Abstract
Nutcracker syndrome is a rare anomaly resulting from compression of the left renal vein between the aorta and the superior mesenteric artery. Open and endovascular interventions have both been performed to relieve the compression. Each of these interventions has strengths and weaknesses. We report two patients in whom a hybrid approach was adopted in the process combining the strengths of each intervention while reducing potential complications.
Nutcracker syndrome results from compression of the left renal vein (LRV) between the aorta and the superior mesenteric artery (SMA). While traditionally treated with open surgery, the last several years have seen increased utilization of an endovascular approach through stenting of the LRV. Both approaches have advantages and drawbacks. In this case report, we describe a hybrid technique that combines the strengths while minimizing the shortcomings of the two approaches.
Case report
Both patients described below gave consent for publication of their data.
Patient 1
A 58-year-old woman presented with severe pelvic pain and abdominal fullness of a few years' duration. She had a brief episode of hematuria in the past. Bleeding from the left ureter was noted on cystoscopy. Computed tomography (CT) venography demonstrated a markedly compressed LRV of 2 mm, and an aortomesenteric angle of 9° (normal >41°1; Fig 1, A). A duplex scan revealed LRV flow velocity at the aortomesenteric window (AMW) of 30 cm/s and at the hilum of 14 cm/s (ratio of 2.1). The left ovarian vein, which measured 8 mm in size, had reversed flow. A diagnosis of nutcracker syndrome and pelvic congestion syndrome was made, and the patient agreed with the surgical plan.
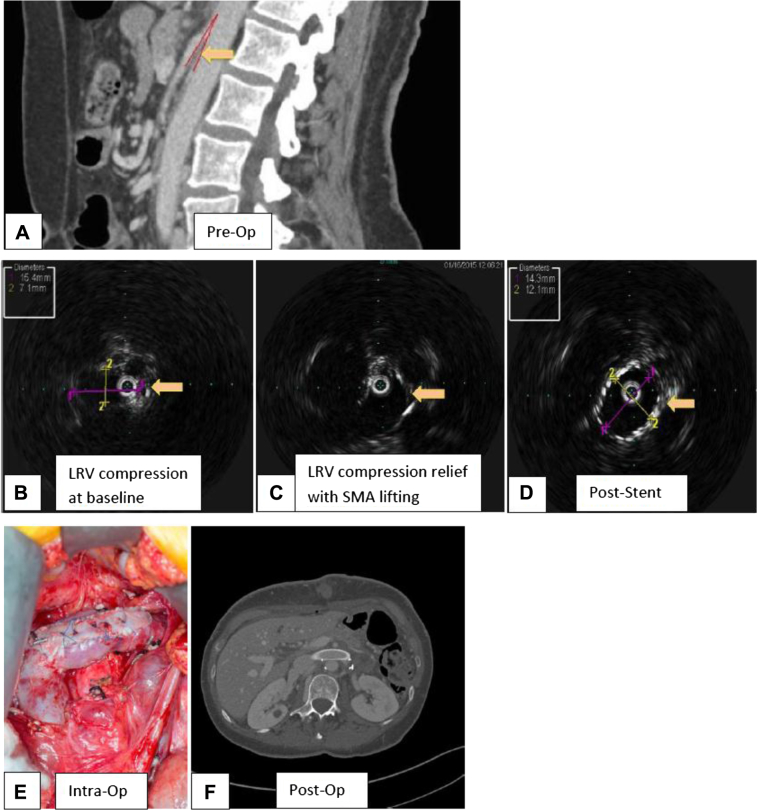
Fig 1.
Patient 1. A, Preoperative computed tomography (CT) venogram (sagittal view) demonstrates compression of the left renal vein (LRV; arrow) in the aortomesenteric window (AMW) and persistent narrowing of the AMW several centimeters distal to the superior mesenteric artery (SMA) origin with consequent risk of compression of the distally transposed LRV. B, Intravascular ultrasound (IVUS) demonstrates LRV compression (arrow) intraoperatively. C, IVUS demonstrates LRV compression relief (arrow) after lifting of the small bowel (SMA). D, IVUS demonstrates a widely patent LRV stent intraoperatively (arrow). E, Intraoperative picture demonstrates the transposed, patched LRV with intraluminal stent. F, Postoperative CT venogram demonstrates a widely patent LRV stent.
