Abstract
Predicting climate change impacts on animal communities requires knowledge of how physiological effects are mediated by ecological interactions. Food‐dependent growth and within‐species size variation depend on temperature and affect community dynamics through feedbacks between individual performance and population size structure. Still, we know little about how warming affects these feedbacks. Using a dynamic stage‐structured biomass model with food‐, size‐ and temperature‐dependent life history processes, we analyse how temperature affects coexistence, stability and size structure in a tri‐trophic food chain, and find that warming effects on community stability depend on ecological interactions. Predator biomass densities generally decline with warming – gradually or through collapses – depending on which consumer life stage predators feed on. Collapses occur when warming induces alternative stable states via Allee effects. This suggests that predator persistence in warmer climates may be lower than previously acknowledged and that effects of warming on food web stability largely depend on species interactions.
Keywords: Allee effects, alternative stable states, climate change, community dynamics, size structure, temperature‐scaling, trophic interactions
Introduction
Predicting the impacts of climate change on natural food webs requires mechanistic understanding of organisms’ physiological responses to warming and how these translate to the population and community level. An individual's metabolism, and related ecological traits including feeding, mortality and population growth rate, depend strongly on body size and temperature (Brown et al. 2004; Savage et al. 2004). Mechanistic models based on metabolic scaling theory have increased our understanding of how warming affects populations and communities in terms of (1) community size structure (Blanchard et al. 2012; O'Gorman et al. 2017), (2) strength of trophic interactions (Rall et al. 2010; Vucic‐Pestic et al. 2011), (3) food chain length (Rall et al. 2010; Fussmann et al. 2014) and (4) stability (Uszko et al. 2017). The effects of temperature on (2)–(4) can largely be predicted from the relative temperature sensitivity of biomass gains (feeding) and losses (metabolism, mortality) – hereafter referred to as energetic efficiency – in combination with the resource carrying capacity (Uszko et al. 2017). Specifically, increased energetic efficiency with temperature is generally predicted to have a destabilising effect on communities and decreased efficiency a stabilising effect, followed by predator extinction from starvation (Uszko et al. 2017). However, while these insights stem from mechanistic models that are typically based on body size dependence of individual‐level processes, two fundamental aspects of body size are commonly overlooked when modelling effects of warming on populations and communities. First, the combination of within‐species size variation and food‐dependent life history processes (e.g. growth, development and reproduction) generates feedbacks between size structure and individual performance, ultimately affecting community dynamics (Persson & De Roos 2013). Second, the effects of warming differ between individuals, depending on life history stage and body size (Angilletta & Dunham 2003; Pörtner & Farrell 2008). Therefore, we need to account for variation in body size within‐ and between‐species to better understand the potential effects of warming on food web structure and stability (Ohlberger 2013).
Within‐species size variation is not only universal in natural systems, but has major implications for the stability and structure of populations and communities because it leads to differences (asymmetry) in net biomass production between individuals of different sizes (De Roos et al. 2003b; Persson & De Roos 2013). This asymmetry means that the least energy efficient life stage forms a bottleneck for population growth, which can lead to phenomena such as biomass overcompensation – an often life stage specific increase in standing stock biomass with mortality (De Roos et al. 2007). This occurs when mortality leads to higher biomass production (greater than losses through mortality) of a life stage due to the increased resource availability. Biomass overcompensation has been identified empirically in several studies (Persson et al. 2007; Schröder et al. 2009, 2014; Huss & Nilsson 2011; Ohlberger et al. 2011b). In the case of predation mortality, predators can thus cultivate biomass density of their own food source by inducing overcompensation in the prey, which can lead to an emergent Allee effect and alternative stable states when predator persistence relies on prey overcompensation (De Roos & Persson 2002; De Roos et al. 2003a). Emergent Allee effects refer to a positive relationship between per capita predator population growth rate and their population density (Allee effect) that emerges from individual‐level assumptions, such as size‐scaling of feeding rates and maintenance costs, instead of predefined population dynamics. As a consequence, predator populations may be exposed to risks of collapses from, for example fishing mortality, from which they may not recover (De Roos & Persson 2002). Emergent Allee effects via food‐dependent body growth have been demonstrated in a natural whole‐lake experiment, where an overharvested predator population (brown trout, Salmo trutta, L.) could not control the size distribution of its then stunted prey (Arctic charr, Salvelinus alpinus, L.). Culling of the stunted prey led to increases in juvenile prey on which predators fed, which shifted the community to a state where abundant predators kept the prey from a stunted state (Persson et al. 2007). The same mechanism has also been proposed to explain the lack of recovery of overfished Atlantic cod (Gadus morhua, L.) stocks, despite reduced fishing pressures (De Roos & Persson 2002; van Leeuwen et al. 2008; Gårdmark et al. 2015). As food‐ and size‐dependent body growth and within‐species size variation are important for understanding dynamics of ecological communities, they are also key factors determining the effects of climate change on food webs.
Body size also shapes the effects of warming on individuals, and this interactive effect is possibly stronger in aquatic compared to terrestrial systems (Pörtner & Farrell 2008; Forster et al. 2012; Horne et al. 2015; Lindmark et al. 2018). Lines of evidence that support size‐dependent temperature effects include directional changes in size composition towards smaller, energetically more efficient individuals (Yvon‐Durocher et al. 2011; Reuman et al. 2014; Malerba et al. 2018), the across taxa observation that size at maturity declines with warming (temperature‐size rule, TSR) (Atkinson 1994), and that optimum temperatures for growth decrease with size (Karås & Thoresson 1992; Björnsson & Steinarsson 2002). Despite the observational evidence of various temperature‐size interactions, only recently have the dynamical consequences of such interactions in warming environments been explored (Ohlberger et al. 2011a; Osmond et al. 2017; Sentis et al. 2017; Lindmark et al. 2018). Theoretical studies suggest that community persistence (in terms of food chain length) generally increases if body sizes decrease in warmer environments, due to weakened interaction strengths between species (Sentis et al. 2017). Smaller mean body sizes can also increase stability (return times) and thus resilience of consumer‐resource systems, which could buffer against extinctions (Osmond et al. 2017). However, it is not known whether the effect of temperature‐size interactions on stability and persistence holds when the change in size (or performance of a given size or life stage) with temperature depends on feedbacks between direct temperature effects, food availability via species interactions and population size structure. This limits our ability to predict how climate change impacts food chains and food webs.
