Summary
Current treatment of diabetic nephropathy is effective; however, substantial gaps in care still remain and new therapies are urgently needed to reduce the global burden of the complication. Desirable properties of an “ideal” new drug should include primary prevention of microalbuminuria, additive/synergistic anti‐proteinuric effect in combination therapy with renin angiotensin system blockers, reduction of chronic kidney disease progression to lower the risk of end‐stage renal disease, and cardiovascular protection. Growing evidence suggests that sodium‐glucose cotransporter 2 inhibitors (SGLT2i) may fulfil many of these criteria and represent novel tools to cover the unmet needs in diabetic nephropathy care. However, the underlying mechanisms of SGLT2i renal benefits are still poorly understood and promising results from cardiovascular outcome trials with SGLT2i need confirmation in dedicated renal outcome trials.
Keywords: albuminuria, diabetic nephropathy, experimental diabetes, GFR, SGLT2
1. INTRODUCTION
Diabetic nephropathy (DN) affects nearly half of patients with type 2 diabetes and is characterized by albuminuria and/or relentless decline in renal function. Today, DN is the leading cause of End Stage Renal Disease (ESRD) in the Western World. In addition, the development of DN enhances the risk of cardiovascular (CV) morbidity and mortality. Subjects who have both diabetes and DN have a 3‐ to 12‐fold higher risk of mortality than those with diabetes only, and most of them will die before ESRD development, predominantly of CV diseases.1, 2, 3
In the nineties, landmark intervention studies proved the efficacy of blockade of the renin angiotensin system (RAS) with either ACE inhibitors (ACE‐I) or angiotensin II receptor antagonists (ARB) in reducing progression of both albuminuria and renal function loss.4, 5, 6, 7 Since then, no drug was licensed for DN treatment despite intensive research to identify new therapeutic strategies. Our understanding of the cellular and molecular mechanisms of DN has significantly improved in the last decades; however, promising preclinical compounds failed to show efficacy in humans. Recently, sodium‐glucose cotransporter 2 inhibitors (SGLT2i) have come on the scene as potential drug candidates for DN. Herein, we will review recent data, supporting the hypothesis that SGLT2i may be a novel tool to address the unmet need for renal protection in patients with diabetes. Moreover, we will discuss potential underlying mechanisms.
2. THE UNMET NEEDS IN DN CARE
The first clinical sign of DN is a moderately increased urinary albumin excretion (UACR 30‐300 mg/g), formerly named microalbuminuria. Intensive glucose control reduces the risk of microalbuminuria in patients with both type 1 (DM1) and type 2 diabetes (DM2). In the BENEDICT (The BErgamo NEphrologic DIabetes Complications Trial) study, treatment with ACE‐I prevented microalbuminuria in hypertensive DM2,8 but this was not confirmed in either normotensive DM1 patients treated with an ACE‐I9 or hypertensive DM2 patients treated with an ARB.10 Therefore, prevention of microalbuminuria is so far solely based on blood glucose control.
Progression from microalbuminuria to macroalbuminuria (UACR >300 mg/g) characteristically occurs over a period of years and is affected by intervention, most notably reduction of blood pressure (BP) and RAS inhibition. Both ACE‐I and ARB reduce albuminuria progression and even induce regression of microalbuminuria to normoalbuminuria. Although these drugs have changed the natural history of DN, a residual risk still remains and around 30% patients progress to macroalbuminuria despite optimum treatment.11
Regardless of the presence of albuminuria, diabetic kidney disease is characterized by a progressive renal function loss that may lead to ESRD or renal death. Both blood glucose control and RAS blockade can slow the glomerular filtration rate (GFR) decline; however, their efficacy in reducing the risk of long‐term renal outcomes is modest compared with that on albuminuria.12 This may partially explain why in the last 30 years albuminuria prevalence has diminished, while that of advanced chronic kidney disease (CKD) has increased threefold, making nonalbuminuric DN the prevailing phenotype.1 Besides renal outcomes, worsening of DN progressively increases the CV risk.13 The nonalbuminuric phenotype is also associated with higher mortality risk, and the presence of both albuminuria and CKD has a synergistic effect.14 A multifactorial intervention targeting not only diabetes but also CV risk factors is beneficial in reducing both all cause and CV mortality but insufficient to abolish the enhanced CV risk associated with DN.15
Taken together these data indicate that current treatment of DN with anti‐hyperglycemic drugs, BP lowering agents, and RAS blockers is effective, but substantial gaps in care still remain. Desirable properties of an “ideal” new drug for DN therapy should include (1) prevention of microalbuminuria, (2) an additive/synergistic effect in combination with RAS blockers on albuminuria, (3) marked reduction of CKD progression to prevent long‐term major renal events, and (4) cardio‐protection.
3. SODIUM‐GLUCOSE COTRANSPORTER 2
SGLT2 is a high‐capacity low‐affinity sodium glucose co‐transporter, present on the luminal surface of proximal tubule (PT) cells. SGLT2 is responsible for the majority of tubular glucose reabsorption (80‐90% of filtered glucose). The residual 10 to 20% is reabsorbed by SGLT1, a low‐capacity high‐affinity co‐transporter, exposed on the distal part of the PT. Entry of sodium and glucose within tubular cells via SGLT1/2 follows a sodium gradient generated by a Na‐K‐ATPase pump. After reabsorption, glucose moves passively into the interstitium using the facilitative glucose transporters GLUT1 and GLUT2, which are expressed on the basolateral membrane of PT cells.16, 17
4. GLYCEMIC AND EXTRA‐GLYCEMIC EFFECTS OF SGLT2 INHIBITORS
Data on the tubular expression of SGLT2 and SGLT1 in human DM2 are conflicting.18, 19, 20 However, the maximal glucose reabsorption capacity of the kidney is increased in DM2 and this contributes to hyperglycaemia, providing a rationale for SGLT2 inhibition.21 Treatment with SGLT2i can improve glucose control in DM2 by reducing renal glucose reabsorption, and today, the SGLT2i canagliflozin, dapagliflozin, empagliflozin, and ertuglifozin are widely used in DM2 therapy, while ipragliflozin, luseogliflozin, and tofogliflozin are available in Japan only.
Hyperglycaemia is a well‐established risk factor for DN both onset and progression; however, SGLT2i are not recommended in DM2 patients with reduced renal function because of their diminished anti‐hyperglycemic effect in people with low eGFR.21 Indeed, the primary determinant of tubular glucose reabsorption (or loss in the presence of SGLT2i) is the amount of filtered glucose that depends not only on blood glucose levels but also on renal function.21
Besides lowering blood glucose, SGLT2i also reduce both body weight22 and uric acid (UA) levels. SGLT2i‐induced glycosuria diminishes weight through calorie loss. GLUT9 exchanges glucose for UA and has been implicated in the uricosuric effect of SGLT2 inhibition.23, 24 However, recent evidence in experimental animals indicates that GLUT9 is dispensable, while URAT1 is required for the uricosuric response to SGLT2i.25 Inhibition of URAT1‐mediated UA tubular reabsorption by glycosuria is considered the predominant mechanism. In addition, as insulin induces URAT1 expression, SGLT2i may also enhance uricosuria by ameliorating glucose control and thus suppressing insulin.25
Treatment with SGLT2i also lowers BP without a compensatory increase in heart rate.26 In a meta‐analysis of 22 528 patients from 43 randomized controlled trials (RCTs), mean difference in systolic and diastolic BP between SGLT2i and placebo/comparators were −2.46 and −1.46 mm Hg, respectively.27 Enhanced natriuresis and osmotic diuresis, leading to sustained reduction in plasma volume, as well as reduced sympathetic tone and arterial stiffness have been proposed as underlying mechanisms.26, 28 At variance with the anti‐hyperglycemic effect, the BP lowering activity of SGLT2i is preserved and even enhanced in advancing CKD.29 The underlying mechanism is unclear; however, patients with CKD are more salt sensitive and the natriuretic effect of SGLT2i may thus result in more pronounced BP diminution in these subjects.26, 28 This anti‐hypertensive effect is highly relevant in the context of DN as BP control is challenging in patients with CKD and hypertension plays a key role in both albuminuria and CKD progression.
