Key Points
Peg-IFNα is tolerated and induces disease response in patients who relapse after allogeneic SCT.
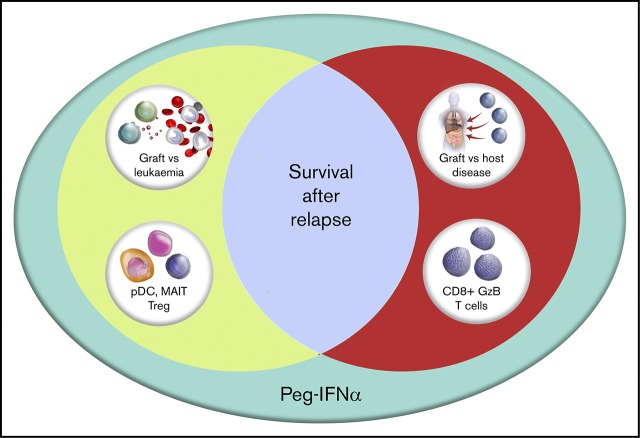
Increased pretreatment MAIT and pDC proportions were associated with better progression-free and overall survival after peg-IFNα treatment.
Abstract
Allogeneic stem cell transplantation (SCT) is a curative therapy for patients with hematological malignancies related largely to an immunological graft-versus-leukemia (GVL) effect mediated by donor T cells and natural killer cells. Relapse of disease after SCT represents failure of GVL and is now the major cause of treatment failure. We sought to augment GVL effects in patients (n = 29) relapsing after SCT in a prospective phase I/II clinical trial of dose-escalated pegylated interferon-2α (peg-IFNα). The administration of peg-IFNα after reinduction chemotherapy, with or without subsequent donor lymphocyte infusion (DLI), resulted in a 2-year overall survival (OS) of 31% (95% confidence interval, 17.3%-49.2%), which rejects the null hypothesis of 7% generated by observations in an institutional historical cohort. As expected, peg-IFNα was associated with graft-versus-host disease (GVHD) and hematological toxicity, which was manageable with scheduled dose modifications. Progression-free survival (PFS) was greatest in patients who experienced GVHD, although the majority of those patients still eventually progressed. Higher PFS and OS were associated with pretreatment proportions of immune cell populations with regulatory function, including mucosal invariant T cells, regulatory T cells, and plasmacytoid dendritic cells, independent of any association with GVHD. Peg-IFNα administration after relapse thus constitutes a logical strategy to invoke GVL effects and should be studied in a larger, multicenter cohort. This trial was registered at www.anzctr.org.au as #ACTRN12612000728831.
Visual Abstract
Introduction
Allogeneic hematopoietic stem cell transplantation (SCT) offers a cure for patients with high-risk hematological malignancies; however, relapse of primary disease remains the major cause of treatment failure and confers a dismal prognosis for survival. Center for International Blood and Marrow Transplant Research data show that beyond 100 days after transplantation, relapse is the cause of death in 58% of recipients of HLA-matched sibling transplants and 47% of unrelated donor transplant recipients.1 Aside from novel and targeted therapies, a core principle in treatment of relapse after SCT is to promote immunological graft-versus-leukemia (GVL) effects. Cessation of posttransplant immune suppression and donor lymphocyte infusion (DLI) are often employed in patients without graft-versus-host disease (GVHD) or a second transplant.2 Reinduction chemotherapy causes cytoreduction of malignant cells but is also highly immunosuppressive.3,4 Despite these strategies, long-term survival after relapse remains the exception rather than the rule. At our institution a historic control cohort treated with a combination of cytoreduction alone or cytoreduction followed by DLI did not result in differential survival between groups, and outcomes were universally poor, with 2-year overall survival of <10%.5 Experience in other institutions6 is similar, highlighting the need for alternative treatments.
