Abstract
Introduction
Stroke is one of the most feared complications during catheter ablation of atrial fibrillation (AF). While symptomatic thromboembolic events are rare, magnetic resonance imaging (MRI) may identify asymptomatic (ie, silent) cerebral lesions (SCLs) following pulmonary vein isolation (PVI) procedures.
Methods and Results
The REDUCE‐TE Pilot was a prospective multicenter, single‐arm observational study investigating the incidence of SCL in patients with symptomatic paroxysmal AF undergoing PVI with a novel gold‐tip, externally irrigated ablation catheter. After ablation, cerebral diffusion‐weighted MRI and a postablation follow‐up were performed at 1 to 3 days after the ablation procedure. A neurocognitive test was done before and after ablation. The primary study endpoint was the occurrence of one or more new SCLs. Secondary study endpoints included neurocognitive status, procedural success rate, and periprocedural complications including symptomatic thromboembolic events. A total of 104 patients were enrolled (69% male, mean age: 61.5 ± 9.7 years, mean CHA2DS 2‐VASc score: 1.7 ± 1.2). Postprocedural MRI examination was performed in 97 patients, and in nine of them (9.3%; 95% CI: 4.3‐16.9%) a total of 11 SCLs were detected. Univariate analyses did not reveal any significant predictor for new SCLs. Nonsignificant trends were observed for low activated clotting time during ablation and for international normalized ratio value outside the range of 2 to 3 at ablation. There was no evidence of significant deterioration of neurocognitive function after PVI. In four patients, a pericardial tamponade was noted but all patients fully recovered during follow‐up.
Conclusions
Ablation of AF using a novel gold‐tip, externally irrigated ablation catheter, resulted in SCLs in approximately one out of 10 patients without a measurable effect on neurocognitive function.
Keywords: ablation, atrial fibrillation, cognitive function, gold‐tip catheter, irrigated catheter, pulmonary vein isolation, silent cerebral embolism
1. INTRODUCTION
Stroke is one of the most feared complications during catheter ablation of atrial fibrillation (AF). While symptomatic thromboembolic events are rare, magnetic resonance imaging (MRI) may identify asymptomatic (ie, silent) cerebral lesions (SCLs) following pulmonary vein isolation (PVI) procedures.1 According to previous reports and reviews, the incidence of SCLs may be as high as 50% depending on the ablation technology and imaging protocol, although their clinical implications remain unclear.2, 3, 4, 5
The electrode‐tissue interface is considered one potential source of thromboembolic debris during radiofrequency current ablation due to excessive heating.6 In an attempt to reduce thermocoagulation at the catheter tip, various irrigated catheter designs have been developed. The improved thermal conductive properties of gold‐tip catheters in comparison to platinum‐iridium‐tip catheters may also contribute to a favorable thromboembolic profile.7
We investigated a novel gold‐tip, externally irrigated ablation catheter (AlCath Flux eXtra Gold) during PVI in patients with paroxysmal AF with focus on the incidence of SCL and the effect of SCL on cognitive function.
2. METHODS
The REDUCE‐TE Pilot was a prospective multicenter single‐arm study in patients with paroxysmal AF scheduled for a PVI procedure. The study was approved by all local ethics committees and was registered at http://www.clinicaltrials.gov (NCT02275260). The study complies with the Declaration of Helsinki. Patients were required to sign the patient informed consent form before enrollment.
2.1. Patient population
Patients with symptomatic paroxysmal AF with an indication for catheter ablation were eligible for enrollment if they were on stable oral anticoagulation (OAC) according to a clinical routine with either a vitamin K antagonist (international normalized ratio [INR]: 2‐3) or a direct OAC.
Patients were excluded if they suffered from nonparoxysmal AF, had previous PVI attempts, or were ineligible for treatment with OAC. Also, a CHA2DS2‐VASc score ≥5, previous transient ischemic attack or stroke, the presence of an intracardiac thrombus, presence of an intracardiac implantable device, moderate or severe valvular heart disease, or symptomatic heart failure led to exclusion from the study. The left atrial size was limited to 55 mm and left ventricular ejection fraction had to be above 35%.
2.2. Study endpoints
The primary endpoint was a new SCL after PVI, assessed by cerebral diffusion‐weighted MRI. Several secondary endpoints were defined. First, the influence on the neurocognitive status was assessed both acutely after PVI and at the 3‐month follow‐up. Second, any periprocedural complication including transient ischemic attack and stroke were documented within 3 months after ablation. Third, efficacy parameters including acute procedural success rate, further procedural characteristics, and the 3‐month success rate defined as freedom from AF recurrence off antiarrhythmic drugs.
2.3. Preablation protocol
To exclude intracardiac thrombi, preprocedural echocardiography (transesophageal or intracardiac) was mandatory within 48 hours before the ablation. In cases of subtherapeutic INR before the procedure, the use of heparin was recommended as per the center's standard. The study protocol did not specify the periprocedural OAC management regarding interruption of OAC and/or bridging with heparin.