5. SGLT2 INHIBITION AND CV OUTCOMES
Since 2008, the Food and Drug Administration (FDA) required new diabetes drugs intended to ameliorate glycaemic control to also show CV safety. The CV outcome trials (CVOT) EMPA‐REG OUTCOME and CANVAS Program reported a reduced risk of major advanced CV events (MACE 3‐points: CV mortality, nonfatal myocardial infarction, or non‐fatal stroke) in SGLT2i‐treated patients, though the striking reduction in CV death observed with empagliflozin was not seen with canagliflozin.30, 31 In contrast, the DECLARE‐TIMI 58 trial only proved noninferiority of dapagliflozin compared with placebo with respect to MACE32 (Table 1). Likely, results varied among trials because of differences in the CV risk of recruited subjects as EMPA‐REG enrolled exclusively patients with established CV diseases, while CANVAS and DECLARE also included subjects in primary prevention. Consistent with this, a recent meta‐analysis has showed that SGLT2i reduced the risk of MACE solely in DM2 patients in secondary CV prevention.33 An important additional finding of these CVOT was a 23% reduced risk of hospitalization for heart failure (HF) in SGLT2i‐treated patients that was consistent across trails regardless of baseline CV risk category and history of HF30, 31, 32, 33 This effect was even greater in patients with reduced renal function, suggesting a specific cardiac benefit in patients with DN.33
Table 1.
Cardiovascular outcome trials with SGLT2i: design and baseline characteristics
| EMPA‐REG OUTCOME | CANVAS Program | DECLARE‐TIMI 58 | |
|---|---|---|---|
| Randomized patients, no. | 7020 | 10 142 | 17 160 |
| Active treatment | Empagliflozin 10 or 25 mg | Canagliflozin 100 or 300 mg | Dapagliflozin 10 mg |
| Follow‐up (median, y) | 3.1 | 2.4 | 4.2 |
| Main inclusion criteria | |||
| Age, y | ≥18 | ≥30 | ≥40 |
| HbA1c, % | 7‐10 | 7‐10.5 | 6.5‐12.0 |
| Body mass index, kg/m2 | ≤45 | ‐ | ‐ |
| eGFR, mL/min/1.73m2 | ≥ 30 | ≥ 30 | ≥ 60 |
| Cardiovascular disease or cardiovascular risk factors | CVD | CVD or age > 50 and ≥ 2 RF | CVD or age ≥ 55 (M) ≥60 (F) and ≥ 1 RF |
| Baseline parameters | |||
| Male, % | 72 | 66 | 63 |
| Age (mean, y) | 63 | 63 | 64 |
| HbA1c (mean, %) | 8.1 | 8.2 | 8.3 |
| Systolic BP, mmHg | 135 | 137 | 135 |
| CV status, % | |||
| Secondary prevention | 99 | 66 | 41 |
| Primary prevention | 1 | 34 | 59 |
| UACR, % | |||
| <30 mg/g | 60 | 70 | 70 |
| 30‐300 mg/g | 29 | 23 | 23 |
| >300 mg/g | 11 | 7 | 7 |
| CKD stage, % | |||
| >60 mL/min/1.73m2 | 74 | 80 | 93 |
| 45‐59 mL/min/1.73m2 | 18 | 15 | 7 |
| <45 mL/min/1.73m2 | 8 | 5 | 0 |
| eGFR (mean, mL/min/1.73m2) | 74.2 | 76.5 | 85.2 |
| RAS blocker therapy, % | 80 | 80 | 81 |
Abbreviations: BP, blood pressure; CKD, chronic kidney disease; CV, cardiovascular; eGFR, estimated glomerular filtration rate; M, male; F, female; RF, cardiovascular risk factors; RAS: renin angiotensin system; SGLT2i, sodium‐glucose cotransporter 2 inhibitors; UACR: urinary albumin excretion rate.
6. RENAL EFFECTS OF SGLT‐2 INHIBITION
CVOT with SGLT2i also included secondary renal outcomes, though definitions of renal endpoints slightly varied among trials, making direct comparisons difficult. In the next paragraphs, we will discuss whether available data support the hypothesis that SGLT2i may cover the specific unmet needs in DN care described above.
6.1. Prevention of microalbuminuria and SGLT2i
Data on the efficacy of SGLT2i in preventing new onset microalbuminuria are conflicting with a 20% risk reduction in CANVAS34 and no effect in EMPA‐REG.35 However, EMPA‐REG findings were based on a single UACR measurement and a subsequent analysis using confirmed UACR data, reported a lower risk of new micro/macroalbuminuria onset in patients with baseline normoalbuminuria.36 Moreover, placebo‐adjusted UACR was significantly reduced in patients with baseline normoalbuminuria in both trials.34, 36 The effect was, however, modest (EMPA‐REG OUTCOME −9% and CANVAS −12%) and difficult to interpret because of the high inherent variability of UACR measurements. In addition, the UACR reduction was no longer present after cessation of treatment in the EMPA‐REG, suggesting a transient hemodynamic underlying mechanism36 (Figure 1).
Figure 1.
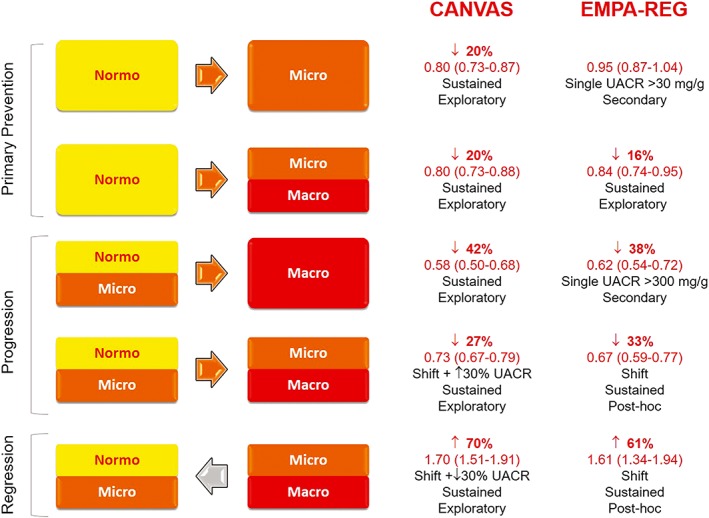
Effects of sodium‐glucose cotransporter 2 (SGLT2) inhibition on primary prevention, progression, and regression of albuminuria in the EMPA‐REG OUTCOME trial and the CANVAS Program. Hazard ratio (95% CI) are shown in red
6.2. Progression and regression of albuminuria and SGLT2i
In CVOT with SGLT2i, treatment with empagliflozin and canagliflozin reduced the risk of new onset macroalbuminuria by ~40% and the shift to higher albuminuria category by ~30%, indicating efficacy of SGLT2 inhibition in reducing albuminuria progression. Moreover, SGLT2 inhibition also enhanced the likelihood of albuminuria regression by approximately 60 to 70%31, 34, 35, 36 (Figure 1).
In agreement with these data, analyses treating UACR as a continuous variable showed that placebo‐adjusted UACR was significantly reduced in patients with either microalbuminuria (EMPA‐REG −30% and CANVAS −34%) or macroalbuminuria (EMPA‐REG −32% and CANVAS −36%).34, 35, 36
The magnitude of these changes in albuminuria is clinically relevant and comparable to that of RAS inhibitors. Furthermore, most patients (~80%) were already on RAS blockers, suggesting a benefit of adding SGLT2i to current therapy. In addition, the reduction in UACR was consistent across eGFR strata and dapagliflozin also displayed anti‐proteinuric properties in DM2 patients with stage 3‐4 CKD.37, 38
Therefore, treatment with SGLT2i may be a valuable approach for providing greater reduction in albuminuria and ultimately renoprotection. Whether microalbuminuria/macroalbuminuria is solely a biomarker or a surrogate end‐point for renal outcomes is still matter of debate; however, a meta‐analysis of large clinical trials suggested that a 30% decline in albuminuria, as reported in CVOT with SGLT2i, might translate in a 24% ESRD risk reduction, assuming that risk factors and other biomarkers are unchanged.39
The underlying mechanism of the SGLT2i anti‐proteinuric effect is unknown. However, in the EMPA‐REG, reduction of UACR in patients with albuminuria persisted, at least in part, after cessation of treatment,36 implying that SGLT2 inhibition may reduce the glomerular injury leading to albuminuria (see below the section on mechanisms).
