Abstract
Background
Several studies demonstrated the adverse effect of milk processing on the allergy‐protective capacity of raw cow's milk. Whether milk processing also affects the allergenicity of raw milk is hardly investigated.
Objective
To assess the allergenicity of raw (unprocessed) and processed cow's milk in a murine model for food allergy as well as in cow's milk allergic children.
Methods
C3H/HeOuJ mice were either sensitized to whole milk (raw cow's milk, heated raw cow's milk or shop milk [store‐bought milk]) and challenged with cow's milk protein or they were sensitized and challenged to whey proteins (native or heated). Acute allergic symptoms, mast cell degranulation, allergen‐specific IgE levels and cytokine concentrations were determined upon challenge. Cow's milk allergic children were tested in an oral provocation pilot with organic raw and conventional shop milk.
Results
Mice sensitized to raw milk showed fewer acute allergic symptoms upon intradermal challenge than mice sensitized to processed milk. The acute allergic skin response was low (103 ± 8.5 µm vs 195 ± 17.7 µm for heated raw milk, P < 0.0001 and vs 149 ± 13.6 µm for shop milk, P = 0.0316), and there were no anaphylactic shock symptoms and no anaphylactic shock‐induced drop in body temperature. Moreover, allergen‐specific IgE levels and Th2 cytokines were significantly lower in raw milk sensitized mice. Interestingly, the reduced sensitizing capacity was preserved in the isolated native whey protein fraction of raw milk. Besides, native whey protein challenge diminished allergic symptoms in mice sensitized to heated whey proteins. In an oral provocation pilot, cow's milk allergic children tolerated raw milk up to 50 mL, whereas they only tolerated 8.6 ± 5.3 mL shop milk (P = 0.0078).
Conclusion and Clinical Relevance
This study demonstrates that raw (unprocessed) cow's milk and native whey proteins have a lower allergenicity than their processed counterparts. The preclinical evidence in combination with the human proof‐of‐concept provocation pilot provides evidence that milk processing negatively influences the allergenicity of milk.
Keywords: allergenicity, cow's milk, cow's milk allergic children, milk processing, mouse model, oral provocation pilot, Vorzugsmilch
1. INTRODUCTION
Thousands of years ago, humans started to consume cow's milk as part of their nutrition. At that time, cow's milk was consumed raw, but since the late 19th century it has been pasteurized and homogenized.1 This industrial milk processing extends shelf life and, more importantly, reduces the risk of milk‐borne infections caused by pathogenic bacteria like Mycobacterium tuberculosis, Listeria, Salmonella, Campylobacter, Enterohemorrhagic Escherichia coli (EHEC) and Shigatoxigenic E coli (STEC).2 However, milk processing can also have disadvantages. Pasteurization, for instance, structurally alters heat‐sensitive milk components, like proteins, which might subsequently lose functionality.3 In addition, homogenization changes the milk fat structure and thereby it might alter allergen presentation to the immune system.4, 5 So even though milk processing ensures microbial safety, it also affects functional milk proteins which might consequently lose their beneficial health properties.
In Germany, a long tradition of the consumption of raw, unprocessed, farm milk is existing. A survey on biodynamic dairy farms reported that (part of the) consumers bought organic raw milk because of a better tolerance and beneficial health effects.6 Beneficial health effects of raw cow's milk consumption are mainly described for asthma and allergic diseases. Raw cow's milk consumption has been associated with a reduced risk of these diseases.7, 8, 9, 10, 11 The body of evidence for this protective effect is accumulating with epidemiological as well as preclinical evidence.12, 13 Interestingly, the asthma‐ and allergy‐protective effect of raw cow's milk seems to be abolished by milk processing. Heat treatment, in particular, appears to impact the protective effect, suggesting the importance of heat‐sensitive milk components, such as whey proteins.7, 12, 13 From a variety of these whey proteins, it is believed that they might contribute to the protective effects of raw cow's milk.14, 15 Whether these components by themselves have the capacity to reduce the asthma and allergy risk remains to be elucidated.
The adverse effect of milk processing on the asthma‐ and allergy‐protective capacity of raw cow's milk is, as mentioned before, demonstrated by several studies.7, 12, 13 Whether milk processing also affects allergen presentation to the immune system and thus influences the allergenic potential of the milk is hardly investigated. Heating of the whey proteins α‐lactalbumin and β‐lactoglobulin induces the formation of aggregates which seem to promote allergic sensitization by shifting uptake from enterocytes to Peyer's patches.16 In addition, homogenization might increase the allergenicity of the milk due to disintegration of casein micelles and milk fat globules.5 However, compelling evidence showing that milk processing affects the allergenicity of milk is still lacking.
In addition to the existing epidemiological evidence showing a tolerogenic feature of raw cow's milk, the present study investigated whether the allergenicity of raw (unprocessed) and processed cow's milk differs in a murine model for food allergy. Since several studies have speculated about the whey fraction of raw cow's milk containing potential allergy‐protective components, we also assessed the allergenicity of native and heated whey proteins. In addition, we performed a proof‐of‐concept provocation pilot using a similar organic raw cow's milk in cow's milk allergic children.
2. METHODS
2.1. Mice
Four‐week‐old, specific pathogen‐free, female C3H/HeOuJ mice were purchased from Charles River Laboratories. Upon arrival, mice were randomly assigned to the control or experimental groups. They were housed at the animal facility of the Utrecht University in filter‐topped makrolon cages (n = 6‐8/cage) on a 12‐hours light/dark cycle with access to food and water ad libitum. All animal procedures were approved by the Ethical Committee for Animal Research of the Utrecht University and conducted in accordance with the European Directive 2010/63/EU on the protection of animals used for scientific purposes (DEC 2014.II.12.107 & AVD108002015346).
2.2. Milk and whey proteins
Raw milk used was an organic raw cow's milk with a fat content between 3.8% and 4.2% (due to seasonal variation) collected from a biodynamic farm legally allowed to sell raw milk (organic “Vorzugsmilch” 17; Hof Dannwisch, Horst, Germany). After collection, part of the raw milk was heated for 10 minutes at 80°C in a water bath to obtain the heated raw cow's milk used. From heating at 80°C, it is known that it will result in structural changes in proteins with immunomodulatory capacities which might subsequently lose functionality, but it will also denature β‐lactoglobulin and α‐lactalbumin. The shop milk (store‐bought milk) used was a conventional pasteurized and homogenized milk standardized at 3.8% fat (EDEKA). All milk types had a protein level of around 3.5 g/100 mL, with no difference between raw and shop milk (as determined by using the Pierce BCA protein assay kit standardized to bovine serum albumin [BSA] according to the manufacturer's protocol [Thermo Fisher Scientific]). Processed cow's milk protein (CMP) was obtained from DMV International. These are solely caseins and whey proteins in an 80:20 ratio. Native whey proteins (nWP; 3% denaturation) were isolated from raw cow's milk. Heated whey proteins (hWP; 73% denaturation; produced for experimental purposes only; Danone Nutricia Research) were obtained by heating nWP for 60 seconds at 100°C.