Here we show how within‐ and between‐species interactions mediate the direct physiological effects of warming on the stability and coexistence of a tri‐trophic food chain, using a dynamic stage‐structured biomass model with temperature‐dependent vital rates. Our analyses generated novel predictions on community responses to changing temperatures that are due to food‐dependent life history processes and species interactions; (i) whether warming stabilises or destabilises communities depends on the life stage preference of the predator, stage structure in the consumer and the current temperature; (ii) warming can cause non‐gradual declines (collapses) in predator populations due to Allee effects, which also induce alternative stable states in which predators are either present or go extinct; (iii) increased energetic costs of being large in a warmer environment reduce the scope for predator persistence and the average community body size. These previously unrecognised temperature responses highlight that food‐dependent life history processes and species interactions mediate the direct effects of warming on the dynamics and structure of ecological communities.
Materials and Methods
Modelling framework
We used a stage‐structured biomass model (eqns (1), (2), (3), (4)) (De Roos et al. 2008), which is derived from – and under equilibrium conditions exactly represents – a size‐structured population model with a continuous size distribution (Metz & Diekmann 1986). Biomass production (used for growth, development and reproduction) is food‐dependent and hence mediated by ecological interactions via exploitation of shared resources. The model is an extension of the temperature‐dependent consumer‐resource model used in Lindmark et al. (2018). This model is empirically parameterised to represent a stage‐structured (juveniles and adults) consumer zooplanktivorous fish (common roach, Rutilus rutilus L.) and its zooplankton prey (Daphnia sp.), here with a stage‐selective top predator (pike, Esox Lucius L.) feeding on the intermediate consumer. We refer to this model as the ‘empirical’ model. To generalise our findings with respect to the stage structure of the consumer population, we also analysed the same model (eqns (1), (2), (3), (4)) using the original parameterisation and formulation (De Roos et al. 2007; De Roos & Persson 2013) to which we added temperature dependence. This parameterisation is more phenomenological, as the type of asymmetry is determined with a single parameter (q). We therefore refer to this as the ‘generic’ model. We present results from analyses using both parameterisations in the main text. The empirical model is described below and in Appendix S1, where we also describe the generic parameterisation.
Juvenile and adult consumers and predators are characterised by a representative body size (c. 4 g, c. 30 g and c. 640 g respectively), which are used to calculate their average mass‐specific rates of metabolism, maximum ingestion, attack and background mortality (Appendix S1). Asymmetrical competition between life stages in the consumer population arises from differences in body size and thus energetic performance. The state variables are biomass densities [g m−3] of a basal resource (R), juvenile (J) and adult (A) consumers feeding on the resource, and a predator (P) feeding with varied preference on the consumer life stages (eqns (1), (2), (3), (4)):
| (1) |
| (2) |
| (3) |
| (4) |
Most terms in eqns (1), (2), (3), (4) are functions of body size, temperature and the basal resource and are described below and in Appendix S1 Table S1‐S2.
We assume that the basal resource grows according to semi‐chemostat dynamics (Persson et al. 1998), with temperature‐dependent turnover rate (δ) and maximum density (). Juvenile biomass increases with adult reproduction (). The +‐superscript refers to positive values of biomass production such that reproduction and maturation only occur when biomass is produced, which ensures that starvation (i.e. when v J,A < 0) in one life stage does not reduce biomass of the other life stage. However, since we analyse equilibrium dynamics, starvation is not possible. Juvenile biomass is lost through maturation (γ) into the adult stage and mortality, μJ (sum of background and predation mortality) and gained by reproduction (). Adult biomass is gained through maturation (γ) and lost by predation and background mortality (μ A). We assume that juveniles invest all biomass into growth and development, whereas adults spend all their energy on reproduction and hence do not grow in size (De Roos et al. 2008).
The net biomass production of consumers and predators (v J,A,P) is the difference between ingested energy, scaled with assimilation efficiency (Appendix S1, Table S1‐S2), and metabolic costs (M J,A,P). Ingestion follows a Holling type II functional response (Holling 1959) for consumers and predators, with size‐ and temperature‐dependent functions describing maximum ingestion (I max,J,A,P) and attack rate (a J,A,P). We vary the feeding preference of predators by scaling their encounter rate of juveniles with parameter p and of adults with 1−p (i.e. p = 1 means juvenile selective predator, and p = 0 means adult selective predator). The predator population is unstructured and its dynamics are therefore determined by its net biomass production (ν P) and losses due to background mortality μ P. These rates are calculated based on its representative size, which was chosen to give equal attack rates on both consumer life stages given published size‐dependent attack rate functions (Appendix 1, Fig. S1).
Size‐ and temperature dependence of vital rates
Temperature dependence is achieved by multiplying allometric functions of individual‐level rates (at reference temperature, T 0, 19 °C) with a Boltzmann‐Arrhenius function, scaled to equal 1 at 19 °C (Appendix S1). The Boltzmann‐Arrhenius function is given by , where T [K] is the temperature, T 0 [K] is the reference temperature, k [eV K−1] is Boltzmann's constant and E Y [eV] is the activation energy of rate or parameter Y (Gillooly et al. 2001). These temperature‐dependent rates and parameters (Y) include: metabolism (M J,A,P), functional response parameters attack rate (a J,A,P) and maximum ingestion rate (I max,J,A,P), basal resource turnover rate (δ), maximum resource density () and background mortality (μ J,A,P).
