Summary
Background
In the U.S.A., an Investigator's Global Assessment (IGA) score of ≤ 1 (clear or almost clear skin) has been the standard measure in regulatory outcomes for registration clinical trials in atopic dermatitis (AD), including those supporting the recent approval of dupilumab.
Objectives
To evaluate the treatment effect of dupilumab in patients with IGA > 1 at the end of treatment, using other validated outcome measures for AD signs, symptoms and quality of life.
Methods
LIBERTY AD SOLO 1 and 2 were two 16‐week, randomized, double‐blind trials enrolling adult patients with moderate‐to‐severe AD (IGA ≥ 3) inadequately controlled with topical treatment. We performed a post hoc analysis in patients receiving dupilumab 300 mg every 2 weeks (q2w) or placebo. Outcome measures in patients with IGA > 1 included Eczema Area and Severity Index (EASI), pruritus numerical rating scale (NRS), affected body surface area (BSA), Patient‐Oriented Eczema Measure (POEM) and Dermatology Life Quality Index (DLQI). The trials were registered at ClinicalTrials.gov: NCT02277743 and NCT02277769.
Results
At week 16, 278 of 449 dupilumab q2w‐treated patients (median age 36·0 years) and 396 of 443 placebo‐treated patients had IGA > 1. Among patients with IGA > 1 at week 16, dupilumab significantly improved several outcome measures compared with placebo: EASI (−48·9% vs. −11·3%, P < 0·001), pruritus NRS (−35·2% vs. −9·1%, P < 0·001), affected BSA (−23·1% vs. −4·5%, P < 0·001), POEM score ≥ 4‐point improvement (57·4% vs. 21·0%, P < 0·001) and DLQI score ≥ 4‐point improvement (59·3% vs. 24·4%, P < 0·001).
Conclusions
In patients with IGA > 1 at week 16, dupilumab induced statistically significant benefits in multiple validated outcome measures compared with placebo. The IGA ≤ 1 end point significantly underestimates clinically relevant dupilumab treatment effects.
Short abstract
What's already known about this topic?
An Investigator's Global Assessment (IGA) score of 0 or 1 (clear or almost clear skin) is considered the regulatory standard for treatment success in trials of patients with atopic dermatitis in the U.S.A.
It is currently unknown whether patients receiving dupilumab treatment for moderate‐to‐severe atopic dermatitis derive clinical and quality‐of‐life benefit even if they have an end‐of‐treatment IGA score > 1.
What's does this study add?
This post hoc analysis of patients with an end‐of‐treatment IGA score > 1 from two randomized, placebo‐controlled trials showed that after 16 weeks of treatment dupilumab significantly improved their outcome measures compared with placebo, including measures of signs, symptoms and quality of life.
These results show that the regulatory IGA ≤ 1 end point used in clinical trials significantly underestimates clinically relevant dupilumab treatment effects, underscoring potential limitations of the IGA scale.
Linked Comment: https://doi.org/10.1111/bjd.18037.
The static Investigator's Global Assessment (IGA) is an important and frequently used outcome measure, included in approximately one‐third of randomized trials for atopic dermatitis (AD).1 Use of the IGA is driven in part by its potential simplicity and by guidance from the U.S. Food and Drug Administration (FDA), which began requiring IGA as a primary end point in all dermatology drug trials in the early 2000s.1, 2 The static IGA scales replaced dynamic global assessments that required investigators to remember results from previous visits to evaluate the change in the patient's dermatological condition. As a static scale, IGA should be less prone to bias and easier to interpret (mild, moderate and severe) than dynamic scales.3 However, IGA scales have not been validated or standardized. A recent systematic review identified several different IGA scales used in AD trials, the validity and comparability of which are unknown.1 Most IGA scales measure only the ‘global’ severity of skin signs (mainly redness and induration) without taking into account the extent of AD skin involvement or patient‐reported symptoms like pruritus. The available IGA scales and their skin morphological descriptors have not been validated for use as standalone outcome measures; therefore, IGA is often used with other validated measures.
Achievement of IGA 0 (clear) or 1 (almost clear) has been the primary definition of treatment success in registration trials. However, improvement in IGA score alone misses clinically meaningful improvement in the extent of AD, and potentially underestimates the effects of treatment on itching, sleep loss and other disease effects.