2.3. Experimental design—Oral sensitization of mice to raw and processed cow's milk
A schematic overview of the experimental design is depicted in Figure 1A. After 1 week habituation, mice (n = 8/group) were sensitized intragastrically (ig) by using a blunt needle with 0.5 mL raw cow's milk, heated raw cow's milk or shop milk using 10 µg cholera toxin (CT; List Biological Laboratories) as an adjuvant. Since sensitization to whole milk was never performed before in this model, a sensitized control group (n = 8) which received 17.5 mg processed CMP (equivalent to amount of protein present in 0.5 mL cow's milk) dissolved in 0.5 mL PBS (17.5 mg CMP/0.5 mL PBS + 10 µg CT) was included.18 Sham‐sensitized control mice (n = 6) received CT alone (10 µg/0.5 mL PBS). Mice were sensitized once a week for five consecutive weeks (on days 0, 7, 14, 21 and 28). Five days after the last sensitization (day 33), all mice were challenged intradermally (id) in the ear pinnae of both ears with 10 µg CMP in 20 µL PBS to determine the acute allergic response. On the same day, mice were challenged ig with 50 mg CMP in 0.5 mL PBS. Sixteen hours after the oral challenge, blood samples were collected via cheek puncture. Mice were subsequently killed by cervical dislocation, and organs were collected for ex vivo analysis. The raw milk used to sensitize mice was obtained from the same farm that was selected five years earlier to deliver the milk for the human provocation pilot. The mouse study was partly based on results of the human study.
Figure 1.
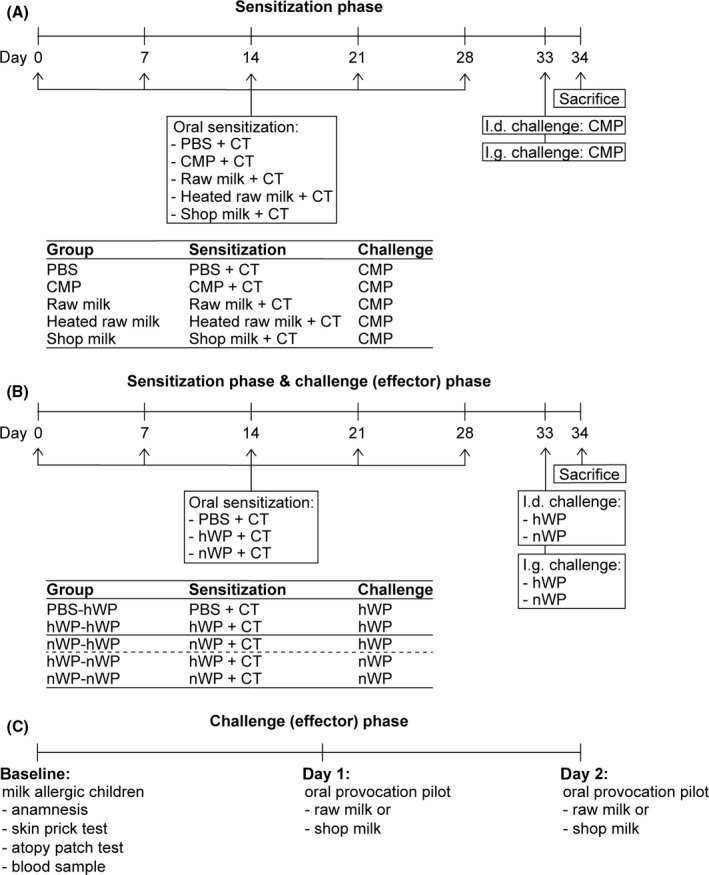
Schematic overview of the in vivo experiments and the proof‐of‐concept provocation pilot. A, Experimental design oral sensitization of mice to raw and processed cow's milk. B, Experimental design oral sensitization and challenge of mice to native and heated whey proteins. To distinguish between effects on the sensitization and challenge phase, groups were split and data were analysed separately. The first two groups, indicated by the solid line, are control groups included in all analyses. The third group, indicated by the dashed line, is only included in analyses on the sensitization phase. The last two groups are only included in analyses on the challenge phase. C, Protocol proof‐of‐concept provocation pilot with organic raw cow's milk and conventional shop milk in cow's milk allergic children. CMP, cow's milk protein; CT, cholera toxin; hWP, heated whey proteins; id, intradermal; ig, intragastric; nWP, native whey proteins
2.4. Experimental design—Oral sensitization and challenge of mice to native and heated whey proteins
In the second experiment, nWP and hWP were used to sensitize and challenge mice (Figure 1B). The experimental design is comparable to the one described above. Shortly, mice (n = 8) were sensitized ig once a week for five consecutive weeks to nWP or hWP (20 mg/0.5 mL PBS + 10 µg CT). Five days after the last sensitization (day 33), mice were challenged both id and ig with nWP or hWP (10 µg/20 µL PBS and 50 mg/0.5 mL PBS, respectively) to assess the allergic response. Mice were killed by cervical dislocation 16 hours after the ig challenge.
2.5. Evaluation of the allergic response
To determine the acute allergic skin response, mice were id challenged with the allergen (10 µg allergen/20 µL PBS) in the ear pinnae of both ears. Ear thickness (in duplicate for each ear) was measured before and 1 hour after the id challenge using a digital micrometre (Mitutoyo). Ear swelling, expressed as Δ µm, was subsequently calculated by subtracting the mean basal ear thickness before id challenge from the mean ear thickness measured 1 hour after the id challenge. The id challenge and the ear measurements were was performed in anesthetized mice (using inhalation of isoflurane; Abbott). Severity of anaphylactic shock symptoms was scored 30 minutes after the id challenge by using a validated scoring table.19 The anaphylactic shock‐induced drop in body temperature was also measured 30 minutes after id challenge using a rectal thermometer. All measurements were performed blinded.
2.6. Measurements of serum allergen‐specific IgE and mMCP‐1
Blood was collected via cheek puncture 16 hours after oral challenge and centrifuged at 10.000 g for 10 minutes. Serum was obtained and stored at −20°C until analysis of allergen‐specific IgE and mouse mast cell protease‐1 (mMCP‐1) levels by means of ELISA. Determination of CMP‐ (ie caseins and whey proteins), hWP‐, nWP‐ and raw cow's milk‐specific IgE antibodies was performed as previously described,20 with few alterations. Briefly, high binding Costar 9018 plates (Corning Inc) were coated with 20 µg/mL caseins or whey proteins in carbonate/bicarbonate coating buffer (0.05 mol/L, pH 9.6; Sigma‐Aldrich) and incubated overnight at 4°C. For the determination of raw cow's milk‐specific IgE antibodies, plates were coated with raw cow's milk (diluted 1750× to obtain a protein concentration of 20 µg/mL). After overnight incubation, plates were washed and blocked for 1 hour with PBS/1% BSA (Sigma‐Aldrich). Serum samples were subsequently incubated for 2 hours. After washing, plates were incubated with biotinylated rat anti‐mouse IgE detection antibody (1 µg/mL; BD Biosciences) for 1.5 hours. Plates were then washed, incubated for 45 minutes with streptavidin‐horseradish peroxidase (0.5 µg/mL; Sanquin), washed again and developed using o‐phenylenediamine (Sigma‐Aldrich). The reaction was stopped by 4 mol/L H2SO4, and absorbance was measured at 490 nm on a microplate reader (Bio‐Rad). Concentrations of mMCP‐1 were measured using a mMCP‐1 Ready‐SET‐Go!® ELISA (eBioscience) according to the manufacturer's instructions.
2.7. Ex vivo antigen‐specific stimulation of splenocytes to determine cytokine profiles
Single‐cell splenocyte suspensions were obtained by passing spleen samples through a 70‐µm nylon cell strainer using a syringe. The splenocyte suspension was rinsed with RPMI 1640 medium (Lonza) and incubated with lysis buffer (8.3 g NH4Cl, 1 g KHC3O and 37.2 mg EDTA dissolved in 1 L demi water, filter sterilized) to remove red blood cells. The reaction was stopped by adding RPMI 1640 medium supplemented with 10% heat‐inactivated fetal bovine serum (FBS; Bodinco), penicillin (100 U/mL)/streptomycin (100 µg/mL; Sigma‐Aldrich) and β‐mercaptoethanol (20 µmol/L; Thermo Fisher Scientific). Splenocytes were subsequently resuspended in this culture medium. For the ex vivo antigen‐specific restimulation assay, splenocytes (8 × 105 cells/well) were cultured in culture medium with or without 500 µg/mL CMP/hWP/nWP. Supernatant was harvested after 4 days of culture (37°C, 5% CO2) and stored at −20°C until cytokine analysis. Measurements of IL‐5, IL‐13, IL‐10 and IFNγ were performed by means of ELISA according to the protocol described above for IgE. Purified rat anti‐mouse antibodies (1 µg/mL for IL‐5 and IFNγ and 2 µg/mL for IL‐13 and IL‐10), recombinant mouse cytokines and biotinylated rat anti‐mouse antibodies (1 µg/mL for IL‐5, IL‐10 and IFNγ and 400 ng/mL for IL‐13), were purchased at BD Biosciences.