Preclinical work carried out by Robb et al7 identified type I interferons (IFNs) as important mediators of both GVL and GVHD, with protection from GVHD effected through reduction of Th1- and Th17-mediated effects in the gastrointestinal tract and augmentation of GVL through CD8+ T cells and natural killer (NK) cells. Type I IFNs are established therapies for treatment of myeloproliferative neoplasms8 and can reverse senescence in hematopoietic and leukemic stem cells.9 Type I IFNs have been used in other settings after allogeneic stem cell transplantation, including in combination with donor lymphocyte infusions for chronic myeloid leukemia and in minimal residual disease states.10-12 Other case reports13 and small series14 have provided clinical grounds for prospective evaluation of IFN in the context of relapse after transplantation; however, use of standard type I IFN preparations have resulted in significant toxicity.15 Given this mechanistic and existing clinical rationale, in addition to availability of a pegylated product (pegylated IFNα [peg-IFNα]) with favorable pharmacokinetic and toxicity profiles, we hypothesized that peg-IFNα would invoke clinically meaningful GVL effects in patients relapsing after SCT.
Methods
A single-arm phase I/II trial was performed at the Royal Brisbane and Women’s Hospital after institutional ethics approval. The study protocol is available in the supplemental Material. Adult patients with relapse of any disease after allogeneic SCT were eligible; 29 patients were needed and recruited, such that the study was powered at 80% to detect an improvement in 2-year overall survival (OS) from a historical institutional level of 7%5 (null hypothesis) with a 2-sided α value of 5%, where the alternate hypothesis was a 2-year OS of 40%. Patients received cytoreduction, typically with fludarabine, cytosine arabinoside, and G-CSF (FLAG),16 followed by addition of a weekly escalating dose of peg-IFNα (Pegasys; Roche) from day 21 or at white blood cell and platelet recovery, in the absence of grades II to IV acute GVHD or progressive/extensive stage chronic GHVD. The initial dose of peg-IFNα was 45 µg, escalating weekly as tolerated, up to 180 µg for 6 months, with dose adjustment in the setting of expected side effects and adverse effects, including cytopenias and GVHD. Systemic steroid (1 mg/kg) was permitted for treatment of GVHD. In the absence of GVHD at 6 weeks, patients were permitted concurrent, monthly DLI in a dose-escalating fashion. Standard institutional, antimicrobial prophylaxis was provided. Details are available in the trial protocol in the supplemental Material.
Secondary end points included progression-free survival (PFS), adverse event (AE), and safety profile and incidence of GVHD.17 PFS was defined as first dose of peg-IFNα to relapse, and OS was defined as first dose to death. Correlative studies included enumeration and functional characterization of immune cell populations, including T, B, dendritic, NK, and mucosal-associated invariant T (MAIT)–cell populations. Kaplan-Meier survival analysis was performed with Prism 7 for Windows (version 7.02; 13 September 2016). Generalized logistic regression and Cox proportional hazard analysis were performed with JMP Pro 14.0.0 (SAS Institute).
Results
Patient characteristics are described in Table 1. Mean age was 46 years, and 66% were male. Primary diseases included acute myeloid leukemia (45%), myelodysplastic syndrome (21%), and acute lymphoblastic leukemia (17%), with myeloproliferative neoplasm and Hodgkin and non-Hodgkin lymphomas, comprising the most of the remainder. Forty-five percent of patients had received a sibling donor transplant, and the remainder volunteer unrelated-donor transplants, including 6 receiving a graft with some degree of mismatch. Some of the patients (58%) were conditioned with fludarabine and melphalan and the remainder with cyclophosphamide and total body irradiation. The average time from SCT to study entry was 1.89 years (range, 0.35-11.6).
Table 1.
Patient characteristics
| Characteristic (n = 29) | Data |
|---|---|
| Sex, n (%) | |
| Female | 10 (34.5) |
| Male | 19 (65.5) |
| Age (mean), y | 20-66 (46.3) |
| Primary disease, n (%) | |
| AML | 13 (44.9) |
| ALL | 5 (17.3) |
| CML | 1 (3.4) |
| MDS | 6 (20.8) |
| HL | 1 (3.4) |
| MF | 1 (3.4) |
| NHL | 1 (3.4) |
| PMF | 1 (3.4) |
| Time to trial entry post HPCT (mean), y | 0.35-11.6 (1.89) |
| <6 mo, n (%) | 6 (20.7) |
| 6-12 mo, n (%) | 6 (20.7) |
| >12 mo, n (%) | 17 (58.6) |
| Source, n (%) | |
| SIB | 13 (45.0) |
| VUD | 16 (55.0) |
| Conditioning, n (%) | |
| Cy TBI | 12 (42.0) |
| Flu Mel | 17 (58.0) |
AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; CML, chronic myeloid leukemia; Cy TBI, cyclophosphamide plus total body irradiation; Flu Mel, fludarabine plus melphalan; HL, Hodgkin lymphoma; MDS, myelodysplastic syndrome; MF, mycosis fungoides; NHL, non-Hodgkin lymphoma; PMF, primary myelofibrosis; SIB, sibling; VUD, volunteer unrelated donor.