Dupilumab is a fully human VelocImmune‐derived monoclonal antibody4, 5 directed against interleukin (IL)‐4 receptor alpha, and inhibits signalling of IL‐4 and IL‐13. Dupilumab has shown efficacy and acceptable safety in clinical trials in AD, asthma, chronic rhinosinusitis with nasal polyposis, and eosinophilic oesophagitis, demonstrating the importance of IL‐4 and IL‐13 in driving multiple type 2 atopic and allergic diseases.6, 7, 8, 9, 10, 11, 12, 13, 14 Dupilumab is approved for the treatment of adults with inadequately controlled moderate‐to‐severe AD. In pivotal trials leading to the approval of dupilumab, a five‐point IGA scale of 0 (clear) to 4 (severe) focusing on clinical signs was used; a primary end point was the proportion of patients achieving an IGA score ≤ 1 at week 16.7, 8 Approximately 40% of patients treated with dupilumab met this end point.7, 8 However, a substantially higher proportion of patients achieved clinically meaningful improvements in other outcome measures, such as Eczema Area and Severity Index (EASI) and pruritus numerical rating scale (NRS),7, 8 underscoring the potential limitations of the IGA scale.
We performed a post hoc analysis of clinical outcomes in patients with IGA > 1 at the end of treatment to evaluate the effects of dupilumab in this subset of patients from two pivotal phase III trials of dupilumab monotherapy in patients with moderate‐to‐severe AD (LIBERTY AD SOLO 1 and LIBERTY AD SOLO 2).
Patients and methods
The study designs and patient populations of SOLO 1 and SOLO 2 have been described elsewhere.7 These trials were conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki, International Conference on Harmonisation Good Clinical Practice guidelines, and applicable regulatory requirements. All of the patients provided signed written informed consent prior to any study procedure. The local institutional review board or ethics committee reviewed and approved the protocol, the informed consent form and patient information. Briefly, eligible patients were aged ≥ 18 years with moderate‐to‐severe AD (severity criteria included IGA 3 or 4, among others) inadequately controlled with topical treatment or for whom topical treatment was inadvisable. All patients had chronic AD for ≥ 3 years before screening. Patients were randomized (1 : 1 : 1) to dupilumab 300 mg weekly, dupilumab 300 mg every 2 weeks (q2w) or placebo; patients assigned to any dupilumab regimen received a loading dose (600 mg) on day 1. Stratification factors included baseline IGA score (3 vs. 4) and geographical region.
End points
A primary end point of the SOLO 1 and SOLO 2 trials was the proportion of patients with IGA ≤ 1 at week 16. Other end points evaluating signs, symptoms and quality of life were EASI, including percentage change in total score and proportions of patients achieving ≥ 50% improvement (EASI 50) or ≥ 75% improvement (EASI 75); weekly average peak pruritus NRS score, including the proportion of patients with ≥ 4‐point improvement and percentage change from baseline; patient‐reported global assessment of treatment effect; five‐dimension, three‐level EuroQoL questionnaire (EQ‐5D), including the proportion of patients reporting ‘no pain or discomfort’; change in the percentage of body surface area (BSA) affected; and changes in Patient‐Oriented Eczema Measure (POEM) and Dermatology Life Quality Index (DLQI).
Analysis
We performed post hoc analyses in patients who did not achieve IGA ≤ 1 (i.e. patients with IGA > 1) at week 16; comparisons were made between placebo and dupilumab 300 mg q2w (approved dose) for key secondary end points. For continuous variables (e.g. percentage change over time in EASI, peak pruritus NRS, and BSA affected by AD), confidence intervals and P‐values were based on treatment differences (dupilumab vs. placebo) of the least squares mean using an ancova model. The baseline measurement was a covariate, and treatment, region and baseline disease severity (IGA 3 vs. IGA 4) were fixed factors. For binary variables (e.g. proportion of patients achieving EASI 75), results were based on the Cochran–Mantel–Haenszel test stratified by region and baseline disease severity (IGA 3 vs. IGA 4). Assessment of the proportion of patients with ≥ 4‐point reduction in DLQI was based on the subset of patients with a baseline DLQI score of ≥ 4; a similar approach was used for POEM.
Patient subgroups were determined by an anchor (i.e. IGA static and categorical scale at week 16), while efficacy outcomes were evaluated cross‐sectionally from baseline to week 16, as reflected in change or percentage change. Two imputation methods were implemented: (i) postbaseline last observation carried forward (LOCF), where IGA anchor categories and efficacy responses were determined with consideration of rescue medication use, and the last assessment values prior to rescue medication use were carried forward; and (ii) observed value, where IGA anchor categories and efficacy responses at week 16 were quantified based on observed values disregarding the use of rescue medication (to reflect a real‐world situation).