2.8. Experimental design—Proof‐of‐concept human provocation pilot with organic raw cow's milk in cow's milk allergic children
This study was a part of a larger research project of the University of Kassel focusing on the difference between organic and conventional milk quality including effects of production and processing of organic (raw) milk on human health. In the current study, the largest contrast in milk quality (organic raw milk vs conventional shop milk) was used to determine tolerance in cow's milk allergic children. The study was reviewed and approved by the ethical committee of the “Ärztekammer” Niedersachsen (Bo/06/2009). In total, 11 children with parent‐reported cow's milk allergy were recruited from the Reha Klinik, Interdisciplinary Centre for Dermatology, Pneumology and Allergology in Neuharlingersiel and informed consent was obtained before enrolment. To confirm milk allergy diagnosis, a skin prick test (expressed as weal diameter in mm; measured after 20 minutes) and an atopy patch test (APT; expressed as negative (−) or positive (+, ++, +++, ++++) reaction, qualified according to the APT reading criteria of the European Task Force on Atopic Dermatitis (ETFAD) 21; measured after 48 hours), were performed with a commercial diagnostic milk‐prick‐solution (ALK‐Abelló Arzneimittel GmbH). In addition, a blood sample was taken to determine total and cow's milk‐specific serum IgE levels. To determine differences in milk tolerance level, each child was subsequently tested in a double‐blind placebo‐controlled oral provocation pilot with raw milk as well as shop milk. All children were tested within a period of one year. The day before testing, fresh raw milk was delivered from the biodynamic farm and shop milk was bought from the local shop. Each child was tested for each milk type in random order on two consecutive days. Since the study was performed double‐blind, milk was offered by a nurse in increasing quantities every 30 minutes. The medical doctor judged the allergic symptoms and gave permission for the next dose at the end of the time interval. Based on this judgement, milk consumption was increased to a maximum of 50 mL.
2.9. Statistical analysis
Data are presented as mean ± SEM or as individual data points when data were not normally distributed. In the first in vivo experiment (oral sensitization of mice to raw and processed cow's milk; Figure 1A), differences between pre‐selected groups were statistically analysed using one‐ or two‐way ANOVA, followed by Bonferroni's multiple comparisons test. For mMCP‐1 concentrations, log‐transformed data were used to obtain normality for one‐way ANOVA. Anaphylactic shock scores and serum IgE levels were analysed using Kruskal‐Wallis test followed by Dunn's multiple comparisons test for pre‐selected groups since data did not obtain normality. For the second in vivo experiment (oral sensitization and challenge of mice to native and heated whey proteins; Figure 1B), groups were split and data were analysed separately to discriminate between effects on the sensitization and challenge (effector) phase. Differences compared to the hWP‐hWP group were statistically analysed using one‐ or two‐way ANOVA, followed by Dunnett's multiple comparisons test. Serum IgE levels were analysed using Kruskal‐Wallis test for non‐parametric data followed by Dunn's multiple comparisons test. Differences in milk tolerance level in the proof‐of‐concept provocation pilot (Figure 1C) were determined using a Wilcoxon signed‐rank test. Results were considered statistically significant when P < 0.05. Analyses were performed using GraphPad Prism software (version 7; GraphPad Software).
3. RESULTS
3.1. Mice sensitized to raw milk show fewer allergic symptoms upon CMP challenge
As expected, mice sensitized to processed cow's milk protein (CMP; sensitized control mice) showed increased allergic symptoms upon id challenge with CMP compared to mice sensitized to PBS (sham‐sensitized control mice). This increase in allergic symptoms was characterized by an increased acute allergic skin response, increased anaphylactic shock symptoms and an anaphylactic shock‐induced drop in body temperature (Figure 2A‐C). To determine whether milk processing affects the capacity of milk to induce allergic symptoms to CMP, mice were sensitized to raw cow's milk, heated raw cow's milk or shop milk. Mice sensitized to raw milk showed little allergic symptoms upon id challenge with CMP: the acute allergic skin response was low, there were no anaphylactic shock symptoms, and the body temperature remained high (Figure 2A‐C). Sensitization to the processed milk types, on the contrary, increased acute allergic skin response and anaphylactic shock symptoms and caused an anaphylactic shock‐induced drop in body temperature (Figure 2A‐C).
Figure 2.
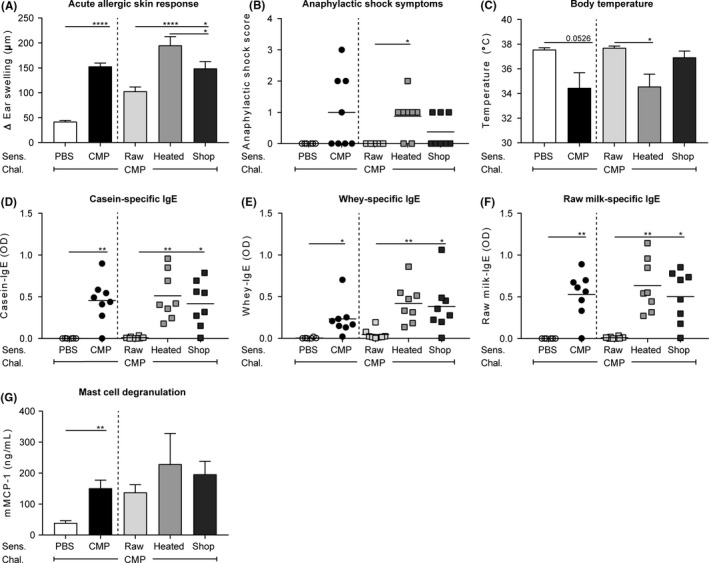
Fewer allergic symptoms and lower IgE levels after sensitization to raw milk. A, The acute allergic skin response (Δ ear swelling) measured 1 hour after id challenge. B, Anaphylactic shock scores and C, body temperature determined 30 minutes after id challenge. D, Serum casein‐ E, whey‐ and F, raw milk‐specific IgE antibody levels and G, serum mMCP‐1 concentrations measured 16 hours after ig challenge. Data are presented as mean ± SEM or as individual data points when data were not normally distributed, n = 6 in PBS group and n = 8 in all other groups. *P < 0.05, **P < 0.01, ****P < 0.0001 as analysed with one‐way ANOVA followed by Bonferroni's multiple comparisons test for pre‐selected groups (A,C,G) or Kruskal‐Wallis test for non‐parametric data followed by Dunn's multiple comparisons test for pre‐selected groups (B,D,E,F). chal., challenge; CMP, cow's milk protein; heated, heated raw cow's milk; id, intradermal; ig, intragastric; mMCP‐1; mucosal mast cell protease‐1; raw, raw cow's milk; Sens., sensitization; shop, shop milk
3.2. Lower allergen‐specific IgE levels in raw milk sensitized mice
To assess whether the reduced allergic symptoms in raw milk sensitized mice coincided with reduced allergic sensitization, serum allergen‐specific IgE levels were measured. Since caseins and whey proteins are the main milk allergens, specific IgE levels against these proteins were determined. Both casein‐ and whey‐specific IgE levels were low in raw milk sensitized mice and increased significantly when processed milk was used to sensitize mice (Figure 2D,E). Since the caseins and whey proteins used to determine these IgE levels were derived from a heated source, one could argue that conformational changes induced by heating limit the detection of IgE antibodies formed to raw milk. Therefore, raw milk‐specific IgE levels were also measured. However, as shown in Figure 2F, raw milk‐specific IgE levels were also low in the raw milk group. In addition, serum mMCP‐1 concentration was measured to assess mucosal mast cell degranulation. Increased mMCP‐1 concentrations were observed in sensitized control mice compared to sham‐sensitized control mice (Figure 2G). mMCP‐1 did, however, not differ between milk groups (Figure 2G).