6.3. Progressive CKD and SGLT2i
Findings from CVOT suggest that SGLT2i may also slow CKD progression and reduce occurrence of renal events of clinical relevance to patients, as renal replacement therapy (RRT) and renal death, and this has generated considerable enthusiasm (Figure 2). In a post‐hoc analysis of the EMPA‐REG, treatment with empagliflozin was associated with a significant 46% risk reduction of a composite of doubling of serum creatinine (44% risk reduction), initiation of RRT (55% risk reduction), and renal death.35 Although renal end‐points were not confirmed or adjudicated in the trial, results were similar using retrospectively confirmed events, though the effect on RRT was no longer significant.40
Figure 2.
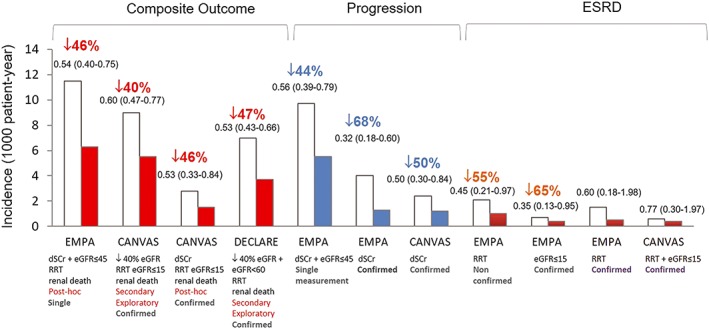
Renal outcomes in the EMPA‐REG OUTCOME (EMPA), CANVAS Program (CANVAS), and DERIVE‐TIMI 58 (DERIVE) trials. Data are expressed as incidence per 1000 patient‐year in SGLT2i‐treated (red bars: composite end‐point; blue bars: progression; brown bars: ESRD) and placebo‐treated patients (white bars). Hazard ratio (95% CI) values are also reported. On the x‐axis, it is specified: end‐point definition, type of variable (post‐hoc, secondary, exploratory) and whether analyses were based on either single or confirmed measurements. Comparison should be taken with caution because of differences in both study design and recruited subjects. ESRD, end‐stage renal disease; dSCr, doubling of serum creatinine; eGFR, estimated glomerular filtration rate (value expressed as mL/min/1.73 m2), RRT, renal replacement therapy
In CANVAS, patients treated with canagliflozin had a 40% risk reduction of a pre‐specified composite of sustained 40% eGFR reduction, ESRD, or renal death. Replacing the 40% eGFR decline with doubling of serum creatinine (~57% eGFR decline) did not change the result.31, 34 However, only a numerical trend for reduced RRT risk was observed during the relatively short follow‐up. The DECLARE trial reported a 47% risk reduction of a similar composite renal outcome in patients treated with dapagliflozin, though the effect on the individual components of the composite was not reported.32
Overall these results look very promising; however, results were driven predominantly by changes in eGFR rather than that by hard renal outcomes and the number of subjects developing ESRD, the most important clinical outcome for CKD progression, was small (27 events in EMPA‐REG and 21 in CANVAS). Moreover, analyses were deemed exploratory and not formally statistically significant in CANVAS and DECLARE because of the pre‐specified hierarchical testing plan. Therefore, these data need to be interpreted with caution and they are meant to generate hypotheses rather than to prove efficacy. Nevertheless, robustness of findings is suggested by the magnitude of the differences between SGLT2i and placebo and the consistency of results across both renal endpoints and trials. In line with this, SGLT2i therapy strongly reduced the composite outcome of worsening of renal function, ESRD, or renal death by 45% in the meta‐analysis of these trials.33 Moreover, renoprotection was the strongest and more consistent effect of SGLT2i and it was equally robust in patients with and without CV disease.33
Most patients in CVOT with SGLT2i had normal baseline renal function because an eGFR below 30 to 60 mL/min/1.73 m2 was an exclusion criterion. In addition, mean eGFR levels at follow‐up were still in the normal range, though significantly different between groups. This raises the question whether SGLT2i are also effective in patients with established CKD. Subgroup analyses did not show difference in renal outcomes across eGFR categories down to 30 mL/min/1.73 m2, 34, 35, 41, 42, 43 but the meta‐analysis found a lesser reduction in CKD progression in patients with worse baseline renal function.33 Data from ongoing SGLT2i trials focusing on patients with established CKD will clarify this relevant point.
On the other hand, the finding that SGLT2 inhibition can slow eGFR decline prior to CKD development may be important as studies in both DM1 and DM2 suggest that the rate of early (before CKD development) eGFR decline is a predictor of renal outcomes.44, 45 Therefore, changing the slope of early eGFR decline with SGLT2i may be a primary prevention strategy to delay CKD onset and delay/avoid renal events. Of interest, the difference in eGFR slopes between empagliflozin and placebo was greater in patients with macroalbuminuria and/or hypertension,43 suggesting that SGLT2i may be particularly effective in stabilizing renal function in these high‐risk subgroups.
7. MECHANISMS OF SGLT2i EFFECT IN THE KIDNEY
The underlying mechanism/s whereby SGLT2i exert their anti‐proteinuric and renoprotective effects are still unclear, and in the following paragraphs, we will summarize the main current hypotheses (Figure 3).
Figure 3.
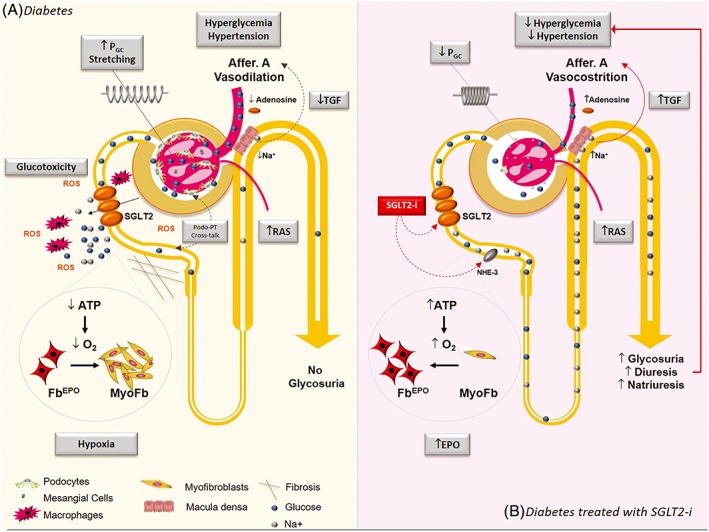
Mechanisms implicated in renal protective effect of sodium‐glucose cotransporter 2 (SGLT2) inhibition. A, In diabetes, both hyperglycaemia and systemic hypertension play a key role in the pathogenesis of the glomerular injury and enhanced SGLT2‐mediated glucose/Na+ reabsorption in the proximal tubule (PT) contribute to both. The reduced delivery of Na+ to the macula densa diminishes adenosine production and leads to afferent arteriole vasodilation. Deactivation of the tubular‐glomerular feedback (TGF) together with renin‐angiotensin‐system (RAS)‐mediated efferent arteriole vasoconstriction results in glomerular capillary hypertension (PGC) that induces hyperfiltration and glomerular volume expansion with cyclic stretching and damage of glomerular cells. Enhanced PT glucose reabsorption may cause inflammation, oxidative stress with reactive oxygen species (ROS) production, and fibrosis leading to tubule‐interstitial injury and possibly contributing to podocyte damage via PT‐podocyte cross talk. Finally, increased oxygen consumption in the renal cortex may contribute to renal fibrosis by inducing hypoxia and trans‐differentiation of erythropoietin‐producing fibroblasts (FBEPO) in profibrotic myofibroblasts (MyoFb). B, In patients treated with SGLT2 inhibitors (SGLT2i), enhanced glycosuria, natriuresis, and osmotic diuresis lower both blood glucose and blood pressure levels. By inhibiting Na+ reabsorption at both SGLT2 and Na+/H+ exchanger‐3 (NHE3) level, SGLT2i reactivate the TGF with lowering of PGC and reduced glomerular cell stretching. Reduced glucose reabsorption diminishes local glucotoxicity. Amelioration of renal cortex hypoxia allows myofibroblasts re‐differentiation in EPO‐producing cells reducing renal fibrosis and enhancing EPO production.