2.4. Neurocognitive testing
All patients underwent neurocognitive testing by trained personnel at baseline, before discharge, and at 90 days after the ablation using the following tests:
-
1.
State‐Trait Anxiety Inventory (STAI‐S) questionnaire8: Evaluates the patient's temporary state anxiety indicated by tension, nervousness, and fright towards future events.
-
2.
d2 Test of Attention9: A cancellation test of attention and concentration to be conducted under time pressure. We calculated the following parameters: total number of processed target items (a measure of speed), total number of correctly crossed target items minus total number of commission errors (concentration performance), and the number of errors (omission and commission errors) divided by the total number of processed target items (error rate).
-
3.
Visual and Verbal Memory (VVM2) test10: Use of a visual part only and an assessment of the visual short‐term recognition.
-
4.
Montreal Cognitive Assessment (MoCA) subtest11: This test consists of a 5‐Word Memory Task (registration, recall, and recognition), a 6‐Item Orientation test, and a 1‐Letter Phonemic Fluency test.
2.5. Ablation procedure
Ablation procedures were carried out according to each investigational site's standard. Unfractionated heparin was administered before or immediately after transseptal access and then repeatedly to maintain an activated clotting time (ACT) between 300 and 400 seconds throughout the procedure. The transseptal sheaths were flushed with heparinized saline. The recommended minimal flush rate was 10 mL/h.
The study catheter (AlCath Flux eXtra Gold; Biotronik, Berlin, Germany) has a novel tip design including material with high thermal conductivity (ie, gold/Au) and a novel configuration of 12 irrigation holes in a three‐dimensional pattern, aiming at optimized cooling of the electrode during ablation. The catheter was introduced into the left atrium to perform mapping and ablation. After defining the left atrial‐to‐PV junction using fluoroscopic and electrical information, antral ablation around the pulmonary veins (PVs) was performed. Electrical PVI was assessed online by the circular mapping catheter placed in one of the PVs. Ablation had to be carried out with a maximum power of 30 W and continuous motorized irrigation with a flush rate of 8 to 30 mL/min. The defined endpoint of acute ablation success was a demonstration of entrance block into all PVs. Monitoring for PV reconduction was recommended for 20 minutes following initial PV isolation.
If needed, restoration of sinus rhythm was attempted by direct current cardioversion. At the discretion of the operator ablation targets beyond PVI could be pursued if deemed necessary or in case of subsequent arrhythmias (ie, atrial flutter).
2.6. Postprocedural care
All patients underwent telemetric monitoring and, in some centers, routine postinterventional transthoracic echocardiography to exclude pericardial effusion. Therapeutic anticoagulation was continued directly after the procedure. Before discharge, an electrocardiogram (ECG) was obtained to exclude arrhythmia recurrence. Neurocognitive testing was performed as described. Brain imaging was performed as follows. No specific recommendation was provided regarding antiarrhythmic drug treatment postablation.
2.7. Diffusion‐weighted magnetic resonance imaging
Between 24 and 72 hours after the ablation, a cerebral diffusion‐weighted MRI was acquired using 1.5 to 3.0 Tesla scanners. An axial T2 weighted turbospin echo sequence with the following parameters was obtained: slice thickness 5 mm, repetition/echo time (TR/TE) 4000/100 ms, flip angle 150°, matrix 448 × 512. Second, an axial fluid‐attenuated inversion recovery sequence was acquired with the following parameters: slice thickness 5 mm, TR/TE/inversion time 9000/104/2500 ms, flip angle 150°, matrix 416 × 512. Then, an axial and a coronal echo planar imaging sequence (diffusion‐weighted sequence) was acquired with the following parameters: slice thickness, 5 mm; TR/TE, 4600/19 and 5500/119 ms; flip angle, 90°; matrix, 128 × 128. Any focal hyperintense area in the diffusion‐weighted MRI images with the corresponding hypointensity in the apparent diffusion coefficient map was defined as SCL. Images were adjudicated by an independent core lab blinded to the patient's clinical symptoms.
For each patient, the number, size, and location of lesions were assessed. The size was classified into three categories (0‐5, 5‐10, >10 mm).
2.8. Follow‐up
The follow‐up was limited to 3 months (allowed range, 9‐17 weeks) and included the assessment of any procedure‐related complaints with emphasis on thromboembolic events, recurrence of symptomatic AF as well as another neurocognitive testing.
2.9. Statistical analysis
The aim of this study was to show that the incidence of SCL using the study device is lower than 15% (one‐sided primary null hypothesis: P ≥ 0.15). The value of 15% is based on the literature, since the upper 95% confidence interval (CI) for the pooled rate of patients with at least one SCL after ablation using a standard irrigated catheter was calculated to be 15%.