Under general anesthesia, the right great saphenous vein was dissected at the groin through a 3-cm-long oblique incision. A 12-cm upper midline minilaparotomy was made just proximal to the umbilicus and the LRV and the inferior vena cava (IVC) exposed by division of the posterior retroperitoneum between the inferior mesenteric vein and duodenum.
A right transfemoral venogram confirmed the LRV stenosis and the reflux through the left ovarian vein. Intravascular ultrasound (Visions PV .035 Digital IVUS Catheter; Volcano Corp, San Diego, Calif) showed the area of the compressed LRV that opened up after we lifted the overlying small bowel with the SMA (Fig 1, B and C). Intravenous unfractionated heparin (5000 units) was given, and the activated clotting time was maintained at >220 seconds. The adrenal vein was double ligated and divided. Double ligation and division of the refluxing incompetent left ovarian vein was performed with excision of an 8-cm segment of the vein. A side-biting clamp was used to partially occlude the IVC. The distal LRV was clamped, and the LRV was excised from the IVC with a 1-mm to 2-mm rim. The IVC was opened more distally, and the LRV was reimplanted about 15 mm distal to its original position using running 5-0 Prolene sutures (Ethicon, Somerville, NJ). The proximal IVC was closed using running 5-0 Prolene sutures. The LRV was enlarged with a 6-cm-long, 8-mm-wide saphenous vein patch using 6-0 Prolene sutures extending the tip of the patch into the IVC. Circulation was re-established.
Intraoperative stenting of the LRV was then performed via the right common femoral vein using an 18-mm × 40-mm Wallstent (Boston Scientific, Marlborough, Mass) to allow 3-mm to 4-mm protrusion into the IVC. Angioplasty with a 14-mm × 40-mm Pacific Plus angioplasty balloon (Medtronic, Minneapolis, Minn) and subsequently with a 16-mm × 40-mm balloon was performed to eliminate the residual waist. Repeat intravascular ultrasound (IVUS) imaging demonstrated a widely patent LRV with excellent flow (Fig 1, D). Transfixation of the Wallstent to the transposed LRV to prevent migration was performed using multiple interrupted 5-0 Prolene sutures (Fig 1, E). Venogram and IVUS imaging confirmed the widely patent stent.
Low-dose perioperative low-molecular-weight heparin was changed to full anticoagulation with enoxaparin at day 2, followed by warfarin anticoagulation with a goal international normalized ratio of 2 to 3 for a total of 3 months.
The patient's postoperative course was uneventful, and a CT angiogram at 3 months demonstrated a widely patent stented and transposed LRV (Fig 1, F). At the 4-month follow-up, she was clinically doing better, with improved pelvic pain.
Patient 2
A 59-year-old woman was referred to us with hematuria of 35 years' duration. Although initially hematuria occurred occasionally, within the last year it increased in frequency to almost every bout of voiding. An examination found no abdominal wall, perineal, or lower extremity varicosities. Bleeding from the left ureter was noted on cystoscopy. CT venography demonstrated a markedly compressed LRV of 2 mm (Fig 2, A), and an aortomesenteric angle of 4.9°. Venous duplex imaging at the AMW revealed a LRV flow velocity of 47 cm/s and at the hilum of 10 cm/, corresponding to a ratio of 4.7. On the basis of these findings, the diagnosis of nutcracker syndrome was made with a plan for intervention.