Results
Patients
In total 892 patients treated with dupilumab 300 mg q2w or placebo had week 16 IGA status available using a postbaseline LOCF with censoring after imputation for rescue medication usage, and were therefore included in the LOCF analyses. At week 16, 171 of 449 (38·1%) dupilumab‐treated patients achieved IGA ≤ 1, compared with 47 of 443 (10·6%) placebo‐treated patients; 278 of 449 (61·9%) dupilumab‐treated patients had IGA > 1, compared with 396 of 443 (89·4%) placebo‐treated patients.
The demographics and baseline characteristics are provided in Table S1 (see Supporting Information). Patients with IGA > 1 at week 16 had higher baseline EASI and POEM scores, higher proportions of BSA affected by AD, and longer disease durations than those who achieved IGA ≤ 1 (Table S2; see Supporting Information). The proportion of patients with IGA 4 at baseline was nearly twice as large in patients with IGA > 1 at week 16 than in those with IGA ≤ 1 (59·4% vs. 31·6%, respectively). Among patients with IGA > 1, baseline characteristics were generally balanced between treatment groups (Table S2; see Supporting Information).
Efficacy outcomes in patients with Investigator's Global Assessment > 1 at week 16
Using LOCF, the least squares mean percentage change in EASI total score from baseline was significantly greater with dupilumab (−48·9%) than with placebo (−11·3%) at week 16 (Fig. 1a and Table S3; see Supporting Information). Compared with placebo, a significantly greater proportion of dupilumab‐treated patients achieved EASI 75 (20·9% vs. 4·8%; Fig. 1b) and EASI 50 (49·6% vs. 16·2%; Fig. 1c). Given the relatively higher baseline EASI score in patients with IGA > 1, achievement of EASI 50 represents a substantial absolute improvement in EASI score, which is reflected in the overall EASI change from baseline (−17·5 vs. −4·3, P < 0·001). At week 16, the decrease in pruritus NRS score from baseline was significantly higher in the dupilumab group than with placebo (−35·2% vs. −9·1%; Fig. 1d and Table S3). The decrease from baseline in the percentage of BSA affected was also significantly greater in the dupilumab group than with placebo (Fig. 1e and Table S3).
Figure 1.
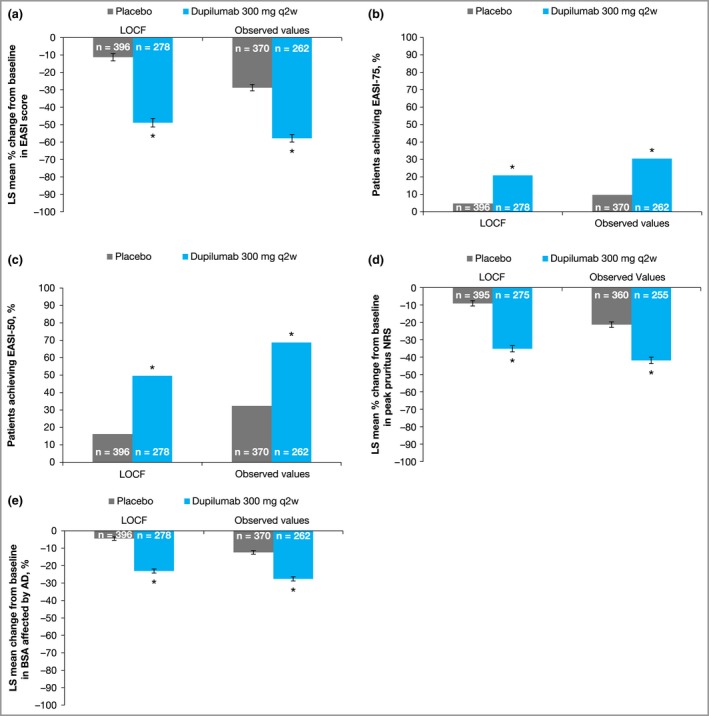
Outcomes in patients with Investigator's Global Assessment > 1 treated with dupilumab or placebo. (a) Percentage change from baseline to week 16 in Eczema Area and Severity Index (EASI) total score. (b, c) Proportions of patients who achieved ≥ 75% improvement in EASI (EASI 75) (b) or EASI 50 (c) at week 16. (d) Percentage change from baseline to week 16 in peak pruritus numerical rating scale (NRS) score. (e) Proportion of body surface area (BSA) affected by atopic dermatitis (AD). Error bars indicate standard error (SE). LOCF, last observation carried forward; LS, least squares; q2w, every 2 weeks. *P < 0·001 vs. placebo.