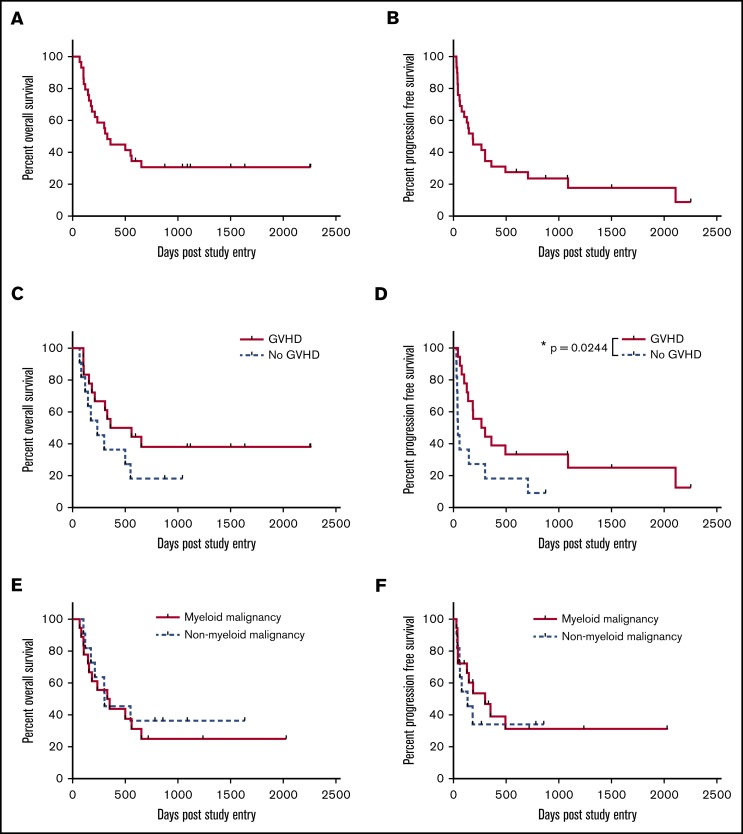
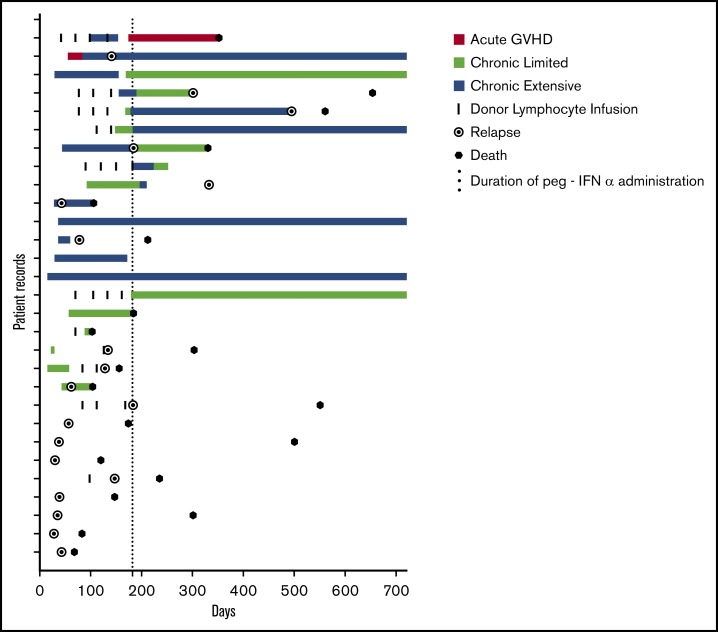
The 2-year OS was 31%, with a median survival of 330 days at a median follow-up of 1119 days (Figure 1A). This result successfully3 rejects the null hypothesis (P < .0001; 95% CI 17.3%-48.2%). The 2-year PFS was 24% (median, 184 days) at a median follow-up of 1502 days (Figure 1B). Eighteen patients developed GVHD, and OS at 2 years for those patients was 38% (Figure 1C), with PFS of 33%, which is significantly better relative to those patients who did not develop GVHD (P = .024; median survival GVHD 284 days vs no GVHD 43 days; Figure 1D). The median time to relapse for patients with GVHD was 187 days and for those without GVHD was 43 days. Thus, patients who relapsed, did so with aggressive disease that was unlikely to be permissive of adequate time in which to generate a meaningful GVHD-associated GVL effect (Figure 2). Figure 2 depicts the chronology of GVHD and relapse after peg-IFNα treatment.
