Summary
Meal replacements (MR) are generally not recommended in clinical guidelines for the management of obesity. The aim of this review is to provide an up‐to‐date systematic evaluation of the effect of weight loss interventions incorporating MR compared with alternative interventions on weight change at 1 year in adults with overweight or obesity. Six electronic databases were searched from inception to the end of August 2018 for randomized controlled trials comparing the effect of MR with interventions that did not include MR on weight at 1 year. We excluded studies using diets providing <3347 kJ/(800 kcal)/day and those which used total diet replacement (TDR) from this review. Risk of bias was assessed using the Cochrane risk of bias tool. Twenty‐three studies with 7884 adult participants were included. Six out of 23 studies were judged at low risk of bias across all domains, and 5/23 studies were judged at high risk of bias in at least one domain. Studies with similar intervention and comparators were grouped into five comparisons for analysis. Mean weight change at 1 year favoured the MR group relative to the control group in each comparison. In those comparisons where we conducted meta‐analysis, in people assigned to a diet incorporating MR, mean difference was −1.44 kg (−2.48 to −0.39 kg; I 2 = 38%) compared with alternative kinds of diets. In those assigned to a MR diet along with support, mean difference was −2.22 kg (−3.99 to −0.45, I 2 = 81%) compared with other diets with support and −3.87 kg (−7.34 to −0.40; I 2 = 60%) compared with other kinds of diet without support. In those assigned a MR diet with an enhanced level of support, mean difference was −6.13 kg (−7.35 to −4.91, I 2 = 19%) compared with alternative diets and regular support. Programmes incorporating meal replacements led to greater weight loss at 1 year than comparator weight loss programmes and should be considered as a valid option for management of overweight and obesity in community and health care settings.
Keywords: diet, meal replacement, obesity, weight loss
Abbreviations
- BOCF
Baseline observation carried forward
- HbA1c
Haemoglobin A1C
- HDL cholesterol
High‐density lipoprotein cholesterol
- LDL cholesterol
Low‐density lipoprotein cholesterol
- MR
Meal replacements
- RCT
Randomized controlled trial
1. INTRODUCTION
Obesity is a condition of excess accumulation of body fat that causes premature mortality. It also causes substantial morbidity, including significantly increased risks of type 2 diabetes, cardiovascular disease, and several nonsmoking‐related cancers, as well as physical impairments linked to excess weight such as breathlessness, joint problems, and back pain.1, 2 Nevertheless, the health risks of obesity are offset by weight loss,3, 4, 5, 6 with greater weight losses associated with greater health benefits.7 The prevalence of excess weight is high and rising throughout the world;8 thus, unless effective obesity treatment and prevention strategies are implemented, the increasing incidence of preventable diseases will place a growing burden on health systems and the global economy.9 There is a pressing need to identify effective interventions to treat obesity that can be delivered at scale.
Weight loss programmes that incorporate self‐management strategies, such as the use of meal replacements (MR), may be a particularly useful approach, as they can be delivered within the community, potentially without support by health care professionals. For the purposes of this review, we defined MR as discrete foods, food products, or drinks that are used to replace foods usually consumed at one or more meals with the intent to reduce daily energy intake for the purposes of achieving weight loss or weight maintenance following weight loss. MR programmes replace at least one meal per day and include at least one meal comprising conventional foods. This definition not only includes products purposefully marketed as MR for weight loss such as soups, shakes, and bars but also includes portion‐controlled ready meals as well as discreet portions of readily available conventional foods such as breakfast cereal or rice. This review does not include very‐low‐energy diets (VLED) providing less than 3347 kJ (800 kcal)/day, or those that use low‐energy total diet replacement (TDR), which use formula food products to replace all meals and snacks, but the impact of these types of programs on weight has been reviewed by others recently.10, 11
A previous systematic review and meta‐analysis of interventions that incorporated MR for weight loss, using a similar definition as here, identified only six studies of MR programmes versus comparator. It is reported that the use of MR significantly increased short‐term weight loss (3 months) compared with control. However, at 1 year, there was no statistically significant difference in weight change between the groups, though the point estimate favoured the intervention.12 Uncertainty over the long‐term effectiveness of MR may explain why many national guidelines do not reference MR,13, 14, 15, 16 or advise against their use17 for the routine management of overweight and obesity.
However, in recent years, several further studies of the effects of MR with longer term follow‐up have been conducted. We therefore set out to conduct an up‐to‐date systematic review of randomized controlled trials to examine the effectiveness of MR in people with overweight and obesity, compared with interventions that do not include MR, on weight loss at 1 year. The effects on cardiometabolic risk and any adverse effects were also investigated. We included interventions intended for the treatment of overweight or obesity and considered suitable for use in the community, without medical supervision.
2. METHODS
A protocol for this review was published in advance and implemented without changes.18
2.1. Search strategy
MEDLINE, Embase, PsycINFO, CINAHL, Web of Science, and the Cochrane Controlled Register of Trials (CENTRAL) were searched from database inception to August 2018. We also screened references from systematic reviews identified through our search and requested papers from authors for those we were unable to obtain or for which we had abstracts only. The searches were not restricted by country or language. The search strategy (MEDLINE) is included in Supplementary Table S1.
Studies were included if they recruited adults (≥18 years) with a body mass index (BMI) ≥25 kg/m2. Studies in pregnant women, people with eating disorders, those who had undergone bariatric surgery, or in those that included concomitant use of pharmacotherapies for the purposes of weight loss were excluded. We included randomized controlled trials of interventions incorporating the use of one or more MR daily, as part of a hypoenergetic diet intended for weight loss. We excluded interventions in which daily energy intake was restricted to <3347 kJ (800 kcal)/day, as these diets (VLEDs) require medical supervision and have been systematically reviewed previously.10 Studies that used formula TDR were also excluded. To be included, studies must have had as a comparator either a weight loss intervention that did not include MR or offered no or minimal intervention. Studies which compared different types of MR but did not include a control arm were excluded. Studies were required to report participants' weight at least 1 year after enrolment.
Covidence (Cochrane) was used for abstract and full‐text screening and data extraction.19 Data were extracted on weight and adverse events at all reported time points as well as on fasting glucose; insulin; HbA1c; total, LDL, and HDL cholesterol; triglycerides; and systolic and diastolic blood pressure at 1 year. Studies were excluded if they did not present sufficient information to allow data extraction or quality assessment and if this information was not available in a trial protocol or provided to us on request by the authors. Initially, titles and abstracts were assessed for inclusion by two independent reviewers with disagreements resolved by discussion or by a third reviewer. Full papers of the included studies were obtained and independently assessed for inclusion by two reviewers, with disagreements resolved by discussion or by consultation with a third reviewer.
2.2. Data extraction
Two reviewers independently used a prespecified and piloted data extraction form to characterize the population, intervention, control groups, and outcomes and to assess risk of bias. Disagreements were resolved by discussion or by a third reviewer. Authors were contacted for missing data and clarifications as required. Risk of bias was assessed using the Cochrane risk of bias tool,20 consistent with the methods used in previous reviews of weight loss interventions.21 If the outcome assessors were not blinded, we judged studies at low risk of detection bias where the outcome was objective (eg, body weight measured by study staff). Studies were judged at high risk of attrition bias if fewer than 50% of participants were measured at follow‐up or if there was a difference of ≥20% drop‐out rate between groups, for the primary outcome of weight at 1 year from baseline.
We categorized interventions including MR, and their comparators, to ensure that we compared effectiveness within meaningful groups. There were three types of active intervention. The first was stand‐alone dietary advice which recommended the use of MR to replace one or more eating occasions with the remainder of the diet provided by conventional food (MR diet). The second included interventions which recommended the use of MR and also provided participants with additional support to lose weight (MR diet + support). If the “support” offered was at a higher intensity than the support offered to the comparator group, we called this enhanced support, and this made up the third intervention group (MR diet + enhanced support). There were three types of comparators. The first comprised advice on dietary changes to aid weight loss that did not include the use of MR (diet only). The second was broader support for weight loss, which include advice on diet and other behavioural strategies, though the details may not be specified (diet + support). The third type of comparator intervention offered a nominal intervention for weight loss, either in the form of a printed leaflet or one‐off educational lecture (minimal intervention).
2.3. Analysis
Meta‐analyses were conducted in Review Manager 5.2 (Cochrane)22 to examine the difference between intervention and comparator groups for weight change at 1 year (primary outcome). Additionally, where available, we extracted interim weight change (~3 months, typically at the end of the main intervention) and weight change at 2 and 4 years after baseline. Further analyses were conducted to examine the changes in biomarkers of diabetes and cardiovascular risk at 1 year. Studies varied in whether and how they imputed data for participants lost to follow‐up, and as the practices adopted for this can impact on the reported effect size, we recalculated weight change using the method of baseline observation carried forward (BOCF) to reduce spurious heterogeneity.23
In studies that did not report baseline weight for the randomized participants, we assumed that there was no difference in baseline weight between those randomized and those who completed the study unless it was stated otherwise. In one study, it was unclear how many participants were lost to follow‐up in each group, and we assumed equal drop‐out for the purposes of the BOCF calculation.24 A sensitivity analysis was conducted removing this study from the comparison.
Because the definitive numbers of participants achieving ≥5% or ≥10% weight loss were inconsistently reported in the included papers, we estimated these numbers based on the mean and standard deviation of the reported weight loss, assuming a normal distribution as a post hoc analysis.
All meta‐analyses used a random effects model to account for differences in the intervention programmes and populations. Pooled results were calculated as mean differences in kilograms (kg) with 95% confidence intervals (CI) for continuous variables; weighted means were used to report individual group means. For the proportion of participants achieving ≥5% or ≥10% weight loss, pooled results are reported as odds ratio with 95% confidence intervals. The I 2 statistic was used to quantify statistical heterogeneity.25 We used Cochrane guidance for interpretation of I 2: 0% to 40% might not be important, 30% to 60% may represent moderate heterogeneity, 50% to 90% may represent substantial heterogeneity, and 75% to 100% considerable heterogeneity.20 Where a study contributed more than one intervention arm to a meta‐analysis, the control group was divided equally between interventions to avoid double counting in the pooled result.