Improvement in DLQI from baseline was significantly greater in the dupilumab group than with placebo at week 16. Dupilumab‐treated patients experienced a mean 7·2‐point improvement in DLQI score, compared with 1·6‐point improvement with placebo (LOCF method; Fig. 2a and Table S3). A significantly greater proportion of dupilumab‐treated patients had ≥ 4‐point reduction (improvement) in DLQI score than with placebo (59·3% vs. 24·4%, P < 0·001; Fig. 2b and Table S3). A similar pattern was observed for POEM scores: among patients with IGA > 1, improvement from baseline POEM score was significantly greater in the dupilumab group than with placebo (Fig. 2c and Table S3). Also, a greater proportion of patients in the dupilumab group had a ≥ 4‐point reduction (improvement) in POEM score (57·4% vs. 21·0%, P < 0·001; Fig. 2d and Table S3). Additionally, at week 16 a significantly greater proportion of dupilumab‐treated patients reported a global treatment effect (Patient's Global Assessment of treatment effect, PGATE) of ‘good’, ‘very good’ or ‘excellent’ compared with placebo (51·5% vs. 17·4%, P < 0·001; Table S3 and Fig. S1; see Supporting Information). A greater proportion of patients treated with dupilumab vs. placebo reported ‘no pain or discomfort’ on the EQ‐5D questionnaire (Table S3 and Fig. S2; see Supporting Information).
Figure 2.
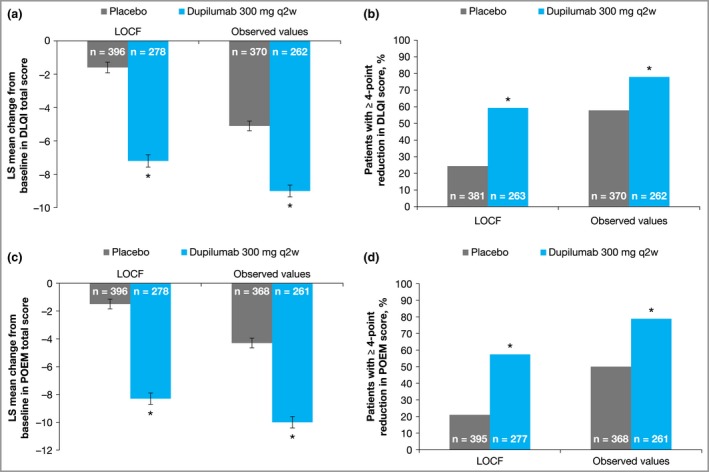
Outcomes in patients with Investigator's Global Assessment > 1 treated with dupilumab or placebo. (a) Change from baseline to week 16 in Dermatology Life Quality Index (DLQI) total score. (b) Proportion of patients with ≥ 4‐point reduction in DLQI score from baseline. (c) Change from baseline to week 16 in Patient‐Oriented Eczema Measure (POEM) score. (d) Proportion of patients with ≥ 4‐point reduction in POEM score from baseline. Error bars indicate the standard error. LOCF, last observation carried forward; LS, least squares; q2w, every 2 weeks. *P < 0·001 vs. placebo.
Similar efficacy results were observed using the observed value method (Table S3). Results using LOCF and observed values are summarized in Figure S3 (see Supporting Information).
Outcomes by discrete (nonaggregated) Investigator's Global Assessment score
Data were also analysed for separate cohorts based on IGA response category at week 16 (IGA 0 vs. 1 vs. 2 vs. 3 vs. 4) to determine whether secondary outcome measures varied among individual IGA categories. Among patients with IGA > 1 at week 16, twice as many achieved IGA 2 (mild disease) with dupilumab compared with placebo (n = 100 vs. n = 52, P < 0·001). Patients requiring rescue medication were considered ‘nonresponders’ for these analyses. Overall, the results using the LOCF or observed value methods were generally consistent in the analysis of patients with IGA > 1, as improvements in secondary outcome measures were seen with dupilumab compared with placebo across most IGA response subgroups (Table 1 and Figs [Link], [Link], [Link], [Link]; see Supporting Information). The magnitude of difference between dupilumab and placebo generally increased as IGA score increased (i.e. small or no difference among IGA 0 patients, progressing to the largest difference among patients with IGA 4).
Table 1.