3.3. Raw milk sensitization inhibits Th2‐related cytokine production after ex vivo stimulation of splenocytes with CMP
To investigate whether sensitization to different milk types affected T‐cell functionality, splenocytes were stimulated ex vivo with CMP and allergen‐induced cytokine concentrations were measured. Low cytokine levels were observed in sham‐sensitized control mice, whereas sensitized control mice showed a, CMP‐specific, increase in IL‐5, IL‐13, IL‐10 and IFNy (Figure 3A‐D). Secretion of Th2‐related cytokines IL‐5 and IL‐13 markedly increased upon CMP stimulation in mice sensitized to processed milk, whereas secretion remained low in mice sensitized to raw milk (Figure 3A,B). A similar pattern was observed for IL‐10 (Figure 3C). IFNy production was not affected by the different milk types (Figure 3D).
Figure 3.
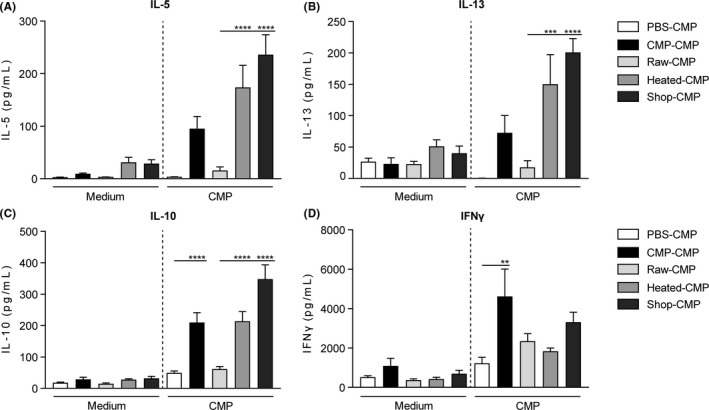
Th2‐related cytokine production after ex vivo stimulation of splenocytes with CMP was inhibited in raw milk sensitized mice. A, IL‐5 (sensitization: P < 0.0001, stimulation: P < 0.0001, interaction: P < 0.0001); B, IL‐13 (sensitization: P < 0.0001, stimulation: P < 0.0001, interaction: P < 0.001); C, IL‐10 (sensitization: P < 0.01, stimulation: P < 0.0001, interaction: P < 0.0001) and D, IFNy (sensitization: P < 0.0001, stimulation: P < 0.0001, interaction: ns) concentrations were measured in supernatant after ex vivo stimulation of splenocytes with medium or CMP for 4 days (37°C, 5% CO2). Data are presented as mean ± SEM, n = 5‐6 in PBS group and n = 7‐8 in all other groups. **P < 0.01, ***P < 0.001, ****P < 0.0001 as analysed with two‐way ANOVA followed by Bonferroni's multiple comparisons test for pre‐selected groups. CMP, cow's milk protein; heated, heated raw cow's milk; ns, not significant; raw, raw cow's milk; shop, shop milk
To determine whether sensitization with the different milk types affected the microbiota composition, metabolic activity of the microbiome was assessed by measuring short‐chain fatty acid concentrations in caecum. However, no differences were observed between groups in acetic acid, propionic acid and butyric acid concentrations (Figures S1A‐C).
3.4. Nativity of whey proteins important to reduce allergic sensitization
Previous studies have speculated the whey protein fraction of raw milk may be a source of the allergy‐protective components.7, 12, 15 To assess whether the reduced sensitizing capacity of raw milk is still present when only looking at the whey protein fraction of the milk, a similar experiment was conducted with native and heated whey proteins (Figure 1B). Figure 4A demonstrates that the acute allergic skin response was indeed reduced in mice sensitized to native whey proteins (nWP) compared to mice sensitized to heated whey proteins (hWP) when challenged with hWP. This reduction coincided with reduced mucosal mast cell degranulation (Figure 4B). Whey protein‐specific IgE antibody levels were increased in hWP sensitized mice, whereas no significant levels were observed in nWP sensitized mice (Figure 4C,D). This effect was observed for both hWP‐ and nWP‐specific IgE (Figure 4C,D), suggesting that heating did not induce conformational changes in these proteins affecting B‐cell epitopes and hence IgE binding. Allergen‐induced IL‐5, IL‐13 and IL‐10 production was also significantly lower in the nWP group compared to the hWP group (Figure 4E‐G). Allergen‐induced IFNy production did not differ between groups (Figure 4H).
Figure 4.
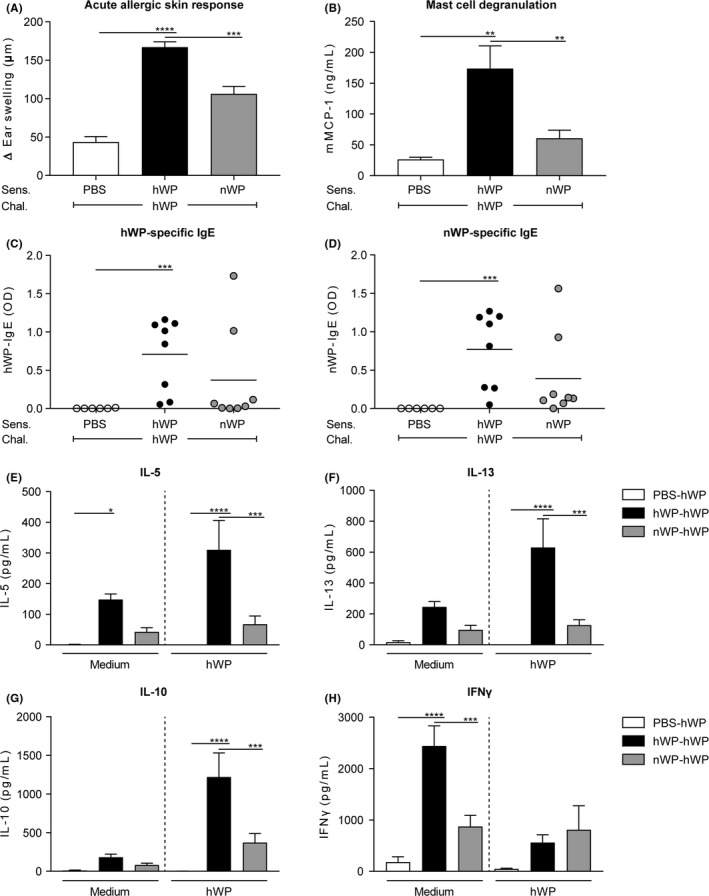
Reduced allergic response after sensitization to native whey proteins. A, The acute allergic skin response (Δ ear swelling) measured 1 hour after id challenge. B, Serum mMCP‐1 concentrations and C, hWP‐ and D, nWP‐specific IgE antibody levels measured 16 h after ig challenge. E, IL‐5 (sensitization: P < 0.0001, stimulation: ns, interaction: ns); F, IL‐13 (sensitization: P < 0.0001, stimulation: ns, interaction: P < 0.05); G, IL‐10 (sensitization: P < 0.0001, stimulation: P < 0.001, interaction: P < 0.01) and H, IFNy (sensitization: P < 0.001, stimulation: P < 0.01, interaction: P < 0.01) concentrations measured in supernatant after ex vivo stimulation of splenocytes with medium or hWP for 4 d (37°C, 5% CO2). Data are presented as mean ± SEM, n = 5‐6 in PBS group and n = 7‐8 in all other groups. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 compared to the hWP‐hWP group as analysed with one‐way ANOVA followed by Dunnett's multiple comparisons test (A,B), Kruskal‐Wallis test for non‐parametric data followed by Dunn's multiple comparisons test (C,D) or two‐way ANOVA followed by Dunnett's multiple comparisons test (E‐H). chal., challenge; hWP, heated whey proteins; id, intradermal; ig, intragastric; ns, not significant; nWP, native whey proteins; Sens., sensitization
3.5. Native whey proteins diminish allergic symptoms in heated whey protein sensitized mice
The data presented (Figure 4) suggest nWP indeed has a lower sensitizing capacity than hWP (comparable to raw milk; Figures 2 and 3). To determine whether nWP also have a lower capacity to induce an allergic response when sensitization to hWP already occurred, hWP sensitized mice were challenged with nWP. Challenge with nWP induced a lower acute allergic skin response than challenge with hWP, in hWP sensitized mice (Figure 5A). It did not reduce mast cell degranulation and whey protein‐specific IgE levels but it did inhibit IL‐5, IL‐13 and IL‐10 production by splenocytes after allergen‐specific stimulation (Figure 5B‐G). Allergen‐specific stimulation did not induce differences between groups in IFNy secretion (Figure 5H). To investigate whether allergic symptoms were induced in mice exclusively exposed to nWP, a group sensitized and challenged to nWP was included. However, this group showed similar protective effects (Figure 5A‐H).
