as a treatment option for BRAF‐positive melanoma in OTRs.14 In murine models, post‐transplant MEK inhibition resulted in delayed onset of graft‐versus‐host disease, associated mortality and a decreased risk of alloreactivity. Therefore, the combination of BRAF and MEK inhibitors in OTRs may prevent acquired resistance to these agents and could help reduce the risk of allograft rejection.14 Regarding oncolytic virotherapy in OTRs, Schvartsman et al. reported successful treatment of a patient with an inoperable, recurrent melanoma after therapy with T‐VEC.7 While it is currently unknown how robust and long lasting this immune response is in OTRs under concurrent immunosuppressive therapy, the 13‐month relapse‐free period observed in our patient treated with T‐VEC is a hint that durable responses can be possible.
We conclude that oncolytic virotherapy can be considered as a potentially effective treatment in OTRs with locoregionally advanced melanoma, but treatment decisions should always be based on a thorough discussion with an interdisciplinary tumour board.
Supporting information
Powerpoint S1 Journal Club Slide Set.
Funding sources
None.
Conflicts of interest
None to declare.
References
- 1. Andtbacka RH, Ross M, Puzanov I et al Patterns of clinical response with talimogene laherparepvec (T‐VEC) in patients with melanoma treated in the OPTiM phase III clinical trial. Ann Surg Oncol 2016; 23:4169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Appleton ES, Turnbull S, Ralph C et al Talimogene laherparepvec in treatment of melanoma. Expert Opin Biol Ther 2015; 15:1517–30. [DOI] [PubMed] [Google Scholar]
- 3. Harrington KJ, Andtbacka RH, Collichio F et al Efficacy and safety of talimogene laherparepvec versus granulocyte–macrophage colony‐stimulating factor in patients with stage IIIB/C and IVM1a melanoma: subanalysis of the phase III OPTIM trial. Onco Targets Ther 2016; 9:7081–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kohlhapp FJ, Kaufmann HL. Molecular pathways: mechanism of action for talimogene laherparepvec, a new oncolytic virus immunotherapy. Clin Cancer Res 2016; 22:1048–54. [DOI] [PubMed] [Google Scholar]
- 5. Long G. Targeted therapy for the adjuvant treatment of stage III BRAF‐mutated melanoma. Clin Adv Hematol Oncol 2018; 16:25–7. [PubMed] [Google Scholar]
- 6. Menshawy A, Eltonob AA, Barkat SA et al Nivolumab monotherapy or in combination with ipilimumab for metastatic melanoma: systematic review and meta‐analysis of randomized controlled trials. Melanoma Res 2018; 28:371–9. [DOI] [PubMed] [Google Scholar]
- 7. Schvartsman G, Perez K, Flynn JE et al Safe and effective administration of T‐VEC in a patient with heart transplantation and recurrent locally advanced melanoma. J Immunother Cancer 2017; 5:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dahlke E, Murray CA, Kitchen J et al Systematic review of melanoma incidence and prognosis in solid organ transplant recipients. Transplant Res 2014; 3:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Spain L, Higgins R, Gopalakrishnan K et al Acute renal allograft rejection after immune checkpoint inhibitor therapy for metastatic melanoma. Ann Oncol 2016; 27:1135–7. [DOI] [PubMed] [Google Scholar]
- 10. Smith A, Niu W, Desai A et al The effect of conversion from a calcineurin inhibitor to sirolimus on skin cancer reduction in post‐renal transplantation patients. Cureus 2017; 9:1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kittai AS, Oldham H, Cetnar J et al Immune checkpoint inhibitors in organ transplant patients. J Immunother 2017; 40:277–81. [DOI] [PubMed] [Google Scholar]
- 12. Winkler JK, Gutzmer R, Bender C et al Safe administration of an anti‐PD‐1 antibody to kidney‐transplant patients: 2 clinical cases and review of the literature. J Immunother 2017; 40:341–4. [DOI] [PubMed] [Google Scholar]
- 13. Min L, Hodi FS, Kaiser UB. Corticosteroids and immune checkpoint blockade. Aging (Albany NY) 2015; 7:521–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chae YK, Galvez C, Anker JF et al Cancer immunotherapy in a neglected population: the current use and future of T‐cell‐mediated checkpoint inhibitors in organ transplant patients. Cancer Treat Rev 2018; 63:116–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Powerpoint S1 Journal Club Slide Set.