Given the assumed incidence of 6% for the study device, a significance level of 2.5%, and a statistical power of 80%, the minimum sample size required was 102 patients. This sample size calculation was based on an exact binomial distribution. Accounting for an 18% drop‐out rate, 125 patients were to be enrolled in the study. For all two‐sided tests, a significance level of 5% was considered statistically significant in an exploratory way. The median and interquartile range (IQR) are given for continuous variables, absolute, and relative frequencies for nominal variables.
The primary endpoint was assessed with an exact binomial test. Incidence and 95% CI are given. The upper limit of this 95% CI is equal to the one‐sided upper 97.5% confidence limit corresponding to the one‐sided primary hypothesis.
The Fisher exact test was used to compare two groups with respect to a binary outcome. Comparison of two time points (eg, preablation and postablation) was done by the Wilcoxon signed‐rank test, sign test, or McNemar test, as appropriate. The Wilcoxon test was used for comparison of two groups with respect to a continuous variable.
3. RESULTS
3.1. Study course
A total of 110 patients were enrolled from December 2014 to February 2017. Mean follow‐up duration was 88 ± 33 days (range, 0‐190 days). Six patients were enrolled but did not receive the PVI procedure (for details, see Figure 1). One patient dropped out before receiving the postprocedural MRI and another six patients did not complete the 3‐month follow‐up.
Figure 1.
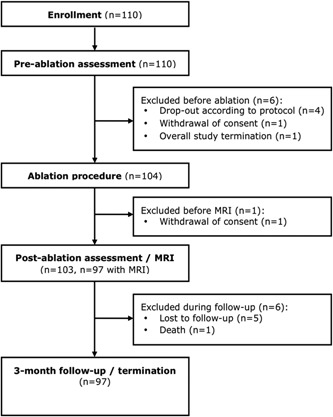
Study flowchart. MRI, magnetic resonance imaging
The sponsor prematurely stopped enrollment due to safety considerations (see data on adverse events below). The available endpoint data were sufficient to draw definite conclusions on the primary endpoint.
3.2. Study population
Baseline characteristics of the study population are presented in Table 1. Briefly, patients were predominantly male with a mean age of 61.5 ± 9.7 years. The mean CHA2DS2‐VASc score of the patients was 1.7 ± 1.2. Oral anticoagulation consisted of a coumarin derivative in 55.8% of cases and novel OACs in 41.3%. Three patients with a CHA2DS2‐VASc score of 0 did not take any anticoagulation at baseline. The mean INR of patients receiving coumarin derivative–based anticoagulation treatment was 2.3 ± 0.4 (range, 1.0‐4.2).
Table 1.
Baseline characteristics of study patients
| Clinical parameter | Value (n = 104) |
|---|---|
| Age, y | 62.5 (55.5‐68.0) |
| Sex, male | 72 (69.2) |
| BMI, kg/m2 | 28.0 (25.7‐30.9) |
| LVEF, % | 58.0 (55.0‐60.0) |
| Time since AF diagnosis, d | 954 (262‐1987) |
| Previous ablation for a different indication | 12 (11.5) |
| Failed antiarrhythmic medication | 73 (70.2) |
| Medical history | |
| Hypertension | 72 (69.2) |
| Diabetes mellitus | 14 (13.5) |
| Vascular disease | 12 (11.5) |
| Sleep apnea | 5 (4.8) |
| Liver or kidney disease | 4 (3.8) |
| Congestive HF/LV dysfunction | 4 (3.8) |
| COPD | 4 (3.8) |
| Myocardial infarction | 1 (1.0) |
| Peripheral arterial embolism | 0 |
| CHA2DS2‐VASc score | |
| 0 | 16 (15.4) |
| 1 | 34 (32.7) |
| 2 | 26 (25.0) |
| 3 | 18 (17.3) |
| 4 | 9 (8.7) |
| 5 | 1 (1.0) |
| Concomitant medication | |
| Antiarrhythmic | 55 (52.9) |
| β‐Blocker | 79 (76.0) |
| Calcium‐channel blocker | 12 (11.5) |
| Digoxin | 4 (3.8) |
| ACEI/ARB | 51 (49.0) |
| Diuretic | 22 (21.2) |
| Aldosterone antagonist | 6 (5.8) |
| Statin | 25 (24.0) |
| Anticoagulation | |
| Coumarin derivatives | 58 (55.8) |
| NOAC | 43 (41.3) |
| None | 3 (2.9) |
| INR | 2.3 (2.0‐2.5) |
Abbreviations: ACEI, angiotensin convertase enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin II receptor blocker; BMI, body mass index; COPD, chronic obstructive pulmonary disease; HF, heart failure; INR, international normalized ratio; NOAC, novel anticoagulant; LV, left ventricular; LVEF, left ventricular ejection fraction.
Data are presented as median (interquartile range) or absolute number (percent).