Figure 5.
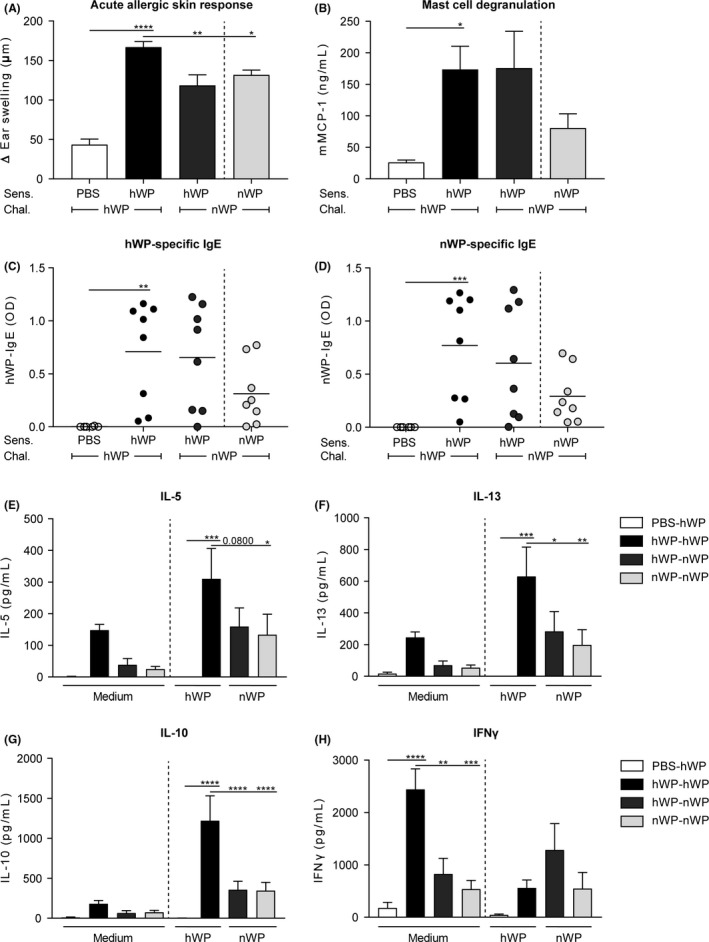
Challenge with native whey proteins diminished the allergic response in heated whey protein sensitized mice. A, The acute allergic skin response measured as Δ ear swelling 1 hour after id challenge. B, Serum mMCP‐1 concentrations 16 h after ig challenge. C, hWP‐ and D, nWP‐specific IgE levels measured 16 h after ig challenge. E, IL‐5 (sensitization: P < 0.001, stimulation: P < 0.01, interaction: ns); F, IL‐13 (sensitization: P < 0.001, stimulation: P < 0.01, interaction: ns); G, IL‐10 (sensitization: P < 0.0001, stimulation: P < 0.0001, interaction: P < 0.01) and H, IFNy (sensitization: P < 0.001, stimulation: ns, interaction: P < 0.01) concentrations measured in supernatant after ex vivo stimulation of splenocytes with medium, hWP or nWP for 4 d (37°C, 5% CO2). Data are presented as mean ± SEM, n = 5‐6 in PBS group and n = 7‐8 in all other groups. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 compared to the hWP‐hWP group as analysed with one‐way ANOVA followed by Dunnett's multiple comparisons test (A,B), Kruskal‐Wallis test for non‐parametric data followed by Dunn's multiple comparisons test (C,D) or two‐way ANOVA followed by Dunnett's multiple comparisons test (E‐H). chal., challenge; hWP, heated whey proteins; id, intradermal; ig, intragastric; ns, not significant; nWP, native whey proteins; Sens., sensitization
3.6. Reduced allergenic potential of organic raw milk in cow's milk allergic children
In addition, children diagnosed with cow's milk allergy were tested in their reaction to organic raw cow's milk and conventional shop milk. Eleven children were included in this proof‐of‐concept double‐blind placebo‐controlled provocation pilot (results presented in Table 1). Two children (patients 6 and 7) were excluded from analysis since their milk allergy could not be confirmed in the skin prick test (0 mm). The remaining nine children (1.5 ± 0.3 [mean ± SEM] years of age) developed a wheel diameter of 6.7 ± 1.0 mm in the skin prick test and showed an average patch test result between ++ and +++ (2.4 ± 0.2). From the six children of which IgE levels were measured, the total serum IgE level was 115.4 ± 43.4 kU/L and the cow's milk‐specific serum IgE level was 10.4 ± 3.4 kU/L. In the oral provocation pilot, all children could tolerate the organic raw milk up to the maximum level of 50 mL (approximately 1750 mg protein). Only one child could tolerate the shop milk to this level, but in all other cases a lower amount of shop milk was tolerated and the provocation had to be stopped due to the development of allergic symptoms. On average, children could only tolerate 8.6 ± 5.3 mL shop milk.
Table 1.