Figure 1.
Survival. (A) Median OS, 330 days, and 2-year 31% OS at median follow-up of 1119 days. (B) Median PFS, 184 days, and 2-year 24% PFS at median follow-up of 1502 days. (C) OS stratified by occurrence of GVHD after treatment with peg-IFNα. P = .166. Median survival 460 days for GVHD and 235 for no GVHD. (D) PFS stratified by occurrence of GVHD after treatment with peg-IFNα. P = .024. Median PFS 283 days for GVHD and 43 for no GVHD. (E) OS stratified by myeloid or nonmyeloid malignancies. P = .6226. Median survival 341 days for myeloid disease and 303 for nonmyeloid. (F) PFS stratified by disease as for panel E. P = .5999. Median survival 301 days for myeloid and 134 for nonmyeloid disease. Kaplan-Meier survival curves, with P values calculated using the log-rank (Mantel-Cox) test. *P < .05.
Figure 2.
GVHD. Swimmer plot demonstrating the development of GVHD during the trial. Onset, duration, and classification of GVHD are depicted in addition to episodes of DLI for each patient on trial. The 6-month period of treatment with peg-IFN is indicated by the dotted line. Relapse and death are indicated if they occurred during the 2-year period depicted.
Adverse events (AEs) of only grade 3 or greater18 were reported that were related to predictable complications in this heavily pretreated population. Hematological toxicity after FLAG was similarly not reported, and hematological toxicity secondary to peg-IFNα was managed by dose adjustment as per protocol. The average duration of therapy was 13 weeks (range, 2-27 weeks; median, 12.5). Only 4 patients tolerated peg-IFNα at maximum dose intensity for the study duration; the remainder required dose reduction or interruption. Cytopenias were the most common indication for dose adjustment, followed by GVHD. Eleven patients (38%) experienced AEs; 2 were fatal: 1 death secondary to sepsis and 1 to GVHD. Seven AEs (63%) were graded as severe (SAEs). Only 4 of the AEs and SAEs were considered to be directly linked to peg-IFNα. GVHD was the most common SAE directly linked to peg-IFNα. Other AEs and SAEs with possible links included fever (n = 4), infection (n = 7), pancreatitis (n = 1), and hemolysis (n = 1). The 7 infectious complications included 3 episodes of lower respiratory tract infection, 1 episode of fatal septicemia, 1 soft tissue infection, and 1 combined Clostridium difficile and Cryptosporidium infection. One patient had reactivation of CMV requiring therapy. AEs thought to be unrelated to peg-IFNα included fever (n = 4), headache (n = 1), and pain (n = 2). Viral infection including parainfluenza 3, norovirus, respiratory syncytial virus, and adenovirus occurred in patients while on peg-IFN, but those infections were not judged to be directly related to therapy. Proven invasive fungal infection was not seen, but 1 patient was treated empirically for such an infection; however, Aspergillus galactomannan (Ag) was not detected on bronchoalveolar lavage. A dose-adjustment schedule was provided for psychiatric AEs related to peg-IFN administration; however, dose adjustment or interruption for this class of AE was not required during the trial.