Key week 16 outcomes according to week 16 Investigator's Global Assessment (IGA) score (last observation carried forward method)
| Outcome | EASI, LS mean % change from baseline | EASI 50, % | Peak pruritus NRS, LS mean change from baselinea | DLQI score, LS mean change from baseline |
|---|---|---|---|---|
| IGA 0 | ||||
| Dupilumab (n = 26) | –99·7 | 100 | –5·0 | –8·4 |
| Placebo (n = 4) | –99 | 100 | –6·0 | –9·2 |
| IGA 1 | ||||
| Dupilumab (n = 145) | –89·9* | 97·9 | –4·1** | –10·4 |
| Placebo (n = 43) | –86·3 | 93 | –3·3 | –9·5 |
| IGA 2 | ||||
| Dupilumab (n = 100) | –75·4** | 78 | –3·3*** | –9·4*** |
| Placebo (n = 52) | –66·9 | 65 | –2·4 | –6·5 |
| IGA 3 | ||||
| Dupilumab (n = 132) | –41·7*** | 42·4*** | –2·5*** | –6·6*** |
| Placebo (n = 174) | –22·8 | 16·7 | –1·1 | –3·0 |
| IGA 4 | ||||
| Dupilumab (n = 46) | –2·7** | 9** | –1·2*** | –3·4*** |
| Placebo (n = 170) | 14·8 | 0·6 | –0·1 | 0·1 |
DLQI, Dermatology Life Quality Index; EASI, Eczema Area and Severity Index; EASI 50, ≥ 50% improvement from baseline in EASI score; LS, least squares; NRS, numerical rating scale. aPeak pruritus NRS scores missing for two patients in the IGA 2 group (both dupilumab) and two patients in the IGA 3 group (one dupilumab, one placebo). *P < 0·05, **P < 0·01, ***P < 0·001 vs. placebo.
Discussion
In this post hoc analysis of data from two phase III trials, patients with IGA > 1 at week 16 had more severe disease at baseline. These patients derived clinically and statistically significant benefits from dupilumab treatment compared with placebo. Benefits included improvements in the extent and severity of AD lesions based on EASI score and the proportion of BSA affected. Patients also had significant reductions in pruritus NRS and scores for pain and discomfort (EQ‐5D), which are important symptoms of AD. Improvement in quality of life (DLQI) in patients with IGA > 1 was greater with dupilumab than with placebo. Improvements in global patient‐reported outcomes, including POEM and PGATE, were also significantly greater in the dupilumab group. Notably, 52% of patients with IGA > 1 rated dupilumab therapy as good, very good or excellent, compared with 17% of patients in the placebo group, reflecting the marked treatment response in these patients, despite not achieving IGA ≤ 1.
Patients treated with dupilumab with IGA > 1 experienced benefits in objective clinical signs, pruritus and quality of life regardless of whether the results were analysed in aggregate (IGA 2–4) or by individual IGA score (IGA 2, 3 or 4). This suggests that achievement of IGA ≤ 1 does not reflect the entire benefit that patients experience from treatment, because IGA is a categorical nonvalidated scale that does not take into account relevant patient symptoms, such as pruritus, sleep and overall quality of life. Patients with IGA > 1 had significantly greater improvement in outcomes when treated with dupilumab compared with placebo. The primary end point of IGA ≤ 1 failed to discern these important differences between treatment groups.
The main advantages of IGAs are that assessment is relatively fast and easy to perform, and the results are readily understood. The IGA scales have been widely used in clinical trials in AD: in a recent review of 317 randomized trials, 101 (32%) included some form of IGA.1 Use of IGA was more common in studies conducted in North America (73%) than in Europe (30%). Currently, the FDA recommends using IGA ≤ 1 as a primary end point for new drug approval trials, although this is not the case for the European Medicines Agency. However, IGA scales have several important limitations. Many variants are in use, with scales ranging from 0–4 to 0–7 points and treatment success defined as score ≤ 1 (clear or almost clear) or ≤ 2 (clear, almost clear or mild).1, 15 The type of skin signs assessed also varies among scales: most assess erythema and induration or papulation, but some scales, such as the one used in the pimecrolimus development programme, also used oozing and crusting to differentiate between ‘severe’ and ‘very severe’ disease.15 Most IGA scales, including the one used in the dupilumab trials, do not account for lesion extent (i.e. the proportion of BSA affected), the location of lesions or symptom burden.
Figure 3 illustrates an example of a patient with IGA > 1 at the end of dupilumab treatment who experienced substantial improvement by several other outcome measures (pruritus NRS, POEM, DLQI and BSA). The ability of most IGA scales to reflect lesion severity but not lesion extent means that it may be acceptable when evaluating topical therapies for localized AD, when only the target lesion is evaluated, but it becomes problematic when evaluating systemic therapies in patients with more extensive and burdensome disease. Another limitation is that the same success criteria (clear or almost clear) are used to demonstrate efficacy in patients with mild, moderate and severe disease, which favours the first category. Lastly, the minimal clinically important difference for any IGA scale, including the five‐point scale used in the SOLO trials, has not been formally determined. Historically, a difference of 2 points has been considered relevant, but this difference was selected empirically, without properly accounting for the inherent variability of the assessment and the apparent nonlinearity of the scale (e.g. the difference between ‘clear’ and ‘mild’ is not necessarily the same as the difference between ‘mild’ and ‘severe’). The limitations of available IGA scales have prompted efforts to refine and validate the IGA.16 However, the performance of these modified scales remains to be confirmed.