We considered two contrasting scenarios of temperature dependence of the maximum basal resource density (), based on mass conservation and metabolic scaling principles (Gilbert et al. 2014; Bernhardt et al. 2018): no effect of temperature on (), or declining with temperature at the same rate as turnover rate increases ( [eV]) (see Appendix S1, Table S5‐S6 for other values, including positive effects). We also relax the assumption of the metabolic theory of ecology (Brown et al. 2004) of independent effects of body size and temperature, by adding a linear temperature dependence (c) to the exponent of metabolic rate, such that , where r M is the temperature scaling factor for metabolism, ρ 1 is the normalisation constant, m J,A,P is mass and is the allometric exponent at T0, and c determines whether metabolism increases faster with temperature for large relative to small individuals (c > 0), or vice versa (c < 0) (Appendix S1, Fig. S2). This linear form of temperature‐size interaction has been shown within species and we assume that both consumers and predators have the same c by default (but see Appendix S1, Fig. S11), but acknowledge that it can vary considerably between species (Ohlberger et al. 2012; Lindmark et al. 2018). A steeper size scaling of metabolism with body size at higher temperatures (c > 0), all else equal, leads to a steeper scaling of the critical resource density needed to meet metabolic demands (R crit) with body size (Appendix S1, Fig. S2). Therefore, c > 0 results in reduced growth performance for larger individuals in warmer environments, as also suggested by Messmer et al. (2016).
Analyses
We analysed equilibrium biomass densities and bifurcations by performing equilibrium continuations using the MATLAB (MATLAB 2014) package MATCONT GUI (Dhooge et al. 2008), and use the same terminology for invasion/persistence as De Roos & Persson (2013). Minimum and maximum of limit cycles were also calculated by running time integrations until equilibrium for selected parameters. We tested the robustness of our results by systematically varying parameter values and by using a temperature‐dependent version of the generic, simpler model formulation (De Roos et al. 2008). Model files, instructions for viewing and implementing the model in MATCONT, and R‐scripts to reproduce the main figures with simulated data have been deposited on https://github.com/maxlindmark/Temperature_Allee (Appendix S2 is a copy of this repository). R version 3.5 (R Core Team 2018) was used to create figures.
Results
Stability and coexistence over temperature depend on predator preference
Whether warming stabilises or destabilises the food chain, and causes predator extinction, depends on which life stage the predator feeds on (Figs 1 and 3). This is due to feedbacks between predation mortality and stage‐structure in the consumer. In the absence of predation, juvenile consumers are competitively superior to adult consumers, because they require lower resource densities to meet basic metabolic demands (Appendix S1, Fig. S2). At equilibrium, the reproductive output by adults is therefore limiting the growth of the population and the adult life stage is a bottleneck. This leads to a potential for biomass overcompensation in response to mortality in juveniles, as mortality increases basal resource levels, which adults can exploit to increase their net biomass production and therefore reproduction (see ‘Introduction’; Appendix S1, Table S4).
Figure 1.
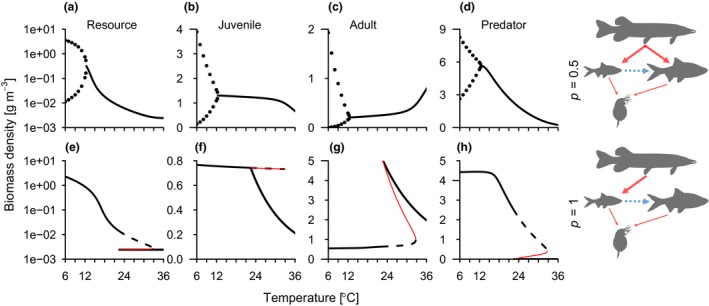
Effects of warming on food chain stability depend on ecological interactions. Equilibrium biomass densities of the resource (a and e), consumer life stages (b, c, f and g) and predator (d and h) as a function of temperature, given a predator feeding with equal intensity on both life stages (a–d) (p = 0.5) or exclusively on juveniles (e–h) (p = 1). Black lines (full and dashed) are stable equilibria and red thin lines are unstable equilibria (connecting the two stable branches in the bistable region), which separate the two stable equilibria when there are alternative stable states. Maximum and minimum biomass density of a stable limit cycle is shown with points (top row below c. 12 °C). Alternative stable states, where predators are either extinct or abundant, occur between c. 22 and 33 °C (e–h). Note the different scales on the y‐axes and the logarithmic y‐axis for resources densities. , all other parameters have default values (Appendix S1, Table S2).
When predators are present and feed mainly on juveniles, the production of juvenile biomass can increase more than what is consumed by the predator (Appendix S1, Section 3). Such biomass overcompensation can ensure predator persistence at higher temperatures, even when they cannot invade a stable consumer‐resource system. These differences in persistence and invasion boundaries over temperature lead to emergent community bistability. The cause of this warming‐induced bistability is that the consumer's top down control of the basal resource increases with temperature, causing the predator population to decline as less biomass is transferred to the top of the food chain. Eventually, predators fall below a critical level where they cannot invade a stable consumer‐resource system. However, predation‐induced biomass overcompensation in the consumer can still allow for persistence if predators are abundant to begin with. For the empirical model using default parameters, this means predator persistence is possible for all temperatures below c. 33 °C, while invasion is only possible below c. 22 °C (Fig. 1h). By contrast, when predators feed with equal intensity on both life stages, they never depend on predation‐induced biomass overcompensation to persist and hence warming does not induce bistability (Fig. 1a–d). Warming can thus have both stabilising effects, by replacing cyclic dynamics with fixed point dynamics (‘inverse enrichment cycles’, Fig. 1a–d), and destabilising effects, by inducing alternative stable states (Fig. 1e–h).