In the preregistered protocol, we planned to use meta‐regression to investigate whether characteristics of the MR interventions impacted the observed effect. However, there were few studies in each meta‐analysis, and the data reporting the details of the programmes were limited such that we judged that the outcomes would not be robust, and this was omitted from our analysis.
3. RESULTS
The literature search identified a total of 2924 papers. One hundred eighteen full papers were retrieved, and 24 papers reporting the results of 23 randomized controlled trials (RCTs) with 8253 participants were eligible for inclusion. The main reason studies were excluded is that they did not report body weight at 1‐year follow‐up or longer, and this information could not be obtained from authors. For some studies, additional data were obtained from the authors.26, 27, 28 The PRISMA flow chart is displayed in Figure 1.
Figure 1.
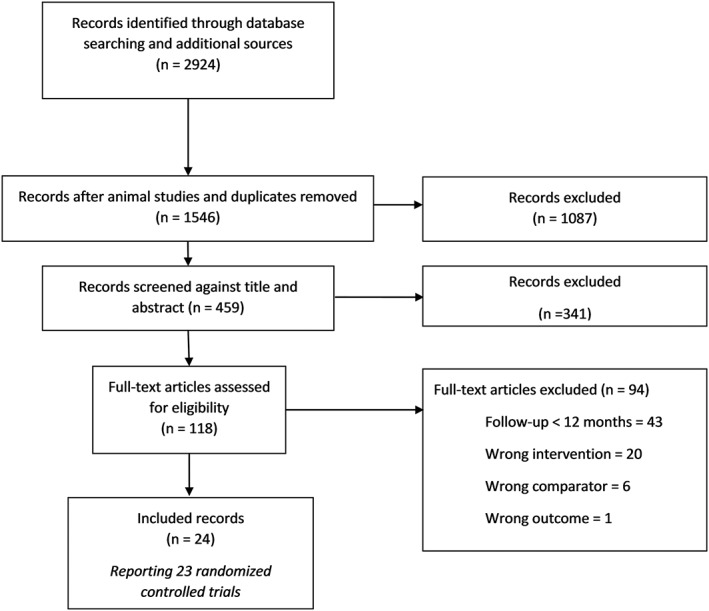
Prisma flow diagram
3.1. Characteristics of included studies
There were 4411 people allocated to an intervention including MR and 3852 people to a comparator in the included studies. One study was conducted in Australia, 1 in China, 3 in Germany, 1 in Malaysia, 1 in Singapore, 1 in Thailand, and 15 in the United States. Most studies were small; the median number of participants per study was 110 [IQR 92.5 to 165.5]. Over three quarters of participants were women, median 79.0% [IQR 54.2 to 94.2]; the mean participant age was 47.7 years [IQR 41.9 to 52.6], and mean baseline BMI was 34.5 kg/m2 [IQR 31.3 to 36.1] (Table 1). There was no significant difference in baseline body weight between intervention and control groups (mean ± SD; http://93.7.kg ± 12.5 and 98.4 ± 12.3 kg in MR and control groups, respectively).
Table 1.
Characteristics of included studies
| Study ID | Location | Funder | Population | Intervention | Comparator | Relevant Outcomes | Follow‐Up Rate |
|---|---|---|---|---|---|---|---|
| MR diet vs diet only | |||||||
| Ahrens 2003 | USA | SlimFast |
N = 95 BMI >25 kg/m2 87% Female |
12‐week weight loss: Replace 2 of 3 meals with a liquid MR plus one self‐selected meal of conventional foods 10‐week weight maintenance: 1 MR and 2 self‐selected meals of conventional foods |
12‐week weight loss: Self‐selected diet, based on diabetic exchange ~5021 kJ (1200 kcal)/day for women, 6276 kJ (1500 kcal)/day for men 10‐week weight maintenance: Healthy self‐selected diet and control of energy intake as desired |
Weight BMI |
20% (Not reported by group) |
| Cheskin 2008 | USA | Medifast Inc. |
N = 119 BMI 25‐40 kg/m2 Type 2 diabetes 56% Female |
34‐week weight loss: 25% energy deficit diet with 50%‐60% energy provided by MR suitable for diabetics, the remainder provided by self‐selected conventional foods. 52‐week weight maintenance: Rerandomized to either 26‐week MR followed by 26‐week control intervention, or 26‐week control intervention followed by 26‐week MR. |
34‐week weight loss: 25% energy‐deficit diet ADA recommendations 52‐week maintenance: Continued at a lower energy restriction for maintenance |
Weight BMI Body fat (%) Waist circumference Fasting glucose Insulin Total cholesterol Systolic blood pressure Diastolic blood pressure Triglycerides HbA1C Medication use |
MR: 57% Control: 29% |
| Ditschuneit 2001 | Germany | SlimFast |
N = 100 BMI 25‐40 kg/m2 79% Female |
3‐month weight loss: 2 MR + regular meal 48‐month weight maintenance: Replace 1 meal and 1 snack with MR. |
3‐month weight loss: Low‐energy diet of conventional foods providing 5021 to 6276 kJ (1200 to 1500 kcal)/day. Three meals (breakfast, lunch, and dinner) and two snacks were recommended. 48‐month weight maintenance: Replace 1 meal and 1 snack with MR. |
Weight Waist circumference Fasting glucose Insulin Total cholesterol LDL cholesterol Systolic blood pressure Diastolic blood pressure |
MR 84% Control: 72% |
| Khoo 2011 | Australia | National Heart Foundation Australia and Medical Benefits Foundation |
N = 31 BMI >30 kg/m2 WC >102 cm Type 2 diabetes 0% Female |
52‐week weight loss: 8 weeks: Energy deficit 2 MR + small meal of self‐selected conventional foods. 44 weeks: Switched to the control diet |
52‐week weight loss: High protein ~2510 kJ (600 kcal) energy‐deficit diet Based on the Commonwealth Scientific Industrial and Research Organisation (CSIRO) total well‐being diet |
Weight Waist circumference Plasma glucose Insulin Triglycerides LDL cholesterol HDL cholesterol |
MR: 47% Control: 58% |
| Khoo 2013 | Singapore | The SingHealth Foundation and Changi General Hospital |
N = 48 BMI >27.5 kg/m2 Men with erectile dysfunction 0% Female |
12‐week weight loss: ~1674 kJ (400 kcal) energy deficit comprising 2 liquid MR + self‐selected meal |
12‐week weight loss: Conventional diet ~1674 kJ (400 kcal) energy deficit |
Weight BMI Waist circumference Body fat (%) Plasma glucose Plasma insulin Systolic blood pressure Diastolic blood pressure |
MR: 58% Control: 54% |
| Rothacker 2001 | USA | Maryland Chapter of the Arthritis Foundation |
N = 75 BMI 25‐32 kg/m2 100% Female |
1‐year weight loss: Replace 1 or more meals with liquid MR |
1‐year weight loss: Low‐energy low‐fat diet of ~5021 kJ (1200 kcal)/day |
Weight Fat mass Lean body mass Fat (%) |
MR: 84% Control: 87% |
| MR diet + support vs diet + support | |||||||
| Ashley 2001 | USA | Slim fast |
N = 113 BMI 25‐50 kg/m2 Premenopausal women 100% Female |
1‐year weight loss: MR (dietician led): Small classes (8‐10 participants per class) Weekly for the first 3 months, biweekly for the next 3 months and monthly for the final 6 months. Diet consisting of replacing 2 of the 3 main meals (breakfast, lunch, or dinner) with MR shakes or bars. MR physician/nurse led: Brief (10/15 min) biweekly visits with a physician or nurse. Diet consisting of replacing 2 of the 3 main meals (breakfast, lunch, or dinner) with MR shakes or bars. |
1‐year weight loss: Small dietician‐led classes (8‐10 participants per class) Weekly for the first 3 months, biweekly for the next 3 months and monthly for the final 6 months. All meals and snacks prepared from self‐selected conventional foods ~5021 kJ (1200 kcal)/day using the US Department of Agriculture food guide pyramid. |
Weight Waist circumference Body fat (skinfold) Glucose Insulin Total cholesterol LDL cholesterol HDL cholesterol Triglycerides Systolic blood pressure Diastolic blood pressure |
65% overall (not reported by group) |
| Ashley 2007 | USA | SlimFast/Unilever |
N = 96 BMI 25‐35 kg/m2 100% Female |
1‐year weight loss: Replace 2 of 3 main meals with MR drinks or bars. Bimonthly classes for 6 months followed by monthly classes for 6 months. |
1‐year weight loss: Diet plan based of ~5021 kJ (1200 kcal)/day based on self‐selection of conventional foods for meals and snacks using the USDA food guide pyramid. Bimonthly classes of 6 months followed by monthly classes for a further 6 months |
Weight (kg) BMI Body fat (%) Waist circumference |
MR: 73% Control: 73% |
| Chaiyasoot 2018 | Thailand | Slimwell |
N = 110 BMI >25 Metabolic syndrome 83% Female |
12 weeks: Replace 2 main meals with one MR for the duration of the 12 week intervention. Group session at baseline followed by 4 individual sessions with dietician. |
12 weeks: Baseline group session and 4 individual sessions with dietitian who advised an energy restriction diet based on ~2092‐4184 kJ (500‐100 kcal)/day energy restriction. |
Weight (kg) |
MR: 72% Control:84% |
| Chee 2017 | Malaysia | Abbott Nutrition Malaysia |
N = 230 BMI >23 kg/m2 Type 2 diabetes 63% Female |
6 months MR (MI): Replace 1 or 2 meals with a diabetes‐specific formula meal replacement. Behavioural support provided using motivational interview. MR (CC): Replace 1 or 2 meals with a diabetes‐specific formula meal replacement. Behavioural support provided using conventional counselling. |
6 months: Followed the clinical care pathway of the Malaysian clinical practice guidelines for type 2 diabetes mellitus (2009) and received advice to follow a conventional low‐calorie diet plan 5021 kJ (1200 kcal) or 6276 kJ (1500 kcal)/day using normal foods with standard diabetes support and lifestyle education. |
Weight (kg) |
MR (MI): 88% MR (CC): 70% Control: 85% |
| Davis 2010 | USA | Medifast Inc. |
N = 90 BMI 30‐50 kg/m2 71% Female |
16‐week weight loss: 5 MR + a self‐selected meal. Biweekly consultations with a dietician for dietary and behavioural counselling. 24‐week weight maintenance: 2 meetings with dietician at 12‐week intervals. |
16‐week weight loss: ~4184 kJ (1000 kcal)/day diet plan plus multivitamin supplement. Biweekly consultations with a dietician for dietary and behavioural counselling. 24‐week weight maintenance: 2 meetings with dietician at 12‐week intervals. |
Weight (kg) Waist circumference Body fat (%) Total cholesterol HDL cholesterol LDL cholesterol Triglycerides Systolic BP Diastolic BP |
MR: 58% Control: 44% |
| Flechtner‐Mors 2010 | Germany | University of Ulm and Herbalife Inc. |
N = 100 Overweight and obese (BMI 27‐45 kg/m2), metabolic syndrome 80% Female |
3‐month weight loss: Diet instructions to follow diet high protein with energy deficit of ~2092 kJ (500 kcal)/day based on consuming 2 protein‐enriched MR plus one conventional meal and 2 snacks. 9‐month weight maintenance: 1 MR two conventional meals and two snacks |
3‐month weight loss: Diet instructions to follow diet with conventional protein content with energy deficit of ~2092 kJ (500 kcal)/day, 3 conventional meals 2 snacks 9‐month weight maintenance: 2 conventional meals 2 conventional snacks and 1 MR |
Weight BMI Body fat (%) Blood glucose Insulin HbA1c Total cholesterol HDL cholesterol Triglycerides Adverse events |
MR: 56% Control: 78% |
| Li 2005 | USA | Not disclosed‐meal replacements provided by SlimFast foods Co. Inc. |
N = 104 BMI 27‐40 kg/m2 Type 2 diabetics 62% Female |