Organic raw cow's milk tolerated by cow's milk allergic children
| Patient | Gender | Age (y) | Skin | Serum | DBPCT | |||
|---|---|---|---|---|---|---|---|---|
| SPT (mm) | APT (class) | Total IgE (kU/L) | Specific IgE (kU/L) | Raw milk (mL) | Shop milk (mL) | |||
| 1 | M | 2.65 | 10 | ++ | 322.0 | 26.3 | 50.0 | 2.0 |
| 2 | M | 3.52 | 4 | ++ | 123.0 | 4.2 | 50.0 | 10.0 |
| 3 | M | 0.55 | 7 | +++ | 37.5 | 8.4 | 50.0 | 0.5 |
| 4 | F | 0.96 | 12 | ++ | 66.8 | 5.6 | 50.0 | 50.0 |
| 5 | M | 1.59 | 3 | +++ | nd | nd | 50.0 | 1.0 |
| 6a | M | 1.65 | 0 | + | nd | nd | 50.0 | 50.0 |
| 7a | M | 1.09 | 0 | + | nd | nd | 50.0 | 50.0 |
| 8 | M | 0.96 | 5 | ++ | 98.6 | 12.4 | 50.0 | 0.5 |
| 9 | F | 0.83 | 7 | +++ | 44.2 | 5.5 | 50.0 | 10.0 |
| 10 | F | 1.28 | 4 | ++ | nd | nd | 50.0 | 2.5 |
| 11 | M | 1.10 | 8 | +++ | nd | nd | 50.0 | 1.0 |
| Mean | 1.49 | 6.7 | 2.4 | 115.4 | 10.4 | 50.0 | 8.6** | |
| SEM | 0.32 | 1.0 | 0.2 | 43.4 | 3.4 | 0.0 | 5.3 | |
Shown are gender, age, skin prick test, atopy patch test and serum total and cow's milk‐specific IgE levels of 11 cow's milk allergic children before oral provocation as well as their level of tolerance to organic raw cow's milk and conventional shop milk during oral provocation.
Abbreviations: APT, atopy patch test; DBPCT, double‐blind placebo‐controlled trial; nd, not determined; SPT, skin prick test.
Patients 6 and 7 were excluded from analysis since their milk allergy could not be confirmed in the skin prick test (0 mm).
**P < 0.01 compared to raw milk tolerance level as analysed with Wilcoxon signed‐rank test.
4. DISCUSSION
In this study, we demonstrate that raw (unprocessed) cow's milk has a lower allergenic potential than processed cow's milk. Similar results were observed when only looking at the whey protein fraction of the milk, suggesting that this fraction contributes to the observed differences. The preclinical evidence was supported by a proof‐of‐concept provocation pilot in which cow's milk allergic children could tolerate raw cow's milk but not commercially available processed milk. These results provide evidence that milk processing negatively influences the allergenicity of milk.
Today's Western society mainly consumes processed milk. Processed milk is safe in terms of pathogens, and the extended shelf life makes it easy to consume in everyday life. However, milk processing also induces unwanted changes in the milk composition. Proteins with potential beneficial health properties (partly) lose functionality, and the context of allergen presentation to the immune system may be altered.3, 4, 5, 15 Current literature mostly demonstrates the adverse effect of milk processing on allergic diseases like asthma and atopy.7, 12, 13 Milk processing, especially heating, abolishes the allergy‐protective effects observed after consumption of raw cow's milk. This loss of protection is often attributed to the denaturation, and subsequent loss of functionality of immunomodulatory proteins present in the whey fraction of the milk.12, 14, 15 However, whether milk processing also affects the allergenicity of the milk is barely researched.
The term allergenicity or allergenic potential is defined in literature as “the potential of a material to cause sensitization and allergic reactions, frequently associated with IgE antibody”.22 The allergenicity of milk is thus determined by two factors: the sensitizing capacity of the milk and the capacity of the milk to bind to IgE antibodies and thereby inducing an allergic reaction. Both factors have been investigated in this study.
When looking at sensitization, our data show that milk processing increases the sensitizing capacity of cow's milk. In a murine model, we observed less acute allergic symptoms in mice sensitized to raw milk compared to mice sensitized to processed milk. Next to reduced acute allergic symptoms, also allergen‐specific IgE levels and Th2 cytokine concentrations were inhibited. The effects seemed to be dependent on processing time and temperature. The strongest sensitizing capacity was observed for heated raw milk which was heated for 10 minutes at 80°C. The shop milk, heated for 15 seconds at 73°C (pasteurization), was less allergenic. However, we should be careful with drawing this conclusion since the shop milk was besides pasteurized also homogenized and was furthermore derived from another milk source. Reduced allergic sensitization was also observed when only looking at the whey protein fraction of the milk. In our murine model, native, raw milk‐derived, whey proteins induced a lower allergic response than heated, processed milk‐derived, whey proteins.
Allergic sensitization can be influenced by many factors, like host genotype, type of allergen, amount, frequency and route of allergen exposure but also whether allergen exposure occurs in combination with components that enhance/reduce the sensitization.23 In addition, milk processing can affect the sensitizing capacity by inducing structural and chemical alterations in milk proteins. Denaturation, aggregation and the Maillard reaction with other molecules, like sugars, are some examples that can have an effect on the sensitizing capacity.24 Unfortunately, little is known about these topics. Few, if any, studies have examined the effect of milk processing on the sensitizing capacity of whole milk in vivo. Roth‐Walter et al 16 did investigate the effect of heating on the main milk allergens: casein, α‐lactalbumin and β‐lactoglobulin. In a murine model, they showed that the whey proteins, α‐lactalbumin and β‐lactoglobulin, form aggregates upon heating and that these aggregates enhanced allergic sensitization, as evidenced by increased IgE and Th2 cytokine responses. Since caseins naturally form micelles and thus already exist as aggregates, their sensitizing capacity was not affected by heat treatment. The enhanced sensitization by aggregated whey proteins was attributed to a shift in uptake from enterocytes to Peyer's patches, thereby increasing immunogenicity. We do not have data to confirm this shift in uptake but our study does confirm increased sensitization upon heat treatment. We observed this effect with whole milk and with the whey protein fraction, confirming that the effect is most likely independent of the caseins. Besides the formation of whey protein aggregates enhancing sensitization, there is also some evidence showing that the whey protein β‐lactoglobulin presents some new epitopes upon heating.25 These epitopes can be uncovered by the unfolding of the protein upon heating or they can be created upon new chemical interactions. At the same time, it is also known that extensive heating can destroy epitopes.24 Whether the net effect is an increased sensitizing capacity has, to the authors knowledge, never been researched. Besides milk allergens, the whey protein fraction also contains a lot of immunomodulatory components. These components, like immunoglobulins, TGF‐β, IL‐10, lactoferrin, lysozyme, osteopontin and lactoperoxidase, are known to enhance mucosal barrier function and to modulate the mucosal immune response. Together they might create an environment that favours unresponsiveness following allergen exposure.3, 14, 26 As most of these components are heat sensitive, they (partly) lose functionality upon processing, providing another potential mechanism for the observed increase in sensitization.
Next to the effect on the sensitizing capacity, we determined whether native whey proteins also have a lower capacity to induce an allergic response when sensitization to heated whey proteins already occurred. We showed that native whey proteins indeed elicited a lower acute allergic skin response than heated whey proteins in heated whey protein sensitized mice. This reduced acute allergic skin response coincided with a reduced Th2 cytokine response. Allergen‐specific IgE levels were, however, not reduced in these mice, most likely because they were sensitized to heated whey proteins so the IgE antibodies were already formed. From clinical practice, it is known that patients can have IgE antibodies against a certain food allergen without having symptoms after exposure to that allergen.27 This indicates that factors other than IgE are playing an important role in the development of an allergic response. Such factors might be the concurrent presence of IgG antibodies, the presence of epithelial derived mediators (eg galectin‐9), the number and sensitivity of mast cells, the threshold of mast cells to induce IgE‐mediated activation but also the sensitivity of target organs to mast cell‐derived mediators.28, 29, 30 We did not see differences in IgG levels (data not shown), and mucosal mast cell degranulation (mMCP‐1) was not affected. However, since we observed a reduction in the acute allergic skin response there seems to be an effect on connective tissue mast cells. Mucosal and connective tissue mast cells were recently shown to underlie different symptoms of food allergy.31 The contribution of these mast cells in our model and whether raw milk and native whey proteins differently affect them should be clarified in future studies.