3.3. Procedure and ablation related data
Complete electrical isolation of all four pulmonary veins using the study ablation catheter was achieved in 101 of 104 patients (97.1%). Mean total procedure time was 126 ± 54 minutes (range, 54‐299 minutes), whereas the mean duration of radiofrequency energy delivery was 37 ± 18 minutes (range, 12‐94 minutes; Table 2). A mean of 56 859 ± 31 069 J was delivered (range, 1170‐149 310 J). Mean maximum delivered power was 30.8±3.6 W, with a range of 25 to 41 W. The prescribed limitation of the delivered power of 30 W was violated in six patients due to clinical necessities to achieve stable lesions. The maximally reached temperature of the catheter tip ranged between 34°C and 46°C (mean, 41°C). Audible steam pops were detected in two patients (1.9%), with one audible steam pop in each patient.
Table 2.
Procedural parameters
| Procedural parameter | Value (n = 104) |
|---|---|
| Total procedure time, min | 115.0 (84.5‐161.5) |
| Total duration of energy delivery, min | 35.9 (23.9‐48.6) |
| Cumulative energy delivered, J | 55 011 (31 336‐73 428), n = 82 |
| Number of RF applications for PVI, n | 35 (16‐54) |
| Maximum power delivered, W | 30 (30‐31) |
| Maximum tip temperature reached, °C | 42 (40‐43) |
| Maximum flow rate of irrigation, mL/min | 20 (17‐25) |
| Total amount of irrigation fluid, mL | 1023 (663‐1500) |
| Periprocedural anticoagulation | |
| Warfarin‐uninterrupted | 22 (21.2) |
| Warfarin‐bridged | 36 (34.6) |
| NOAC‐uninterrupted | 15 (14.4) |
| NOAC‐interrupted | 28 (26.9) |
| No anticoagulation at (pre)ablation | 3 (2.9) |
Abbreviations: NOAC, novel anticoagulant; PVI, pulmonary vein isolation; RF, radiofrequency.
Data are presented as median (interquartile range) or absolute number (percent).
During the ablation, 47.4% of all patients had all ACT values above 300 seconds and 52.7% of patients had at least one value below 300 seconds. The mean minimal ACT value was 204 ± 55 seconds.
3.4. Primary endpoint
In nine of 97 patients undergoing postablation MRI, 11 SCLs were detected by MRI (9.3% incidence; 95% CI: 4.3‐16.9%; the null hypothesis could not be rejected). Eight patients showed only one lesion, whereas one patient showed three new lesions.
The majority of lesions (9 of 11, 81.8%) were small, up to 5 mm. Only two of nine (22.2%) patients with lesions had medium‐size SCLs (5‐10 mm). Most SCLs were located in the frontal lobe (7 of 11, 63.6%). One lesion was located in each of the following regions: cerebellum, occipital lobe, parietal lobe, and posterior limb of the internal capsule.
3.5. Per‐protocol analysis
In seven patients, violations of the clinical investigational plan were observed including retrospectively detected violation of inclusion or exclusion criteria (n = 6) and use of a nonapproved ablation system (n = 1). Thus, a per‐protocol analysis of the primary endpoint was performed in 90 patients, showing SCLs in six patients (6.7% incidence; 95% CI: 2.5‐13.9%). The result of the per‐protocol analysis would have rejected the null hypothesis. Univariate analyses did not detect any significant predictor for new SCLs. Nonsignificant trends were observed for low ACT values during ablation and for INR outside the range of 2 to 3, at ablation (Table 3).
Table 3.
Subgroup analyses
| Subgroup | Patients | Odds ratio | 95% CI | P value | |
|---|---|---|---|---|---|
| With SCL (n = 9) | Without SCL (n = 88) | ||||
| Age ≥ median (62 y) | 5 (56) | 48 (55) | 1.04 | 0.21‐5.61 | 1.00 |
| Sex, male | 7 (78) | 59 (67) | 1.72 | 0.30‐17.9 | 0.71 |
| Hypertension | 7 (78) | 60 (68) | 1.63 | 0.28‐17.0 | 0.72 |
| CHADS2‐VASc score ≥3 | 2 (22) | 23 (26) | 0.81 | 0.08‐4.68 | 1.00 |
| Heparin bridging | 6 (67) | 54 (64), n = 85 | 1.15 | 0.23‐7.58 | 1.00 |
| Novel anticoagulant preablation and postablation | 4 (44) | 36 (42), n = 85 | 1.08 | 0.20‐5.45 | 1.00 |
| Additional lesions performed | 1 (11) | 16 (18) | 0.56 | 0.01‐4.76 | 1.00 |
| Method of left atrial access = double puncture | 6 (67) | 70 (80), n = 87 | 0.49 | 0.09‐3.33 | 0.39 |
| Mean ACT < median (311 s) | 5 (56) | 38 (49), n = 78 | 1.32 | 0.26‐7.13 | 0.74 |
| Minimum ACT (<250 s) | 7 (78) | 41 (47) | 4.01 | 0.70‐41.1 | 0.09 |
| INR at ablation out of range 2‐3 | 1 (50), n = 2 | 3 (18), n = 17 | 4.67 | 0.04‐391.7 | 0.39 |
| INR at postablation <2 | 1 (33), n = 3 | 6 (27), n = 22 | 1.33 | 0.02‐30.0 | 1.00 |
| Electrical cardioversion performed | 1 (11) | 15 (17) | 0.61 | 0.01‐5.17 | 1.00 |
| Procedure time > median (120 min) | 5 (56) | 44 (50) | 1.25 | 0.25‐6.72 | 1.00 |
| Patient suffers tamponade a | 1 (11) | 2 (2) | 5.38 | 0.08‐111.1 | 0.26 |
Abbreviations: ACT, activated clotting time; CI, confidence interval; INR, international normalized ratio; SCL, silent cerebral lesion.