7.1. Beneficial effects on risk factors
In CVOT trials, body weight and both HbA1c and BP levels were lower in SGLT2i‐ than in placebo‐treated patients. However, the effect on both HbA1c (~0.5%) and body weight (~1.7 kg) was modest. Canagliflozin slowed progression of renal function decline compared with glimepiride in the CANTATA‐SU study despite similar HbA1c levels in the two arms of the study.46 Moreover, adjustment for changes in these variables did not modify the results on various renal end‐points,34, 36, 37 making unlikely the possibility that the renal benefit of SGLT2i is fully explained by their positive effects on DN risk factors.
7.2. The hemodynamic hypothesis
Diabetes induces glomerular capillary hypertension and hyperfiltration. The greater efficacy of RAS blockers as compared with other BP lowering agents in their anti‐proteinuric and renoprotective action has been ascribed to their capacity to reduce glomerular capillary pressure by removing the constrictor effect of angiotensin II on the efferent arteriole. According to the hemodynamic hypothesis, SGLT2i also reduce glomerular capillary pressure, but at variance with RAS blockers, they act on the afferent arteriole by restoring the tubule‐glomerular feedback (TGF).
In diabetes, hyperglycaemia increases SGLT2‐mediated tubular reabsorption of glucose and sodium and thus diminishes sodium delivery to the macula densa, mimicking kidney hypo‐perfusion. In macula densa cells, lower sodium entry reduces basolateral release of adenosine, a potent vasoconstrictor, and thus causes afferent arteriolar vasodilation.47 This leads to an enhanced glomerular capillary pressure and also allows transmission of any rise in systemic BP to the glomerular capillaries.48 Inhibition of SGLT2 induces opposing effects, resulting in afferent arteriolar vasoconstriction and reduction of both intraglomerular pressure and glomerular filtration.47
In keeping with this hypothesis, hyperfiltration is reduced in streptozotocin‐induced diabetic mice knockout for SGLT249 and a study performed in patients with DM1 and hyperfiltration has demonstrated that empagliflozin attenuates diabetes‐induced changes in renal hemodynamics, leading to glomerular hyperfiltration.50
Changes in eGFR over time in CVOT with SGLT2i are also consistent with the hemodynamic hypothesis.32, 40 SGLT2i treatment causes an acute decline in eGFR during the first weeks of treatment likely due to a reduction in glomerular capillary pressure and thus glomerular filtration. This acute eGFR loss is a transient/reversible phenomenon as it is no longer present after SGLT2i discontinuation, and it is similar to that observed in response to RAS blockade. Following the initial drop, eGFR tends to return toward the baseline over several weeks, remaining stable thereafter, and at the end of the study is significantly higher than in placebo‐treated patients, who show a gradual eGFR decline over time. Likely, the reduction in glomerular capillary pressure induced by SGLT2i protects glomerular cells from the hemodynamic insult with long‐term advantageous effects. In keeping with this notion, an acute fall in eGFR induced by losartan predicted a less steep decline of renal function slope.51
The underlying mechanisms are still unclear; however, glomeruli are highly compliant structures and an increase in glomerular capillary pressure results in glomerular volume expansion. Studies in isolated glomeruli have shown that as the intraglomerular pressure rises to levels similar to those seen in the diabetic kidney, glomerular volume increases by ~30%.52, 53 Under normal conditions, renal auto‐regulation allows tight control of intraglomerular pressure and glomeruli are exposed to small pulse pressure changes and their volume remains stable. By contrast, loss of auto‐regulation, as it occurs in the diabetic glomeruli, results in wide wings in glomerular volume up to 18‐fold changes.52, 53 In addition, at any given intraglomerular pressure level, larger hypertrophied glomeruli are more distensible than smaller glomeruli. Therefore, compensatory glomerular hypertrophy can magnify the deleterious effects of altered glomerular hemodynamics.53 During glomerular expansion, glomerular cells undergo centrifugal displacement and experience substantial mechanical stretching and cyclic changes in glomerular volume are associated with repeated episodes of cell stretch and relaxation.
Studies on glomerular cells exposed in vitro to mechanical stretching to mimic glomerular hypertension in vivo have shown that the mechanical insult can lead to changes in cell phenotype. Specifically, mesangial cells enhance production of extracellular matrix components and deleterious cytokines (monocyte chemoattractant protein‐1, transforming growth factor‐β1, and vascular endothelial growth factor), thus favouring sclerosis and inflammation.54, 55, 56 In addition, they overexpress both GLUT‐1 and the angiotensin AT1 receptor and this results in enhanced glucotoxicity and responsiveness to angiotensin II.57, 58 Furthermore, stretching of podocytes, which are crucial component of the glomerular filtration barrier, induces both nephrin downregulation59 and apoptosis60 that are key determinants in the pathogenesis of albuminuria.
Glomerular capillary hypertension and hyperfiltration are a well‐established mechanism of progression in all CKD. Whether SGLT2i may also be beneficial in other kidney diseases besides DN is undefined. Treatment with SGLT2i failed to show renoprotective effects in the 5/6 nephrectomy animal model61 and in oxalate‐induced CKD,62 while a benefit was reported in a model of proteinuric non‐DN.63 Recently, the TRANSLATE study has shown that short‐term treatment with dapagliflozin did not modify renal hemodynamic function or attenuate proteinuria in either humans or in experimental focal segmental glomerulosclerosis (FSGS). SGLT2 expression was reduced in FSGS, and the absence of diabetes‐induced both hyperglycaemia and SGLT2 overexpression/hyperactivity may explain the lack of efficacy.64
7.3. Tubular glucotoxicity
Oxidative stress, inflammation, and fibrosis play a key role in the pathogenesis of DN. Studies in experimental DN have shown that SGLT2i ameliorate oxidative stress, inflammation, and fibrosis, predominantly in the tubule‐interstitium, indicating a role of enhanced tubular glucose reabsorption through SGLT2 in their pathogenesis. However, available experimental data are highly heterogeneous, as different animal models, compounds, doses, and length of treatment were used, making difficult to draw final conclusions. In addition, many studies did not control for blood glucose and BP levels and it is difficult to distinguish between the direct beneficial effects of SGLT2i on the kidney and their indirect effect due to improved metabolism/hemodynamics. Table 2 summarizes the study design and the results of the main studies on SGLT2i in experimental DN that control for blood glucose levels.
Table 2.
SGLT2 inhibitors in experimental diabetic nephropathy
| Active Treatment | Animal Model | Study Design | Study Duration | Functional and structural effects | Mechanisms |
|---|---|---|---|---|---|
| Empagliflozin84 | Type 1 DM (eNOS‐KO STZ mice) | Empaglifozin 10 mg/kg/day vs. insulin | 19 weeks | = Albuminuria | |
| = Glomerulosclerosis | |||||
| = Tubular atrophy | |||||
| Empagliflozin85 | Type 2 DM (db/db mice) | Empaglifozin 10 mg/kg/day vs. metformin | 10 weeks | = Albuminuria | ↓ Fibrosis (fibronectin TGF‐β) |
| = Glomerulosclerosis | |||||
| = Kidney growth | |||||
| Dapaglifozin86 | Type 2 DM (OLEFT rats) | Dapaglifozin 1 mg/kg/day vs. voglibose | 12 weeks | ↓ Albuminuria | ↓ RAS activation |
| ↓ Oxidative stress | |||||
| ↓ Mesangial | ↓ Inflammation | ||||
| ↓ Interstitial fibrosis | |||||
| Dapaglifozin87 | Type 1 DM (Akita mice) | Dapaglifozin 1 mg/kg/day vs. insulin | 12 weeks | ↓ Albuminuria | ↓ Interstitial |
| ↓ Interstitial fibrosis | inflammation | ||||
| ↓ Fibrosis (TGF‐β1) | |||||
| ↓ Oxidative stress | |||||
| Dapaglifozin88 | Type 2 DM (db/db mice) | Dapaglifozin 2 mg/kg/day vs. pioglitazone | 9 weeks | = Albuminuria | |
| = Mesangial Expansion | |||||
| = Foot process width | |||||
| Luseoglifozin89 | Type 1 DM and hypertension (STZ‐Dahl Salt‐sensitive rats) | Luseoglifozin 10 mg/kg/day vs. insulin | 8 weeks | = Albuminuria | |
| = Hyperfiltration | |||||
| = Renal injury | |||||
| Luseoglifozin90 | Type 2 DM (T2DN rats) | Luseoglifozin 10 mg/kg food vs. insulin | 12 weeks | ↓ eGFR decline | ↓ Nephrin excretion |
| ↓ Glomerulosclerosis, | |||||
| ↓ Renal fibrosis | |||||
| Ipraglifozin91 | Type 2 DM (BTBR ob/ob mice) | Ipraglifozin 4 mg/kg/day vs. 30% calorie restriction | 18 weeks | ↓ Albuminuria | ↓ TCA cycle |
| ↓ Hyperfiltration | ↓ Oxidative stress | ||||
| ↓ Mesangial expansion |
Abbreviations: DM, diabetes; eGFR, glomerular filtration rate; KO. knockout; RAS, renin angiotensin system; SGLT2i, sodium‐glucose cotransporter 2 inhibitors; STZ: streptozotocin; TCA, tricarboxylic acid cycle; TGF‐β1, transforming growth factor‐β1.