GVHD occurred in 18 patients (62%). Onset, duration, and classification of GVHD are depicted in Figure 2. Fifteen patients had experienced GVHD before study entry, and 8 of those (53%) experienced GVHD on trial. Of the 14 patients who had not had any GVHD before study entry, 10 (71%) developed GVHD during the trial. GVHD occurred at a median of 43.5 days (range, 15-168) after the first dose of peg-IFN. Only 2 patients (7%) developed acute GVHD, and 1 succumbed to this SAE in the context of disease relapse. Of the 16 patients who developed chronic GVHD, the majority had extensive-stage disease (n = 11; 69%), with the remainder having only limited stage (n = 5; 31%). The skin was the most commonly involved organ (n = 16; 55%), and the liver was also seen to be frequently involved, (n = 13; 45%), with infrequent involvement of the gut (n = 2; 7%) and lung (n = 1; 3%). Two thirds of patients who developed extensive chronic GVHD responded to dose adjustment of peg-IFNα and steroid therapy, with resolution to limited-stage disease in 8 patients (72%). Comparable rates of GVHD were seen in patients treated with peg-IFN who had not experienced GVHD after their primary transplant (10 of 14; 71%) compared with patients who had experienced GVHD after their primary transplant (8 of 15; 53%). Eleven patients were eligible for and received DLI; of those, 5 (45%) subsequently developed grade II or more severe GVHD. Twenty-three of 29 patients had disease identifiable by a minimal residual disease (MRD) target by flow cytometry, cytogenesis, fluorescence in situ hybridization, or polymerase chain reaction. Of these patients, 12 (41%) had residual disease after reinduction chemotherapy, and disease responses to peg-IFNα were noted in 6 patients (50%), including 3 (25%) who became MRD negative. These observations are consistent with the induction of a GVL effect by peg-IFN. There was no significant difference in OS or PFS for the 23 patients with an MRD marker if outcomes were stratified by MRD positivity or MRD negativity after FLAG chemotherapy (OS: MRD+ median survival 235 days, MRD− median survival 315.5 days, P = .5072; PFS: MRD+ median survival 134 days, MRD− median survival 156 days, P = .6731).
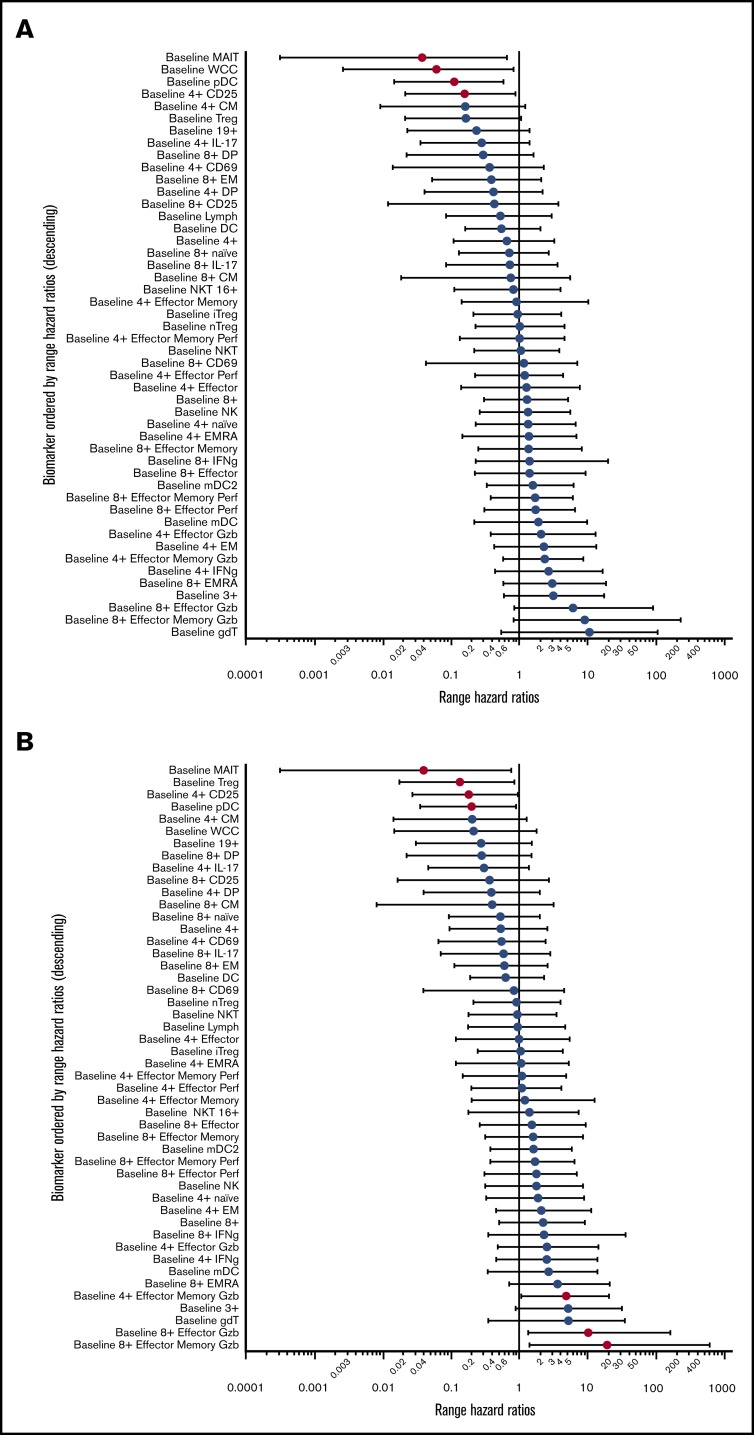