One patient with a pericardial tamponade did not undergo MRI scanning.
3.6. Subgroup analyses
Univariate analysis did not identify any predictor for SCL (Table 3). A trend towards more SCLs was observed in patients with a minimum ACT < 250 seconds (odds ratio: 4.0; 95% CI: 0.7‐41.0; P = 0.09). However, the incidence of SCL was found to be significantly different across centers ranging from 0% to 100% (P < 0.01; Table 4).
Table 4.
Incidence of silent cerebral lesions (SCLs) according to center
| Center | No. of patients without SCL (%) | No. of patients with SCL (%) |
|---|---|---|
| 1 | 2 (67) | 1 (33) |
| 2 | 18 (100) | 0 (0) |
| 3 | 5 (100) | 0 (0) |
| 4 | 7 (78) | 2 (22) |
| 5 | 4 (66) | 2 (33) |
| 6 | 2 (100) | 0 (0) |
| 7 | 4 (100) | 0 (0) |
| 8 | 6 (100) | 0 (0) |
| 9 | 0 (0) | 2 (100) |
| 10 | 28 (93) | 2 (7) |
| 11 | 4 (100) | 0 (0) |
| 12 | 8 (100) | 0 (0) |
| Total | 88 (91) | 9 (9) |
A significant difference between the center's SCL incidence was found (the Fisher exact test; P < 0.01).
3.7. Neurocognitive scores
In the d2 Test of Attention, there was a significant intraindividual improvement in the concentration performance and in the error rate from preablation to postablation assessment (median increase in concentration performance: +12 [IQR: 1‐25]; median decrease in error rate: −3% [IQR: −13‐1]; Table 5). There was an even more pronounced significant intraindividual improvement in all parameters from preablation assessment to the 3‐month follow‐up visit (median increase in concentration performance: +23 [IQR: 9‐49]; median decrease in error rate: −5% [IQR: −17‐1]; median increase in the total number of processed target items: +15 [IQR: −7‐32]).
Table 5.
Results of neurocognitive tests: intraindividual changes compared to preablation assessment
| Time of assessment | All patients, median (IQR) | P value for change since preablation (signed‐rank test) | Median (IQR) | ||
|---|---|---|---|---|---|
| With silent lesions | Without silent lesions | ||||
| d2 Target items | Postablation | 3 (−18‐16), n = 91 | NS | −12 (−13‐18), n = 7 | 3 (−18.5‐16), n = 84 |
| 3‐mo FU | 15 (−7‐32), n = 79 | <0.001 | 25 (5‐33), n = 5 | 14.5 (−7‐31), n = 74 | |
| d2 Concentration performance | Postablation | 12 (1‐25), n = 91 | <0.001 | 4 (−11‐16), n = 7 | 13 (1.5‐25.5), n = 84 |
| 3‐mo FU | 23 (9‐49), n = 79 | <0.001 | 38 (10‐40), n = 5 | 22.5 (9‐49), n = 74 | |
| d2 Error rate, % | Postablation | −3 (−13‐1), n = 91 | <0.001 | −2 (−14‐4), n = 7 | −3 (−13‐0), n = 84 |
| 3‐mo FU | −5 (−17‐1), n = 79 | <0.001 | −7 (−15–5), n = 7 | −4 (−17‐1), n = 74 | |
| MoCA memory | Postablation | 0 (0‐1), n = 96 | <0.001 | 0 (0‐0), n = 9 | 0 (0‐1), n = 87 |
| 3‐mo FU | 0 (0‐1), n = 82 | 0.008 | 0 (−1‐0), n = 6 | 0 (0‐1), n = 76 | |
| VVM2 | Postablation | 1 (−3‐5), n = 95 | NS | 2 (−1‐4.5), n = 8 | 1 (−4‐5), n = 87 |
| 3‐mo FU | 0 (−2.5‐5.5), n = 80 | NS | 5 (4‐6), n = 5 | 0 (−3‐5), n = 75 | |
| STAI‐S | Postablation | −2 (−7.5‐1), n = 96 | <0.001 | −1 (−4‐1), n = 9 | −2 (−8‐1), n = 87 |
| 3‐mo FU | −2 (−7‐3), n = 81 | 0.022 | −0.5 (−15‐6), n = 6 | −2 (−7‐3), n = 75 | |
Abbreviations: FU, follow‐up; IQR, interquartile range; MoCA, Montreal Cognitive Assessment subtest; NS, nonsignificant; STAI‐S, State‐Trait Anxiety Inventory questionnaire; VVM2, Visual and Verbal Memory test.