7.4. The hypoxia hypothesis
Diabetes increases oxygen requirement of the PTs due to excessive glucose reabsorption by SGLT2. According with the hypothesis by Sano M et al, the resulting tubulointerstitial hypoxia causes trans‐differentiation of EPO‐producing interstitial fibroblasts in myofibroblasts, thus contributing to interstitial fibrosis. As trans‐differentiation is reversible, treatment with SGLT2i can induce differentiation of myofibroblasts back to erythropoietin (EPO)‐producing cells, enhancing EPO production and more importantly limiting renal fibrosis.65 In keeping with this notion, patients with DN are nearly twice as likely to develop anaemia compared with patients with non‐diabetic CKD and similar eGFR.66 Moreover, treatment with SGLT2i increases both haematocrit values and EPO synthesis.65, 67, 68
In experimental diabetes, inhibition of SGLT returned to normal renal cortex O2 tension, but worsened hypoxia in the medulla.69 This likely reflects increased delivery of sodium to the distal nephron that enhances medullary transport and hence consumption of oxygen. Indeed, in the PT, SGLT2i inhibits not only SGLT2 but also Na+/H+ exchanger‐3‐dependent sodium uptake, preventing compensation of diminished Na+ reabsorption by SGLT.70 This raises the hypothesis that the increased EPO production may be important in limiting the potential deleterious effects of SGLT2i‐induced medullary hypoxia.26
Moreover, in subjects treated with SGLT2i increased oxygen consumption in the medulla can also be balanced by a metabolic shift toward more energy‐efficient fuels. According with the “thrifty substrate hypothesis,”71 treatment with SGLT2i induces persistent hyperketonemia and tissues that are more susceptible to hypoxia, as the renal medulla, can oxidize β‐hydroxybutyrate instead of fatty acids. Compared with fatty acid, ketone bodies are more energetically efficient, yielding more energy available for ATP synthesis per molecule of oxygen. Therefore, this shift in substrate energetics may reduce the risk of hypoxia within the renal medulla. In addition, a rise in circulating β‐hydroxybutyrate levels increases the acetylation of renal histones H3K9 and H3K14 that induce expression of genes promoting resistance to oxidative stress.72 Therefore, SGLT2‐i not only may prevent hypoxia but also reduce the deleterious effects of hypoxia‐induced oxidative stress. However, further studies are required to confirm these fascinating hypotheses.
7.5. Alterative RAS pathways
SGLT2i can induce RAS activation by enhancing both diuresis and natriuresis.73 However, most patients enrolled in CVOT with SGLT2i were on RAS blockers, and hence, the classical renin‐angiotensin II‐AT1 receptor pathway was blocked. In this context, angiotensin processing may occur via the ACE homologue ACE2, resulting in formation of Ang‐(1‐7) and Ang‐(1‐9), and angiotensin II may bind to the AT2 receptor instead of AT1. Signalling of Ang‐(1‐7) and Ang‐(1‐9) as well as AT2 receptor activation has anti‐oxidative and anti‐fibrotic effects that may contribute to renoprotection in patients under dual treatment with SGLT2 and RAS blockers.74, 75
7.6. Podocyte damage
Podocytes play a key role in the pathogenesis of albuminuria; therefore, there is increasing interest on potential mechanism/s, whereby SGLT2i may affect this cell type. A cross talk may occur between PT cells and podocytes. For instance, downregulation of SIRT1 in PT, causing reduced NAD production, induces both podocyte damage and albuminuria in experimental diabetes.76 Of interest, tubular SIRT1 downregulation in db/db mice is mediated by enhanced PT glucose entry through SGLT2 followed by SGLT2 overexpression.77
Although SGLT2 is not present within the glomeruli in normal conditions, SGLT2 is expressed by podocytes in a non‐diabetic model of proteinuric glomerulopathy and dapagliflozin treatment ameliorated albuminuria, glomerular lesions, and podocyte dysfunction/loss in this model, suggesting a functional role of SGLT2 in podocytes.63
8. SGLT2 INHIBITION AND RENAL/GENITAL SIDE EFFECTS
Treatment with SGLT2i enhances the risk of genital mycotic infections, particularly in women. By contrast, an increased risk of urinary tract infections was not found in CVOT with SGLT2i.30, 31, 32 Volume depletion may occur as a consequence of both osmotic diuresis and natriuresis. Volume depletion‐related events did not differ in patients treated with either empagliflozin or placebo in the EMPA‐REG, while a higher rate of volume depletion was observed in CKD patients treated with the highest canagliflozin dose in phase III clinical studies.30, 78 Volume depletion together with impaired auto‐regulation of renal hemodynamics may enhance the likelihood of acute kidney injury (AKI). Neither CVOT with SGLT2i nor real‐world data showed an increased risk of AKI, and AKI events were even reduced in the EMPA‐REG.30, 79 However, SGLT2 inhibition should be used with caution in hemodynamic unstable patients or in combination with medications that may induce AKI and the FDA has requested to include AKI among the SGLT2i potential side effects following an excess of acute renal failure adverse reports.80 In the CANVAS Program, canagliflozin increased the risk of both overall bone fractures and lower limb amputations, though the latter was not confirmed in real life studies and experimental animals.31, 81, 82 Whether this is a real and drug‐specific effect remains unclear. Despite these adverse events do not directly involve the kidney, they are relevant to DN as mineral bone disorders complicate CKD and patients with DN have a high risk and prevalence of peripheral vascular disease.
9. CONCLUSIONS AND FUTURE PERSPECTIVES
In conclusion, there is emerging evidence that SGLT2i have an anti‐proteinuric and renoprotective activity independent of the presence or absence of established CV disease. This benefit appears a class effect, and it is also observed in patients who are already under treatment with RAS inhibitors. This raises hope that dual RAS and SGLT2 blockade may represent a new therapy for DN, filling unmet needs for care and effectively tackling the cardio‐renal vicious cycle.
However, properly powered and dedicated studies are needed to clarify SGLT2i role in DN. There is thus great interest for ongoing clinical trials (Table 3) that will test the efficacy of SGLT2i on primary renal outcomes in people with established DN. In this regard, it is noteworthy that the CREDENCE trial, designed to test the effect of canagliflozin compared with standard care in DM2 patients with established DN,78 was stopped early on July 2018 based on the achievement of pre‐specified efficacy criteria for the primary composite of doubling of serum creatinine, ESRD, and renal or CV death. The DAPA‐CKD and the EMPA‐KIDNEY trials will also clarify if SGLT2i may have renal benefits in other kidney diseases as they have enrolled non‐diabetic patients with CKD and they are powered to show a renal benefit in this subgroup. Results from these trials have thus the potential to extend SGLT2i application beyond DN.
Table 3.