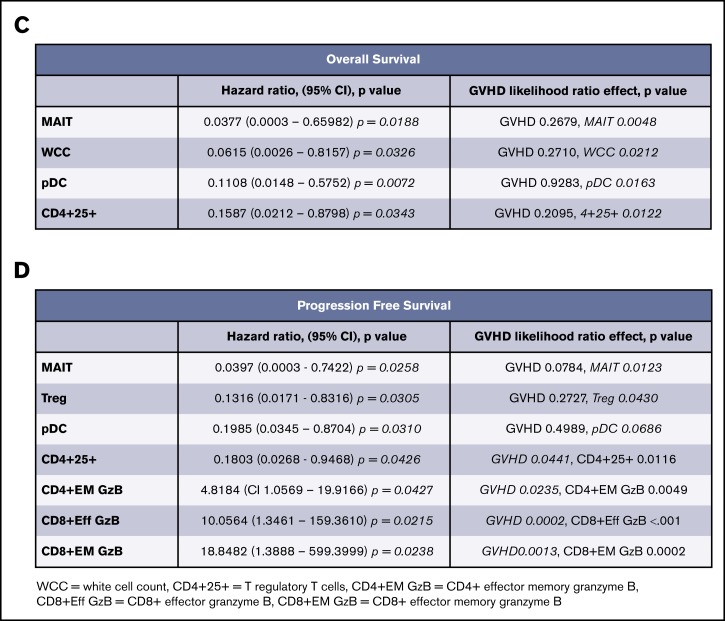
We sought to determine predictors for response through enumeration and functional characterization of immune cell populations. Peripheral blood was collected before treatment and at 30-day intervals until the end of therapy. Analysis of hazard ratios demonstrated that increased pretreatment proportions of MAIT cells and plasmacytoid dendritic cells (pDCs) conferred improved PFS and OS (Figure 3A-B). These hazard ratios retained significance, independent of the development of GVHD. In addition, the proportions of Treg (CD4+CD25+CD127lo) and total white cell count had independent predictive hazard ratios for PFS or OS, respectively, and the proportion of all CD4+ T cells expressing CD25 had prognostic value for OS that was independent of GVHD. An interval increase in values of MAITs, pDCs, and CD25+CD4+ T cells from days 0 to 30 of treatment (data not shown) were also predictive of improved survival, independent of GVHD. In contrast, the negative prognostic impact of increased pretreatment proportions of conventional T-cell subsets (CD4+ effector memory, CD8+ effector, and effector memory granzyme+ populations) displayed significant interaction with GVHD (Figure 3C-D), consistent with the induction of a mixed GVL/GVHD effect. Although the biomarkers associated with development of GVHD displayed significant correlation with each other, the values for pDCs, MAITs, and Tregs were mutually exclusive of each other in variable clustering analysis.
Figure 3.
Logistic regression for biomarkers. (A) Range hazard ratios and confidence intervals for OS and baseline proportions of immune biomarkers before therapy with peg-IFNα. Those with P < .05 are marked in red. (B) Range hazard ratios for PFS as for panel A. (C) Summary table including significant range hazard ratios for biomarkers associated with OS and interaction with GVHD. Biomarkers with a nonsignificant P value for GVHD are predictive of outcome independent of GVHD, and those with a significant P value for GVHD display significant interaction with this clinical outcome. (D) Summary table for PFS as for panel C. Generalized logistic regression and Cox proportional hazard analysis were performed with JMP Pro 14.0.0 (SAS Institute).