Value for MoCA fluency were 0 and 1 only, values for MoCA orientation were 5 and 6 only, both precluding descriptive statistics.
There was a trend towards a poorer performance of patients with SCLs at the postablation assessment in term of fewer target items processed and a poorer concentration performance. However, none of these differences were statistically significant. This trend was no longer visible at the 3‐month follow‐up visit.
There was a significant improvement in the performance of the MoCA memory subtest from the preablation assessment to the postablation assessment and to the 3‐month follow‐up visit, with no difference between patients with and without new cerebral ischemic lesions. No significant changes for the MoCA orientation test, the MoCA fluency test, and the VVM2 test have been observed.
There was a significant decline in the STAI‐S test score from preablation assessment to the postablation assessment and the 3‐month follow‐up visit, indicating less anxiety after the ablation procedure, with no difference between patients with and without new cerebral ischemic lesions.
3.8. Rhythm outcome during follow‐up
In 21 of 96 patients (21.9%), a recurrence of AF had been detected within a follow‐up period of at least 9 weeks. Another 27 patients received continuous antiarrhythmic drug treatment and were therefore also counted as “nonsuccess”. A total of 41 of 97 patients (42.7%) received antiarrhythmic medication at the 3‐month follow‐up visit. At that last documented follow‐up visit, 90 patients (92.8%) were reported to be in sinus rhythm, six patients (6.2%) were in AF, and one patient (1.0%) had left atrial tachycardia.
3.9. Adverse events
In six patients, procedure‐related adverse events occurred, including vagal reaction (n = 1), atrioventricular block (n = 1), and pericardial tamponade (n = 4). Three patients with pericardial tamponade were managed by subxiphoidal puncture and the fourth patient underwent cardiac surgery to suture perforation of the left atrial appendage and a laceration of the right superior pulmonary vein ostium. All patients recovered during follow‐up. None of these adverse events had occurred in patients in whom the maximum delivered power exceeded 30 W.
One patient experienced an ischemic stroke (weakness of right arm) 18 days after the ablation procedure. The computed tomography scan was negative. One day later, the patient developed left‐sided hemiplegia and computed tomography revealed a subdural hematoma. After clinical deterioration 10 days later, multiple cerebral embolizations were diagnosed by MRI. The patient died 2 weeks later.
4. DISCUSSION
In this prospective multicenter study, the incidence of SCL after PVI using a novel extra irrigated gold‐tip catheter was 9.3%. The null hypothesis could not be rejected; however, this SCL incidence is well comparable to contemporary ablation devices using irrigated radiofrequency catheters. No significant differences in any of the neurocognitive tests were observed between patients with and without SCLs.
4.1. Procedural parameters associated with SCL
The exact pathophysiologic mechanisms that cause SCL have not been fully elucidated. The most likely reasons remain air, thrombus/coagulum‐char, and tissue debris.6 Repeated catheter exchanges via the steerable sheath have been identified as a risk factor for SCL.12 We observed a trend towards fewer SCLs when two transseptal sheaths had been used as compared to one sheath.
Stringent anticoagulation remains a key factor to prevent thromboembolic complications during catheter‐based AF ablation. Therefore, uninterrupted OAC using vitamin K antagonists is unequivocally recommended.13 Alternatively, direct OAC may be used uninterruptedly, without increased rates of bleeding.14, 15 In the present study, 55% and 40% of patients had been on OAC using vitamin K antagonists or direct OACs, respectively. Due to this heterogeneous management, results may be easily extrapolated to various clinical settings.
Intraprocedurally, the ACT management was suboptimal in a considerable number of patients, with ACT values less than 300 seconds. Univariate analysis identified a trend towards a higher likelihood for SCLs in patients with lower ACT values (<250 seconds), which is in accordance with previous observations.16 This again highlights the importance of proper anticoagulation management during the ablation procedure.