1 year: Individual consultation with a dietician at baseline and weekly for 2 months, then monthly for the remainder First 5 days: 3 soy MR and advice to add fruits and vegetables to their intake Day 6‐3 months: 2 MR plus one meal of self‐selected foods 3 months‐1 year: 1 or 2 MR plus 2 sensible meals. |
1 year: Individual consultation with a dietician at baseline and weekly for 2 months, then monthly for the remainder. Individualized meal plan based on ADA exchanges, target reduction in intake of ~2092 kJ (500 kcal)/day. |
Weight BMI Glucose Insulin HbA1c Total cholesterol LDL cholesterol HDL cholesterol Triglycerides |
MR: 81% Control: 67% |
| Lowe 2018 | USA | National Institutes of Health (NIH) |
N = 181 BMI 27‐45 kg/m2 84% Female |
1 year: Weekly groups for 6 mo, then biweekly for 6 mo. During months 1‐6 participants were advised to replace 2 meals with MR product. In months 7‐12 switched to replacing 1 meal and 1 snack with MR product. |
1 year: Weekly groups for 6 mo, then biweekly for 6 mo. Following materials from lifestyle, exercise, attitude relationships and nutrition (LEARN) and diabetes prevention program manual. Focus on weight loss months 1‐6 and weight maintenance months 7‐12. |
Weight (kg) |
MR: 73% Control: 81% |
| Ptomey 2017 | USA | National Institutes of Diabetes, Digestive and Kidney Diseases (NIDDK) |
N = 150 BMI >25 kg/m2 Intellectual developmental disabilities 57% Female |
18‐month weight loss: Participants and study partners taught how to prepare and consume 2 portion‐controlled main meal MR and 2 MR shakes a day with additional meal of self‐selected conventional foods according to a guide. Health educators conducted monthly home visits. |
18‐month weight loss: Participants and study partners taught how to follow a 2092‐2929 kJ (500‐700 kcal)/day energy deficit diet following Myplate approach. Health educators conducted monthly home visits. |
Weight BMI Waist circumference |
MR: 69% Control: 65% |
| Rolls 2017 | USA | National Institutes of Diabetes, Digestive and Kidney Diseases (NIDDK) |
N = 124 BMI 28‐45 kg/m2 100% Female |
1‐year weight loss: Participants met individually with a dietician for 19 sessions. They were instructed to eat preportioned main dishes daily for lunch and dinner during months 1‐3 of the study and were encouraged to continue this practice subsequently. |
1‐year weight loss: Participants met individually with a dietician for 19 sessions. Instructed to follow US dietary guidelines emphasizing eating less whilst making healthy choices from all food groups. |
Weight Glucose HbA1c Total cholesterol LDL cholesterol HDL cholesterol Triglycerides Systolic blood pressure Diastolic blood pressure |
MR: 82% Control:82% |
| MR diet + support vs diet only | |||||||
| Rock 2007 | USA | Jenny Craig Inc. |
N = 70 BMI 25‐40 kg/m2 Minimum 15 kg overweight 100% F |
12‐month weight loss: Weekly one‐to‐one contacts with a counsellor, with follow‐up telephone and e‐mail contacts and web site/message board availability. Energy reduced diet with prepackaged prepared foods typically providing 3347 kJ (800 kcal)/day, with the remainder provided by self‐selected conventional food. A goal is 30 minutes of physical activity on 5 or more days of the week. |
1‐year weight loss: Consultation with a dietician at baseline and 16 weeks who provided a diet and physical activity plan to promote weight loss. |
Weight BMI Waist circumference Hip circumference Insulin Total cholesterol LDL cholesterol HDL cholesterol Triglycerides |
MR: 91% Control: 94% |
| Shikany 2013 | USA | Medifast Inc. |
N = 120 BMI 30‐50 kg/m2 |
26‐week weight loss: Quick start guide for dietary plan 5 MR (3347‐4184 kJ (800‐1000 kcal)/day) plus 1 self‐selected meal of conventional food. Online access to resources, dietician trainers, message boards, chat rooms. 26‐week weight maintenance: Energy intake to achieve weight maintenance with the option to include 0‐3 MR a day. |
26‐week weight loss: 4184 kJ (1000 kcal)/day mean plan based on self‐selected, self‐prepared conventional foods. Referral to the http://MyPyramid.gov website for nutritional information. Advised to take multivitamin supplement. 26‐week weight maintenance: Energy intake to achieve weight maintenance based on self‐selected conventional foods |
Weight BMI Waist circumference Fat mass Fat‐free mass Systolic BP Glucose Total cholesterol LDL cholesterol HDL cholesterol Triglycerides Diastolic BP |
MR: 83% Control: 75% |
| MR diet + enhanced support vs diet + support | |||||||
| Rock 2010 | USA | Jenny Craig Inc. |
N = 442 BMI >25 kg/m2 Minimum 15 kg overweight 100% Female |
2‐year weight loss: Weekly contacts for up to 2 years. Meal plan consisting of low‐fat reduced energy diet, with prepackaged foods providing up to 70% energy requirements, with remainder from self‐selected conventional foods. Group was split into 2 groups: Telephone advice: Counselling provided over the telephone or via email In‐person advice: Counselling provided face‐to face |
2‐year weight loss: Consultation with a research dietetic professional at baseline and 6 months. Provided with print material describing dietary and physical activity guidelines to promote weight loss. |
Weight BMI Waist circumference Total cholesterol LDL cholesterol HDL cholesterol Triglycerides |
MR (in person): 99% MR (telephone): 100% Control: 98% |
| Rock 2014 | USA | Jenny Craig Inc. |
N = 70 BMI 25‐40 kg/m2 Minimum 15 kg overweight 100% F |
9‐month weight loss: Months 1‐6: Three main meals and one to two snack MR provided for 7 days/week Months 7‐9: Three main meals and one to two snack MR provided for And for 5 days/week 3‐month weight maintenance: One main meal and one snack MR provided each day for use as desired. Weekly consultations with train counsellors were recommended during the first 9 months after which participants had the option to move to biweekly or monthly consultations. |
1‐year weight loss: Consultation at baseline and at 6 months with a research dietician who provided print materials on dietary and physical activity guidelines for weight loss and weight loss maintenance |
Weight BMI Waist circumference Total cholesterol LDL cholesterol HDL cholesterol Triglycerides Insulin |
MR: 91% Control: 94% |
| MR diet + support vs minimal intervention | |||||||
| Kempf 2017 | Germany | Almased |
N = 409 BMI ≥27 kg/m2 Poorly controlled type 2 diabetes (HbA1c ≥ 7.5%) 42% Female |
52 weeks: MR (stringent use of MR): Week 1: Replace 3 meals/day with MR and include 45 g oil rich omega 3 fatty acids and 750 mL vegetable juice. Weeks 2‐4: Replace 2 meals/day with MR and low‐carb other meals Weeks 5‐52: Replace 1 meal/day with MR MR (moderate use of MR): Week 1: Replace 2 meals/day with MR and include 45 g oil rich omega 3 fatty acids and 750 mL vegetable juice. Weeks 2‐4: Replace 2 meals/day with MR and low‐carb other meals Weeks 5‐52: Replace 1 meal/day with MR |
Usual care: Quarterly visits with physician for routine care as defined by the disease management programs (DMP) for type 2 diabetes in Germany. |
Weight (kg) Blood glucose Total cholesterol LDL cholesterol HDL cholesterol Triglycerides Systolic blood pressure Diastolic blood pressure |
MR (stringent):69% MR (moderate):70% Control:74% |
| Look Ahead Research Group 2007 | USA | NIH |
N = 5145 BMI >25 kg/m2 (BMI >27 kg/m2 if taking insulin) Type 2 diabetic 60% F |
1‐year weight loss: Months 1‐6: weekly session plus 3 group sessions per month Months 7‐12: biweekly group sessions plus one individual session. All sessions comprised of advice and support from dieticians, registered psychologists and exercise specialists to encourage >10% weight loss from initial body weight. |
Diabetes support and education: Diabetes education session and invited to attend 3 additional group sessions during the 1st year. |
Weight BMI Waist circumference HbA1c LDL cholesterol HDL cholesterol Triglycerides Systolic blood pressure Diastolic blood pressure |
MR: 97% Control: 96% |
| Xu 2013 | China | The Science and Technology Commission of Shanghai Municipality (07ZR14036) and the Public Health Bureau of Shanghai (2007168) |
N = 88 BMI >18.5 kg/m2 Impaired glucose regulation 52% F |
12‐week weight loss: Educational lecture on balanced diet, regular exercise, and behavioural strategies. Encouraged to follow the “2007 Chinese guidelines for the management of type 2 diabetes” and “Dietary Guidelines for Chinese” Weekly intensive lifestyle meetings and weekly medical evaluation by a physician. |
Educational lecture: Educational lecture on balanced diet, regular exercise, and behavioural strategies. Encouraged to follow the “2007 Chinese guidelines for the management of type 2 diabetes” and “Dietary Guidelines for Chinese” |
Weight BMI Waist circumference Total cholesterol HDL cholesterol LDL cholesterol Triglycerides Fasting plasma glucose HbA1c Systolic blood pressure Diastolic blood pressure |
MR: 89% Control: 95% |
Fourteen studies were conducted in people who were overweight but with no specific clinical condition,24, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39 five in people with type 2 diabetes,40, 41, 42, 43, 44 two in participants with metabolic syndrome or impaired glucose regulation,45, 46 one in men with type 2 diabetes and erectile dysfunction,47 and one in adults with developmental disabilities and obesity.48
From the 23 studies reporting weight change data at 1 year (Figure 2), 17 of these studies reported weight change at a point earlier than 1 year (Figure 3), which was collected at a median 18 weeks (range 8 to 34 weeks). Six studies also reported weight outcomes at 2 years (range 64 to 108 weeks) (Figure 4), and two studies reported weight outcomes at 4 years (range 204‐208 weeks) (Figure 5). Twelve studies reported information on biomarkers of cardiovascular and diabetes risk at 1 year (Table 3 and Figures S1‐S9).