Discussion
Our results demonstrate the efficacy of peg-IFNα as an adjunctive therapy for relapse after SCT. Peg-IFNα was delivered with predictable and manageable toxicity. The development of GVHD was associated with improved PFS, although GVL effects were not clearly separable from GVHD. The GVHD induced was predominantly chronic. Notably, GVHD was seen in a number of patients who had not developed it after primary transplant. The disease responses seen, including MRD negativity after administration of peg-IFN, support the clinical activity of peg-IFN in amplification of GVL effects. Although a clinically meaningful improvement, the 31% 2-year OS suggests only subsets of patient benefit. Pretreatment proportions of MAIT cells and pDCs appear to be novel biomarkers for response to peg-IFNα in this dataset and should be explored in subsequent studies. pDCs are known to promote tolerance via Treg expansion,19 whereas MAIT cells enhance gastrointestinal tract barrier function and microbiome diversity and inhibit alloantigen presentation after SCT.20,21 To our knowledge, neither population has been linked to GVL effects previously. It is possible that temporal modulation of these innate cell populations can modify leukemia and alloantigen presentation in a way that favors GVL over GVHD. We observed agnostic responses with respect to myeloid and lymphoid malignancies and concluded that peg-IFNα may be useful in sequence with other therapies. Contextually, cellular therapies for patients with lymphoid malignancies and small-molecule inhibitors, including FLT-3 and IDH, for myeloid malignancies are not mutually exclusive of concurrent use of peg-IFNα, such that the effects may be additive. In summary, we have demonstrated that the use of peg-IFNα in relapsed disease after SCT is associated with disease responses and can result in long-term survival in a subset of patients.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
This study was supported by a QLD Health Senior Research Fellowship and a National Health Medical and Research Council Australia Fellowship (G.R.H.), and by a Leukaemia Foundation clinical scholarship, the Royal Brisbane and Women’s Hospital Foundation, and a Haematology Society of Australia and New Zealand New Investigator Scholarship (A.S.H.).
Authorship
Contribution: G.R.H. developed the concept; G.K. and G.R.H. developed the methodology; A.S.H., A.V., S.O., and L.S. conducted the investigations; G.H. provided statistical analysis; S.D., J.B., A.J.M., A.M., S.-K.T., E.S., C.C., G.K., and G.R.H. participated in patient care; J.L., E.S., J.A., and J.K. provided trial support; A.S.H. conducted formal data analysis and wrote the original draft; and G.R.H. reviewed and edited the final draft and supervised the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andrea S. Henden, Department of Haematology and Bone Marrow Transplantation, Cancer Care Services, Royal Brisbane and Women’s Hospital, Butterfield St, Herston, QLD 4029, Australia; e-mail: andrea.henden@health.qld.gov.au; and Geoffrey R. Hill, Clinical Research Division, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave, Seattle, WA 98109; e-mail: grhill@fredhutch.org.
References
- 1.D’Souza A, Fretham C. Current Uses and Outcomes of Hematopoietic Cell Transplantation (HCT): Summary CBIMTR Slides. Milwaukee, WI: Center for International Blood and Marrow Transplant Research; (CIBMTR); 2017. [Google Scholar]
- 2.Apperley J, Carreras E, Gluckman E, Masszi T, eds. The EBMT Handbook, 6th ed London, United Kingdom: Springer; 2012. [Google Scholar]
- 3.Schietinger A, Delrow JJ, Basom RS, Blattman JN, Greenberg PD. Rescued tolerant CD8 T cells are preprogrammed to reestablish the tolerant state. Science. 2012;335(6069):723-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maury S, Lemoine FM, Hicheri Y, et al. . CD4+CD25+ regulatory T cell depletion improves the graft-versus-tumor effect of donor lymphocytes after allogeneic hematopoietic stem cell transplantation. Sci Transl Med. 2010;2(41):41ra52. [DOI] [PubMed] [Google Scholar]