Figure 3.
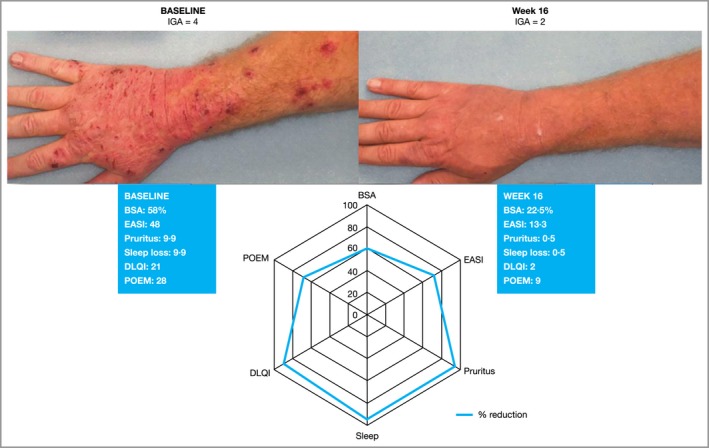
Illustration of a patient who improved from Investigator's Global Assessment (IGA) 4 (severe) at baseline to IGA 2 (mild) after 16 weeks of dupilumab treatment. Change in IGA score was accompanied by clinically meaningful improvement in BSA, symptoms (sleep, pruritus, POEM) and quality of life. BSA, body surface area; DLQI, Dermatitis Life Quality Index; EASI, Eczema Area and Severity Index; POEM, Patient‐Oriented Eczema Measure.
The fact that patients treated with dupilumab with IGA > 1 had improvements in other outcomes suggests that a dichotomized treatment success criterion based on IGA may be too limited to capture the full spectrum of treatment benefits. While IGA ≤ 1 remains a valid measure of an exceptionally good response, these findings suggest that the traditional IGA scale starts to lose sensitivity above a value of 1 and fails to capture the full spectrum of clinical benefits that patients with moderate‐to‐severe AD may derive from effective treatments, especially for symptoms and quality of life. This underscores the importance of using multiple outcome measures, including validated measures of AD lesion severity and extent (e.g. EASI) and patient‐reported outcomes, to assess treatment effects adequately.
There were limitations to this analysis. The results should be interpreted with caution, given the post hoc nature of the analysis. It is possible that week 16 was too early for optimal IGA assessment; however, in the long‐term study of dupilumab treatment with topical corticosteroids, there was no significant difference between week 16 and week 52 values for all efficacy end points.8 We included two analytical methods (LOCF and observed value), and the results were generally similar with both approaches. Differences in outcomes using the two methods could reflect the impact of rescue medication (accounted for in the LOCF method). In the analysis by individual IGA score, the number of patients in each subgroup was relatively small; nevertheless, the differences observed between dupilumab and placebo were nominally significant. We chose a priori not to examine the EASI 90 outcome because it overlaps with IGA 0–1 and is therefore too stringent to apply to this population of patients achieving IGA > 1.
In summary, the benefits of dupilumab in patients with moderate‐to‐severe AD are clinically meaningful and statistically significant beyond the strict success criterion of IGA ≤ 1. This is supported by multiple validated clinician‐ and patient‐reported outcome measures, such as EASI, DLQI, pruritus NRS, POEM and patient‐reported outcomes. The findings highlight the limitations of the conventional IGA scale in general, and the IGA ≤ 1 end point in particular.
Supporting information
Fig S1. Proportions of patients who, at week 16, rated the effects of treatment as ‘good’, ‘very good’ or ‘excellent’.
Fig S2. Proportions of patients who, at week 16, reported ‘no pain or discomfort’ on the EuroQol‐5D questionnaire.
Fig S3. Forest plot summary of results using the last observation carried forward method and observed value method.
Fig S4. Percentage change in Eczema Area and Severity Index score from baseline to week 16 according to Investigator's Global Assessment score at week 16.
Fig S5. Proportion of patients achieving ≥ 50% improvement in Eczema Area and Severity Index according to Investigator's Global Assessment score at week 16.
Fig S6. Change in peak pruritus numerical rating scale score from baseline to week 16 according to Investigator's Global Assessment score at week 16.
Fig S7. Change in Dermatology Life Quality Index score from baseline to week 16 according to Investigator's Global Assessment score at week 16.