Figure 2.
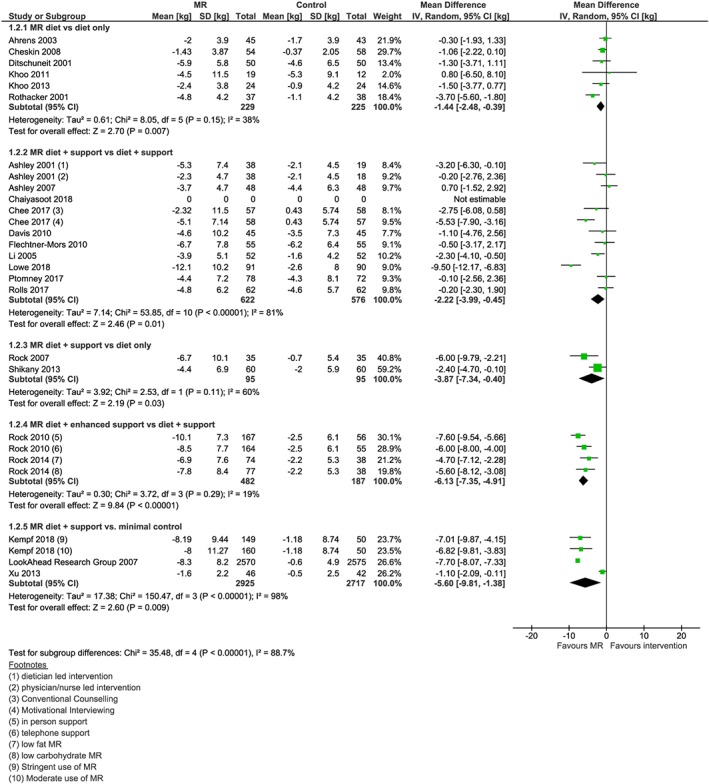
Forest plot of mean weight change (kg) from baseline at 1 year between interventions incorporating meal replacements (MR) and control interventions by comparison group [Colour figure can be viewed at http://wileyonlinelibrary.com]
Figure 3.
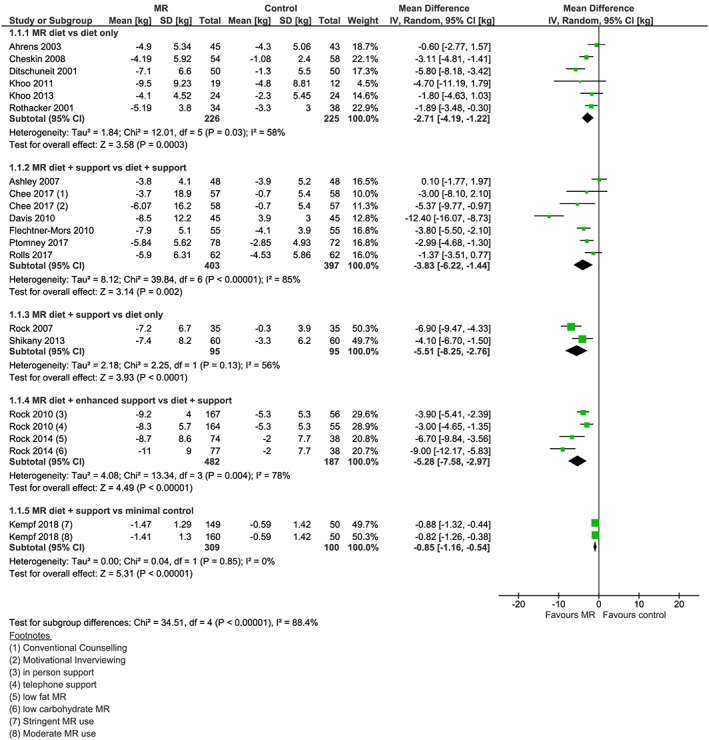
Forest plot of interim (>1 year) mean weight change (kg) from baseline between interventions incorporating meal replacements (MR) and control interventions by comparison group [Colour figure can be viewed at http://wileyonlinelibrary.com]
Figure 4.
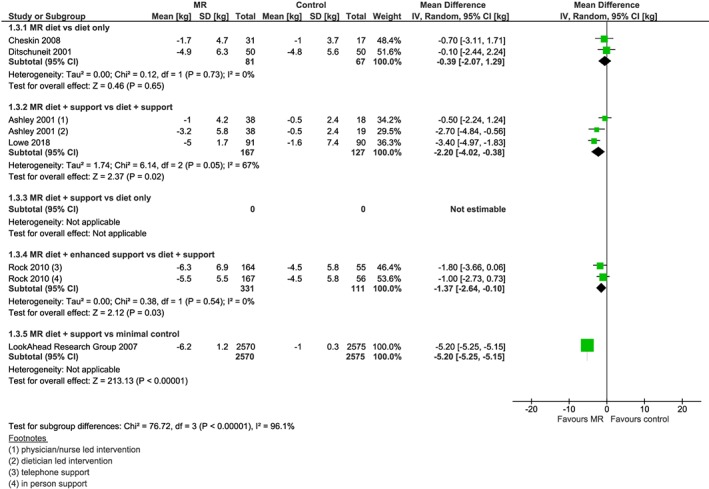
Forest plot of mean weight change (kg) from baseline at 2 years between interventions incorporating meal replacements (MR) and control interventions by comparison group [Colour figure can be viewed at http://wileyonlinelibrary.com]
Figure 5.
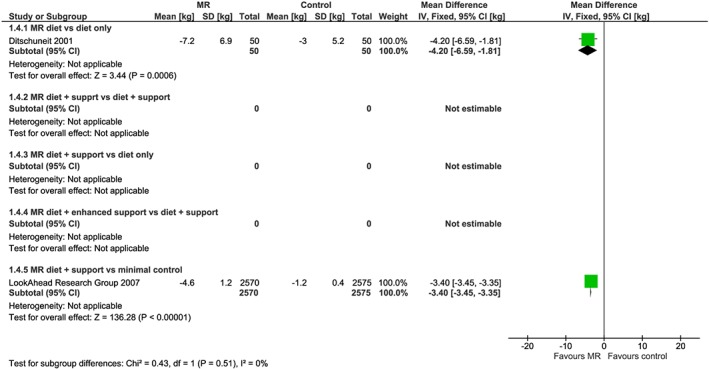
Forest plot of mean weight change (kg) from baseline at 4 years between interventions incorporating meal replacements (MR) and control interventions by comparison group [Colour figure can be viewed at http://wileyonlinelibrary.com]
Table 3.