Varying temperature‐scaling scenarios
Warming‐induced alternative stable states emerge both when maximum density of the basal resource is temperature‐independent () and when it declines with temperature () for most of the R max,T19 values analysed at reference temperature, 19 °C (Fig. 2; see Appendix S1, Fig. S7 for biomass densities). However, R max regulates predator biomass density such that both Rmax and its temperature dependence affect at which specific temperature transitions between different types of dynamics occur, and higher R max favours predator persistence. They also affect food chain structure, such that predator persistence decreases faster with warming when R max declines with temperature () (Fig. 2b and d).
Figure 2.
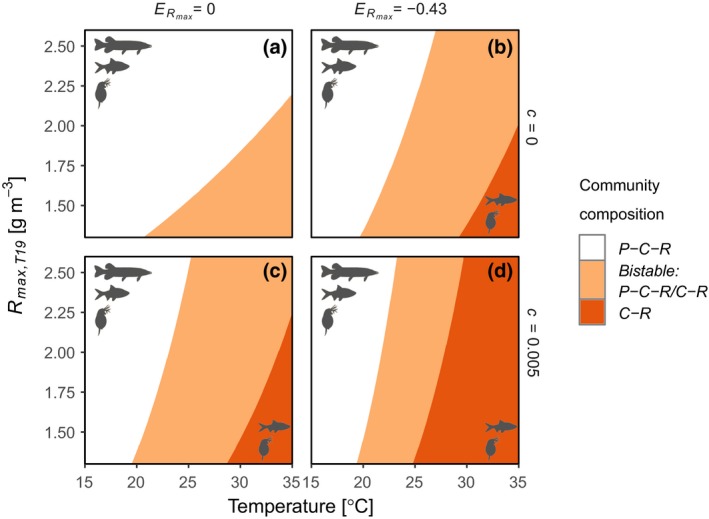
Effects of temperature on community structure depend on temperature scaling of maximum resource density (R max,T19) and whether metabolism scales with body size and temperature independently (c = 0) or interactively (c ≠ 0) in the consumer (C) and predator (P). With warming, the tri‐trophic food‐chain changes from stable (white space), to exhibiting alternative stable states (orange space; with predators either present or absent), to being reduced to two trophic levels following predator extinction (dark orange space). The figure shows how the species composition and dynamics of the food‐chain change with temperature and R max,T19, given no () (a and c) or negative () (b and d) effects of temperature on R max,T19, with independent (a and b) or interactive (c and d) effects of body size and temperature on metabolism. The predator feeds exclusively on juveniles (p = 1), all other parameters have default values (Appendix S1, Table S2).
When temperature affects the size dependence of metabolic rate (c > 0), energetic costs increase faster with warming in large compared to small individuals. Therefore, the food density needed for a consumer or predator to grow (R crit) has a steeper scaling with body size compared to scenarios assuming independent effects of temperature and body size (Appendix S1, Fig. S2). In these scenarios (Fig. 2c and d), predators go extinct at lower temperatures than if there is no temperature‐size interaction (Fig. 2a and b). This is because c > 0 leads to reduced reproductive output and thus a lower juvenile to adult biomass ratio (Appendix S1, Fig. S5). Therefore, temperature‐size interactions here negatively affect predators that feed predominantly on juveniles via two mechanisms: reductions in prey availability due to shifts in the stage structure of the consumer, and increased metabolic costs. Consequently, a predator species feeding on both consumer life stages can persist at higher temperatures than a predator specialised on juveniles in the empirical parameterisation (Appendix S1, Fig. S10 and 11).
Predator biomass declines with warming, and eventually causes predator extinction, in most temperature‐scaling scenarios (Figs 1, 2, 3, Appendix S1, Table S5‐S6). This decline can be gradual or in the form of a collapse, depending on which consumer life stage the predator mainly feeds on. However, a few specific scenarios can lead to increases in predator biomass density over temperature. For this to occur, R max must not decrease with temperature, resource turnover rate must increase faster with temperature than metabolic and feeding rates, and energetic efficiencies must not decline with temperature (i.e.) (Appendix S1, Table S5‐S6).
Figure 3.
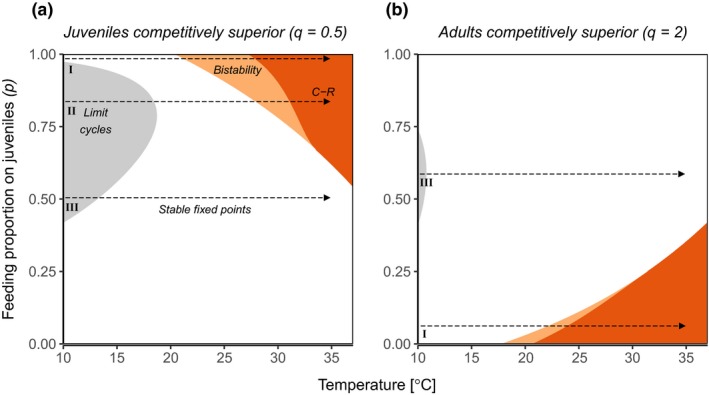
Shifts in structure and stability with increasing temperature depend on the predators’ feeding preference (p), current temperature and which life stage is competitively inferior and therefore limits growth of the consumer population. Shown here are results for the generic model parameterisation with added temperature dependence (Appendix S1, Table S7 and S8). Panel (a) is analogous to the empirical model used for Figs 1 and 2 (juveniles competitively superior) and in (b) adults are superior competitors. In grey regions all species in the food‐chain exhibit stable population cycles, white corresponds to stable tri‐trophic states, orange shows bistable regions where the food chain exhibits alternative stable states with predators being either extinct or abundant (here the lower temperature boundary of the region corresponds to the invasion boundary and the upper is the persistence boundary), and dark orange is the stable consumer‐resource system where predators cannot persist. Roman numerals correspond to three distinct regions of predator feeding preference (p) that lead to different stability‐temperature relationships with warming (indicated by arrows along the temperature axis): I) warming destabilises the food chain by inducing bistability (alternative stable states), II) warming initially stabilises the food chain by switching the state from limit cycles to fixed point dynamics but eventually induces bistability III) warming stabilises the food chain by replacing cyclic dynamics with fixed points. , all other parameters have default values (Appendix S1, Table S7 and S8).