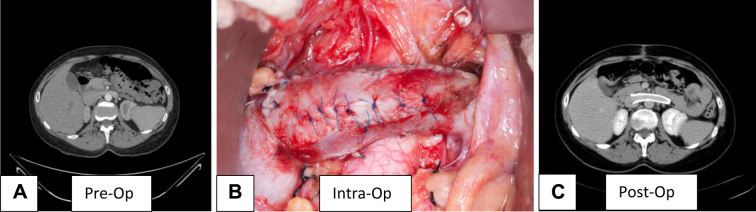
Fig 2.
Patient 2. A, Preoperative computed tomography (CT) venogram demonstrates compression of the left renal vein (LRV). B, Intraoperative picture demonstrates the transposed, patched LRV with an intraluminal stent. C, Postoperative CT venogram demonstrates a widely patent LRV stent.
The operation was performed under general anesthesia and in a manner similar to what was noted for patient 1, with transfixation of the Wallstent to the transposed vein (Fig 2, B). Anticoagulation in the postoperative period was with prophylactic heparin administered subcutaneously while in the hospital, and aspirin and Xarelto (Bayer HealthCare AG, Leverkusen, Germany) as an outpatient for 3 months.
The patient's postoperative course was uneventful, and a CT venogram at 4 months showed a widely patent LRV (Fig 2, C). Her only symptom, hematuria, decreased significantly but was not completely cured at 4 months.
Discussion
The diagnosis of nutcracker syndrome, which usually presents with flank pain and hematuria, is made using a combination of modalities, including CT venogram, renal vein duplex imaging, and left renal venogram. The diagnostic criteria include bird beak sign, defined as compression of the LRV in the AMW, decreased aortomesenteric angle (normal >41°), decreased diameter ratio of the LRV in the AMW to the LRV at the hilum (normal, <1:5), increased velocity ratio of LRV in the AMW to the LRV at the hilum (normal, <5:1), presence of collateralization around the proximal left renal vein with or without reflux down the ovarian vein, and a pressure gradient of >1 mm Hg across the LRV.1 The use of IVUS imaging in the diagnosis of nutcracker syndrome intraoperatively has not been performed previously. We report the first instance of use of IVUS to make the diagnosis intraoperatively and assess repair after stenting.
The most frequent treatment options include open surgery with transposition of the LRV2 distally into the IVC or an endovascular approach with stenting of the LRV.3, 4 Open repair may result in anastomotic restenosis or persistent compression of the transposed vein. In our recently reported experience with 34 operated-on patients, 11 (32%) required a vascular reintervention.5 This included open revisions in three and endovascular intervention in the remainder. Stenting presents the potential risk of migration, fracture, or, rarely, in-stent thrombosis. Instances of stent migration into the IVC4, 6, 7 or heart3, 8, 9, 10 have been reported requiring endovascular procedures, open cavotomy, or sternotomy to retrieve the stent.
A hybrid technique using a combination of open repair and stenting combines the strengths of the two approaches while reducing potential complications. The strengths of the distal LRV transposition and patch angioplasty are the removal of the vein from the position of external compression and enlargement of the vein with a patch to assure good outflow. The strength of stenting is to expand the vein to better resist any external compression that may persist. The weakness of the open procedure is restenosis due to persistent external pressure by the distal SMA or by the tension cause by the aorta on the posterior wall of the transplanted vein, or restenosis due to intimal hyperplasia or thrombosis. The greatest weakness of currently used stents has been migration.
Transposition of the LRV with placement of a patch allows placement of a large and short Wallstent (abutting for 3 to 4 mm into the IVC; Fig 3, A-C). Significant oversizing, however, is not recommended because that prevents complete expansion of the stent, leading to decreased resistance to external compression and increased risk of protrusion of the stent into the IVC. Additional placement of sutures into the stent prevents migration.
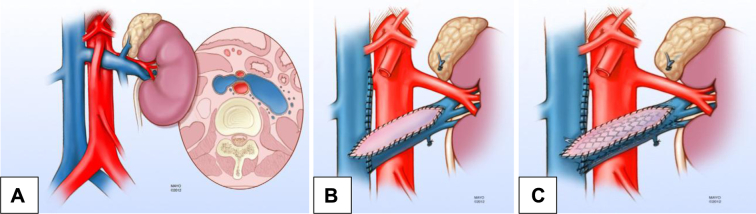
Fig 3.