4.2. Technical parameters associated with SCL
Thrombus‐char formation was frequently observed with nonirrigated radiofrequency ablation devices and was also associated with a considerable rate of stroke or transient ischemic attack in the tailored treatment of persistent atrial fibrillation (TTOP‐AF) study that investigated duty‐cycled multipolar ablation.17 Among other technical refinements, the implementation of gold electrodes limited the rate of SCL with this particular ablation device.7 The likely reason for this favorable performance lies in its biophysical properties, that is, its almost four times higher thermal conductivity resulting in lower temperatures at the electrode‐tissue interface. In vitro studies as well as clinical investigations have shown that with the use of gold‐tip catheters, more energy can be delivered to the tissue while the tip temperature remains lower as compared to conventional platinum‐iridium catheters.18, 19
In addition, the novel irrigation design of the study catheter may have contributed to the low rate of SCL.
4.3. Serious adverse events
In the present study, four patients (4%) experienced pericardial tamponade. Although this by far exceeded the rate reported in contemporary real‐world registries, similar rates were observed in other randomized multicenter studies.20, 21 Operators complied with the recommended power settings and did not add extra ablation lesions beyond PVI. Of note, the catheter was not equipped with contact‐force sensing which may have helped to avoid excessive forces.
4.4. Neurocognitive testing
Despite being considered “silent”, there is an ongoing discussion whether the detected lesions or the AF ablation procedure itself may cause neurocognitive impairment. While a study found a subtle neurocognitive decline in up to 20% of patients undergoing AF ablation,22 others could not establish a causal relationship between the presence of SCL and cognitive impairment.5 In the present study, various tests were applied before, immediately after, and during short‐term follow‐up, without showing any significant performance decline in patients with SCL. In this context, it is noteworthy that the vast majority of SCL was small in size (<5 mm). This may explain the absence of even subtle neurocognitive changes.
4.5. Limitations
The present study was designed without a control arm, preventing a direct comparison of SCL rates to a standard ablation catheter. However, in comparison to historical controls using irrigated radiofrequency catheters, the detected SCL rate was well comparable. Since the study protocol did not mandate a preprocedural MRI, the reported rate could be overestimated; on the other hand, prior studies on SCL using preablation MRI reported negligible preprocedural SCL rates. Due to its multicentric nature, the study procedures showed marked differences as per the center's standard, in particular regarding the periprocedural anticoagulation. This may result in relevant local differences regarding SCLs. Scientifically, a clinical study should be as much standardized as possible, but the present study may well reflect clinical routine. The total number of patients and the number of patients with SCL were too low to draw definite conclusions on the absence of risk parameters for SCL and on the absence of any neurological implications. Nonetheless, previously described factors for low thromboembolic events, in particular, uninterrupted OAC and therapeutic ACT levels before transseptal puncture should be implemented into clinical routine.
Last, the reported success rate may be interpreted with caution given the short follow‐up without multiple days Holter ECG recordings.
5. CONCLUSION
AF ablation using a novel gold‐tip, externally irrigated ablation catheter, resulted in SCLs in approximately one out of 10 patients without a measurable effect on the neurocognitive function.
ACKNOWLEDGMENT
This study was supported by Biotronik SE & Co KG, Berlin, Germany.
Schmidt B, Széplaki G, Merkely B, et al. Silent cerebral lesions and cognitive function after pulmonary vein isolation with an irrigated gold‐tip catheter: REDUCE‐TE pilot study. J Cardiovasc Electrophysiol. 2019;30:877‐885. 10.1111/jce.13902
GS received speaker fee and honorary for consultancy from Biotronik and Biosense Webster. BW is an employee of Biotronik. DS received consultancy fees from Biosense Webster and Boston Scientific as well as research grants from Biosense Webster, Biotronik, Boston Scientific and Abbott. Other authors: No disclosures.