Generalising the model: effect of asymmetry in net biomass production of consumer life stages
How stage‐specific predation affects the stability‐persistence relationship with temperature depends on the specific type of asymmetry in net biomass production between juvenile and adult consumers. We used the generic and more phenomenological model parameterisation (competitiveness is here modified with parameter q, scaling the relative ingestion rates of the life stages) to generalise these findings. When juveniles are competitively superior (same asymmetry as in the empirical model; Fig. 3a; Appendix S1, Fig. S10) and predators feed predominantly on juveniles (c. 0.72 < p < c. 0.98), the effect of warming on community stability depends on the current temperature. This is because at low temperatures, warming initially stabilises the community but induces bistability with additional warming. Over the whole range of feeding preferences (p), there are three distinct parameter regions corresponding to different effects on food chain stability along the entire temperature axis: I) destabilising, II) stabilising followed by destabilising or III) stabilising (see arrows in Fig. 3).
When adults are competitively superior (opposite asymmetry to empirical model) (Fig. 3b) and juveniles are the energetic bottleneck in the population in the absence of predation, there is instead a potential for biomass overcompensation in the adult life stage. In this case, the system exhibits warming‐induced bistability when the predator feeds on the adult life stage rather than the juvenile life stage. Hence, the effect of stage‐specific predation on the stability and persistence response to warming is reversed when the asymmetry is reversed. This is shown in Fig. 3, where persistence of an adult‐specialised predator is more limited than a juvenile‐specialised predator in warmer environments. This demonstrates that independent of the type of asymmetry in net biomass production between consumer stages, warming induces bistability in predators that feed selectively on the competitively inferior consumer stage.
Discussion
Here we show that food dependence of life history processes, such as maturation and reproduction, and stage preference of predator feeding can explain previously unrecognised community‐wide responses to warming, including alternative stable states due to emergent Allee affects, and both stabilisation and destabilisation of communities. These diverse community responses result from feedbacks between food, size and temperature dependence of individual performance and life history processes. Warming generally results in reduced potential for predator persistence and declines in average community body size. Importantly, predator persistence in warmer waters is determined by whether or not the predator feeds on the competitively superior life stage of the consumer. Our main results hold across a wide range of assumptions of temperature dependencies on maximum basal resource density (R max), feeding and metabolism, and interactive effects of body size and temperature on metabolic rate. Much of the research on how temperature shapes population and community dynamics has focused on the role of relative temperature sensitivities of vital rates (Fussmann et al. 2014; Gilbert et al. 2014; Uszko et al. 2017). Our findings demonstrate that even under large variation in thermal sensitivities of vital rates, food‐ and size dependence of ecological interactions can determine the outcome of warming on community structure and dynamics. This study highlights the importance of size‐based interactions and food‐dependent life history processes for the energetic performance of individuals in changing climates, and how that translates to shifts in community dynamics and structure.
The general prediction of reduced predator persistence with increasing temperatures corroborates earlier studies (Rall et al. 2010; Fussmann et al. 2014). Moreover, the conditions that cause predators to decline, that is a combination of negative temperature dependence of maximum resource density, slower resource growth rates relative to feeding and metabolic rates, and reduced energetic efficiency of consumers and predators, have also been found in both terrestrial and aquatic systems (Vasseur & McCann 2005; Rall et al. 2010; Vucic‐Pestic et al. 2011; O'Gorman et al. 2017). Our novel finding is that predator declines with increasing temperatures are not always gradual, but can be sudden and collapses can occur at temperatures much lower than those that would cause starvation. This happens when warming induces bistability due to Allee effects, which emerge from feedbacks between individual performance and population size structure (De Roos & Persson 2002), given food‐dependent growth – all of which are ubiquitous in natural food webs. Therefore, our results suggest that warming may expose predators to an additional, previously overlooked risk of collapse and/or impaired recovery potential in warmer environments. This increased risk could manifest itself in systems where strong interactions among and within species shape community size structure (the prerequisites for emergent Allee effects) as has been suggested for populations of Atlantic cod, brown trout and Arctic charr (De Roos & Persson 2002; Persson et al. 2007; van Leeuwen et al. 2008; Gårdmark et al. 2015). The model predicts non‐gradual declines in biomass when predators feed mainly on smaller consumers (or larger, if these limit consumer population growth). Such feeding behaviour has empirical support, for example in Atlantic salmon (Salmo salar L.) (Jacobson et al. 2018) and Atlantic cod (van Leeuwen et al. 2008; Gårdmark et al. 2015). Thus, the model used here generally predicts lower persistence of predators in warmer environments, and importantly, that both predator densities and mean community body size do not necessarily decline gradually with temperature but can exhibit collapses.