- 5.Curley C, Hill GR, McLean A, Kennedy GA. Immunotherapy following relapse of acute leukaemia after T-cell-replete allogeneic peripheral blood progenitor cell transplantation: importance of new onset chronic graft-versus-host disease. Int J Lab Hematol. 2014;36(2):197-204. [DOI] [PubMed] [Google Scholar]
- 6.Fathi AT, Chen YB. Treatment of relapse of acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation. Curr Hematol Malig Rep. 2014;9(2):186-192. [DOI] [PubMed] [Google Scholar]
- 7.Robb RJ, Kreijveld E, Kuns RD, et al. . Type I-IFNs control GVHD and GVL responses after transplantation. Blood. 2011;118(12):3399-3409. [DOI] [PubMed] [Google Scholar]
- 8.Talpaz M, Mercer J, Hehlmann R. The interferon-alpha revival in CML. Ann Hematol. 2015;94(suppl 2):195-207. [DOI] [PubMed] [Google Scholar]
- 9.Essers MA, Offner S, Blanco-Bose WE, et al. . IFNalpha activates dormant haematopoietic stem cells in vivo. Nature. 2009;458(7240):904-908. [DOI] [PubMed] [Google Scholar]
- 10.Mo XD, Zhang XH, Xu LP, et al. . IFN-α Is Effective for Treatment of Minimal Residual Disease in Patients with Acute Leukemia after Allogeneic Hematopoietic Stem Cell Transplantation: Results of a Registry Study. Biol Blood Marrow Transplant. 2017;23(8):1303-1310. [DOI] [PubMed] [Google Scholar]
- 11.Bezerra ED, Flowers ME, Onstad LE, Chielens D, Radich J, Higano CS. A phase 2 study of alpha interferon for molecularly measurable residual disease in chronic myeloid leukemia after allogeneic hematopoietic cell transplantation [published online ahead of print 24 April 2019]. Leuk Lymphoma. doi:10.1080/10428194.2019.1605508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin XJ, Dai HP, Wang AJ, et al. . Effects of preemptive interferon-α monotherapy in acute leukemia patients with relapse tendency after allogeneic hematopoietic stem cell transplantation: a case-control study. Ann Hematol. 2018;97(11):2195-2204. [DOI] [PubMed] [Google Scholar]
- 13.Volodin L, Peddi P, Takalkar A, Comeau JM, Shahan JL, Hildebrandt GC. Successful treatment of first and second recurrence of acute lymphoblastic leukemia after related allogeneic bone marrow transplant at unusual sites using single-dose vincristine followed by interferon-α2b and granulocyte-macrophage colony-stimulating factor. Leuk Lymphoma. 2013;54(5):1107-1109. [DOI] [PubMed] [Google Scholar]
- 14.Arellano ML, Langston A, Winton E, Flowers CR, Waller EK. Treatment of relapsed acute leukemia after allogeneic transplantation: a single center experience. Biol Blood Marrow Transplant. 2007;13(1):116-123. [DOI] [PubMed] [Google Scholar]
- 15.Cooper N, Rao K, Goulden N, Amrolia P, Veys P. Alpha interferon augments the graft-versus-leukaemia effect of second stem cell transplants and donor lymphocyte infusions in high-risk paediatric leukaemias. Br J Haematol. 2012;156(4):550-552. [DOI] [PubMed] [Google Scholar]
- 16.Jackson G, Taylor P, Smith GM, et al. . A multicentre, open, non-comparative phase II study of a combination of fludarabine phosphate, cytarabine and granulocyte colony-stimulating factor in relapsed and refractory acute myeloid leukaemia and de novo refractory anaemia with excess of blasts in transformation. Br J Haematol. 2001;112(1):127-137. [DOI] [PubMed] [Google Scholar]
- 17.Glucksberg H, Storb R, Fefer A, et al. . Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18(4):295-304. [DOI] [PubMed] [Google Scholar]
- 18.Common Terminology Criteria for Adverse Events (CTCAE), version 4.03. Bethesda, MD: U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute; NIH publication no. 09-5410; 2009, revised June 2010. [Google Scholar]
- 19.Ochando JC, Homma C, Yang Y, et al. . Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol. 2006;7(6):652-662. [DOI] [PubMed] [Google Scholar]
- 20.Kawaguchi K, Umeda K, Hiejima E, et al. . Influence of post-transplant mucosal-associated invariant T cell recovery on the development of acute graft-versus-host disease in allogeneic bone marrow transplantation. Int J Hematol. 2018;108(1):66-75. [DOI] [PubMed] [Google Scholar]
- 21.Varelias A, Gartlan KH, Wilkinson AN, et al. . Expansion of IL-17A-secreting CD8+ mucosa-associated invariant T cells in peripheral blood following stem cell mobilization. Blood Adv. 2019;3(5):718-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.