Major ongoing SGLT2 inhibitor trials with renal outcomes
| Study Name | EMPA‐ KIDNEY | CREDENCE | DAPA‐ CKD | VERTIS CV |
|---|---|---|---|---|
| Registration number | NCT03594110 | NCT02065791 | NCT03036150 | NCT01986881 |
| Intervention | Empaglifozin vs. placebo | Canaglifozin 100 mg vs. placebo | Dapaglifozin 5 and 10 mg vs. placebo | Ertuglifozin 5 and 15 mg vs. placebo |
| No. of patients | 5000 (estimated) | 4401 | 4000 (estimated) | 8000 (estimated) |
| Study population | DM2 and non‐DM2 with CKD | DM2 with CKD and high CV risk | DM2 and non‐DM2 with CKD and high CV risk | DM2 with CVD |
| Renal inclusion criteria | eGFR 20‐44 mL/min/1.73m2 or eGFR <90 mL/min/1.73m2 with UACR ≥200 mg/g | eGFR 30‐90 mL/min/1.73m2 with UACR 300‐5000 mg/g | eGFR 25‐75 mL/min/1.73m2 with UACR 200‐5000 mg/g | eGFR ≥30 mL/min/1.73m2 |
| Estimated FU duration | 3.1 y | 5.5 y | 4 y | 6.1 y |
| Primary composite outcomes | CKD progression (↓ ≥ 40% eGFR, ESRDa, eGFR <10 mL/min/1.73m2, renal death) or CV death | ESRDb, dSCr, or CV or renal death | ESRDb, ↓ ≥ 50% eGFR or CV or renal death | MACE |
| Secondary renal outcomes | CKD progression, CV death or ESRD | Renal composite outcome (ESRDb, dSCr, or renal death) | Individual components of the primary outcome | Renal composite outcome (dSCr, RRT, or renal death) |
| Estimated completion date | June 2022 | June 2019c | November 2020 | September 2019 |
ESRD initiation of maintenance dialysis or receipt of a kidney transplant.
ESRD initiation of maintenance dialysis or receipt of a kidney transplant or sustained <15 mL/min/1.73 m2.
The trial was stopped study early on July 2018 based on the achievement of pre‐specified efficacy criteria for the primary composite.
Abbreviations: CKD, chronic kidney disease; CV, cardiovascular; CVD, cardiovascular disease; DM2, type 2 diabetes; dSCr, doubling of serum creatinine from baseline; eGFR, estimated glomerular filtration rate; ESRD; end‐stage renal disease; FU, follow‐up; MACE, major advanced cardiovascular events; UACR, urinary albumin excretion. EMPA‐KIDNEY, The Study of Heart and Kidney Protection With Empagliflozin; CREDENCE, Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants With Diabetic Nephropathy; DAPA‐CKD, A Study to Evaluate the Effect of Dapagliflozin on Renal Outcomes and Cardiovascular Mortality in Patients With Chronic Kidney Disease; VERTIS CV, Cardiovascular Outcomes Following Ertugliflozin Treatment in Type 2 Diabetes Mellitus Participants With Vascular Disease.
A recent consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) recommends considering the use of SGLT2i as add‐on therapy to metformin in DM2 patients with CKD with or without CVD.83 Although, SGLT2i are currently not recommended in patients with reduced renal function, the beneficial effect of SGLT2i on BP and both CV and renal outcomes are retained in patients with reduced eGFR. Re‐evaluating current limitations on SGLT2i use in patients with CKD would allow more individuals to benefit from SGLT2 inhibition. Notably, SGLT2i may also be advantageous in the treatment of non‐albuminuric DN, in which specific therapies are still lacking.
Although our knowledge of SGLT2i mechanisms of renoprotection is gradually improving, basic science research is still left behind and we need to undertake a reverse “bedside to bench” approach to explain mechanistically exciting data coming from clinical trials.
CONFLICT OF INTEREST
Gruden G. has received a speaker fee from MundiPharma.
AUTHOR'S CONTRIBUTION
Gruden, G., wrote the manuscript and revised the final version. Barutta, F., wrote the manuscript; Bernardi, S,. wrote the manuscript; Gargiulo, G., wrote the manuscript; Durazzo, M., edited the manuscript.
Barutta F, Bernardi S, Gargiulo G, Durazzo M, Gruden G. SGLT2 inhibition to address the unmet needs in diabetic nephropathy. Diabetes Metab Res Rev. 2019;35:e3171 10.1002/dmrr.3171
F. Barutta and S. Bernardi contributed equally to this work.
REFERENCES
- 1. Afkarian M, Zelnick LR, Hall YN, et al. Clinical manifestations of kidney disease among US adults with diabetes. 1988‐2014. JAMA. 2016;316(6):602‐610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Saran R, Robinson B, Abbott KC, et al. US renal data system 2017 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2018;71(3S1):A7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Levin A, Tonelli M, Bonventre J, et al. Global kidney health 2017 and beyond: a roadmap for closing gaps in care, research, and policy. Lancet. 2017;390(10105):1888‐1917. [DOI] [PubMed] [Google Scholar]
- 4. Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861‐869. [DOI] [PubMed] [Google Scholar]
- 5. Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin‐converting‐enzyme inhibition on diabetic nephropathy. The collaborative study group. N Engl J Med. 1993;329(20):1456‐1462. [DOI] [PubMed] [Google Scholar]
- 6. Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin‐receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851‐860. [DOI] [PubMed] [Google Scholar]
- 7. Heart Outcomes Prevention Evaluation Study Investigators . Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO‐HOPE substudy. Lancet. 2000;355(9200):253‐259. [PubMed] [Google Scholar]
- 8. Ruggenenti P, Fassi A, Ilieva AP, et al. Preventing microalbuminuria in type 2 diabetes. N Engl J Med. 2004;351(19):1941‐1951. [DOI] [PubMed] [Google Scholar]
- 9. Mauer M, Zinman B, Gardiner R, et al. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med. 2009;361(1):40‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Haller H, Ito S, Izzo JL Jr, et al. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med. 2011;364(10):907‐917. [DOI] [PubMed] [Google Scholar]
- 11. Gaede P, Lund‐Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358(6):580‐591. [DOI] [PubMed] [Google Scholar]
- 12. Oellgaard J, Gæde P, Rossing P, Persson F, Parving HH, Pedersen O. Intensified multifactorial intervention in type 2 diabetics with microalbuminuria leads to long‐term renal benefits. Kidney Int. 2017;91(4):982‐988. [DOI] [PubMed] [Google Scholar]
- 13. Adler AI, Stevens RJ, Manley SE, et al. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int. 2003;63(1):225‐232. [DOI] [PubMed] [Google Scholar]
- 14. Penno G, Solini A, Orsi E, et al. Non‐albuminuric renal impairment is a strong predictor of mortality in individuals with type 2 diabetes: the Renal Insufficiency And Cardiovascular Events (RIACE) Italian multicentre study. Diabetologia. 2018;61(11):2277‐2289. [DOI] [PubMed] [Google Scholar]
- 15. Gæde P, Oellgaard J, Carstensen B, et al. Years of life gained by multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: 21 years follow‐up on the Steno‐2 randomised trial. Diabetologia. 2016;59(11):2298‐2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vallon V, Platt KA, Cunard R, et al. SGLT2 mediates glucose reabsorption in the early proximal tubule. J Am Soc Nephrol. 2011;22(1):104‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ghezzi C, Loo DDF, Wright EM. Physiology of renal glucose handling via SGLT1, SGLT2 and GLUT2. Diabetologia. 2018;61(10):2087‐2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Solini A, Rossi C, Mazzanti CM, Proietti A, Koepsell H, Ferrannini E. Sodium‐glucose co‐transporter (SGLT)2 and SGLT1 renal expression in patients with type 2 diabetes. Diabetes Obes Metab. 2017;19(9):1289‐1294. [DOI] [PubMed] [Google Scholar]
- 19. Norton L, Shannon CE, Fourcaudot M, et al. Sodium‐glucose co‐transporter (SGLT) and glucose transporter (GLUT) expression in the kidney of type 2 diabetic subjects. Diabetes Obes Metab. 2017;19(9):1322‐1326. [DOI] [PubMed] [Google Scholar]
- 20. Wang XX, Levi J, Luo Y, et al. SGLT2 protein expression is increased in human diabetic nephropathy: SGLT2 protein inhibition decreases renal lipid accumulation, inflammation, and the development of nephropathy in diabetic mice. J Biol Chem. 2017;292(13):5335‐5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Abdul‐Ghani MA, DeFronzo RA. Lowering plasma glucose concentration by inhibiting renal sodium‐glucose cotransport. J Intern Med. 2014;276(4):352‐363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cai X, Yang W, Gao X, et al. The association between the dosage of SGLT2 inhibitor and weight reduction in type 2 diabetes patients: a meta‐analysis. Obesity. 2018;26(1):70‐80. [DOI] [PubMed] [Google Scholar]