Recent studies have aimed to reconcile the diverse effects warming can have on community stability by deriving general principles based on the relative temperature‐sensitivities of resource energetic efficiency (i.e. biomass gains vs losses) and resource carrying capacity (Vasseur & McCann 2005; Fussmann et al. 2014; Uszko et al. 2017). However, we do not know if these predictions apply to size‐ or stage‐structured populations with individuals exhibiting food‐dependent growth, development and reproduction. This is a key question to address, as both size variation within species and food dependence of development and reproduction are widespread in nature, and often govern ecological dynamics (Persson & De Roos 2013). We account for food dependence of growth, development and reproduction mechanistically, such that these processes depend on both interactions within and among species, and on direct physiological effects of warming. The model analyses suggest that for a predator feeding on both consumer life stages, warming shifts the community from exhibiting limit cycles to stable point dynamics, similar to findings in previous studies using models without stage‐structure (Binzer et al. 2012; Sentis et al. 2017). In contrast to models that do not account for stage‐structure within populations, we show that the qualitative shifts in community structure and stability with increased temperatures are not primarily driven by the relative temperature dependence of energetic efficiency. Instead, we found that the effect of body size on competitive interactions within the consumer species and the effect of predation on them can determine the effects of warming on community structure and stability.
As the effects of temperature on performance tend to vary with body size or life stage, a single activation energy parameter cannot describe the entire temperature dependence of a given vital rate (Ohlberger et al. 2012; Lindmark et al. 2018). Such interactive effects of temperature and body size are reflected in empirical patterns such as the temperature‐size rule (TSR) (increased juvenile growth‐ or developmental rates but smaller adult body size in warmer environments) (Atkinson 1994), which is especially strong in aquatic environments (Forster et al. 2012; Horne et al. 2015). Still, the implications of such temperature‐size interactions for population and community dynamics are poorly understood (Lindmark et al. 2018). Recent studies suggest that when the average body size of species declines with warming, stability, in terms of return times after perturbations, generally increases (Osmond et al. 2017). Also, persistence of species in a food chain was found to increase when warming causes reductions in size, though this depends on the trophic level at which the reductions occur (Sentis et al. 2017). Our approach to address temperature‐size interactions differs from those previous studies, because we account for stage structure within species and do not assume temperature effects on body size based on empirical temperature‐size patterns. Instead, we assess how the energetic performance of an individual of a specific size changes with temperature, based on the scaling of individual‐level rates and species interactions. Thus, any changes in average body size within species or in the food chain emerge dynamically in our model. We find that predator persistence is always lower with temperature‐size interactions that more negatively affect the energy budget of large individuals, compared to independent temperature and body size effects.
Within‐species phenotypic variation is increasingly recognised as an important driver of ecological dynamics (Bolnick et al. 2011; Miller & Rudolf 2011; Persson & De Roos 2013), and there is vast empirical evidence for size‐dependent responses to warming (Atkinson 1994; Angilletta & Dunham 2003; Pörtner & Farrell 2008). Accordingly, warming should influence the outcome of size‐dependent interactions. However, the ecological implications of within‐species variation in the context of responses to global warming have been largely overlooked [but see (Ohlberger et al. 2011a; Lindmark et al. 2018)]. Our results demonstrate that approaches based on species‐averaged traits (such as mean body size) cannot accurately represent the full range of dynamics and shifts in community size or stage structure that warming can cause. We show that even simple stage structure within a population can result in unexpected community‐level responses to rising temperatures, including alternative stable food web states and potential collapses of predators due to emergent Allee effects. Thus, feedbacks between food dependence of life history processes and population stage structure, both ubiquitous in natural food webs, can alter the effects of temperature on food web stability and species persistence.
Authorship
AG conceived the study. All authors contributed to the formulation of research questions and study design. ML performed modelling analyses and wrote the first draft. All the authors contributed to the revision of the manuscript.
Supporting information
Acknowledgements
We thank Sebastian Diehl, Wojciech Uszko and Viktor Thunell for inspiring and helpful discussions, André De Roos for assistance in implementing the maturation function and phylopic (http://phylopic.org/) for the pike silhouette, available for reuse under the Public Domain Mark 1.0 license (the other silhouettes were drawn by ML) and three anonymous reviewers for insightful and constructive comments that helped improve the manuscripts. This work was supported by grants from the Swedish Research Council FORMAS (no. 217‐2014‐474 to MH), the Swedish Research Council (no. 2015‐03752 to AG), and the Swedish Research Council FORMAS (no. 217‐ 2013‐1315 to AG).