Another explanation for the lower capacity of native whey proteins to induce an allergic reaction in heated whey protein sensitized mice is perhaps a difference in protein conformation which prevents IgE binding. However, in all experiments performed, we observed that IgE antibodies formed (whether this was against native or heated whey proteins) bound as well to native as to heated whey proteins. This suggests that our heat treatments did not induce major differences in protein conformation affecting B‐cell epitopes and hence IgE binding. The effect of milk processing on protein conformation and IgE‐binding capacity is contradictory in current literature. Some studies report an increased IgE‐binding capacity of α‐lactalbumin and β‐lactoglobulin heated at temperatures between 50 and 90°C, while others showed a decrease.32, 33, 34, 35 These effects were, however, observed in in vitro studies. Little in vivo research has been performed. There is some evidence showing that homogenized milk induces a stronger allergic reaction in milk allergic mice than raw milk,5, 36 but these findings could not be confirmed in clinical studies.37, 38
The reduced allergenicity of raw milk was confirmed in a proof‐of‐concept provocation pilot. Cow's milk allergic children tolerated organic raw cow's milk up to the maximum level of 50 mL (approximately 1750 mg protein), whereas in most cases provocation with conventional shop milk had to be stopped earlier because of the development of allergic symptoms. To our knowledge, this is the first human pilot trial showing that traditional milk processing increases the allergenicity of raw cow's milk. Human trials have been performed with extensively heat treated (baked) milk. These trials show that the majority of cow's milk allergic children tested could tolerate the extensively heated milk.39, 40, 41 This might indicate that the allergenicity of cow's milk is following a parabolic form, with a low allergenic potential at low (<50°C, eg raw cow's milk) and extremely high temperatures (>180°C, eg baked milk) and an increasing allergenic potential with temperatures in between. In the case of raw cow's milk, the lower allergenic potential could be caused by the fact that native, non‐heated, proteins might be taken up differently than aggregated proteins thereby reducing immunogenicity, and/or by immunomodulatory components present in raw cow's milk that might create an environment favouring unresponsiveness upon allergen exposure. In the case of baked milk, the lower allergenic potential could be caused by destruction of conformational epitopes.39
The effect of the origin of the milk (organic vs conventional) on the allergenicity needs to be assessed in future studies. Different production and feeding methods on organic farms impact among others the fatty acid composition and antioxidant concentrations of the milk and might have contributed to the observed tolerance to organic raw milk in cow's milk allergic children.42, 43 In addition, the inclusion of children based on parent‐reported cow's milk allergy potentially leading to a heterogeneous group of children (two children were left out because of the absence of a positive skin prick test), the limited number of children, the lack of IgE levels for some children and the fact that the oral challenge did not include the full range of up to 3000 mg protein as recommended by the PRACTALL guidelines 44 represent the main limitations of this study.
In summary, in this study we demonstrated a lower allergenic potential of raw (unprocessed) cow's milk and native whey proteins as compared to their processed counterparts. These findings were extensively shown in a murine model and were confirmed in a human proof‐of‐concept provocation pilot. The observed effects were most likely not caused by an altered IgE binding. Instead, a change in allergen uptake and/or the formation of an environment favouring unresponsiveness upon allergen exposure might underlie the beneficial effects, although these are speculations which should be investigated in future studies. Risks from the certified, strictly controlled, raw milk used in this study are low, but a zero‐risk can never be attained. The consumption of raw milk is therefore not recommended by the WHO. However, this study does add to the evidence on allergy‐protective capacities of raw cow's milk and emphasizes once more the need for minimally processed milk. Besides, elucidating the raw milk components responsible for the allergy‐protective effects and understanding the underlying mechanisms might help the development of new dietary concepts aimed at safe allergy management.
CONFLICT OF INTEREST
GH is employed at Danone Nutricia Research. JG and BvE are partly employed at Danone Nutricia Research. All other authors report no potential conflicts of interest.
Supporting information
ACKNOWLEDGEMENTS
The preclinical studies were financially supported by Danone Nutricia Research. The human proof‐of‐concept provocation pilot was funded by Dr. C. Langen.
Abbring S, Kusche D, Roos TC, et al. Milk processing increases the allergenicity of cow’s milk—Preclinical evidence supported by a human proof‐of‐concept provocation pilot. Clin Exp Allergy. 2019;49:1013–1025. 10.1111/cea.13399
REFERENCES
- 1. Steele JH. History, trends, and extent of pasteurization. J Am Vet Med Assoc. 2000;217(2):175‐178. [DOI] [PubMed] [Google Scholar]
- 2. Oliver SP, Boor KJ, Murphy SC, Murinda SE. Food safety hazards associated with consumption of raw milk. Foodborne Pathog Dis. 2009;6(7):793‐806. [DOI] [PubMed] [Google Scholar]
- 3. Chatterton DE, Nguyen DN, Bering SB, Sangild PT. Anti‐inflammatory mechanisms of bioactive milk proteins in the intestine of newborns. Int J Biochem Cell Biol. 2013;45(8):1730‐1747. [DOI] [PubMed] [Google Scholar]
- 4. Michalski MC, Januel C. Does homogenization affect the human health properties of cow's milk? Trends Food Sci Technol. 2006;17(8):423‐437. [Google Scholar]
- 5. Poulsen OM, Hau J, Kollerup J. Effect of homogenization and pasteurization on the allergenicity of bovine milk analysed by a murine anaphylactic shock model. Clin Exp Allergy. 1987;17(5):449‐458. [DOI] [PubMed] [Google Scholar]
- 6. Kusche D, Sahm H, Baars T. Konsum ökologischer Milch aus gesundheitlichen Gründen‐Eine qualitative Erhebung auf deutschen Demeter Milchviehbetrieben und bei ihren. Kunden.2009.
- 7. Loss G, Apprich S, Waser M, et al. The protective effect of farm milk consumption on childhood asthma and atopy: the GABRIELA study. J Allergy Clin Immunol. 2011;128(4):766–773. e4. [DOI] [PubMed] [Google Scholar]
- 8. Riedler J, Braun‐Fahrländer C, Eder W, et al. Exposure to farming in early life and development of asthma and allergy: a cross‐sectional survey. Lancet. 2001;358(9288):1129–1133. [DOI] [PubMed] [Google Scholar]
- 9. Waser M, Michels KB, Bieli C, et al. Inverse association of farm milk consumption with asthma and allergy in rural and suburban populations across Europe. Clin Exp Allergy. 2007;37(5):661–670. [DOI] [PubMed] [Google Scholar]
- 10. Perkin MR, Strachan DP. Which aspects of the farming lifestyle explain the inverse association with childhood allergy? J Allergy Clin Immunol. 2006;117(6):1374–1381. [DOI] [PubMed] [Google Scholar]
- 11. Ege MJ, Frei R, Bieli C, et al. Not all farming environments protect against the development of asthma and wheeze in children. J Allergy Clin Immunol. 2007;119(5):1140–1147. [DOI] [PubMed] [Google Scholar]
- 12. Abbring S, Verheijden KAT, Diks MAP, et al. Raw cow's milk prevents the development of airway inflammation in a murine house dust mite‐induced asthma model. Front Immunol. 2017;8:1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brick T, Schober Y, Bocking C, et al. Omega‐3 fatty acids contribute to the asthma‐protective effect of unprocessed cow's milk. J Allergy Clin Immunol. 2016;137(6):1699–1706. e13. [DOI] [PubMed] [Google Scholar]
- 14. van Neerven RJ, Knol EF, Heck JM, Savelkoul HF. Which factors in raw cow's milk contribute to protection against allergies? J Allergy Clin Immunol. 2012;130(4):853–858. [DOI] [PubMed] [Google Scholar]
- 15. Brick T, Ege M, Boeren S, et al. Effect of processing intensity on immunologically active bovine milk serum proteins. Nutrients. 2017;9(9):963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roth‐Walter F, Berin MC, Arnaboldi P, et al. Pasteurization of milk proteins promotes allergic sensitization by enhancing uptake through Peyer's patches. Allergy. 2008;63(7):882–890. [DOI] [PubMed] [Google Scholar]
- 17. Bundesministerium der Justiz und für Verbraucherschutz . Verordnung über die Güteprüfung und Bezahlung der Anlieferungsmilch (Milch‐Güteverordnung) 1980. [Available from: http://www.gesetze-im-internet.de/milchg_v/index.html.