- 23. Cheeseman C. Solute carrier family 2, member 9 and uric acid homeostasis. Curr Opin Nephrol Hypertens. 2009;18(5):428‐432. [DOI] [PubMed] [Google Scholar]
- 24. Lytvyn Y, Skrtic M, Yang GK, Yip PM, Perkins BA, Cherney DZ. Glycosuria‐mediated urinary uric acid excretion in patients with uncomplicated type 1 diabetes mellitus. Am J Physiol Renal Physiol. 2015;308(2):F77‐F83. [DOI] [PubMed] [Google Scholar]
- 25. Novikov A, Fu Y, Huang W, et al. SGLT2 inhibition and renal urate excretion: role of luminal glucose, GLUT9, and URAT1. Am J Physiol Renal Physiol. 2019;316(1):F173‐F185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thomas MC, Cherney DZI. The actions of SGLT2 inhibitors on metabolism, renal function and blood pressure. Diabetologia. 2018;61(10):2098‐2107. [DOI] [PubMed] [Google Scholar]
- 27. Mazidi M, Rezaie P, Gao HK, Kengne AP. Effect of sodium‐glucose cotransport‐2 inhibitors on blood pressure in people with type 2 diabetes mellitus: a systematic review and meta‐analysis of 43 randomized control trials with 22 528 patients. J Am Heart Assoc. 2017;6(6):e004007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Heerspink HJL, Kosiborod M, Inzucchi SE, Cherney DZI. Renoprotective effects of sodium‐glucose cotransporter‐2 inhibitors. Kidney Int. 2018;94(1):26‐39. [DOI] [PubMed] [Google Scholar]
- 29. Cherney DZI, Cooper E, Tikkanen I, et al. Pooled analysis of phase III trials indicate contrasting influences of renal function on blood pressure, body weight, and HbA1c reductions with empagliflozin. Kidney Int. 2018;93(1):231‐244. [DOI] [PubMed] [Google Scholar]
- 30. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117‐2128. [DOI] [PubMed] [Google Scholar]
- 31. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644‐657. [DOI] [PubMed] [Google Scholar]
- 32. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2018. 10.1056/NEJMoa1812389;380(4):347‐357. [DOI] [PubMed] [Google Scholar]
- 33. Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta‐analysis of cardiovascular outcome trials. Lancet. 2019;393(10166):31‐39. [DOI] [PubMed] [Google Scholar]
- 34. Perkovic V, de Zeeuw D, Mahaffey KW, et al. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol. 2018;6(9):691‐704. [DOI] [PubMed] [Google Scholar]
- 35. Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(18):323‐334. [DOI] [PubMed] [Google Scholar]
- 36. Cherney DZ, Zinman B, Inzucchi SE, et al. Effects of empagliflozin on the urinary albumin‐to‐creatinine ratio in patients with type 2 diabetes and established cardiovascular disease: an exploratory analysis from the EMPA‐REG OUTCOME randomised, placebo‐controlled trial. Lancet Diabetes Endocrinol. 2017;5(8):610‐621. [DOI] [PubMed] [Google Scholar]
- 37. Fioretto P, Stefansson BV, Johnsson E, Cain VA, Sjöström CD. Dapagliflozin reduces albuminuria over 2 years in patients with type 2 diabetes mellitus and renal impairment. Diabetologia. 2016;59(9):2036‐2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dekkers CCJ, Wheeler DC, Sjöström CD, Stefansson BV, Cain V, Heerspink HJL. Effects of the sodium‐glucose co‐transporter 2 inhibitor dapagliflozin in patients with type 2 diabetes and stages 3b‐4 chronic kidney disease. Nephrol Dial Transplant. 2018;33(11):2005‐2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Heerspink HJ, Kröpelin TF, Hoekman J, de Zeeuw D, Reducing Albuminuria as Surrogate Endpoint (REASSURE) Consortium . Drug‐induced reduction in albuminuria is associated with subsequent renoprotection: a meta‐analysis. J Am Soc Nephrol. 2015;26(8):2055‐2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wanner C, Inzucchi SE, Zinman B. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(18):1801‐1802. [DOI] [PubMed] [Google Scholar]
- 41. Wanner C, Lachin JM, Inzucchi SE, et al. Empagliflozin and clinical outcomes in patients with type 2 diabetes mellitus, established cardiovascular disease, and chronic kidney disease. Circulation. 2018;137(2):119‐129. [DOI] [PubMed] [Google Scholar]
- 42. Neuen BL, Ohkuma T, Neal B, et al. Cardiovascular and renal outcomes with canagliflozin according to baseline kidney function. Circulation. 2018;138(15):1537‐1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wanner C, Heerspink HJL, Zinman B, et al. Empagliflozin and kidney function decline in patients with type 2 diabetes: a slope analysis from the EMPA‐REG OUTCOME trial. J Am Soc Nephrol. 2018;29(11):2755‐2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Skupien J, Warram JH, Smiles AM, et al. The early decline in renal function in patients with type 1 diabetes and proteinuria predicts the risk of end‐stage renal disease. Kidney Int. 2012;82(5):589‐597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pavkov ME, Knowler WC, Lemley KV, Mason CC, Myers BD, Nelson RG. Early renal function decline in type 2 diabetes. Clin J Am Soc Nephrol. 2012;7(1):78‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Heerspink HJ, Desai M, Jardine M, Balis D, Meininger G, Perkovic V. Canagliflozin slows progression of renal function decline independently of glycemic effects. J Am Soc Nephrol. 2017;28(1):368‐375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes: cardiovascular and kidney effects, potential mechanisms and clinical applications. Circulation. 2016;134(10):752‐772. [DOI] [PubMed] [Google Scholar]
- 48. Hostetter TH, Rennke H, Brenner BM. The case for intra‐renal hypertension in the initiation and progression of diabetic and other glomerulopathies. Am J Med. 1982;72(3):375‐380. [DOI] [PubMed] [Google Scholar]
- 49. Vallon V, Rose M, Gerasimova M, et al. Knockout of Na‐glucose transporter SGLT2 attenuates hyperglycemia and glomerular hyperfiltration but not kidney growth or injury in diabetes mellitus. Am J Physiol Renal Physiol. 2013;304(2):F156‐F167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Skrtić M, Yang GK, Perkins BA, et al. Characterisation of glomerular haemodynamic responses to SGLT2 inhibition in patients with type 1 diabetes and renal hyperfiltration. Diabetologia. 2014;57(12):2599‐2602. [DOI] [PubMed] [Google Scholar]
- 51. Holtkamp FA, de Zeeuw D, Thomas MC, et al. An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long‐term renal function. Kidney Int. 2011;80(3):282‐287. [DOI] [PubMed] [Google Scholar]
- 52. Cortes P, Riser BL, Zhao X, Narins RG. Glomerular volume expansion and mesangial cell mechanical strain: mediators of glomerular pressure injury. Kidney Int. 1994;45:S11‐S16. [PubMed] [Google Scholar]
- 53. Cortes P, Zhao X, Riser BL, Narins RG. Regulation of glomerular volume in normal and partially nephrectomized rats. Am J Physiol. 1996;270(2 Pt 2):F356‐F370. [DOI] [PubMed] [Google Scholar]
- 54. Giunti S, Pinach S, Arnaldi L, et al. The MCP‐1/CCR2 system has direct proinflammatory effects in human mesangial cells. Kidney Int. 2006;69(5):856‐863. [DOI] [PubMed] [Google Scholar]
- 55. Gruden G, Zonca S, Hayward A, et al. Mechanical stretch‐induced fibronectin and transforming growth factor‐beta1 production in human mesangial cells is p38 mitogen‐activated protein kinase‐dependent. Diabetes. 2000;49(4):655‐661. [DOI] [PubMed] [Google Scholar]
- 56. Gruden G, Thomas S, Burt D, et al. Mechanical stretch induces vascular permeability factor in human mesangial cells: mechanisms of signal transduction. Proc Natl Acad Sci U S A. 1997;94(22):12112‐12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gnudi L, Viberti G, Raij L, et al. GLUT‐1 overexpression: link between hemodynamic and metabolic factors in glomerular injury? Hypertension. 2003;42(1):19‐24. [DOI] [PubMed] [Google Scholar]