Biomarkers of cardiovascular disease risk and glycaemic control
| Studies n | Participants n | Mean Difference | 95% CI | I 2 | |
|---|---|---|---|---|---|
| Fasting blood glucose, mmol/L | |||||
| MR diet vs diet only | 4 | 291 | −0.14 | −0.57, 0.29 | 79 |
| MR diet + support vs diet + support | 3 | 336 | −0.03 | −0.24, 0.19 | 29 |
| MR diet + support vs diet only | 1 | 120 | −0.07 | −0.2, 0.06 | ‐ |
| MR diet + enhanced support vs diet + support | 1 | 227 | −0.96* | −1.84, −0.07 | 73 |
| MR diet + support vs minimal intervention | 3 | 5822 | −0.60* | −0.85, −0.35 | 98 |
| Fasting insulin, pmol/L | |||||
| MR diet vs diet only | 4 | 291 | −12.37* | −17.93, −6.82 | 94 |
| MR diet + support vs diet + support | 3 | 336 | −6.9* | −7.74, −6.06 | 91 |
| MR diet + support vs diet only | 1 | 70 | −36.8* | −53.05, −20.55 | ‐ |
| MR diet + enhanced support vs diet + support | 1 | 227 | 1.25 | −40.32, 42.83 | 0 |
| MR diet + support vs minimal intervention | ‐ | ‐ | ‐ | ‐ | ‐ |
| HbA1c, %a | |||||
| MR diet vs diet only | 1 | 112 | −0.8* | −1.19, −0.41 | ‐ |
| MR diet + support vs diet + support | 2 | 214 | −0.18* | −0.22, −0.14 | 90 |
| MR diet + support vs diet only | ‐ | ‐ | ‐ | ‐ | ‐ |
| MR diet + enhanced support vs diet + support | 1 | 227 | −0.55* | −0.74, −0.36 | ‐ |
| MR diet + support vs minimal intervention | 3 | 5822 | −0.49* | −0.52, −0.46 | 81 |
| Total cholesterol, mmol/L | |||||
| MR diet vs diet only | 2 | 212 | −0.11* | −0.18, −0.03 | 0 |
| MR diet + support vs diet + support | 5 | 541 | −0.07* | −0.13, −0.01 | 23 |
| MR diet + support vs diet only | 2 | 190 | −0.05 | −0.18, 0.08 | 0 |
| MR diet + enhanced support vs diet + support | 2 | 669 | 0.04 | −0.03,0.12 | 0 |
| MR diet + support vs minimal intervention | 2 | 499 | −0.28 | −0.52, 0.05 | 91 |
| LDL cholesterol, mmol/L | |||||
| MR diet vs diet only | 2 | 143 | 0.11 | −0.37, 0.59 | 77 |
| MR diet + support vs diet + support | 4 | 429 | −0.14 | −0.29, 0.01 | 88 |
| MR diet + support vs diet only | 2 | 190 | −0.08 | −0.28, 0.12 | 62 |
| MR diet + enhanced support vs diet + support | 2 | 667 | 0.07 | −0.08, 0.21 | 67 |
| MR diet + support vs minimal intervention | 3 | 5642 | −0.12 | −0.45, 0.20 | 99 |
| HDL Cholesterol, mmol/L | |||||
| MR diet vs diet only | 3 | 243 | 0.00 | −0.03, 0.03 | 0 |
| MR diet + support vs diet + support | 4 | 426 | −0.01 | −0.07, 0.05 | 87 |
| MR diet + support vs diet only | 2 | 190 | 0.11 | −0.06, 0.28 | 89 |
| MR diet + enhanced support vs diet + support | 2 | 667 | 0.06 | 0.00, 0.11 | 62 |
| MR diet + support vs minimal intervention | 3 | 5822 | 0.06* | 0.05, 0.07 | 11 |
| Triglycerides, mmol/L | |||||
| MR diet vs diet only | 3 | 243 | −0.14 | −0.49, 0.21 | 75 |
| MR diet + support vs diet + support | 3 | 316 | 0.02 | −0.10, 0.15 | 77 |
| MR diet + support vs diet only | 2 | 190 | −0.09 | −0.17, 0.00 | 0 |
| MR diet + enhanced support vs diet + support | 2 | 669 | −0.22* | −0.43, −0.02 | 90 |
| MR diet + support vs minimal intervention | 2 | 5233 | 0.00 | −0.28, 0.29 | 99 |
| Systolic blood pressure, mmHg | |||||
| MR diet vs diet only | 2 | 212 | −8.30* | −11.83, −4.76 | 23 |
| MR diet + support vs diet + support | 4 | 435 | −0.33* | −0.6, −0.06 | 0 |
| MR diet + support vs diet only | 1 | 120 | −1.60 | −3.86, 0.66 | ‐ |
| MR diet + enhanced support vs diet + support | 1 | 227 | 0.34 | 0.15, 0.53 | ‐ |
| MR diet + support vs minimal intervention | 3 | 5642 | −3.76* | −4.18, −3.34 | 0 |
| Diastolic blood pressure, mmHg | |||||
| MR diet vs diet only | 3 | 260 | −2.81* | −5.01, −0.60 | 55 |
| MR diet + support vs diet + support | 3 | 325 | −1.23* | −2.42, −0.04 | 20 |
| MR diet + support vs diet only | 1 | 120 | 0.07 | −1.20, 1.34 | ‐ |
| MR diet + enhanced support vs diet + support | 1 | 227 | −0.58 | −3.28, 2.12 | 69 |
| MR diet + support vs minimal intervention | 3 | 5642 | −1.22* | −1.53, −0.90 | 11 |
HbA1C % units used cannot convert to mmol/mol.
Statistically significant P < 0.05.
3.2. Interventions and comparators
In 15 studies (65%), the MR used consisted of specially formulated shakes, soups, or bars.24, 27, 29, 30, 32, 33, 34, 37, 38, 39, 41, 43, 44, 45, 47 In four studies (17%), the MR consisted of prepackaged portion‐controlled meals which participants reconstituted or cooked at home,28, 35, 36, 42 and in four studies (17%), the MR was a combination of prepackaged portion‐controlled meals and specially formulated shakes, soups, or bars.31, 38, 40, 48
The median duration of the active weight loss intervention in all 23 included studies was 20 weeks [IQR 12 to 52 weeks]. Twelve studies also included a second MR phase for continued weight loss or weight maintenance where continued use of MR was recommended, generally at a reduced level. The median duration of this phase was 42 weeks [IQR 30 to 52 weeks].28, 30, 31, 32, 33, 34, 38, 42, 44, 46, 47, 48
During the weight loss phase, 13 studies advised participants to replace two eating occasions each day with a MR.24, 27, 28, 29, 30, 32, 33, 34, 41, 43, 44, 45, 47 One study advised participants to replace one eating occasion,39 and one, two, and four studies advised participants to replace three,37 four,26, 48 or five31, 38, 40, 42 eating occasions (meals or snacks) with one MR, respectively. Two studies (four intervention arms) did not specify the number of MR products participants were advised to consume, but rather recommended that participants replace a proportion of their daily energy intake with the MR products.35, 36
In six of the included studies, the active intervention consisted of advice to use and/or provision of MR products along with dietary guidance on the quality and quantity of food to be consumed in the remainder of the diet (MR diet).4, 12, 19, 33, 47, 49 In 15 studies, in addition to the MR diet, participants also received support for weight loss during the active intervention. This support consisted of 12 sessions [IQR 7 to 19] over 24 weeks, ranging from 12 to 52 weeks [IQR 12 to 52 weeks] (MR diet + support).27, 28, 29, 30, 31, 34, 36, 38, 39, 41, 43, 44, 45, 46, 48 In two studies (four intervention arms), an enhanced level of support was offered to the MR group (73 sessions [IQR 57.5 to 88.5 sessions] over 78 weeks [IQR 65 to 91 weeks]) compared with the support provided to the control group which consisted of two sessions [IQR 2 to 2] over 78 weeks [IQR 65 to 91 weeks]) (MR diet + enhanced support).35, 42
In eight studies, the control intervention was dietary advice or a diet plan providing guidance on what to eat to achieve weight loss (diet only).24, 32, 33, 36, 37, 38, 40, 47 In a further 12 studies, the control diet was combined with support for weight loss, consisting of 11.5 sessions [IQR 4.75 to 18.25] over 52 weeks ranging from 12 to 52 weeks [IQR 45 to 52 weeks] (diet + support).26, 27, 28, 29, 30, 31, 34, 35, 42, 43, 45, 48 In three studies, the control intervention consisted of an information leaflet, educational lecture, or usual care (minimal control).39, 41, 44 Accordingly, the included studies fell into one of five distinct comparisons for analysis:
A full explanation and examples of the interventions that make up the comparison groups are provided in Table S2. Details of the individual interventions in the included studies by comparison group are presented in Table 1.
3.3. Risk of bias
Summary risk of bias for the included studies is provided in Table 2, and justifications for any unclear of high judgments are provided in the supplementary material (Table S3). Six of the included studies were judged to be at low risk of bias across all domains.33, 38, 41, 44, 45, 48 Five studies were judged to be at high risk of bias in at least one domain,24, 26, 31, 34, 40 with all other studies judged to be at unclear risk of bias in at least one domain.27, 28, 29, 30, 32, 34, 35, 36, 37, 39, 40, 42, 47 Twelve studies were judged to be at low risk of sequence generation bias,28, 32, 33, 34, 38, 40, 41, 42, 43, 44, 45, 48 with the remaining studies at unclear risk,24, 26, 27, 29, 30, 31, 35, 36, 37, 39, 47 mainly due to insufficiently detailed reporting. Six studies were judged to be at low risk of allocation concealment bias,33, 38, 41, 44, 45, 48 with the other 17 studies judged to be at unclear risk of this bias because they did not report whether allocation was concealed or not. Five studies were judged to be at high risk of attrition bias,24, 31, 34, 40, 46 mainly because of unequal follow‐up rates between groups, with all other studies judged to be at low risk of attrition bias (Table 3).27, 28, 29, 30, 32, 33, 35, 36, 37, 38, 39, 41, 42, 43, 44, 45, 47, 48
Table 2.
Risk of bias for included studies
| Sequence Generation | Allocation Concealment | Blinding of Participants and Personnel | Incomplete Outcome Data | Other Sources of Bias | |
|---|---|---|---|---|---|
| MR diet vs. diet‐only | |||||
| Ahrens 2003 | Unclear | Unclear | Low | High | Low |
| Cheskin 2008 | Low | Unclear | Low | High | Low |
| Ditschuneit 2001 | Low | Unclear | Low | Low | Low |
| Khoo 2011 | Unclear | Unclear | Low | Low | Low |
| Khoo 2013 | Low | Low | Low | Low | Low |
| Rothacker 2001 | Unclear | Unclear | Low | Low | Low |
| MR diet + support vs diet + support | |||||
| Ashley 2001 | Unclear | Unclear | Low | Low | Low |
| Ashley 2007 | Unclear | Unclear | Low | Low | Low |
| Chaiasoot 2017 | Low | Low | Low | Low | Low |
| Chee 2018 | Low | Unclear | Low | Low | Low |
| Davis 2010 | Unclear | Unclear | Low | High | Low |
| Flechtner‐Mors 2010 | Unclear | Unclear | Low | High | Low |
| Li 2005 | Low | Unclear | Low | High | Low |
| Lowe 2018 | Unclear | Unclear | Low | Low | Low |
| Ptomey 2017 | Low | Low | Low | Low | Low |
| Rolls 2017 | Low | Unclear | Low | Low | Low |
| MR diet + support vs diet only | |||||
| Rock 2007 | Unclear | Unclear | Low | Low | Low |
| Shikany 2013 | Low | Low | Low | Low | Low |
| MR diet + enhanced support vs diet + support | |||||
| Rock 2010 | Unclear | Unclear | Low | Low | Low |
| Rock 2014 | Low | Unclear | Low | Low | Low |
| MR diet + support vs minimal control | |||||
| Kempf 2017 | Low | Low | Low | Low | Low |
| Look Ahead 2007 | Low | Low | Low | Low | Low |
| Xu 2013 | Unclear | Unclear | Low | Low | Low |
3.4. Weight change
Twenty‐three studies were included in the primary analysis (weight change at 1 year) across the five comparisons, defined based on intervention and comparator type (Figure 2). Where reported, the difference in weight change reported earlier than 1 year consistently favoured the MR treatment (Figure 3). Results for 1 year or longer follow‐up are reported narratively below. Odds ratios of achieving weight change ≥5% and ≥10% baseline weight at 1 year were calculated for each comparison (Table 4).