- 18. van Esch B, van Bilsen J, Jeurink PV, et al. Interlaboratory evaluation of a cow's milk allergy mouse model to assess the allergenicity of hydrolysed cow's milk based infant formulas. Toxicol Lett. 2013;220(1):95–102. [DOI] [PubMed] [Google Scholar]
- 19. Li XM, Schofield BH, Huang CK, Kleiner GI, Sampson HA. A murine model of IgE‐mediated cow's milk hypersensitivity. J Allergy Clin Immunol. 1999;103(2 Pt 1):206–214. [DOI] [PubMed] [Google Scholar]
- 20. Schouten B, van Esch BC, Hofman GA, van den Elsen LW, Willemsen LE, Garssen J. Acute allergic skin reactions and intestinal contractility changes in mice orally sensitized against casein or whey. Int Arch Allergy Immunol. 2008;147(2):125–134. [DOI] [PubMed] [Google Scholar]
- 21. Turjanmaa K, Darsow U, Niggemann B, Rance F, Vanto T, Werfel T. EAACI/GA2LEN position paper: present status of the atopy patch test. Allergy. 2006;61(12):1377–1384. [DOI] [PubMed] [Google Scholar]
- 22. Verhoeckx KCM, Vissers YM, Baumert JL, et al. Food processing and allergenicity. Food Chem Toxicol. 2015;80:223–240. [DOI] [PubMed] [Google Scholar]
- 23. Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008;454(7203):445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bu G, Luo Y, Chen F, Liu K, Zhu T. Milk processing as a tool to reduce cow's milk allergenicity: a mini‐review. Dairy Sci Technol. 2013;93(3):211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Davis PJ, Williams SC. Protein modification by thermal processing. Allergy. 1998;53(46 Suppl):102–105. [DOI] [PubMed] [Google Scholar]
- 26. Perdijk O, van Splunter M, Savelkoul H, Brugman S, vanNeerven R . Cow’s milk and immune function in the respiratory tract: potential mechanisms. Front Immunol. 2018;9:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sampson HA, Aceves S, Bock SA, et al. Food allergy: a practice parameter update‐2014. J Allergy Clin Immunol. 2014;134(5):1016–1025. e43. [DOI] [PubMed] [Google Scholar]
- 28. Renz H, Allen KJ, Sicherer SH, et al. Food allergy. Nat Rev Dis Primers. 2018;4:17098. [DOI] [PubMed] [Google Scholar]
- 29. Kivit S, Saeland E, Kraneveld AD, et al. Galectin‐9 induced by dietary synbiotics is involved in suppression of allergic symptoms in mice and humans. Allergy. 2012;67(3):343–352. [DOI] [PubMed] [Google Scholar]
- 30. Niki T, Tsutsui S, Hirose S, et al. Galectin‐9 is a high affinity IgE‐binding lectin with anti‐allergic effect by blocking IgE‐antigen complex formation. J Biol Chem. 2009;284(47):32344–32352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Benede S, Berin MC. Mast cell heterogeneity underlies different manifestations of food allergy in mice. PLoS ONE. 2018;13(1):e0190453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bu G, Luo Y, Zheng Z, Zheng H. Effect of heat treatment on the antigenicity of bovine α‐lactalbumin and β‐lactoglobulin in whey protein isolate. Food Agric Immunol. 2009;20(3):195–206. [Google Scholar]
- 33. Ehn BM, Ekstrand B, Bengtsson U, Ahlstedt S. Modification of IgE binding during heat processing of the cow's milk allergen beta‐lactoglobulin. J Agric Food Chem. 2004;52(5):1398–1403. [DOI] [PubMed] [Google Scholar]
- 34. Taheri‐Kafrani A, Gaudin JC, Rabesona H, et al. Effects of heating and glycation of beta‐lactoglobulin on its recognition by IgE of sera from cow milk allergy patients. J Agric Food Chem. 2009;57(11):4974–4982. [DOI] [PubMed] [Google Scholar]
- 35. Kleber N, Krause I, Illgner S, Hinrichs J. The antigenic response of beta‐lactoglobulin is modulated by thermally induced aggregation. Eur Food Res Technol. 2004;219(2):105–110. [Google Scholar]
- 36. Poulsen OM, Nielsen BR, Basse A, Hau J. Comparison of intestinal anaphylactic reactions in sensitized mice challenged with untreated bovine milk and homogenized bovine milk. Allergy. 1990;45(5):321–326. [DOI] [PubMed] [Google Scholar]
- 37. Pelto L, Rantakokko HK, Lilius EM, Nuutila J, Salminen S. No difference in symptoms and receptor expression in lactose‐intolerant and in milk‐hypersensitive subjects following intake of homogenized and unhomogenized milk. Int Dairy J. 2000;10(11):799–803. [Google Scholar]
- 38. Host A, Samuelsson EG. Allergic reactions to raw, pasteurized, and homogenized/pasteurized cow milk: a comparison. A double‐blind placebo‐controlled study in milk allergic children. Allergy. 1988;43(2):113–118. [DOI] [PubMed] [Google Scholar]
- 39. Nowak‐Wegrzyn A, Bloom KA, Sicherer SH, et al. Tolerance to extensively heated milk in children with cow's milk allergy. J Allergy Clin Immunol. 2008;122(2):342–347. e1–2. [DOI] [PubMed] [Google Scholar]
- 40. Mehr S, Turner PJ, Joshi P, Wong M, Campbell DE. Safety and clinical predictors of reacting to extensively heated cow's milk challenge in cow's milk‐allergic children. Ann Allergy Asthma Immunol. 2014;113(4):425–429. [DOI] [PubMed] [Google Scholar]
- 41. Caubet JC, Nowak‐Wegrzyn A, Moshier E, Godbold J, Wang J, Sampson HA. Utility of casein‐specific IgE levels in predicting reactivity to baked milk. J Allergy Clin Immunol. 2013;131(1):222–224. e1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kusche D, Kuhnt K, Ruebesam K, et al. Fatty acid profiles and antioxidants of organic and conventional milk from low‐ and high‐input systems during outdoor period. J Sci Food Agric. 2015;95(3):529–539. [DOI] [PubMed] [Google Scholar]
- 43. Srednicka‐Tober D, Baranski M, Seal CJ, et al. Higher PUFA and n‐3 PUFA, conjugated linoleic acid, alpha‐tocopherol and iron, but lower iodine and selenium concentrations in organic milk: a systematic literature review and meta‐ and redundancy analyses. Br J Nutr. 2016;115(6):1043–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sampson HA, Gerth van Wijk R, Bindslev‐Jensen C, et al. Standardizing double‐blind, placebo‐controlled oral food challenges: American Academy of Allergy, Asthma & Immunology‐European Academy of Allergy and Clinical Immunology PRACTALL consensus report. J Allergy Clin Immunol. 2012;130(6):1260–1274. [DOI] [PubMed] [Google Scholar]
- 45. Bakker‐Zierikzee AM, Alles MS, Knol J, Kok FJ, Tolboom JJ, Bindels JG. Effects of infant formula containing a mixture of galacto‐ and fructo‐oligosaccharides or viable Bifidobacterium animalis on the intestinal microflora during the first 4 months of life. Br J Nutr. 2005;94(5):783–790. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