References
REFERENCES
- 1. Gaita F, Caponi D, Pianelli M, et al. Radiofrequency catheter ablation of atrial fibrillation: A cause of silent thromboembolism?: magnetic resonance imaging assessment of cerebral thromboembolism in patients undergoing ablation of atrial fibrillation. Circulation. 2010;122:1667‐1673. [DOI] [PubMed] [Google Scholar]
- 2. Schmidt B, Gunawardene M, Krieg D, et al. A prospective randomized single‐center study on the risk of asymptomatic cerebral lesions comparing irrigated radiofrequency current ablation with the cryoballoon and the laser balloon. J Cardiovasc Electrophysiol. 2013;24:869‐874. [DOI] [PubMed] [Google Scholar]
- 3. Deneke T, Jais P, Scaglione M, et al. Silent cerebral events/lesions related to atrial fibrillation ablation: a clinical review. J Cardiovasc Electrophysiol. 2015;26:455‐463. [DOI] [PubMed] [Google Scholar]
- 4. Herrera Siklódy C, Deneke T, Hocini M, et al. Incidence of asymptomatic intracranial embolic events after pulmonary vein isolation. J Am Coll Cardiol. 2011;58:681‐688. [DOI] [PubMed] [Google Scholar]
- 5. Haeusler KG, Koch L, Herm J, et al. 3 Tesla MRI‐detected brain lesions after pulmonary vein isolation for atrial fibrillation: results of the MACPAF study. J Cardiovasc Electrophysiol. 2013;24:14‐21. [DOI] [PubMed] [Google Scholar]
- 6. Haines DE, Stewart MT, Barka ND, et al. Microembolism and catheter ablation II: effects of cerebral microemboli injection in a canine model. Circ Arrhythmia Electrophysiol. 2013;6:23‐30. [DOI] [PubMed] [Google Scholar]
- 7. Greef YDe, Dekker L, Boersma L, et al. Low rate of asymptomatic cerebral embolism and improved procedural efficiency with the novel pulmonary vein ablation catheter GOLD: results of the PRECISION GOLD trial. Europace. 2016;18:687‐695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Spielberger CD, Gorsuch RL, Lushene RE. The State‐Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- 9. Bates ME, Lemay EP. The d2 test of attention: Construct validity and extensions in scoring techniques. J Int Neuropsychol Soc. 2004;10:392‐400. [DOI] [PubMed] [Google Scholar]
- 10. Woods AT, Newell FN. Visual, haptic and cross‐modal recognition of objects and scenes. J Physiol. 2004;98:147‐159. [DOI] [PubMed] [Google Scholar]
- 11. Hachinski V, Iadecola C, Petersen RC, et al. National Institute of Neurological Disorders and Stroke‐Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;37:2220‐2241. [DOI] [PubMed] [Google Scholar]
- 12. Miyazaki S, Watanabe T, Kajiyama T, et al. Thromboembolic risks of the procedural process in second‐generation cryoballoon ablation procedures: analysis from real‐time transcranial Doppler monitoring. Circ Arrhythmia Electrophysiol. 2017;10:10. [DOI] [PubMed] [Google Scholar]
- 13. Di Biase L, Burkhardt JD, Santangeli P, et al. Periprocedural stroke and bleeding complications in patients undergoing catheter ablation of atrial fibrillation with different anticoagulation management: results from the role of coumadin in preventing thromboembolism in atrial fibrillation (AF) patient. Circulation. 2014;129:2638‐2644. [DOI] [PubMed] [Google Scholar]
- 14. Calkins H, Willems S, Gerstenfeld EP, et al. RE‐CIRCUIT investigators. Uninterrupted dabigatran versus warfarin for ablation in atrial fibrillation. N Engl J Med. 2017;376:1627‐1636. [DOI] [PubMed] [Google Scholar]
- 15. Cappato R, Marchlinski FE, Hohnloser SH, et al. Uninterrupted rivaroxaban vs. uninterrupted vitamin K antagonists for catheter ablation in non‐valvular atrial fibrillation. Eur Heart J. 2015;36:1805‐1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Doi A, Takagi M, Kakihara J, et al. Incidence and predictors of silent cerebral thromboembolic lesions after catheter ablation for atrial fibrillation in patients treated with direct oral anticoagulants. Heart Vessels. 2017;32:1227‐1235. [DOI] [PubMed] [Google Scholar]
- 17. Hummel J, Michaud G, Hoyt R, et al. Phased RF ablation in persistent atrial fibrillation. Hear Rhythm. 2014;11:202‐209. [DOI] [PubMed] [Google Scholar]
- 18. Linhart M, Liberman I, Schrickel JW, et al. Superiority of gold versus platinum irrigated tip catheter ablation of the pulmonary veins and the cavotricuspid isthmus: a randomized study comparing tip temperatures and cooling flow requirements. J Cardiovasc Electrophysiol. 2012;23:717‐721. [DOI] [PubMed] [Google Scholar]
- 19. Balázs T, Laczkó R, Bognár E, et al. Ablation time efficiency and lesion volume—in vitro comparison of 4 mm, non irrigated, gold‐ and platinum‐iridium‐tip radiofrequency ablation catheters. J Interv Card Electrophysiol. 2013;36:13‐18. [DOI] [PubMed] [Google Scholar]
- 20. Arbelo E, Brugada J, Blomström‐Lundqvist C, et al. Contemporary management of patients undergoing atrial fibrillation ablation: in‐hospital and 1‐year follow‐up findings from the ESC‐EHRA atrial fibrillation ablation long‐term registry. Eur Heart J. 2017;38:1303‐1316. [DOI] [PubMed] [Google Scholar]
- 21. Morillo CA, Verma A, Connolly SJ, et al. RAAFT‐2 Investigators. Radiofrequency ablation vs antiarrhythmic drugs as first‐line treatment of paroxysmal atrial fibrillation (RAAFT‐2). JAMA. 2014;311:692. [DOI] [PubMed] [Google Scholar]
- 22. Medi C, Evered L, Silbert B, et al. Subtle post‐procedural cognitive dysfunction after atrial fibrillation ablation. J Am Coll Cardiol. 2013;62:531‐539. [DOI] [PubMed] [Google Scholar]