Table S1 Demographics and baseline characteristics.
Table S2 Demographics and baseline characteristics within subsets of patients with Investigator's Global Assessment (IGA) ≤ 1 vs. IGA > 1 at week 16.
Table S3 Key outcomes at week 16 in patients who achieved Investigator's Global Assessment > 1.
Supplementary Info S1 Supplementary figure legends.
Supplementary Info S2 Plain Language Summary.
Video S1 Author video.
Powerpoint S1 Journal Club Slide Set.
Acknowledgments
We would like to thank Ferdinando Giacco, PhD, of Excerpta Medica for medical writing, editorial and submission support; and Linda Williams (Regeneron Pharmaceuticals, Inc.) and El‐Bdaoui Haddad (Sanofi Genzyme) for their contributions.
Conflicts of interest: J.I.S. is an investigator for AbbVie, Celgene, Chugai, Eli Lilly, GlaxoSmithKline, Regeneron Pharmaceuticals, Inc. and Sanofi; a consultant for AbbVie, Anacor, Eli Lilly, Galderma, GlaxoSmithKline, Incyte, Kiniksa, LEO Pharma, Menlo, Pfizer, Realm, Regeneron Pharmaceuticals, Inc. and Sanofi; and a speaker for Regeneron Pharmaceuticals, Inc. and Sanofi. E.L.S. has received honoraria for consulting services from AbbVie, Anacor, Celgene, Dermira, Eli Lilly, Galderma, Genentech, GlaxoSmithKline, LEO Pharma, Menlo Therapeutics, Pfizer, Regeneron Pharmaceuticals, Inc., Sanofi Genzyme and Valeant; and provided study support for Anacor, Eli Lilly, GlaxoSmithKline, MedImmune, Novartis, Regeneron Pharmaceuticals, Inc., Roivant Sciences, Tioga and Vanda. M.A., Z.C., J.C., A.K., Y.L., N.M.H.G. and B.A. are all employees and shareholders of Regeneron Pharmaceuticals, Inc. D.T. has provided research support for AbbVie, Almirall, Amgen, Biogen‐Idec, Boehringer Ingelheim, BMS, Celgene, Dignity, Eli Lilly, GlaxoSmithKline, Janssen‐Cilag, LEO Pharma, Novartis, Pfizer, Regeneron, Sandoz, Sanofi and UCB; has received honoraria from AbbVie, BMS, Boehringer Ingelheim, Celgene, GSK, Janssen, LEO Pharma, Novartis, Pfizer, Roche‐Possay and UCB; is a consultant for AbbVie, Celgene, Galapagos, Novartis, Pfizer and UCB; and is a scientific advisory board member for AbbVie, Amgen, Biogen‐Idec, BMS, Celgene, Eli Lilly, GlaxoSmithKline, Janssen, LEO Pharma, Novartis, Pfizer, Sandoz and UCB. S.B. is an advisory board member for and has received research grants (to the institution) from Pierre Fabre Laboratory; is a speaker for Bioderma; and is an advisory board member for Sanofi Genzyme. J.B. is a consultant or investigator for AbbVie, Celgene, Eli Lilly, Janssen, Novartis, Pfizer and Regeneron; a speaker for AbbVie, Celgene, Eli Lilly, Janssen, LEO Pharma, Novartis and Regeneron; and an investigator for AbbVie, Celgene, DS Biopharma, Eli Lilly, Janssen, LEO Pharma, Novartis and Regeneron. L.E., E.R., A.B.R., T.H. and G.P. are all employees of and may hold stock and/or stock options in Sanofi.
Funding sources This research was sponsored by Sanofi Genzyme and Regeneron Pharmaceuticals, Inc.
Conflicts of interest statements can be found in the Appendix.
J.I.S. and E.L.S. are joint first authors and contributed equally to this work.