- 58. Gruden G, Thomas S, Burt D, et al. Interaction of angiotensin II and mechanical stretch on vascular endothelial growth factor production by human mesangial cells. J Am Soc Nephrol. 1999;10(4):730‐737. [DOI] [PubMed] [Google Scholar]
- 59. Miceli I, Burt D, Tarabra E, Camussi G, Perin PC, Gruden G. Stretch reduces nephrin expression via an angiotensin II‐AT(1)‐dependent mechanism in human podocytes: effect of rosiglitazone. Am J Physiol Renal Physiol. 2010;298(2):F381‐F390. [DOI] [PubMed] [Google Scholar]
- 60. Durvasula RV, Petermann AT, Hiromura K, et al. Activation of a local tissue angiotensin system in podocytes by mechanical strain. Kidney Int. 2004;65(1):30‐39. [DOI] [PubMed] [Google Scholar]
- 61. Zhang Y, Thai K, Kepecs DM, Gilbert RE. Sodium‐glucose linked Cotransporter‐2 inhibition does not attenuate disease progression in the rat remnant kidney model of chronic kidney disease. PLoS ONE. 2016;11(1):e0144640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ma Q, Steiger S, Anders HJ. Sodium glucose transporter‐2 inhibition has no renoprotective effects on non‐diabetic chronic kidney disease. Physiol Rep. 2017;5(7):pii:e13228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cassis P, Locatelli M, Cerullo D, et al. SGLT2 inhibitor dapagliflozin limits podocyte damage in proteinuric nondiabetic nephropathy. JCI Insight. 2018;3(15):pii:98720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rajasekeran H, Reich HN, Hladunewich MA, et al. Dapagliflozin in focal segmental glomerulosclerosis: a combined human‐rodent pilot study. Am J Physiol Renal Physiol. 2018;314(3):F412‐F422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sano M, Takei M, Shiraishi Y, Suzuki Y. Increased hematocrit during sodium‐glucose cotransporter 2 inhibitor therapy indicates recovery of Tubulointerstitial function in diabetic kidneys. J Clin Med Res. 2016;8(12):844‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Thomas MC, Cooper ME, Rossing K, Parving H‐H. Anaemia in diabetes: is there a rationale to TREAT? Diabetologia. 2006;49(6):1151‐1157. [DOI] [PubMed] [Google Scholar]
- 67. Ferrannini E, Baldi S, Frascerra S, et al. Renal handling of ketones in response to sodium‐glucose cotransporter 2 inhibition in patients with type 2 diabetes. Diabetes Care. 2017;40(6):771‐776. [DOI] [PubMed] [Google Scholar]
- 68. Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose‐regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. 2013;15(9):853‐862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. O'Neill J, Fasching A, Pihl L, Patinha D, Franzén S, Palm F. Acute SGLT inhibition normalizes O2 tension in the renal cortex but causes hypoxia in the renal medulla in anaesthetized control and diabetic rats. Am J Physiol Renal Physiol. 2015;309(3):F227‐F234. [DOI] [PubMed] [Google Scholar]
- 70. Layton AT, Vallon V, Edwards A. Modeling oxygen consumption in the proximal tubule: effect of NHE and SGLT2 inhibition. Am J Physiol Renal Physiol. 2015;308(12):F1343‐F1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ferrannini E, Mark M, Mayoux E. CV protection in the EMPA‐REG OUTCOME trial: a “thrifty substrate” hypothesis. Diabetes Care. 2016;39(7):1108‐1114. [DOI] [PubMed] [Google Scholar]
- 72. Shimazu T, Hirschey MD, Newman J, et al. Suppression of oxidative stress by beta‐hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 2013;339(6116):211‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Vallon V, Thomson SC. Targeting renal glucose reabsorption to treat hyperglycaemia: the pleiotropic effects of SGLT2 inhibition. Diabetologia. 2017;60(2):215‐225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Cherney DZ, Perkins BA, Soleymanlou N, et al. Sodium glucose cotransport‐2 inhibition and intrarenal RAS activity in people with type 1 diabetes. Kidney Int. 2014;86(5):1057‐1058. [DOI] [PubMed] [Google Scholar]
- 75. Muskiet MH Muskiet MH, van Raalte DH, van Bommel EJ, Smits MM, Tonneijck L. Understanding EMPA‐REG OUTCOME. Lancet Diabetes Endocrinol. 2015;3(12):928‐929. [DOI] [PubMed] [Google Scholar]
- 76. Hasegawa K, Wakino S, Simic P, et al. Renal tubular Sirt1 attenuates diabetic albuminuria by epigenetically suppressing Claudin‐1 overexpression in podocytes. Nat Med. 2013;19(11):1496‐1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Umino H, Hasegawa K, Minakuchi H, et al. High basolateral glucose increases sodium‐glucose cotransporter 2 and reduces Sirtuin‐1 in renal tubules through glucose Transporter‐2 detection. Sci Rep. 2018;8(1):6791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Jardine MJ, Mahaffey KW, Neal B, et al. The canagliflozin and renal endpoints in diabetes with established nephropathy clinical evaluation (CREDENCE) study rationale, design, and baseline characteristics. Am J Nephrol. 2017;46(6):462‐472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Nadkarni GN, Ferrandino R, Chang A, et al. Acute kidney injury in patients on SGLT2 inhibitors: a propensity‐matched analysis. Diabetes Care. 2017;40(11):1479‐1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. U.S. Food and Drug Administration . FDA drug safety communication: FDA strengthens kidney warnings for diabetes medicines canagliflozin (Invokana, Invokamet) and dapagliflozin (Farxiga, Xigduo XR) 2016. https://www.fda.gov/downloads/Drugs/DrugSafety/UCM506772.pdf.
- 81. Yuan Z, DeFalco FJ, Ryan PB, et al. Risk of lower extremity amputations in people with type 2 diabetes mellitus treated with sodium‐glucose co‐transporter‐2 inhibitors in the USA: a retrospective cohort study. Diabetes Obes Metab. 2018;20(3):582‐589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sherman SE, Bell GI, Teoh H, et al. Canagliflozin improves the recovery of blood flow in an experimental model of severe limb ischemia. JACC Basic Transl Sci. 2018;3(2):327‐329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Davies MJ, D'Alessio DA, Fradkin J. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of diabetes (EASD). Diabetes Care. 2018;41(12):2669‐2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Gangadharan Komala M, Gross S, Mudaliar H, et al. Inhibition of kidney proximal tubular glucose reabsorption does not prevent against diabetic nephropathy in type 1 diabetic eNOS knockout mice. PLoS ONE. 2014;9(11):e108994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gallo LA, Ward MS, Fotheringham AK, et al. Once daily administration of the SGLT2 inhibitor, empagliflozin, attenuates markers of renal fibrosis without improving albuminuria in diabetic db/db mice. Sci Rep. 2016;6(1):26428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Shin SJ, Chung S, Kim SJ, et al. Effect of sodium‐glucose co‐transporter 2 inhibitor, dapagliflozin, on renal renin‐angiotensin system in an animal model of type 2 diabetes. PLoS ONE. 2016;11(11):e0165703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hatanaka T, Ogawa D, Tachibana H, et al. Inhibition of SGLT2 alleviates diabetic nephropathy by suppressing high glucose‐induced oxidative stress in type 1 diabetic mice. Pharmacol Res Perspect. 2016;4(4):e00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Han E, Shin E, Kim G, et al. Combining SGLT2 inhibition with a thiazolidinedione additively attenuate the very early phase of diabetic nephropathy progression in type 2 diabetes mellitus. Front Endocrinol (Lausanne). 2018;9:412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kojima N, Williams JM, Slaughter TN, et al. Renoprotective effects of combined SGLT2 and ACE inhibitor therapy in diabetic Dahl S rats. Physiol Rep. 2015;3(7):e12436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kojima N, Williams JM, Takahashi T, Miyata N, Roman RJ. Effects of a new SGLT2 inhibitor, luseogliflozin, on diabetic nephropathy in T2DN rats. J Pharmacol Exp Ther. 2013;345(3):464‐472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Tanaka S, Sugiura Y, Saito H, et al. Sodium‐glucose cotransporter 2 inhibition normalizes glucose metabolism and suppresses oxidative stress in the kidneys of diabetic mice. Kidney Int. 2018;94(5):912‐925. [DOI] [PubMed] [Google Scholar]