Table 4.
Estimated odds of achieving weight change ≥5% and ≥10% lower than baseline weight at 1 year in MR group compared with control group by comparison
| ≥5% Weight Loss | ≥10% Weight Loss | |
|---|---|---|
| OR [95% CI] | OR [95% CI] | |
| MR diet vs diet only | 2.83* [1.37, 5.86] I 2 = 40 | 1.73 [0.92, 3.26] I 2 = 0 |
| MR diet + support vs diet + support | 1.49* [1.08, 2.06] I 2 = 44 | 1.80* [1.12, 2.87] I 2 = 56 |
| MR diet + support vs diet only | 2.83* [1.37, 5.86] I 2 = 25 | 5.95* [2.12, 16.67] I 2 = 1 |
| MR diet + enhanced support vs diet + support | 4.32* [3.01, 6.20] I 2 = 0 | 6.63* [4.01, 10.94] I 2 = 0 |
| MR diet + support vs minimal control | 4.03* [1.87, 8.69] I 2 = 82 | 8.32* [2.02, 34.16] I 2 = 93 |
Statistically significant P < 0.05.
3.4.1. MR diet vs diet only
Six studies compared a MR diet with diet only.24, 32, 33, 37, 40, 47 Mean weight change among participants randomized to an intervention incorporating the use of MR (n 229) was −3.0 kg at 1 year compared with participants in the diet only intervention (n 225) who had weight change of −1.5 kg (mean difference = −1.44 kg; 95% CI −2.48, −0.39, I 2 = 38%)24, 32, 33, 37, 40, 47 (Figure 2).
Two of the studies in this comparison also reported weight change at 2 years32, 40 where mean weight change in participants randomized to the MR diet intervention (n 81) was −3.4 kg while those randomized to the diet only intervention (n 67) lost −3.0 kg (mean difference = −0.39 kg; 95% CI −2.07, 1.29, I 2 = 0%) (Figure 4). One of these studies32 reported that 4 years after randomization, mean weight change in those allocated to the MR diet intervention (n 50) was −7.2 kg, while in the comparator group (n 50), it was −3.0 kg (mean difference = −4.2 kg; 95% CI −6.59, −1.81), reflecting continued weight loss in the MR group but little change in weight since 1 year in the comparator group (Figure 5).
3.4.2. MR diet + support vs diet + support
Ten studies (12 intervention arms and 10 control arms) were included in the MR diet + support versus diet + support comparison.27, 28, 29, 30, 31, 34, 43, 45, 46, 48 One study was omitted from the meta‐analysis as weight data were not normally distributed, were reported in the paper as median and interquartile ranges, and we could not obtain further data.45 In the nine studies included in the meta‐analysis, mean weight change among participants randomized to a MR diet plus support programme (n 622) was −2.4 kg at 1 year, compared with −1.34 kg in a comparator group (n 576) with a diet but no MR and a similar support programme (mean difference = −2.22 kg; 95% CI −3.99 to −0.45, I 2 = 81%) (Figure 2). Statistical heterogeneity was high, but attributable to one small outlier,27 which showed greater weight loss favouring the intervention. In the study omitted from the meta‐analysis, weight change was consistent with the other included studies, in that the median weight change was greater in the MR group (median −1.8 kg, IQR −4.13, 0.43) compared with the control group (median −0.8 kg, IQR −4.0, 1.05).45 Two of the included studies also reported weight at 2 years,27, 30 with weight change favouring MR over control interventions (mean difference −2.2 kg, 95% CI −4.02 to −0.38, I 2 = 67%) (Figure 4).
3.4.3. MR diet + support vs diet only
Two studies compared interventions involving a MR diet and support programme (n 95) with a diet‐only comparator (n 95).36, 38 Mean weight change at 1 year was −5.3 kg for the MR diet + support and −1.5 kg in the diet only group (mean difference = −3.87 kg; 95% CI −7.34 to −0.40, I 2 = 60%) (Figure 2). Neither of these studies reported weight beyond 1 year.
3.4.4. MR diet + enhanced support vs diet + support
Two studies tested a MR diet together with an enhanced support programme.35, 42 At 1 year, mean weight change among participants randomized to MR diet plus enhanced support (n 482) was −8.5 kg while among those receiving the diet + support (n 187), it was −2.4 kg (mean difference = −6.13 kg; 95% CI −7.35, to −4.91, I 2 = 19%) (Figure 2). One study (two intervention arms) followed up participants at 2 years,35 with weight change (−5.5 kg MR with in person support, −6.3 kg MR with telephone support, and −4.5 kg in control group) continuing to favour the MR diet (n 331) over the comparator (n 111) (mean difference = −1.37 kg; 95% CI −2.64 to −0.10) (Figure 4).
3.4.5. MR diet + support vs minimal intervention
Three studies (four intervention arms) compared a MR diet plus support with a minimal intervention control.39, 41, 44 Statistical heterogeneity was considerable (I 2 = 98%); therefore, we do not present pooled results here. In all three studies, participants in the intervention arm lost significantly more weight than those in the control arm at 1 year. In a study in China (n = 88), the mean difference was −1.10 kg (95% CI −2.09 to −0.11), favouring the MR group. In a study in Germany (n = 409), mean difference in weight change from baseline was −7.01 kg (95% CI −9.87, to −4.15) in the moderate use of MR group and −6.82 kg (95%CI −9.81 to −3.83) in the stringent use of MR groups compared with control group, respectively.44 In the Look Ahead study in the USA (n = 5145), mean difference in weight change between participants in the MR diet + support group compared with the control (usual care) group was −7.70 kg (95% CI −8.07 to −7.33) (Figure 2). In the latter study, the mean difference in weight change was also significantly greater for MR diet plus support group at 2 years (mean difference = −5.20 kg, 95% CI −5.25 to −5.15) and 4 years (mean difference −3.4 kg, 95% CI −3.45 to −3.35) (Figures 4 and 5). The other two studies did not report weight change beyond a year.
3.5. Participants achieving >5% and >10% weight loss at 1 year
For each comparison, we calculated the odds ratio that participants would achieve a weight loss ≥5% and ≥10% from baseline at 1 year in the MR group compared with the control (Table 4).
For weight change of ≥5% baseline weight, all comparisons showed that the odds of achieving this were statistically significantly greater in the MR group compared with control. For weight change ≥10%, four out of the five showed that participants allocated to MR intervention had statistically greater odds of achieving the cut‐point compared with those assigned to the control.
3.6. Biochemical outcomes
There were very limited data on biochemical outcomes with only 12 studies reporting at least one biochemical outcome.28, 32, 33, 34, 38, 39, 40, 41, 42, 44, 46, 47 Across all studies that reported outcomes, results consistently favoured meal replacement groups for HbA1C. For the results for all other biochemical outcomes (glucose, insulin, lipids, and blood pressure), results were mixed and rarely reached statistical significance. Further detail can be found in Table 3 and Figures S1 to S9.
3.7. Adverse events
Only two studies reported information on adverse events (AE).26, 41 Neither of these studies gave details on the total number of AEs reported by intervention arm, but the Look Ahead Research Group 2007 reported that there were no between‐group differences in the frequency of hypoglycaemia, fractures, amputations, congestive heart failure, or occurrence of gallstones among 5145 participants.50 Flechtner‐Mors et al reported that there were no adverse events that could be attributed to the intervention.26 We also considered nutritional deficiencies to be an AE. Five studies assessed diet quality during the interventions, and these studies reported that diet quality was improved in those randomized to MR.29, 30, 32, 46, 48
4. DISCUSSION
The use of discrete foods, food products, or drinks a replacement for usual foods on some occasions during the day leads to significantly greater weight loss at 1 year than comparator interventions based wholly on conventional foods, with commensurate improvements in HbA1c and mixed findings for markers of diabetes and cardiovascular disease risk. All of the included studies individually reported that interventions including MR products were either equally as effective or superior to the comparator interventions for interim weight change as well as weight change at 1 year. Only two studies reported outcomes at 4 years, but both reported that weight change from baseline to 4 years favoured the MR groups over control. Only two studies assessed adverse events; there was no evidence from these studies of any excess adverse events associated with the use of MR products.26, 41
A strength of this review is the rigorous methodology, including use of a consistent approach to address missing data (BOCF).23 Given the scope of this review, the included studies are inherently clinically diverse. We tried to overcome this by grouping studies according to a predefined protocol to offer a meaningful insight into some of the variation in weight losses seen in studies using different treatment or comparator protocols. However, there is still moderate to substantial statistical heterogeneity in some of the groups. It is plausible that the effect of MR on weight could be affected by the factors that vary between studies, most obviously variations between interventions (nature, frequency, duration, or composition of the MR used) or differences in participant characteristics. However, there were insufficient studies in each comparison to fully explore study characteristics which may explain the observed heterogeneity between studies through meta‐regression or subgroup analyses.
For each domain of bias, the majority of studies were judged at low or unclear risk of bias. Five studies were judged at high risk of attrition bias.24, 31, 34, 40, 46 These studies were judged at high risk of bias because fewer than 50% of participants were measured at follow‐up or if there was a difference of ≥20% drop‐out rate between groups, and in these cases, it is possible that there is a plausible intervention effect among participants with missing data.
We endeavoured to identify unpublished studies by searching study registries and grey literature and contacting experts in the field, but we cannot rule out publication bias. We are not aware of unpublished studies with negative results, but given the nature of this research and the fact that many studies were funded or supported by industry, it is possible that smaller studies with nonsignificant results may be less likely to be published. Reporting of the content of the interventions was poor, and there were insufficient studies within each comparison to enable us to conduct a meta‐regression to identify the effective components of the interventions. There was no information reported on the cost‐effectiveness of these programmes or the costs to individuals compared with diets based on conventional food.