References
- Angilletta, M.J. & Dunham, A.E. (2003). The temperature‐size rule in ectotherms: simple evolutionary explanations may not be general. Am. Nat., 162, 332–342. [DOI] [PubMed] [Google Scholar]
- Atkinson, D. (1994). Temperature and organism size—A biological law for ectotherms? Adv. Ecol. Res., 25, 1–58. [Google Scholar]
- Bernhardt, J.R. , Sunday, J.M. & O'Connor, M.I. (2018). Metabolic theory and the temperature‐size rule explain the temperature dependence of population carrying capacity. Am. Nat., 192, 687–697. [DOI] [PubMed] [Google Scholar]
- Binzer, A. , Guill, C. , Brose, U. & Rall, B.C. (2012). The dynamics of food chains under climate change and nutrient enrichment. Philos. Trans. R. Soc. Lond. B Biol. Sci., 367, 2935–2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björnsson, B. & Steinarsson, A. (2002). The food‐unlimited growth rate of Atlantic cod (Gadus morhua). Can. J. Fish Aquat. Sci., 59, 494–502. [Google Scholar]
- Blanchard, J.L. , Jennings, S. , Holmes, R. , Harle, J. , Merino, G. , Allen, J.I. et al (2012). Potential consequences of climate change for primary production and fish production in large marine ecosystems. Philos. Trans. R. Soc. Lond. B Biol. Sci., 367, 2979–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolnick, D.I. , Amarasekare, P. , Araújo, M.S. , Bürger, R. , Levine, J.M. , Novak, M. et al (2011). Why intraspecific trait variation matters in community ecology. Trends Ecol. Evol., 26, 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, J.H. , Gillooly, J.F. , Allen, A.P. , Savage, V.M. & West, G.B. (2004). Toward a metabolic theory of ecology. Ecology, 85, 1771–1789. [Google Scholar]
- De Roos, A.M. & Persson, L. (2002). Size‐dependent life‐history traits promote catastrophic collapses of top predators. Proc. Natl Acad. Sci. USA, 99, 12907–12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Roos, A.M. & Persson, L. (2013). Population and Community Ecology of Ontogenetic Development. Princeton University Press, Princeton, NJ. [Google Scholar]
- De Roos, A.M. , Persson, L. & Thieme, H.R. (2003a). Emergent Allee effects in top predators feeding on structured prey populations. Proc. R. Soc. Lond. B Biol. Sci., 270, 611–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Roos, A.M.D. , Persson, L. & McCauley, E. (2003b). The influence of size‐dependent life‐history traits on the structure and dynamics of populations and communities. Ecol. Lett., 6, 473–487. [Google Scholar]
- De Roos, A.M. , Schellekens, T. , van Kooten, T. , van de Wolfshaar, K.E. , Claessen, D. & Persson, L. (2007). Food‐dependent growth leads to overcompensation in stage‐specific biomass when mortality increases: the influence of maturation versus reproduction regulation. Am. Nat., 170, E59–E76. [DOI] [PubMed] [Google Scholar]
- De Roos, A.M. , Schellekens, T. , van Kooten, T. , van De Wolfshaar, K.E. , Claessen, D. & Persson, L. (2008). Simplifying a physiologically structured population model to a stage‐structured biomass model. Theor. Popul. Biol., 73, 47–62. [DOI] [PubMed] [Google Scholar]
- Dhooge, A. , Govaerts, W. , Kuznetsov, Y.A. , Meijer, H.G.E. & Sautois, B. (2008). New features of the software MatCont for bifurcation analysis of dynamical systems. Math. Comput. Model. Dyn. Syst., 14, 147–175. [Google Scholar]
- Forster, J. , Hirst, A.G. & Atkinson, D. (2012). Warming‐induced reductions in body size are greater in aquatic than terrestrial species. Proc. Natl Acad. Sci. USA, 109, 19310–19314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fussmann, K.E. , Schwarzmüller, F. , Brose, U. , Jousset, A. & Rall, B.C. (2014). Ecological stability in response to warming. Nat. Clim. Change, 4, 206–210. [Google Scholar]
- Gårdmark, A. , Casini, M. , Huss, M. , van Leeuwen, A. , Hjelm, J. , Persson, L. et al (2015). Regime shifts in exploited marine food webs: detecting mechanisms underlying alternative stable states using size‐structured community dynamics theory. Philos. Trans. R. Soc. Lond. B Biol. Sci., 370, 20130262. [Google Scholar]
- Gilbert, B. , Tunney, T.D. , McCann, K.S. , DeLong, J.P. , Vasseur, D.A. , Savage, V.M. et al (2014). A bioenergetic framework for the temperature dependence of trophic interactions. Ecol. Lett., 17, 902–914. [DOI] [PubMed] [Google Scholar]
- Gillooly, J.F. , Brown, J.H. , West, G.B. , Savage, V.M. & Charnov, E.L. (2001). Effects of size and temperature on metabolic rate. Science, 293, 2248–2251. [DOI] [PubMed] [Google Scholar]
- Holling, C.S. (1959). Some characteristics of simple types of predation and parasitism. Can. Entomol., 91, 385–398. [Google Scholar]
- Horne, C.R. , Hirst, A.G. & Atkinson, D. (2015). Temperature‐size responses match latitudinal‐size clines in arthropods, revealing critical differences between aquatic and terrestrial species. Ecol. Lett., 18, 327–335. [DOI] [PubMed] [Google Scholar]
- Huss, M. & Nilsson, K.A. (2011). Experimental evidence for emergent facilitation: promoting the existence of an invertebrate predator by killing its prey. J. Anim. Ecol., 80, 615–621. [DOI] [PubMed] [Google Scholar]
- Jacobson, P. , Gårdmark, A. , Östergren, J. , Casini, M. & Huss, M. (2018). Size‐dependent prey availability affects diet and performance of predatory fish at sea: a case study of Atlantic salmon. Ecosphere, 9, e02081. [Google Scholar]
- Karås, P. & Thoresson, G. (1992). An application of a bioenergetics model to Eurasian perch (Perca fluviatilis L.). J. Fish Biol., 41, 217–230. [Google Scholar]
- van Leeuwen, A. , De Roos, A.M. & Persson, L. (2008). How cod shapes its world. J. Sea Res., 60, 89–104. [Google Scholar]
- Lindmark, M. , Huss, M. , Ohlberger, J. & Gårdmark, A. (2018). Temperature‐dependent body size effects determine population responses to climate warming. Ecol. Lett., 21, 181–189. [DOI] [PubMed] [Google Scholar]
- Malerba, M.E. , White, C.R. & Marshall, D.J. (2018). Eco‐energetic consequences of evolutionary shifts in body size. Ecol. Lett., 21, 54–62. [DOI] [PubMed] [Google Scholar]
- MATLAB . (2014). version 8.4.0.150421 (2014b), Natick, Ma.