Illustration of the hybrid repair (reproduced with permission from the Mayo Foundation). A, Left renal vein (LRV) compression by the superior mesenteric artery (SMA; nutcracker anatomy). B, The LVR has been transposed and patched with great saphenous vein. C, Stenting of the transposed and patched LRV using a Wallstent (Boston Scientific, Marlborough, Mass). Reproduced by permission of the Mayo Foundation for Medical Education and Research. All rights reserved. The Mayo Clinic does not endorse specific products, advertisers, or services included in this article.
Conclusions
Until reliable stents with a structure that prevents migration into the IVC are available, a hybrid approach of LRV transposition with patch angioplasty and stenting as a one-stage procedure can be successful in patients with nutcracker syndrome. Longer follow-up in more patients is necessary to confirm the safety, efficacy, and durability of this hybrid intervention.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Kim K.W., Cho J.Y., Kim S.H., Kim S.H., Yoon J.H., Kim D.S. Diagnostic value of computed tomographic findings of nutcracker syndrome: correlation with renal venography and renocaval pressure gradients. Eur J Radiol. 2011;80:648–654. doi: 10.1016/j.ejrad.2010.08.044. [DOI] [PubMed] [Google Scholar]
- 2.Reed N.R., Kalra M., Bower T.C., Vrtiska T.J., Ricotta J.J., 2nd, Gloviczki P. Left renal vein transposition for nutcracker syndrome. J Vasc Surg. 2009;49:386–393. doi: 10.1016/j.jvs.2008.09.051. [DOI] [PubMed] [Google Scholar]
- 3.Chen S., Zhang H., Shi H., Tian L., Jin W., Li M. Endovascular stenting for treatment of nutcracker syndrome: report of 61 cases with long-term followup. J Urol. 2011;186:570–575. doi: 10.1016/j.juro.2011.03.135. [DOI] [PubMed] [Google Scholar]
- 4.Wang X., Zhang Y., Li C., Zhang H. Results of endovascular treatment for patients with nutcracker syndrome. J Vasc Surg. 2012;56:142–148. doi: 10.1016/j.jvs.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Erben Y., Gloviczki P., Kalra M., Bjarnason H., Reed N.R, Duncan A.A. Treatment of nutcracker syndrome with open and endovascular interventions. J Vasc Surg Venous Lymphat Disord. 2015;3:389–396. doi: 10.1016/j.jvsv.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Rana M.A., Oderich G.S., Bjarnason H. Endovenous removal of dislodged left renal vein stent in a patient with nutcracker syndrome. Semin Vasc Surg. 2013;26:43–47. doi: 10.1053/j.semvascsurg.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Hartung O., Grisoli D., Boufi M., Marani I., Hakam Z., Barthelemy P. Endovascular stenting in the treatment of pelvic vein congestion caused by nutcracker syndrome: lessons learned from the first five cases. J Vasc Surg. 2005;42:275–280. doi: 10.1016/j.jvs.2005.03.052. [DOI] [PubMed] [Google Scholar]
- 8.Chen S., Zhang H., Tian L., Li M., Zhou M., Wang Z. A stranger in the heart: LRV stent migration. Int Urol Nephrol. 2009;41:427–430. doi: 10.1007/s11255-008-9478-9. [DOI] [PubMed] [Google Scholar]
- 9.Zhang H., Li M., Jin W., San P., Xu P., Pan S. The left renal entrapment syndrome: diagnosis and treatment. Ann Vasc Surg. 2007;21:198–203. doi: 10.1016/j.avsg.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y., Mou Y., Cheng Y., Wang H., Zheng Z. Late stent migration into the right ventricle in a patient with nutcracker syndrome. Ann Vasc Surg. 2015;29:839.e1–839.e4. doi: 10.1016/j.avsg.2014.12.003. [DOI] [PubMed] [Google Scholar]