This review expands on the indicative findings of a previous review.12 By including more studies, and reducing the confidence interval around the point estimate, the present findings demonstrate that the weight loss at 1 year in interventions that include MR products is statistically significantly greater than in interventions that do not use these products. Other recent reviews have included weight loss interventions incorporating meal replacements.49, 51 but these reviews have considered the use of meal replacements alongside other weight loss interventions, and have therefore been unable to determine the specific effect of meal replacements per se. TDR have also been reviewed in various populations.10, 11, 52 Although TDR products are like MR in that they are designed to replace usual food‐based meals, TDR products are formulated to be used as the sole source of nutrition, that is, to replace all food in the diet. MR aims to replace one or more usual meals, but the instructions for use suggest eating a food‐based meal at least once a day to ensure adequate intake of nutrients and vitamins. MR can be purchased over the counter and used without guidance from a health professional, whereas weight loss programmes incorporating TDR are advised to be undertaken under supervision. Given these differences between MR and TDR programmes, it is important to consider their effectiveness independently.
The key finding of this review is that participants assigned to a MR diet compared with a diet only approach (akin to a self‐directed weight loss attempt) lose an additional 1.44 kg at 1 year, and this difference appears to be maintained up to 4 years. Likewise, many people join behavioural weight loss programmes to provide support to lose weight. The results of the present study suggest that all other things being equal, incorporating meal replacement products into such behavioural weight loss programmes enhances their effect (reflected in the MR diet + support versus diet + support comparison), with a 2.22 kg greater weight loss at 1 year in the MR group. Although these differences may appear to be modest, currently around 70% of the UK population with a BMI of 30 kg/m2 are trying to lose weight,53 and a sustained weight loss of even 1 kg will bring substantial benefits to public health.54, 55 Moreover, since the comparative interventions also lead to weight loss, by and large incorporating MR into weight loss programmes increases the proportion of participants who achieve ≥5% and ≥10% weight loss at 1 year.
Meal replacement products are consumer food products (as opposed to medical devices or drugs), widely available to be purchased over the counter without prescription, but at present are infrequently used by those attempting weight loss in their weight loss attempts.56 Advice and guidance provided by clinicians to encourage their use could deliver benefits at minimal cost to health care providers. Few studies included in the review reported adverse events, including the impact on the nutritional quality of the data. Two studies found no evidence of adverse events arising from the use of these products,26, 41 and five studies report that they may improve the overall nutritional quality of the diet.5, 6, 18, 19, 35
The greater weight loss observed in programmes incorporating MR suggests that this approach makes it easier to adhere to a reduced energy diet. However, there has been little detailed study of eating behaviours. It is possible that these fixed‐energy, portion‐controlled, or prepacked foods contain less energy than most self‐selected meals and snacks, or that the structure and external control associated with their use facilitate adherence. Further work could usefully examine the behavioural processes that facilitate weight loss which would be of use when designing effective weight loss programmes to enhance effectiveness and reach.
These findings provide important new evidence to inform clinical guidelines. At present in many countries, clinical recommendations for the treatment of obesity advise that individuals attempting weight loss should be advised to aim for an energy deficit of 2092 to 4184 kJ/day (500‐1000 kcal), but they do not suggest that MR could be used to help individuals achieve this deficit nor do they recommend MR as an effective dietary strategy for weight loss.13, 15 Guidelines in some countries do refer to meal replacement programmes. In the United States, the guidelines state that the strength of evidence on the longer term effect on weight of MR is low.57 Singaporean guidelines note that meal replacements may induce greater acute reductions in weight but are not advised for the long‐term management of overweight and obesity.17 Australian guidelines do not recommend meal replacements for weight loss beyond their use as part of a VLED.16
In conclusion, this review provides evidence that MR is an effective intervention for the treatment of overweight and obesity at a year, especially when used together with a support programme. MR could be recommended for inclusion in weight management programmes offered by professionals or as part of a self‐management strategy for people with overweight or obesity.
CONFLICTS OF INTEREST
NMA, PA, and SAJ are investigators on a research grant from Cambridge Weight Plan UK Ltd. PA and SAJ are investigators on a research grant from Weight Watchers Inc.
Supporting information
Table S1: Example of search strategy (MEDLINE)
Table S2: Descriptions of the intervention and control groups in each of the comparisons
Table S3: Justification of studies judged at high or unclear risk of bias in one or more domain
Figure S1: Forest plot of mean change in fasting blood glucose concentrations (mmol/L) from baseline to 1 year between interventions incorportaing meal replacments (MR) and control interventions
Figure S2: Forest plot of mean change in fasting serum insulin concentrations (pmol/L) from baseline to 1 year between interventions incorportaing meal replacments (MR) and control interventions
Figure S3: Forest plot of mean change in HbA1c (%) from baseline to 1 year between interventions incorportaing meal replacments (MR) and control interventions
Figure S4: Forest plot of mean change in total cholesterol concentrations (mmol/L) from baseline to 1 year between interventions incorportaing meal replacments (MR) and control interventions
Figure S5: Forest plot of mean change in LDL cholesterol concentrations (mmol/L) to 1 year between interventions incorportaing meal replacments (MR) and control interventions
Figure S6: Forest plot of mean change in HDL cholesterol concentrations (mmol/L) from baseline to 1 year between interventions incorportaing meal replacments (MR) and control interventions
Figure S7: Forest plot of mean change in triglyceride concentrations (mmol/L) from baseline to 1 year between interventions incorportaing meal replacments (MR) and control interventions
Figure S8: Forest plot of mean change in systolic blood pressure (mmHg) from baseline to 1 year between interventions incorportaing meal replacments (MR) and control interventions
Figure S9: Forest plot of mean change in diastolic blood pressure (mmHg) from baseline to 1 year between interventions incorportaing meal replacments (MR) and control interventions
ACKNOWLEDGEMENTS
This review was funded by National Institute of Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care Oxford at Oxford Health NHS Foundation Trust. NMA, JHB, PA, and SAJ are supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care.
Astbury NM, Piernas C, Hartmann‐Boyce J, Lapworth S, Aveyard P, Jebb SA. A systematic review and meta‐analysis of the effectiveness of meal replacements for weight loss. Obesity Reviews. 2019;20:569–587. 10.1111/obr.12816
REFERENCES
- 1. World Health Organization , Global health risks: mortality and burden of disease attributable to selected major risks. 2009.
- 2. Butland B, Jebb SA, McPherson K, et al. Tackling obesities: foresight report. 2007, Government Office for Science
- 3. Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34(7):1481‐1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Knowler WC, Barrett‐Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Birks S, Peeters A, Backholer K, O’Brien P, Brown W. A systematic review of the impact of weight loss on cancer incidence and mortality. Obes Rev. 2012;13(10):868‐891. [DOI] [PubMed] [Google Scholar]
- 6. Christensen R, Bartels EM, Astrup A, Bliddal H. Effect of weight reduction in obese patients diagnosed with knee osteoarthritis: a systematic review and meta‐analysis. Ann Rheum Dis. 2007;66(4):433‐439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Poobalan A, Aucott L, Smith WCS, et al. Effects of weight loss in overweight/obese individuals and long‐term lipid outcomes—a systematic review. Obes Rev. 2004;5(1):43‐50. [DOI] [PubMed] [Google Scholar]
- 8. Seidell JC. Prevalence and time trends of obesity in Europe. J Endocrinol Invest. 2002;25(10):816‐822. [DOI] [PubMed] [Google Scholar]
- 9. Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011;378(9793):815‐825. [DOI] [PubMed] [Google Scholar]
- 10. Parretti HM, Jebb SA, Johns DJ, Lewis AL, Christian‐Brown AM, Aveyard P. Clinical effectiveness of very‐low‐energy diets in the management of weight loss: a systematic review and meta‐analysis of randomized controlled trials. Obes Rev. 2016;17(3):225‐234. [DOI] [PubMed] [Google Scholar]
- 11. w?>Leslie WS, Taylor R, Harris L, Lean MEJ. Weight losses with low‐energy formula diets in obese patients with and without type 2 diabetes: systematic review and meta‐analysis. Int J Obes (Lond). 2017;41(1):96‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heymsfield SB, van Mierlo CAJ, van der Knaap HCM, Heo M, Frier HI. Weight management using a meal replacement strategy: meta and pooling analysis from six studies. Int J Obes Relat Metab Disord. 2003;27(5):537‐549. [DOI] [PubMed] [Google Scholar]
- 13. National Institute for Health and Clinical Excellence (NICE) . Obesity: Identification, Assesment and Management. London; 2014. [Google Scholar]
- 14. Guidelines for managing overweight and obesity in adults. Obesity. 2014;22(S2):i‐xvi. [Google Scholar]
- 15. Malaysia, M.o.H . Clinical practice guidelines on the management of obesity. 2004. [cited 2018 4th July]; Available from: http://www.moh.gov.my/penerbitan/CPG2017/3932.pdf.
- 16. National Health & Medical Research Council . Clinical Practice Puidelines for the Management of Overweight & Obesity In Adults, Adolescents & Children in Australia (N57). Melbourne; 2013. [Google Scholar]
- 17. MOH, H . Obesity Clinical Practice Guidelines 1/2016. Singapore; 2016. [Google Scholar]
- 18. Astbury NM, J.S., Hartmann‐Boyce J, Piernas‐Sanchez C. A Systematic Review and Meta‐Analysis on the Effect of Using Meal Replacements on Weight Loss in Overweight and Obese Adults. 2016; PROSPERO: International prospective register of systematic reviews:[Available from: http://www.crd.york.ac.uk/PROSPERO/display_record.asp? ID=CRD42016027048. [Google Scholar]
- 19. Covidence systematic review software. Available from: http://www.covidence.org.
- 20. Cochrane Handbook for Systematic Reviews of Interventions. 2011. updated March 2011; Version 5.1.0 [Available from: http://www.cochrane-handbook.org.