- Messmer, V. , Pratchett, M.S. , Hoey, A.S. , Tobin, A.J. , Coker, D.J. , Cooke, S.J. et al (2016). Global warming may disproportionately affect larger adults in a predatory coral reef fish. Glob. Change Biol., 23, 2230–2240. [DOI] [PubMed] [Google Scholar]
- Metz, J.A.J. & Diekmann, O. (1986). The Dynamics of Physiologically Structured Populations. Springer‐Verlag, Heidelberg, Germany, Springer Lect. Biomatchematics. [Google Scholar]
- Miller, T.E.X. & Rudolf, V.H.W. (2011). Thinking inside the box: community‐level consequences of stage‐structured populations. Trends Ecol. Evol., 26, 457–466. [DOI] [PubMed] [Google Scholar]
- O'Gorman, E.J. , Zhao, L. , Pichler, D.E. , Adams, G. , Friberg, N. , Rall, B.C. et al (2017). Unexpected changes in community size structure in a natural warming experiment. Nat. Clim. Change, 7, 659–663. [Google Scholar]
- Ohlberger, J. (2013). Climate warming and ectotherm body size ‐ from individual physiology to community ecology. Funct. Ecol., 27, 991–1001. [Google Scholar]
- Ohlberger, J. , Edeline, E. , Vollestad, L.A. , Stenseth, N.C. & Claessen, D. (2011a). Temperature‐driven regime shifts in the dynamics of size‐structured populations. Am. Nat., 177, 211–223. [DOI] [PubMed] [Google Scholar]
- Ohlberger, J. , Langangen, Ø. , Edeline, E. , Claessen, D. , Winfield, I.J. , Stenseth, N.C. et al (2011b). Stage‐specific biomass overcompensation by juveniles in response to increased adult mortality in a wild fish population. Ecology, 92, 2175–2182. [DOI] [PubMed] [Google Scholar]
- Ohlberger, J. , Mehner, T. , Staaks, G. & Hölker, F. (2012). Intraspecific temperature dependence of the scaling of metabolic rate with body mass in fishes and its ecological implications. Oikos, 121, 245–251. [Google Scholar]
- Osmond, M.M. , Barbour, M.A. , Bernhardt, J.R. , Pennell, M.W. , Sunday, J.M. & O'Connor, M.I. (2017). Warming‐induced changes to body size stabilize consumer‐resource dynamics. Am. Nat., 189, 718–725. [DOI] [PubMed] [Google Scholar]
- Persson, L. & De Roos, A.M. (2013). Symmetry breaking in ecological systems through different energy efficiencies of juveniles and adults. Ecology, 94, 1487–1498. [DOI] [PubMed] [Google Scholar]
- Persson, L. , Leonardsson, K. , De Roos, A.M. , Gyllenberg, M. & Christensen, B. (1998). Ontogenetic scaling of foraging rates and the dynamics of a size‐structured consumer‐resource model. Theor. Popul. Biol., 54, 270–293. [DOI] [PubMed] [Google Scholar]
- Persson, L. , Amundsen, P.‐A. , De Roos, A.M. , Klemetsen, A. , Knudsen, R. & Primicerio, R. (2007). Culling prey promotes predator recovery ‐ alternative states in a whole‐lake experiment. Science, 316, 1743–1746. [DOI] [PubMed] [Google Scholar]
- Pörtner, H.O. & Farrell, A.P. (2008). Physiology and climate change. Science, 322, 690–692. [DOI] [PubMed] [Google Scholar]
- R Core Team . (2018). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria, Availbale at: https://www.R-project.org/\. Last accessed 23 February 2018. [Google Scholar]
- Rall, B.C. , Vucic‐Pestic, O. , Ehnes, R.B. , Emmerson, M. & Brose, U. (2010). Temperature, predator‐prey interaction strength and population stability. Glob. Change Biol., 16, 2145–2157. [Google Scholar]
- Reuman, D.C. , Holt, R.D. & Yvon‐Durocher, G. (2014). A metabolic perspective on competition and body size reductions with warming. J. Anim. Ecol., 83, 59–69. [DOI] [PubMed] [Google Scholar]
- Savage, V.M. , Gillooly, J.F. , Brown, J.H. , West, G.B. & Charnov, E.L. (2004). Effects of body size and temperature on population growth. Am. Nat., 163, 429–441. [DOI] [PubMed] [Google Scholar]
- Schröder, A. , Persson, L. & de Roos, A.M. (2009). Culling experiments demonstrate size‐class specific biomass increases with mortality. Proc. Natl Acad. Sci. USA, 106, 2671–2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder, A. , van Leeuwen, A. & Cameron, T.C. (2014). When less is more: positive population‐level effects of mortality. Trends Ecol. Evol., 29, 614–624. [DOI] [PubMed] [Google Scholar]
- Sentis, A. , Binzer, A. & Boukal, D.S. (2017). Temperature‐size responses alter food chain persistence across environmental gradients. Ecol. Lett., 20, 852–862. [DOI] [PubMed] [Google Scholar]
- Uszko, W. , Diehl, S. , Englund, G. & Amarasekare, P. (2017). Effects of warming on predator‐prey interactions: a resource‐based approach and a theoretical synthesis. Ecol. Lett., 20, 513–523. [DOI] [PubMed] [Google Scholar]
- Vasseur, D.A. & McCann, K.S. (2005). A mechanistic approach for modelling temperature‐dependent consumer‐resource dynamics. Am. Nat., 166, 184–198. [DOI] [PubMed] [Google Scholar]
- Vucic‐Pestic, O. , Ehnes, R.B. , Rall, B.C. & Brose, U. (2011). Warming up the system: higher predator feeding rates but lower energetic efficiencies. Glob. Change Biol., 17, 1301–1310. [Google Scholar]
- Yvon‐Durocher, G. , Montoya, J.M. , Trimmer, M. & Woodward, G.U.Y. (2011). Warming alters the size spectrum and shifts the distribution of biomass in freshwater ecosystems. Glob. Change Biol., 17, 1681–1694. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