References
- 1. Futamura M, Leshem YA, Thomas KS et al A systematic review of Investigator Global Assessment (IGA) in atopic dermatitis (AD) trials: many options, no standards. J Am Acad Dermatol 2016; 74:288–94. [DOI] [PubMed] [Google Scholar]
- 2. Chalmers JR, Schmitt J, Apfelbacher C et al Report from the third international consensus meeting to harmonise core outcome measures for atopic eczema/dermatitis clinical trials (HOME). Br J Dermatol 2014; 171:1318–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Feldman SR, Krueger GG. Psoriasis assessment tools in clinical trials. Ann Rheum Dis 2005; 64 (Suppl. II):ii65–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. MacDonald LE, Karow M, Stevens S et al Precise and in situ genetic humanization of 6 Mb of mouse immunoglobulin genes. Proc Natl Acad Sci U S A 2014; 111:5147–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Murphy AJ, Macdonald LE, Stevens S et al Mice with megabase humanization of their immunoglobulin genes generate antibodies as efficiently as normal mice. Proc Natl Acad Sci U S A 2014; 111:5153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gandhi NA, Pirozzi G, Graham NMH. Commonality of the IL‐4/IL‐13 pathway in atopic diseases. Expert Rev Clin Immunol 2017; 13:425–37. [DOI] [PubMed] [Google Scholar]
- 7. Simpson EL, Bieber T, Guttman‐Yassky E et al Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med 2016; 375:2335–48. [DOI] [PubMed] [Google Scholar]
- 8. Blauvelt A, de Bruin‐Weller M, Gooderham M et al Long‐term management of moderate‐to‐severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1‐year, randomised, double‐blinded, placebo‐controlled, phase 3 trial. Lancet 2017; 389:2287–303. [DOI] [PubMed] [Google Scholar]
- 9. de Bruin‐Weller M, Thaçi D, Smith CH et al Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to ciclosporin A or when this treatment is medically inadvisable: a placebo‐controlled, randomized phase III clinical trial (LIBERTY AD CAFÉ). Br J Dermatol 2018; 178:1083–101. [DOI] [PubMed] [Google Scholar]
- 10. Wenzel S, Castro M, Corren J et al Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium‐to‐high‐dose inhaled corticosteroids plus a long‐acting β2 agonist: a randomised double‐blind placebo‐controlled pivotal phase 2b dose‐ranging trial. Lancet 2016; 388:31–44. [DOI] [PubMed] [Google Scholar]
- 11. Castro M, Corren J, Pavord I et al Dupilumab efficacy and safety in moderate‐to‐severe uncontrolled asthma. N Engl J Med 2018; 378:2486–96. [DOI] [PubMed] [Google Scholar]
- 12. Rabe K, Nair P, Brusselle G et al Efficacy and safety of dupilumab in glucocorticoid‐dependent severe asthma. N Engl J Med 2018; 378:2475–85. [DOI] [PubMed] [Google Scholar]
- 13. Bachert C, Mannent L, Naclerio RM et al Effect of subcutaneous dupilumab on nasal polyp burden in patients with chronic sinusitis and nasal polyposis: a randomized clinical trial. JAMA 2016; 315:469–79. [DOI] [PubMed] [Google Scholar]
- 14. Hirano I, Dellon ES, Hamilton JD et al Dupilumab efficacy and safety in adult patients with active eosinophilic oesophagitis: a randomised double‐blind placebo‐controlled phase 2 trial. United Eur Gastroenterol J 2017; 5:1138–50. [Google Scholar]
- 15. Barbier N, Paul C, Luger T et al Validation of the Eczema Area and Severity Index for atopic dermatitis in a cohort of 1550 patients from the pimecrolimus cream 1% randomized controlled clinical trials programme. Br J Dermatol 2004; 150:96–102. [DOI] [PubMed] [Google Scholar]
- 16. International Eczema Council . Validated Investigator Global Assessment Scale for Atopic Dermatitis (vIGA‐AD™). Available at: http://www.eczemacouncil.org/research/investigator-global-assessment-scale (last accessed 19 February 2019).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. Proportions of patients who, at week 16, rated the effects of treatment as ‘good’, ‘very good’ or ‘excellent’.
Fig S2. Proportions of patients who, at week 16, reported ‘no pain or discomfort’ on the EuroQol‐5D questionnaire.
Fig S3. Forest plot summary of results using the last observation carried forward method and observed value method.
Fig S4. Percentage change in Eczema Area and Severity Index score from baseline to week 16 according to Investigator's Global Assessment score at week 16.
Fig S5. Proportion of patients achieving ≥ 50% improvement in Eczema Area and Severity Index according to Investigator's Global Assessment score at week 16.
Fig S6. Change in peak pruritus numerical rating scale score from baseline to week 16 according to Investigator's Global Assessment score at week 16.
Fig S7. Change in Dermatology Life Quality Index score from baseline to week 16 according to Investigator's Global Assessment score at week 16.
Table S1 Demographics and baseline characteristics.
Table S2 Demographics and baseline characteristics within subsets of patients with Investigator's Global Assessment (IGA) ≤ 1 vs. IGA > 1 at week 16.
Table S3 Key outcomes at week 16 in patients who achieved Investigator's Global Assessment > 1.
Supplementary Info S1 Supplementary figure legends.
Supplementary Info S2 Plain Language Summary.
Video S1 Author video.
Powerpoint S1 Journal Club Slide Set.