- 21. Hartmann‐Boyce J, Johns D, Jebb S, Aveyard P, Behavioural Weight Management Review Group . Effect of behavioural techniques and delivery mode on effectiveness of weight management: systematic review, meta‐analysis and meta‐regression. Obes Rev. 2014;15(7):589‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. The Cochrane Collaboration . Review Manager (RevMan). Copenhagen: The Nordic Cochrane Centre. p. Computer Programme; 2014. [Google Scholar]
- 23. Kaiser KA, Affuso O, Beasley TM, Allison DB. Getting carried away: a note showing baseline observation carried forward (BOCF) results can be calculated from published complete‐cases results. Int J Obes (Lond). 2012;36(6):886‐889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ahrens RA, Hower M, Best AM. Effects of weight reduction interventions by community pharmacists. J Am Pharm Assoc. 2003;43(5):583‐589. [DOI] [PubMed] [Google Scholar]
- 25. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21(11):1539‐1558. [DOI] [PubMed] [Google Scholar]
- 26. Flechtner‐Mors M, Ditschuneit HH, Johnson TD, Suchard MA, Adler G. Metabolic and weight loss effects of long‐term dietary intervention in obese patients: four‐year results. Obes Res. 2000;8(5):399‐402. [DOI] [PubMed] [Google Scholar]
- 27. Lowe MR, Butryn ML, Zhang F. Evaluation of meal replacements and a home food environment intervention for long‐term weight loss: a randomized controlled trial. Am J Clin Nutr. 2018;107(1):12‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rolls BJ, Roe LS, James BL, Sanchez CE. Does the incorporation of portion‐control strategies in a behavioral program improve weight loss in a 1‐year randomized controlled trial? Int J Obes (Lond) (2005). 2017;41(3):434‐442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ashley JM, Herzog H, Clodfelter S, Bovee V, Schrage J, Pritsos C. Nutrient adequacy during weight loss interventions: a randomized study in women comparing the dietary intake in a meal replacement group with a traditional food group. Nutr J. 2007;6(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ashley JM, Jeor STS, Perumean‐Chaney S, Schrage J, Bovee V. Meal replacements in weight intervention. Obes Res. 2001;9(Suppl 4):312S‐320S. [DOI] [PubMed] [Google Scholar]
- 31. Davis LM, Coleman C, Kiel J, et al. Efficacy of a meal replacement diet plan compared to a food‐based diet plan after a period of weight loss and weight maintenance: a randomized controlled trial. Nutr J. 2010;9(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ditschuneit HH, Flechtner‐Mors M. Value of structured meals for weight management: risk factors and long‐term weight maintenance. Obes Res. 2001;9(Suppl 4):284S‐289S. [DOI] [PubMed] [Google Scholar]
- 33. Khoo J, Chen RYT, Cheong M, et al. Comparing effects of meal replacements with isocaloric diet plan on voiding and storage lower urinary tract symptoms, insulin resistance and nutrient intake in obese men. Obes Facts. 2013;6:103‐104.23493065 [Google Scholar]
- 34. Li Z, Hong K, Saltsman P, et al. Long‐term efficacy of soy‐based meal replacements vs an individualized diet plan in obese type II DM patients: relative effects on weight loss, metabolic parameters, and C‐reactive protein. Eur J Clin Nutr. 2005;59(3):411‐418. [DOI] [PubMed] [Google Scholar]
- 35. Rock CL, Flatt SW, Sherwood NE, Karanja N, Pakiz B, Thomson CA. Effect of a free prepared meal and incentivized weight loss program on weight loss and weight loss maintenance in obese and overweight women: a randomized controlled trial. JAMA. 2010;304(16):1803‐1810. [DOI] [PubMed] [Google Scholar]
- 36. Rock CL, Pakiz B, Flatt SW, Quintana EL. Randomized trial of a multifaceted commercial weight loss program. Obesity (Silver Spring). 2007;15(4):939‐949. [DOI] [PubMed] [Google Scholar]
- 37. Rothacker DQ, Staniszewski BA, Ellis PK. Liquid meal replacement vs traditional food: a potential model for women who cannot maintain eating habit change. J Am Diet Assoc. 2001;101(3):345‐347. [DOI] [PubMed] [Google Scholar]
- 38. Shikany JM, Thomas AS, Beasley TM, Lewis CE, Allison DB. Randomized controlled trial of the Medifast 5 & 1 plan for weight loss. Int J Obes (Lond). 2013;37(12):1571‐1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xu DF, Sun JQ, Chen M, et al. Effects of lifestyle intervention and meal replacement on glycaemic and body‐weight control in Chinese subjects with impaired glucose regulation: a 1‐year randomised controlled trial. Br J Nutr. 2013;109(3):487‐492. [DOI] [PubMed] [Google Scholar]
- 40. Cheskin LJ, Mitchell AM, Jhaveri AD, et al. Efficacy of meal replacements versus a standard food‐based diet for weight loss in type 2 diabetes: a controlled clinical trial. Diabetes Educ. 2008;34(1):118‐127. [DOI] [PubMed] [Google Scholar]
- 41. Look ARG, Pi‐Sunyer X, Blackburn G, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one‐year results of the look AHEAD trial. Diabetes Care. 2007;30(6):1374‐1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rock CL, Flatt SW, Pakiz B, et al. Weight loss, glycemic control, and cardiovascular disease risk factors in response to differential diet composition in a weight loss program in type 2 diabetes: a randomized controlled trial. Diabetes Care. 2014;37(6):1573‐1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chee WSS, Gilcharan Singh HK, Hamdy O, et al. Structured lifestyle intervention based on a trans‐cultural diabetes‐specific nutrition algorithm (tDNA) in individuals with type 2 diabetes: a randomized controlled trial. BMJ Open Diabetes Res Care. 2017;5(1):e000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kempf K, Rohling M, Niedermeier K, Gärtner B, Martin S. Individualized Meal Replacement Therapy Improves Clinically Relevant Long‐Term Glycemic Control in Poorly Controlled Type 2 Diabetes Patients. Nutrients. 2018;10(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chaiyasoot K, Sarasak R, Pheungruang B, et al. Evaluation of a 12‐week lifestyle education intervention with or without partial meal replacement in Thai adults with obesity and metabolic syndrome: a randomised trial. Nutrition Diabetes. 2018;8(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Flechtner‐Mors M, Boehm BO, Wittmann R, Thoma U, Ditschuneit HH. Enhanced weight loss with protein‐enriched meal replacements in subjects with the metabolic syndrome. Diabetes Metab Res Rev. 2010;26(5):393‐405. [DOI] [PubMed] [Google Scholar]
- 47. Khoo J, Piantadosi C, Duncan R, et al. Comparing effects of a low‐energy diet and a high‐protein low‐fat diet on sexual and endothelial function, urinary tract symptoms, and inflammation in obese diabetic men. J Sex Med. 2011;8(10):2868‐2875. [DOI] [PubMed] [Google Scholar]
- 48. Ptomey LT, Saunders RR, Saunders M, et al. Weight management in adults with intellectual and developmental disabilities: a randomized controlled trial of two dietary approaches. J Appl Res Intellect Disabil. 2018;31(Suppl 1):82‐96. [DOI] [PubMed] [Google Scholar]
- 49. Franz MJ, VanWormer JJ, Crain AL, et al. Weight‐loss outcomes: a systematic review and meta‐analysis of weight‐loss clinical trials with a minimum 1‐year follow‐up. J Am Diet Assoc. 2007;107(10):1755‐1767. [DOI] [PubMed] [Google Scholar]
- 50. Chyun D. An intensive lifestyle intervention reduced weight and cardiovascular disease risk factors in overweight and obese people with type 2 diabetes. Evid Based Nurs. 2008;11(1):16‐16. [DOI] [PubMed] [Google Scholar]
- 51. McEvedy SM, Sullivan‐Mort G, McLean SA, Pascoe MC, Paxton SJ. Ineffectiveness of commercial weight‐loss programs for achieving modest but meaningful weight loss: systematic review and meta‐analysis. J Health Psychol. 2017;22(12):1614‐1627. [DOI] [PubMed] [Google Scholar]
- 52. Rehackova L, Arnott B, Araujo‐Soares V, Adamson AA, Taylor R, Sniehotta FF. Efficacy and acceptability of very low energy diets in overweight and obese people with type 2 diabetes mellitus: a systematic review with meta‐analyses. Diabet Med. 2016;33(5):580‐591. [DOI] [PubMed] [Google Scholar]
- 53. Piernas C, Aveyard P, Jebb SA. Recent trends in weight loss attempts: repeated cross‐sectional analyses from the health survey for England. Int J Obes (Lond). 2016;40(11):1754‐1759. [DOI] [PubMed] [Google Scholar]
- 54. Prospective Studies, C , Whitlock G, Lewington S, et al. Body‐mass index and cause‐specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373(9669):1083‐1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hartmann‐Boyce J, Johns DJ, Aveyard PA, et al. Managing Overweight and Obese Adults: The Clinical effectiveness of Long‐Term Weight Management Schemes for Adults. Oxford: University of Oxford; 2013. [Google Scholar]
- 56. Hartmann‐Boyce J, Aveyard P, Koshiaris C, Jebb SA. Development of tools to study personal weight control strategies: OxFAB taxonomy. Obesity (Silver Spring). 2016;24(2):314‐320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines and the Obesity Society. Circulation. 2014;129(25 Suppl 2):S102‐S138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Example of search strategy (MEDLINE)
Table S2: Descriptions of the intervention and control groups in each of the comparisons
Table S3: Justification of studies judged at high or unclear risk of bias in one or more domain
Figure S1: Forest plot of mean change in fasting blood glucose concentrations (mmol/L) from baseline to 1 year between interventions incorportaing meal replacments (MR) and control interventions
Figure S2: Forest plot of mean change in fasting serum insulin concentrations (pmol/L) from baseline to 1 year between interventions incorportaing meal replacments (MR) and control interventions
Figure S3: Forest plot of mean change in HbA1c (%) from baseline to 1 year between interventions incorportaing meal replacments (MR) and control interventions
Figure S4: Forest plot of mean change in total cholesterol concentrations (mmol/L) from baseline to 1 year between interventions incorportaing meal replacments (MR) and control interventions
Figure S5: Forest plot of mean change in LDL cholesterol concentrations (mmol/L) to 1 year between interventions incorportaing meal replacments (MR) and control interventions
Figure S6: Forest plot of mean change in HDL cholesterol concentrations (mmol/L) from baseline to 1 year between interventions incorportaing meal replacments (MR) and control interventions
Figure S7: Forest plot of mean change in triglyceride concentrations (mmol/L) from baseline to 1 year between interventions incorportaing meal replacments (MR) and control interventions
Figure S8: Forest plot of mean change in systolic blood pressure (mmHg) from baseline to 1 year between interventions incorportaing meal replacments (MR) and control interventions
Figure S9: Forest plot of mean change in diastolic blood pressure (mmHg) from baseline to 1 year between interventions incorportaing meal replacments (MR) and control interventions


